Abstract
Current thinking views mild head impact (i.e., subconcussion) as an underrecognized phenomenon that has the ability to cause significant current and future detrimental neurological effects. Repeated mild impacts to the head, however, often display no observable behavioral deficits based on standard clinical tests, which may lack sensitivity. The current study investigates the effects of subconcussive impacts from soccer heading with innovative measures of vestibular function and walking stability in a pre- 0–2 h, post- 24 h post-heading repeated measures design. The heading group (n = 10) executed 10 headers with soccer balls projected at a velocity of 25 mph (11.2 m/sec) over 10 min. Subjects were evaluated 24 h before, immediately after, and 24 h after soccer heading with: the modified Balance Error Scoring System (mBESS); a walking stability task with visual feedback of trunk movement; and galvanic vestibular stimulation (GVS) while standing with eyes closed on foam. A control group (n = 10) followed the same protocol with no heading. The results showed significant decrease in trunk angle, leg angle gain, and center of mass gain relative to GVS for the heading group compared with controls. Medial-lateral trunk orientation displacement and velocity during treadmill walking increased immediately after mild head impact for the heading group compared with controls. Controls showed an improvement in mBESS scores over time, indicating a learning effect, which was not observed with the heading group. These results suggest that mild head impact leads to a transient dysfunction in vestibular processing, which deters walking stability during task performance.
Keywords: : behavioral assessments, head trauma, human studies, outcome measures, sensory function
Introduction
Subconcussion, defined as a head impact that does not result in clinically observable deficits, is an underrecognized phenomenon resulting from low levels of head impact that have the potential to cause significant long-term neurological damage.1 While the focus over the last 20 years has been the diagnosis and management of mild traumatic brain injury (mTBI), emerging evidence suggests that subconcussive head impacts are often neglected and underreported in spite of potential risks.2–5 We provide evidence of transient behavioral deficits using sensitive measures to identify deficits from repetitive mild head impact. Because we observe recovery within 24 h, the “soccer heading” paradigm we use may serve to better understand mechanisms underlying injury and recovery from subconcussive head impacts that may share common mechanisms of injury/recovery with more severe head impacts (e.g., concussion).
Severe impacts to the head can instantly cause conditions such as dizziness, confusion, and postural instability.6,7 In contrast, symptoms resulting from mild head impact have proven to be subtle and more difficult to consistently identify. Soccer heading has been the primary method to investigate the effects of mild head impact, because one can control the level and frequency of impact in a research setting. Several studies have shown significant changes in motor performance after repeated subconcussive head impacts,4,8,9 while others have shown no significant changes in behavior.10–15 It is likely that the clinical tests used may not be sensitive to the subtle changes in response to subconcussion.1,3,11,16,17
In the current study, we used the soccer-heading paradigm along with sophisticated measures of vestibular function to characterize how mild head impact may influence standing and walking stability. Because of its anatomical location in the head, the vestibular system is particularly vulnerable to disruption from head impact, and the disruption in the integration of vestibular processing could be an underlying basis for balance problems after head impact.18
Estimation of body position/velocity (i.e., self-motion) for postural stability is heavily dependent on the integration of information from multiple sensory modalities including visual, vestibular, and somatosensory (touch, pressure, proprioception). Vestibular input is unique because it provides information relative to motion of the head/body, whereas visual and proprioceptive inputs provide information about body orientation that is relative to the external scene/support surface. Because in everyday life the visual scene and/or support surface can move independently of the body, visual and proprioceptive inputs do not necessarily provide veridical information about body orientation. Therefore, vestibular input is thought to provide veridical, albeit noisy information about self-motion,19 serving as a reference against which other sensory inputs are evaluated when conflicts arise.20
The aim of the study is to investigate possible sensory-motor dysfunction after subconcussive impact on vestibular processing and walking stability. We use galvanic vestibular stimulation21 to test whether changes in vestibular function can be detected during quiet standing, which has proven inconsistent as a task to detect behavioral deficits in previous soccer heading studies.13–15 We also investigated the control of balance during walking using visual feedback of the trunk while walking on a treadmill.22 Our hypothesis is that these techniques are more sensitive to the subtle, transient effects of mild head impact with regard to the processing of vestibular information and postural stability under static and dynamic conditions.
Methods
Subjects
Twenty healthy adults with at least 5 years of soccer playing experience and no neurological injury in the 6 months before and during the duration of the study, volunteered to participate in the study. All participants were active members of an organized soccer team (i.e., collegiate, intramural, club) at the time of the study. They were divided in two groups: heading (EXP) and control (CON). EXP performed soccer heading using a controlled soccer heading model (see Methods), while CON did not perform any soccer heading. Demographic information is provided in Table 1 (EXP: 8 males, 2 females, mean age = 21 ± 1.2 years old; CON: 7 males, 3 females, mean age = 20 ± 1.5 years old).
Table 1.
Demographic Data
| EXP | CON | |
|---|---|---|
| # of participants | 10 | 10 |
| Agea | 20.7 ± 1.1 | 18.9 ± 1.1 |
| Height (cm) | 178.3 ± 9.2 | 175.8 ± 5.3 |
| Weight (kg) | 74.6 ± 9.5 | 74.1 ± 5.7 |
| Years of experienceb | 14.9 ± 2.2 | 11.4 ± 4.2 |
| # of participants reported being diagnosed with at least a concussion in the past | 4 | 3 |
| Average # of concussions reported per participant | 0.5 ± 0.7 | 0.5 ± 1 |
| Average linear acceleration when performing soccer heading (g)c | 14.5 ± 4.6 | None |
EXP, Heading Group; CON, Control Group.
