Abstract
Objective. To investigate the effect of negative pressure conditions induced by NPWT on P. aeruginosa. Methods. P. aeruginosa was cultured in a Luria–Bertani medium at negative pressure of −125 mmHg for 24 h in the experimental group and at atmospheric pressure in the control group. The diameters of the colonies of P. aeruginosa were measured after 24 h. ELISA kit, orcinol method, and elastin-Congo red assay were used to quantify the virulence factors. Biofilm formation was observed by staining with Alexa Fluor® 647 conjugate of concanavalin A (Con A). Virulence-regulated genes were determined by quantitative RT-PCR. Results. As compared with the control group, growth of P. aeruginosa was inhibited by negative pressure. The colony size under negative pressure was significantly smaller in the experimental group than that in the controls (p < 0.01). Besides, reductions in the total amount of virulence factors were observed in the negative pressure group, including exotoxin A, rhamnolipid, and elastase. RT-PCR results revealed a significant inhibition in the expression level of virulence-regulated genes. Conclusion. Negative pressure could significantly inhibit the growth of P. aeruginosa. It led to a decrease in the virulence factor secretion, biofilm formation, and a reduction in the expression level of virulence-regulated genes.
1. Introduction
Infection is considered one of the most critical factors in impeding wound healing [1]. When the skin or tissue is compromised, bacteria can easily access the underlying tissues, which are believed to be the optimal places for colonization and growth of bacteria. It is reported that the infection rate was as much as 12% in acute wounds and 38% in chronic wounds [2], posing a challenge to clinical doctors. Pseudomonas aeruginosa (P. aeruginosa), a kind of gram-negative bacteria, is one of the most common pathogens isolated from wound infections [3]. It has been widely used in wound infection-related studies [4–6] owing to its virulence factor secretion and biofilm formation. P. aeruginosa can secrete various exotoxins, such as exotoxin A, rhamnolipid, and elastase, which play an important role in impeding wound healing and inflammatory reaction [7–9]. Moreover, exotoxin A and elastase are encoded by ToxA and LasB and the RhlA gene encodes a rhamnolipid synthase involved in the biosynthetic pathway [10, 11]. P. aeruginosa expresses two types of quorum sensing (QS) systems, LasI and RhlI, which contribute to the pathology of cutaneous wound infections [12, 13]. Based on this fact, the search for measures to inhibit toxin production and biofilm formation is an active area of clinical research. Recently, as an effective management of contaminated wounds, negative pressure wound therapy (NPWT) has been widely used in clinical laboratories [14, 15]. However, whether NPWT could reduce the bacterial load of wounds is still controversial. Weed reported that bacterial colonization increased significantly with NPWT [16]. Lalliss found that NPWT showed a significant and sustained decrease in the P. aeruginosa levels compared to WTD dressings [17]. However, the mechanism underlying the action of NPWT in the reduction of P. aeruginosa levels is still unknown. It is well known that both the immune status of host and bacterial invasiveness play important roles in the infection process [18]. Thus, the mechanism explaining the change in P. aeruginosa levels could not be confirmed under NPWT in vivo. Besides, few studies have reported the bacteria in wounds, secondary to negative pressure treatment, particularly with regard to P. aeruginosa proliferation, virulence, and gene expression. Previous studies have indicated that negative pressure induced by NPWT could alter the gene expression and proliferation of bone marrow mesenchymal stem cells [19, 20]. Our previous work had shown that negative pressure had an effect on the growth, secretion, and biofilm formation of Staphylococcus aureus [21].
The aim of this study was to evaluate the influence of negative pressure on the proliferation, virulence factor secretion, biofilm formation, and the virulence-regulated gene expression of P. aeruginosa in vitro.
2. Materials and Methods
2.1. Bacterial Strain and Preparation
P. aeruginosa laboratory strain PAO1 carrying the gene encoding the green fluorescent protein (GFP) was obtained from the laboratory of the Chinese PLA Institute for Disease Control and Prevention (Beijing, China). P. aeruginosa was grown overnight and cultured in Luria broth at 37°C until log-phase was achieved. Optical density at 600 nm wavelength was measured. An optical density of 1.0 was equivalent to 105 colony-forming units per microliter, as determined by a standard curve.