Data are presented as mean ± standard deviation of the mean.
Significant difference between groups from independent t tests (p < 0.05).
Significant difference between groups from independent t tests (p < 0.005).
Mean and standard deviation values are reported from 9 of the 10 subjects in the EXP group. One of the subject's acceleration data was not recorded because the measurement device was not turned on during that subject's heading performance.
Four participants in EXP and three participants in CON self-reported a history of at least one concussion; however, the time of the concussion was not collected. Independent t test reveal no differences for self-reported concussion between groups. Subjects were told to refrain from substances that could affect nervous system function (e. g., stimulants and/or depressants) during the period of testing. The Temple University Institutional Review Board approved the procedures, and all participants signed informed consent and Health Insurance Portability and Accountability Act forms before the start of the study.
Experiment design
The experiment used a repeated measures design with three time sessions (a pre-heading, 0-hour post-heading, 24-h post-heading). In each time session, a clinical measurement and two different assessments (standing posture control assessment and walking posture control assessment) were conducted with both groups in randomized order. All measurements were completed within a 2-h window. The pre-heading session (Pre) was a baseline measurement. After approximately 24 h, the subject performed 10 headers following the protocol described in the Soccer heading model section. The same measurements were performed within 2 h of heading (Hr0) and then approximately 24 h later (Hr24).
Soccer heading model
A human head impact model was used to provide standardized and reliable soccer heading testing.4 A triaxial accelerometer (Gforce Tracker, Gforcetracker Inc., Markham, ON) was positioned and secured on the back of the head with pre-wrap and tape to measure linear head impact acceleration (g-force; Table 1). A JUGS soccer machine (JPS Sports, Tualatin, OR) was used to simulate a soccer kick and standardize ball speed across subjects. A size 5 soccer ball was inflated to 8 psi and launched from the JUGS machine at speeds of 25 mph (11.2 m/sec). Participants stood approximately 40 feet away from the machine to perform the headers. These experimental parameters were used, because it has a low level of impact and has created significant results post-heading using robust assessment techniques in a previous study.4 EXP subjects performed 10 standing headers over a 10 min period, whereas CON subjects did not perform soccer heading. Acceleration data of the headers is reported in Table 1.
Clinical measurement
The Sport Concussion Assessment Tool, 3rd edition (SCAT3) is a standardized tool for evaluating injured athletes for concussion and is mostly used in the field for sideline clinical concussion measurement. We used a modified Balance Error Scoring System (mBESS), which is a subsection of the SCAT3, to examine the subconcussive head impact as a clinical measurement.7 The mBESS consists of three different tasks including: single leg (nondominant), double leg, and tandem stance (nondominant foot at back). All tasks are performed on a firm surface without shoes. Subjects were asked to close their eyes, put their hands on the iliac crests, and maintain upright stance for 20 sec for each task. There was a 1-min break between tasks.
An error is credited to the subject when any of the following occurred: moving the hands from the iliac crests, opening the eyes, step stumble or fall, abduction or flexion of the hip beyond 30 degrees, lifting the forefoot or heel off the ground, and remaining out of the proper testing position for greater than 5 sec. Two experienced scorers performed the assessment in the current study; however, each subject was assessed by the same scorer throughout all testing sessions.
Standing posture control assessment
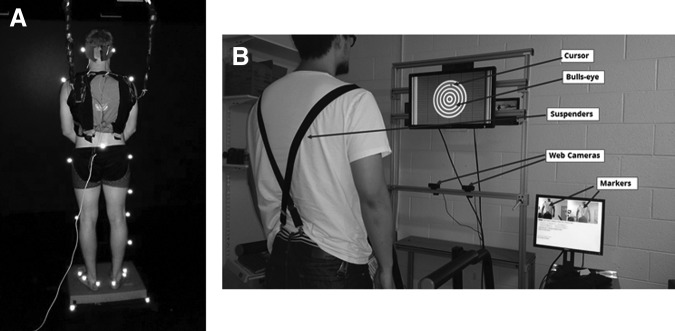
Galvanic vestibular stimulation (GVS) was applied while subjects stood on a foam pad with eyes closed to diminish reliable foot/ankle proprioceptive input and maximize the vestibular sensory modality for postural control of upright stance, as pictured in Figure 1A. Two linear isolated stimulators (Biopac Systems, Inc., Goleta, CA) generated the binaural-monopolar GVS. Independent stimuli were delivered to each side via a pair of circular electrodes secured over the mastoid process with an elastic headband and 2 cm ipsilateral to the T2 spinous process.21 The electrodes were secured using adhesive tape, and electrode gel was applied at the electrode-skin interface to improve conductance.
FIG. 1.
Experimental setup for standing and walking posture control assessment. (A) Standing on foam with eyes-closed during galvanic vestibular stimulation. (B) Schematic of a subject walking the treadmill with visual feedback system.
GVS consisted of a ±1 mA sinusoidal galvanic stimulus at 0.36 Hz.20 The polarity of stimulation was always the same for the two sides (binaural-monopolar GVS), inducing an illusion of pitch rotation at the ankle joint (i.e., anterior-posterior sway) in the sagittal plane. The response to the GVS stimulus was measured by gain and phase. Gain represents the amplitude of response to the GVS stimulation, while phase represents the response time to the GVS stimulation. Calculations for gain and phase is further described under the Frequency response function analysis section.
The instruction to the subjects was to look straight ahead and stand upright comfortably. The subjects were instructed to place their feet on the foam so that they could stand comfortably for the length of a trial, but no wider than shoulder width apart. The feet were aligned symmetrically in the frontal plane, and foot position was marked by tape to be reproducible throughout the experiment. Eight trials were repeated for each subject, and the length of each trial was 125 sec with 5 sec added at the beginning and end of each trial (total 135 sec) to allow vestibular stimulation to ramp up and ramp down.