2.2. Growth Conditions
The bacterial culture protocol was based on our previously published model of in vitro negative pressure condition [21]. In brief, negative pressure condition was created for bacterial growth and an airtight chamber was used as the incubator. The air was sucked from the chamber by a vacuum pump device (provided by Professor Hu Lei, Beihang University, Beijing, China), which could automatically produce and maintain the negative pressure at −125 mmHg. The O2 concentration was constantly maintained at 20%, as adequate amount of room air was introduced into the incubator every 15 min. Bacterial culture was performed in culture dishes (Corning Life Sciences, USA) with a diameter of 35 mm at 37°C. Each of the dishes contained 2 mL LB medium and 106 P. aeruginosa (in a volume of 10 μL) was added. Bacteria in the control group were grown under atmospheric pressure, and other conditions were the same as that of the experimental group.
2.3. Morphological Characterization of Bacterial Colony
LB agar plates were inoculated with 2 μL of bacterial culture (OD at 600 nm = 1.0). P. aeruginosa was grown under aforementioned culture conditions for 24 h. To evaluate the colonial morphology, including the shape, color, size, and surface, a digital camera (IXUSi, Canon, Japan) was used to capture images of the bacterial colonies. The colony diameter was independently measured by two observers and the results were averaged.
2.4. Growth Curves
Bacteria were grown in 2 mL LB broth, with an inoculation of 106 P. aeruginosa in culture dishes at 37°C under a static condition. The growth of the bacteria exposed and unexposed to negative pressure was measured by reading the OD values at 600 nm after every 60 min with adequate mixing.
2.5. Virulence Factor Assays
Exotoxin A was measured according to the method of Shigematsu et al. [22] and was determined using a commercially available Human Pseudomonas Exotoxin A (PEA) ELISA Kit (Cusabio Biotech Co., Ltd., Hubei, PR China, product code: CSB-E11252 h), according to the manufacturer's instructions. The data were recorded as ng/mL.
Rhamnolipid was quantified by orcinol method, as previously described with a few modifications [23]. Briefly, 400 μL supernatant from the bacterial culture was extracted twice using 600 μL diethyl ether. The ether layer was transferred to a fresh tube for evaporation. Residues were dissolved in 150 μL H2O, 100 μL 1.6% orcinol (Sigma), and 750 μL 60% sulphuric acid (H2SO4). After heating for 30 min at 80°C, all the tubes were cooled at room temperature for 30 min and absorbance was recorded at 421 nm. The concentrations of rhamnolipid were calculated by multiplying rhamnose values by a coefficient of 2.5, as previously described [24].
The elastase activity was measured by the elastin-Congo red assay, as previously described [23]. Briefly, 100 μL supernatant from 24 h LB cultures was added to tubes containing 10 mg of elastin-Congo red (Sigma) and 900 μL Na2HPO4 (pH 7.0). Tubes were incubated for 4 h at 37°C under shaking conditions and the absorbance was recorded at 495 nm after removing the precipitate by centrifugation.
2.6. Static Biofilm Assays
To observe the influence of negative pressure on biofilm formation, 18 × 18 mm cover glass was put into a 35 mm culture dish, and each dish was incubated with 2 mL LB broth containing 106 P. aeruginosa for 24 h in a constant temperature incubator at 37°C. After 24 h, each cover glass was washed three times with phosphate buffered saline (PBS) to remove planktonic bacteria. The P. aeruginosa glycocalyx was visualized by staining with 50 μg/mL of Alexa Fluor 647 conjugate of Con A (Life Technologies, USA) for 15 min at room temperature in the dark as previously described with a few modifications [4]. Biofilm formation was observed through fluorescence microscopy (Olympus BX51).