During each trial, kinematics were captured by a six-camera motion capture system (Motion Analysis, Santa Rosa, CA). The head (temple), shoulder (scapula), hip (greater trochanter), knee (lateral femoral condyle), ankle (lateral malleolus), and foot (fifth metatarsal head) were measured by attaching 12 reflective markers on both sides of the subject to measure subject's anterior-posterior movement in the sagittal plane. An extra marker was placed on the sacrum to approximate body center of mass (COM) based on the sacral marker method.23 The leg angle θ1(t), trunk angle θ2(t), and head angle θ3(t) with respect to vertical were determined by the anterior-posterior (AP) and vertical displacement of the head, shoulder, hip, and ankle markers. Kinematics were sampled at 120 Hz.
Walking posture control assessment
For the walking balance assessment, subjects walked on a treadmill (56 cm × 160 cm, TMX425, Full Vision, Newton, KS) while two webcams (Logitech HD Pro Webcam C920, Logitech, Newark, CA) tracked the trunk orientation angle with respect to a self-calibrated vertical reference axis of each subject's standing posture (Fig. 1B). Trunk orientation was based on the three-dimensional (3D) coordinates of three flat green rectangular markers, affixed to an elastic suspender, at the acromioclavicular joints and the navel. The 3D coordinates over the walking space of the treadmill were reconstructed from two consecutive sequences of live images from the webcams calibrated using a modified open source code.24 The visual display was created with custom scripts using Vizard (WorldViz, Santa Barbara, CA), on a desktop computer (Dell PWS650, Dell, Round Rock, TX). The current system setup has been described in detail in a previous aticle.22
Trunk orientation was represented to the subject as the position of a moving cursor within a bull's-eye target on a 68.58 cm (27-inch) wide screen TV (ViewSonic VA2703, ViewSonic,Walnut, CA) mounted at standing eye level. Trunk orientation relative to vertical here is defined as AP and medial-lateral (ML) angular displacement of the segment defined by the naval marker to the midpoint of the acromion process markers. The center of the bull's-eye corresponded to the subject's standing upright vertical position. The bull's-eye was displayed as 10 concentric 2.54 cm wide rings with each expanding ring corresponding to 1 degree of angular displacement from the vertical. The center circle of the bull's-eye is 2.54 cm in diameter. The radius of the center circle and the width of each expanding ring is 1.27 cm. The length of 1.27 cm is represented as 1 degree of trunk angular deviation.
Cursor motion was scaled as a 1:1 ratio to trunk segment motion. Up-down movement of the cursor represented fore-aft angular deviations of the trunk from vertical, and left-right movement of the cursor represented side-to-side angular deviations of the trunk from vertical. During each time session, subjects were instructed to walk for three trials on the treadmill at 1.4 m/sec for 2 min while maintaining the cursor within the center of the bull's-eye. The amount of time the cursor maintained within the center circle of the bull's-eye was converted to a performance score out of a maximum of 1200 points (e.g., 1 sec maintained at the center was given as 10 points), displayed after each trial. Trunk kinematics was measured with the same two webcams used to display the cursor, sampled at 15 Hz.
Frequency response function analysis
Frequency response function (FRF) analysis was applied to the standing posture control assessment to investigate the relationship between two signals: GVS (input) and body movement from segment angles and COM (output). For any two signals x(t) and y(t), the power spectral densities (PSDs) pxx( f ) and pyy( f ) and cross spectral density (CSD) pxy( f ), where f is frequency, are computed using the Welch method25 with 50-sec Hanning windows and 50% overlap and then averaged across trials. FRF is the CSD divided by the PSD of the input. Gain is the absolute value of the FRF, describing the strength of the relationship between output and input signals. Phase, calculated from the angle of FRF, is a measure of the temporal relationship between the input and output; the output may lead the input (positive values) or lag behind it (negative values).
Trunk orientation
To investigate dynamic stability involving sensory feedback during walking, trunk orientation displacement was calculated as the difference between the midpoint of the top two acromion process markers and the navel marker in the frontal plane (for ML direction), to approximate small angular deviations.26 Trunk orientation velocity was calculated from v(t) = (x(t + 1) − x(t − 1))/2, where t is the time/frame, v(t) is the velocity as a function of time, x(t) is the position as function of time. This method reduces higher-frequency noise from the tracking system when calculating velocity from displacement data.27 Root mean square (RMS) displacement and velocity was calculated as a measure of overall trunk motion variability.
Statistical analysis
Independent t tests were used to compare subjects' characteristics (age, weight, height, years of soccer playing experience, and number of past concussions) between EXP and CON groups. Significant demographic difference in age and soccer playing experience existed between groups as shown in Table 1. Because of significant demographic difference between groups (Table 1) and the repeated measures design, a conservative approach using analysis of covariance (ANCOVA) was applied to determine group differences in all outcome measurements with respective baseline values of each measurement set as covariate to account for any potential differences of task performance by the subjects.
Values for experiences were self-reported and their playing and practicing habits were not accounted for. Therefore controlling for the baseline performances of each measurement created a more empirical comparison by creating a state of equality for the subject's performance capabilities at the time of testing. Moreover, the current study's aim is to investigate behavioral changes across time. Thus, to control for potential validity threats when comparing between groups during pre- and post-heading, baseline values were set as covariates to account for potential changes caused by subconcussive impact. The level of statistical significance for ANCOVA analysis was set to p < 0.05.
Paired t tests (two-tailed) were used to determine significant differences within subjects across testing sessions. The level of statistical significance for the paired t tests was set to p < 0.0175 after adjusting for Bonferroni correction. Because of the limitation of having 10 subjects per group, t tests that resulted in significant difference at p < 0.017 were further investigated by calculating the Cohen d to identify effect size for the observed change. All data analyses were performed in SPSS 20 statistical software (SPSS Inc, Chicago, IL) and MATLAB R2014b (The MathWorks Inc, Natick, MA).
Results
mBESS
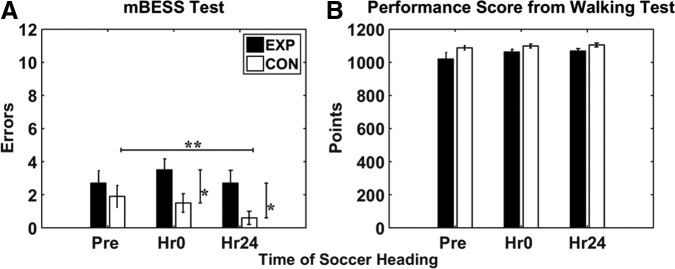
Figure 2A shows mBESS scores across time sessions in the EXP group and the CON group. ANCOVA analysis revealed group differences at Hr0 (p = 0.017) and Hr24 (p = 0.002). Two-tailed paired t test for the CON group indicate a significant decrease in error scores from Pre to Hr24 (p = 0.013, Cohen d = 0.29). It is possible that the CON group displayed a learning effect for the mBESS; however, the low effect size hints at minimal practical significance.
FIG. 2.
(A) Modified Balance Error Scoring System (mBESS) error scores and (B) performance scores on walking task across sessions for the experimental group (EXP) and control group (CON). Error bars represent standard error of the mean. *Represents significant group effect from analysis of covariance with pre-test set as covariate (p < 0.05). **Represents significant time effect within group from two-tailed paired t test (p < 0.017).
Body segment-GVS gain/phase
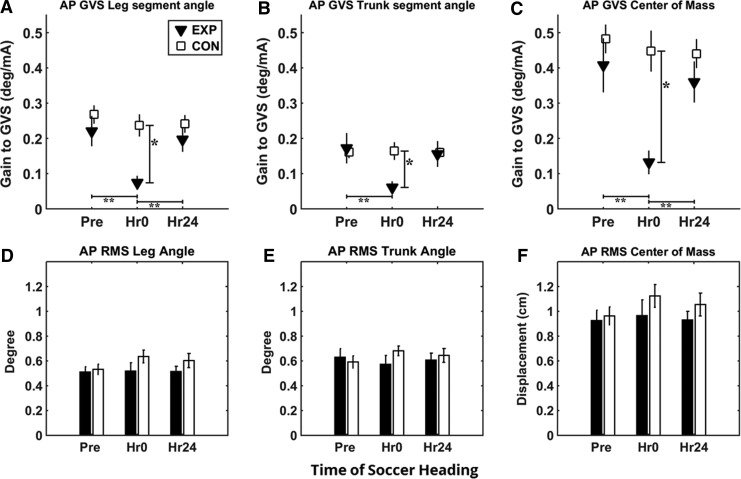
Mean gain of segment angles and the COM relative to GVS are shown in Figure 3. Gain decreased after the subconcussive head impact (Hr0) in the EXP group and then returned to pre-heading levels 24 h later. Control subjects showed no change in gain/phase across the three sessions. ANCOVA analysis showed significant group differences at Hr0 for gain response to GVS in all segment angles relative to GVS (p < 0.005), as shown in Figure 3A–C. No significant group effect was observed 24 h after heading.
FIG. 3.
Gain response and root mean square (RMS) from galvanic vestibular stimulation (GVS) in anterior/posterior (AP) direction. Experimental (EXP) group shows significant decrease in gain from Pre to Hr0 and significant increase in gain from Hr0 to Hr24 for leg angle, trunk angle, and center of mass (COM). RMS across all conditions shows no changes in overall sway across time and group. (A) Gain of leg segment angle relative to GVS. (B) Gain of trunk segment angle relative to GVS. (C) Gain of COM relative to GVS. (D) Overall sway of leg angle in RMS during GVS. (E) Overall sway of trunk angle in RMS during GVS. (F) Overall sway of COM in RMS during GVS in EXP and control (CON) groups. Error bars represent standard error of the mean. *Represents significant group effect from analysis of covariance with pre-test set as covariate (p < 0.05). **Represents significant time effect from two-tailed paired t test (p < 0.017).
In addition, paired t test indicated a significant gain decrease from Pre to Hr0 in leg segment angle (p = 0.004, Cohen d = 1.378), trunk segment angle (p = 0.016, Cohen = 1.067), and COM (p = 0.003, Cohen = 1.433). There is also significant gain increase from Hr0 to Hr24 in the EXP group for leg segment angle (p = 0.003, Cohen d = 1.367) and COM (p = 0.002, Cohen = 1.488). Trunk segment angle showed a difference from Hr0 to Hr24 (p = 0.026); however, it was suggested to be insignificant after adjusting for Bonferroni correction suggesting that majority of gain response returned to baseline after 24 h when compared across measurement times (Fig. 3). Overall RMS for leg angle, trunk angle, and COM showed no significant interactions effect in ANCOVA, suggesting no changes in overall gross movement during standing (Fig. 3D–F).