2.7. Quantitative RT-PCR
Bacteria were isolated from the LB medium for quantitative RT-PCR analysis as previously described [21]. Primers used to amplify ToxA, RhlA, LasB, LasI, and RhlI, as well as the reference gene, RpoD, are shown in Table 1. Briefly, total RNA was extracted using an RNAprep Pure Cell/Bacteria Kit (TIANGEN, China) according to the manufacturer's instructions. Total RNA was treated with Recombinant DNase I (TAKARA, Japan) and reverse-transcribed using the TIANScript RT Kit (TIANGEN) according to the manufacturer's instructions. Real-time PCR analyses using the SYBR FAST qPCR Kit Master Mix Universal (KAPA, USA) were performed with an ABI7900HT sequence detection system (ABI, USA). The reaction procedures were as follows: incubation at 95°C for 3 min, 40 cycles at 95°C for 3 s, 60°C for 20 s, and one dissociation step at 95°C for 15 s, 60°C for 15 s, and 95°C for 15 s. All samples were analyzed in triplicate and normalized against RpoD expression. Results were shown as the fold change of gene expression relative to the control.
Table 1.
Primer sequences for quantitative RT-PCR.
| Gene | Primer | Amplicon (bp) |
|---|---|---|
| ToxA | Forward: GCCGATCTACACCATCGAGA Reverse: CATCTCGTTGCTCTCGTGC |
94 |
| RhlA | Forward: TGATCACCAAGGACGACGAG Reverse: GCCAGCAGCGTGGAGATAC |
106 |
| LasB | Forward: GACCCACAAGCTGTACATGAAG Reverse: CCAGCGGATAGAACATGGTG |
110 |
| LasI | Forward: ACTCAGCCGTTTCGCCAT Reverse: TCATCTTCTCCACGCCTACG |
152 |
| RhlI | Forward: ATTCTGGTCCAGCCTGCAA Reverse: CTGGAGGATCACGCCGTT |
109 |
| RpoD | Forward: AGAGAAGGACGACGAGGAAGAAG Reverse: GGCCAGGCCGGTGAGTTC |
193 |
2.8. Statistical Analysis
SPSS 17.0 was used for the statistical analysis. The measurement data were expressed as mean ± SD and compared between the two groups using Student's t-test. A p value less than 0.05 was considered to be statistically significant.
3. Results
3.1. Growth of P. aeruginosa under Negative Pressure
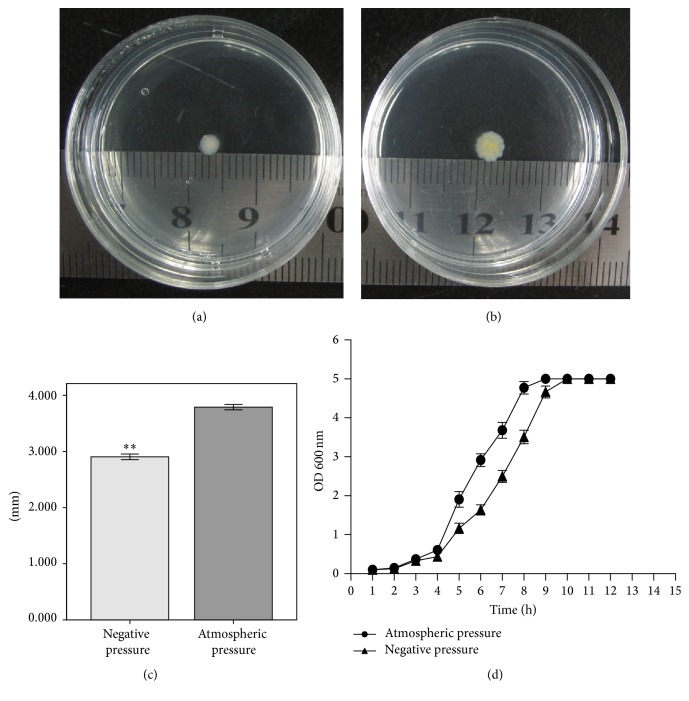
The diameters of the colonies of the two groups are shown in Figures 1(a) and 1(b). Colonies in both groups were round in shape. However, colonies under negative pressure were light in color. Moreover, the size of colonies under negative pressure was significantly smaller than that of the controls (p < 0.01, Figure 1(c)). The OD for the growth curve of P. aeruginosa was measured at 600 nm and it is shown in Figure 1(d). The growth rate of bacteria under negative pressure was less than that under atmospheric pressure from the third hour. Besides, the time to reach maximum OD (OD at 600 nm, 5.0) was 1 hour longer in the experimental group than that in the control group.