ANCOVA analysis of the phase response showed significance only for trunk segment angle group differences at Hr0 (p = 0.04). This was the product on high variance across subjects and difficult to interpret, however. Moreover, paired t tests revealed no significant difference across time for any of the segment outcomes, indicating no significant change in phase relative to GVS after soccer heading.
Walking balance assessment
Trunk orientation
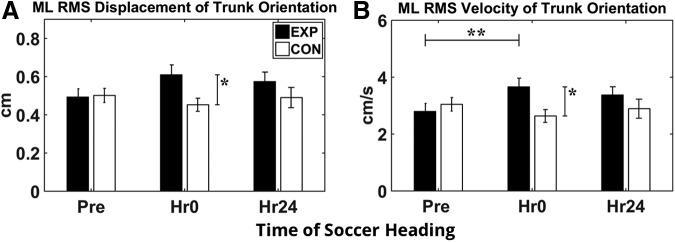
Figure 4A shows the ML trunk orientation displacement variability and Figure 4B shows velocity variability in both groups across three time sessions. In the EXP group, ML trunk orientation displacement and velocity variability increased immediately after subconcussive head impact at Hr0. These results were supported by ANCOVA analysis, which showed significant group differences at Hr0 for ML RMS trunk orientation displacement (p = 0.007) and ML trunk orientation velocity (p = 0.005). No significant group effect was present at Hr24, suggesting that trunk motion variability returned to normal after 24 h when compared across groups. Paired t tests indicated a significant increase in ML trunk velocity from Pre to Hr0 (p = 0.016; Cohen d = 0.881) within the EXP group.
FIG. 4.
Trunk orientation during walking posture assessment in experimental group (EXP) and control group (CON). (A) Root mean square (RMS) of displacement of trunk orientation in medial-lateral (ML) direction. (B) RMS velocity of trunk orientation in ML direction. Error bars represent standard error of the mean. *Represents significant group effect from analysis of covariance with pre-test set as covariate (p < 0.05). **Represents significant time effect from two-tailed paired t test (p < 0.017).
Task performance
Figure 2B shows performance measure for each group at each session. The EXP group scored an average of 1020, 1062, and 1068 points at each of the respective testing times, while the CON group scored 1088, 1099, 1100 at each respective times. ANCOVA analysis resulted in no group effect at Hr0 and Hr24 with Pre as a covariate.
Discussion
We investigated the effect of repeated mild head impact on standing and walking using precise assessment of sensory-motor function in a pre, 0–2 h post-, 24 h post-heading repeated measures design. After performing 10 soccer headers at an average linear acceleration of 14.5g (Table 1), changes in sensory-motor function were observed. As expected, changes in mBESS scores were not observed in the EXP group. GVS to probe vestibular function during standing, however, revealed a consistent deficit in vestibular processing immediately after the subconcussive head impact, which recovered back to pre-heading levels after 24 h. This deficit in vestibular processing potentially contributed to an increase in ML trunk orientation displacement and velocity variability during walking while maintaining trunk position at vertical after subconcussive impact, which also recovered back to pre-heading levels after 24 h.
Subconcussive head impacts transiently alter vestibular processing for standing postural control
Controlled soccer heading has not consistently led to differences in clinical assessments of posture such as the BESS or the foam and dome test,13–15 suggesting that the levels of impact may be too small to cause brain injury and disrupt central nervous system function related to balance control. While current results from mBESS show group differences at Hr0 and Hr24 after accounting for baseline differences, this group difference is driven by the decrease in mBESS values for the control group across time, which could suggest that the mBESS is prone to a learning effect, consistent with previous findings of learning effect for the BESS test.28
It is important to note that the EXP group did not show any mBESS changes across time, suggesting that subconcussive head impacts could potentially inhibit the observed mBESS learning effect. Nonetheless, mBESS is still limited in detecting any immediate changes from subconcussive head impacts, and it is dependent on the judgment of the tester. In the current study, mBESS was performed by two different scorers; thus, this could be a potential confounding factor. Changes over time were controlled by the same scorer, however, because each subject was assessed by the same scorer in all testing sessions.
We tested subjects while standing on a foam pad (to diminish reliable foot/ankle proprioceptive input) with eyes closed to bias the nervous system toward reliance on vestibular information for self-motion estimation while standing.29 By applying a small electrical current to the mastoid process to stimulate the vestibular nerve (i.e., GVS), we could then measure the body sway response when the vestibular system is perturbed. The results showed that gains of all segment angles and COM relative to GVS significantly decreased after the subconcussive head impact in the EXP group and then recovered back to pre-heading levels after 24 h, whereas the control group showed no change in gain across the three time sessions (Fig. 3A–C).
Diminished gain response to GVS after subconcussive head impact suggests that the EXP group's standing postural control became less responsive to GVS and that vestibular processing is disrupted from subconcussive impacts. While trunk phase relative to GVS showed significant decrease in the EXP group immediately after soccer heading, this observation is difficult to interpret at the low levels of gain at Hr0 (0.06). Estimates of phase are unreliable when gain is small.
Interestingly, overall body sway amplitude show no significant difference across group and time from this vestibular deficit (Fig. 3D–F). This may not be surprising, considering the low frequencies and amplitudes of head movement typical during quiet standing. Body sway velocities during quiet standing are approximately 1 deg/sec or lower.30 This is on the order of 1% of the dynamic range of the semicircular canals. It would be reasonable to expect that the signal-to-noise ratio of the canal signal would be fairly low during operation in the restricted range of motions associated with spontaneous body sway, although compensation for this deficit may be achieved by combining otolith and canal information,31 and through proprioceptive feedback from muscles and joints.
In contrast, the “noisy” vestibular signal may not contribute substantially to self-motion estimation and subsequent corrective postural responses with the low head accelerations observed during quiet standing.19 Thus, transient disruption of vestibular processing because of mild head impact may only be observed at the larger head accelerations during walking.
Subconcussive head impact diminishes lateral balance control during walking
Given the ambiguous relationship between vestibular function and quiet standing, our strategy was to impose a task that would generate larger head velocities/accelerations to test behavioral deficits that may be related to vestibular processing. During walking, all subjects exhibited no change in the performance measure across testing sessions, suggesting that subconcussive impacts did not hinder the task of using visual feedback to stabilize trunk orientation at vertical while walking on a treadmill. This is again consistent with the previous studies indicating that relatively short bouts of soccer heading do not impact the performance of tasks such as control of upright stance.
More detailed analysis, however, indicated behavioral changes in trunk motion during walking in the current study, suggesting potential disruption in the sensory-motor control system to achieve equivalent performance. Subjects were not instructed specifically to minimize their trunk movement as much as possible but rather control their trunk within a 2.54 diameter (approximately 2 degrees of angular deviation) of the bull's-eye's center to achieve the highest score.
Both groups were able to maintain their trunk within the center of the bull's-eye for an extended period across all sessions, as presented by performance scores (Fig. 2B) and trunk orientation displacement (Fig. 4A). The EXP group, however, had significantly higher levels of ML trunk orientation displacement and velocity immediately after heading, suggesting more rapid and robust adjustments of the trunk to maintain equivalent performance.
This behavioral change in the EXP group in the ML direction could be the consequence of altered processing in active lateral balance control during walking, implying that subjects may not able to use the visual feedback to maintain a vertical trunk position as effectively immediately after subconcussive impacts. Such results are consistent with previous research on balance control during walking, indicating that step width variability in the ML direction is more prone to visual influence.32
Although no stepping data were collected in the current study, it is feasible that ML trunk variability observed is strongly correlated with the step width variability,33–35 potentially leading to an increase in metabolic cost as well.34,36 This finding is also in line with previous studies on gait/balance after grade 2 concussion, revealing an increase in ML sway during walking with divided attention within the first 48 h after experiencing neurological stress from head impact.37,38 While the performance task in the current study was not dual in nature, our results suggest that similar mechanisms may underlie the deficits observed after both subconcussive and concussive impact.
As mentioned previously, studies using the soccer heading model have reported mixed results for postural sway. The majority of past studies on the effects of “soccer heading” focused on the variability of body sway during upright stance. This is not considered a particularly challenging task, because of the lack of damping required by the body to minimalize head acceleration during standing when compared with walking.39 In addition, at low levels of head acceleration, the vestibular signal is too noisy to be useful to correct sway deviations.19
Considering the noisy nature of vestibular signal and that vestibular processing is believed to be disrupted after mild head impact,40,41 RMS sway during quiet stance may not be discerning. Instead, we measured trunk sway variability during a walking task, which entails much larger head accelerations, requiring a larger role for vestibular processing. Thus, by instructing subjects to perform a dynamic task requiring head/trunk stabilization while inducing head acceleration from the motion of walking, we emphasized vestibular input.
TBI has been shown to cause alterations in timing of inputs and interactions of visual and vestibular processing resulting in irregular motion perception and motor control.18 Although the current experimental protocol only induces 10 soccer headers within the span of 10 min, it is possible that the interaction of visual and vestibular processing is affected at a subtle and transient level during walking that could potentially resemble functional and physiologic changes of a concussive impact.
The current results suggest that subconcussive impacts to the head within the span of 10 min do not exhibit any long-term changes to balance. While trunk sway during the walking task did significantly increase immediately after subconcussive impact, the performance of the walking task did not suffer. This may be because the walking task we imposed is fairly simple and other degrees of freedom can be recruited to compensate for diminished trunk stability. Under the more rigorous conditions of high-level athletic play requiring precise timing, even subconcussive effects may be enough to hinder performance.
Central/peripheral vestibular processing
To isolate the effects of head impact on a specific brain area is a challenge. The vestibular modality interconnects with many areas of the brain, such as the brainstem, cerebellum, and cortex. One possible explanation is that afferent signals to vestibular processing centers are abated. In the current study, GVS is applied at the mastoid process to stimulate the vestibular nerve. The vestibular nerve is a predominantly sensory nerve that terminates at the brainstem. From the brainstem, second-order neurons projected to the cortex and cerebellum are crucial for postural control.
A modeling study of subconcussive head impacts from the National Football League demonstrated highest strain forces at the midbrain level.42 It is possible that the vestibular signal influenced by GVS traveling up to the cortex or cerebellum from the vestibular nerve was disrupted, starting at the midbrain level, leading to the decrease in GVS gain. Another possible explanation is that the central processing down-weighted the vestibular signal to cause the decrease in GVS gain. The current experimental setup, however, is not able to identify whether it is peripheral, central, or both that is disrupted after the applied subconcussive impacts.
Interestingly, the EXP group did not exhibit an increase in overall variability of COM or body segment kinematics (e.g., trunk angle) during eyes-closed standing on foam immediately after mild head impact (Fig. 3D–F), but only a decrease in gain relative to GVS stimulation (Fig. 3A–C). This suggests that gross motor output of the head, trunk, and leg during standing was relatively intact, implying that efferent pathways, such as the vestibulospinal tract, were not affected by the subconcussive impact. The lack of change in overall postural sway while standing on foam can also suggest that central and peripheral processing of proprioception also remained relatively unimpaired. Thus, despite the transient disruption in vestibular processing that may emanate at either the brainstem level or cortex level, compensatory mechanisms could mask its effects on postural control.