Figure 1.
Colony of P. aeruginosa under negative pressure (a) and atmospheric pressure (b) at 24 h. (c) Diameters of colony of P. aeruginosa in two groups at 24 h, N = 10, ∗∗ p < 0.01. (d) Growth curve of P. aeruginosa. OD 600 nm value was recorded per hour (N = 3).
3.2. Effect of Negative Pressure on the Production of Virulence Factors
Bacteria were cultured under negative pressure or atmospheric pressure for 24 h. The content of exotoxin A, rhamnolipid, and elastase secreted by P. aeruginosa was measured to evaluate the effect of negative pressure on the main virulence factors. Exotoxin A in the negative pressure group was significantly less than that in the control group (p < 0.01) (Figure 2(a)). A similar effect was observed for rhamnolipid and elastase (p < 0.01 and p < 0.05, resp.) (Figures 2(b) and 2(c)).
Figure 2.
Detection of virulence factors following negative pressure and atmospheric pressure at 24 h. Production of exotoxin A (a), rhamnolipid (b), and elastase (c) in negative pressure group was significantly less than that in atmospheric pressure group. ∗ p < 0.05, ∗∗ p < 0.01, and N = 10.
3.3. Biofilm Formation
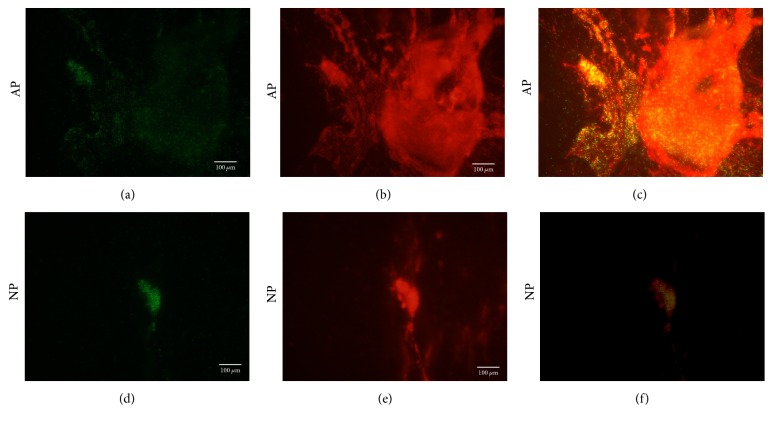
Biofilm formation was observed in both the atmospheric pressure (AP) group and the negative pressure (NP) group through fluorescence microscopy at 24 h. P. aeruginosa (green) were observed to be big aggregates with excessive biofilm (red) under atmospheric pressure (Figures 3(a)–3(c)). However, P. aeruginosa (green) were observed to be small aggregates with a small amount of biofilm (red) under negative pressure (Figures 3(d)–3(f)).
Figure 3.
The P. aeruginosa glycocalyx was visualized by staining with 50 μg/mL of Alexa Fluor 647 conjugate of Con A. Static biofilm assays under atmospheric pressure (AP) and negative pressure (NP) were shown. P. aeruginosa (green) under negative pressure conditions (d–f) were apt to be small aggregates and exhibited a reduced capacity for biofilm (red) adherence on the cover glass relative to the control (a–c) at 24 h.