Conclusion
There is growing concern, particularly with regard to the developing brain, that even low levels of head impact—i.e., subconcussive head impact—can cause significant injury if sustained repetitively. Physical responses to subconcussive impacts, however, are difficult to identify and highly varied from clinical assessments and traditional standing balance tests. We provide experimental evidence of transient behavioral changes related to balance control and vestibular processing from 10 subconcussive impacts over a period of 10 min. While these behavioral changes returned to baseline levels after 24 h, understanding the mechanisms underlying these transient deficits may provide a window into understanding the relationship between brain injury and behavioral function under conditions of more severe head trauma.
Acknowledgments
This study was partially supported by NIH grant R21 AG041714-01A1, J. Jeka, PI. The authors thank Eric Anson, PhD and Peter Agada, MS for their input and assistance.
Author Disclosure Statement
John Jeka is one of the inventors of the sensory treadmill described in this experiment. United States Patent# 8,900,165. For the remaining authors, no competing financial interests exist.
References
- 1.Bailes J.E., Petraglia A.L., Omalu B.I., Nauman E., and Talavage T. (2013). Role of subconcussion in repetitive mild traumatic brain injury. J. Neurosurg. 119, 1235–1245 [DOI] [PubMed] [Google Scholar]
- 2.Bauer J.A., Thomas T.S., Cauraugh J.H., Kaminski T.W., and Hass C.J. (2001). Impact forces and neck muscle activity in heading by collegiate female soccer players. J. Sports Sci. 19, 171–179 [DOI] [PubMed] [Google Scholar]
- 3.Dashnaw M.L., Petraglia A.L., and Bailes J.E. (2012). An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg. Focus 33, E5: 1–9 [DOI] [PubMed] [Google Scholar]
- 4.Haran F.J., Tierney R., Wright W.G., Keshner E., and Silter M. (2013). Acute changes in postural control after soccer heading. Int. J. Sports Med. 34, 350–354 [DOI] [PubMed] [Google Scholar]
- 5.Talavage T.M., Nauman E., Breedlove E.L., Yoruk U., Dye A.E., Morigaki K., Feuer H., and Leverenz L.J. (2014). Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J. Neurotrauma 31, 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guskiewicz K.M., Weaver N.L., Padua D.A., and Garrett W.E. (2000). Epidemiology of concussion in collegiate and high school football players. Am. J. Sports Med. 28, 643–650 [DOI] [PubMed] [Google Scholar]
- 7.McCrory P., Meeuwisse W.H., Aubry M., Cantu R.C., Dvorák J., Echemendia R.J., Engebretsen L., Johnston K.M., Kutcher J.S., Raftery M., Sills A., Benson B.W., Davis G.A., Ellenbogen R., Guskiewicz K.M., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D.L., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H., and Turner M. (2013). Consensus Statement on Concussion in Sport—The 4th International Conference on Concussion in Sport held in Zurich, November 2012. PM R 5, 255–279 [DOI] [PubMed] [Google Scholar]
- 8.Riemann B.L., and Guskiewicz K.M. (2000). Effects of mild head injury on postural stability as measured through clinical balance testing. J. Athl. Train. 35, 19–25 [PMC free article] [PubMed] [Google Scholar]
- 9.Webbe F.M., and Ochs S.R. (2003). Recency and frequency of soccer heading interact to decrease neurocognitive performance. Appl. Neuropsychol. 10, 31–41 [DOI] [PubMed] [Google Scholar]
- 10.Clark D. (2012). Effects of repeated mild head impacts in contact sports: adding it up. Neurology 78, e140–e142 [DOI] [PubMed] [Google Scholar]
- 11.Kaminski T.W., Cousino E.S., and Glutting J.J. (2008). Examining the relationship between purposeful heading in soccer and computerized neuropsychological test performance. Res. Q. Exerc. Sport 79, 235–244 [DOI] [PubMed] [Google Scholar]
- 12.Putukian M., Echemendia R.J., and Mackin S. (2000). The acute neuropsychological effects of heading in soccer: a pilot study. Clin. J. Sport Med. 10, 104–109 [DOI] [PubMed] [Google Scholar]
- 13.Broglio S.P., Guskiewicz K.M., Sell T.C., and Lephart S.M. (2004). No acute charges in postural control after soccer heading. Br. J. Sports Med. 38, 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangus B.C., Wallmann H.W., and Ledford M. (2004). Analysis of postural stability in collegiate soccer players before and after an acute bout of heading multiple soccer balls. Sports Biomech. 3, 209–220 [DOI] [PubMed] [Google Scholar]
- 15.Schmitt D.M., Hertel J., Evans T.A., Olmsted L.C., and Putukian M. (2004). Effect of an acute bout of soccer heading on postural control and self-reported concussion symptoms. Int. J. Sport Med. 25, 326–331 [DOI] [PubMed] [Google Scholar]
- 16.Guskiewicz K.M., Ross S.E., and Marshall S.W. (2001). Postural stability and neuropsychological deficits after concussion in collegiate athletes. J. Athl. Train. 36, 263–273 [PMC free article] [PubMed] [Google Scholar]
- 17.Drezner J.A., Gammons M., Guskiewicz K.M., Halstead M., Herring S.A., Kutcher J., Pana A., Putukian M., and Roberts W.O. (2013). American Medical Society for Sports Medicine position statement: concussion in sport. Br. J. Sports Med. 47, 15–26 [DOI] [PubMed] [Google Scholar]
- 18.Franke L.M., Walker W.C., Cifu D.X., Ochs A.L., and Lew H.L. (2012). Sensorintegrative dysfunction underlying vestibular disorders after traumatic brain injury: a review. J. Rehabil. Res. Dev. 49, 985–94 [DOI] [PubMed] [Google Scholar]
- 19.Peterka R.J., and Benolken M.S. (1995). Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp. Brain Res. 105, 101–110 [DOI] [PubMed] [Google Scholar]
- 20.Hwang S., Agada P., Kiemel T., and Jeka J.J. (2014). Dynamic reweighting of three modalities for sensor fusion. PLoS One 9, e88132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day B.L., Marsden J.F., Ramsay E., Mian O.S., and Fitzpatrick R.C. (2010). Non-linear vector summation of left and right vestibular signals for human balance. J. Physiol. 588, 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anson E., Agada P., Kiemel T., Ivanenko Y., Lacquaniti F., and Jeka J. (2014). Visual control of trunk translation and orientation during locomotion. Exp. Brain Res. 232, 1941–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gard S.A., Miff S.C. and Kuo A.D. (2004). Comparison of kinematic and kinetic methods for computing the vertical motion of the body center of mass during walking. Human Movement Science 22, 597–610 [DOI] [PubMed] [Google Scholar]
- 24.Bouguet J.Y. (2013). Camera Calibration Toolbox for Matlab. 2015
- 25.Bendat J.S., and Piersol A.G. (2000). Random Data: Analysis and Measurement Procedures. New York, NY:Wiley [Google Scholar]
- 26.Logan D., Kiemel T., Dominici N., Cappellini G., Ivanenko Y., Lacquaniti F., and Jeka J.J. (2010). The many roles of vision during walking. Exp. Brain Res. 206, 337–350 [DOI] [PubMed] [Google Scholar]
- 27.Winter D.A. (2009). Biomechanics and Motor Control of Human Movement. John Wiley & Sons: Hoboken, NJ [Google Scholar]
- 28.Valovich McLeod T.C., Perrin D.H., Guskiewicz K.M., Shultz S.J., Diamond R., and Gansneder B.M. (2004). Serial administration of clinical concussion assessments and learning effects in healthy young athletes. Clin. J. Sport Med. 14, 287–295 [DOI] [PubMed] [Google Scholar]
- 29.Peterka R.J., and Loughlin P.J. (2004). Dynamic regulation of sensorimotor integration in human postural control. J. Neurophysiol. 91, 410–423 [DOI] [PubMed] [Google Scholar]
- 30.Jeka J., Kiemel T., Creath R., Horak F., and Peterka R. (2004). Controlling human upright posture: velocity information is more accurate than position or acceleration. J. Neurophysiol. 92, 2368–2379 [DOI] [PubMed] [Google Scholar]
- 31.Schmid-Priscoveanu A., Straumann D., and Kori A.A. (2000). Torsional vestibulo-ocular reflex during whole-body oscillation in the upright and the supine position: I. Responses in healthy human subjects. Exp. Brain Res. 134, 212–219 [DOI] [PubMed] [Google Scholar]
- 32.O'Connor S.M., and Kuo A.D. (2009). Direction-dependent control of balance during walking and standing. J. Neurophysiol. 102, 1411–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacKinnon C.D., and Winter D.A. (1993). Control of whole body balance in the frontal plane during human walking. J. Biomech. 26, 633–644 [DOI] [PubMed] [Google Scholar]
- 34.Donelan J.M., Shipman D.W., Kram R., and Kuo A.D. (2004). Mechanical and metabolic requirements for active lateral stabilization in human walking. J. Biomech. 37, 827–835 [DOI] [PubMed] [Google Scholar]
- 35.Hurt C.P., Rosenblatt N., Crenshaw J.R., and Grabiner M.D. (2010). Variation in trunk kinematics influences variation in step width during treadmill walking by older and younger adults. Gait Posture 31, 461–464 [DOI] [PubMed] [Google Scholar]
- 36.Ijmker T., Houdijk H., Lamoth C.J.C., Beek P.J., and van der Woude L.H. (2013). Energy cost of balance control during walking decreases with external stabilizer stiffness independent of walking speed. J. Biomech. 46, 2109–2114 [DOI] [PubMed] [Google Scholar]
- 37.Parker T.M., Osternig L.R., Lee H.J., Donkelaar P., and Chou L.S. (2005). The effect of divided attention on gait stability following concussion. Clin. Biomech. (Bristol, Avon) 20, 389–395 [DOI] [PubMed] [Google Scholar]
- 38.Parker T.M., Osternig L.R., VAN Donkelaar P., and Chou L.S. (2006). Gait stability following concussion. Med. Sci. Sports Exerc. 38, 1032–1040 [DOI] [PubMed] [Google Scholar]
- 39.Winter D.A. (1995). Human balance and posture control during standing and walking. Gait Posture 3, 193–214 [Google Scholar]
- 40.Guskiewicz K.M., Riemann B.L., Perrin D.H., and Nashner L.M. (1997). Alternative approaches to the assessment of mild head injury in athletes. Med. Sci. Sports Exerc. 29, Suppl 7, S213–S221 [DOI] [PubMed] [Google Scholar]
- 41.Guskiewicz K.M., and Mihalik J.P. (2011). Biomechanics of sport concussion: quest for the elusive injury threshold. Exerc. Sport Sci. Rev. 39, 4–11 [DOI] [PubMed] [Google Scholar]
- 42.Pellman E.J., Lovell M.R., Viano D.C., and Casson I.R. (2006). Concussion in professional football: Recovery of NFL and high school athletes assessed by computerized neuropsychological testing—Part 12. Neurosurgery 58, 263–272 [DOI] [PubMed] [Google Scholar]