3.4. Negative Pressure Changes Virulence and Biofilm-Regulated Genes in P. aeruginosa
To investigate the mechanism of negative pressure induction in reducing virulence of P. aeruginosa, quantitative real-time PCR was used to assess relative expression levels of ToxA, RhlA, LasB, LasI, and RhlI genes. Negative pressure was found to significantly inhibit the transcription of ToxA, RhlA, LasB, LasI, and RhlI and the expression of these genes in the negative pressure group was 0.3-, 0.7-, 0.68-, 0.21-, 0.11-fold that of the control group, respectively (Figure 4). The repression of these genes under negative pressure supports the observed reduction in virulence factors and biofilm formation of P. aeruginosa.
Figure 4.
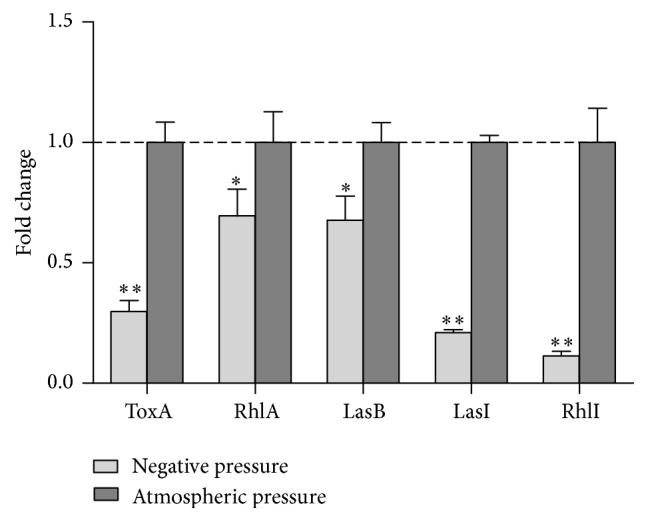
Analysis of virulence and biofilm-regulated genes, ToxA, RhlA, LasB, LasI, and RhlI, under negative pressure and atmospheric pressure. The atmospheric pressure group was used as calibrator with a value of 1, ∗ p < 0.05, ∗∗ p < 0.01, and N = 3. Expression of ToxA, RhlA, LasB, LasI, and RhlI in negative pressure group was 0.3-, 0.7-, 0.68-, 0.21-, and 0.11-fold that of the control group, respectively.
4. Discussion
In recent years, physical therapies have been increasingly popular in the management of contaminated wounds owing to their satisfying wound closure and low risk of microbial resistance [25]. In particular, NPWT has been shown to promote the healing rates and prevent wound infections by multiple mechanisms, including decreasing edema, removal of wound exudates, and translating physical stimulation to signal transduction in cells [26, 27]. Previous studies indicated that negative pressure conditions caused by NPWT could alter the gene expression and the function of host cells in vitro, such as bone marrow MSCs and keratinocytes [19, 20, 28]. However, its potential effects on P. aeruginosa and virulence factors have not been studied yet. In this study, we investigated the effect of negative pressure on the proliferation, virulence factor secretion, and virulence-regulated gene expression of P. aeruginosa, which is one of the most frequently isolated pathogens during wound infections [3].
In this study, the negative pressure value (−125 mmHg) was consistent with the clinical use of negative pressure in NPWT, and the O2 tension was kept at 20% during bacterial culture in order to reduce interference from low oxygenation [29]. Colony diameter and growth curve indicated that negative pressure conditions could significantly inhibit the proliferation and growth rate of P. aeruginosa. Physical stimulations caused by pressure variation may contribute to this inhibition. Similarly, Liu et al. found that NPWT could decrease proliferation of P. aeruginosa within the burn wound and reduce mortality in a murine model [6]. Previous studies have found that physical stimulations, such as shear stress and hydrostatic pressure, could decrease the growth rate of S. aureus, attenuate bacterial virulence, and increase susceptibility to antimicrobial treatment [30, 31]. Furthermore, significant decrease in metabolic functions, such as carbohydrate metabolism and protein synthesis, was also observed under shear stress conditions [32]. Thus, it is hypothesized that negative pressure might inhibit the growth of P. aeruginosa by altering the metabolic rate.
Exotoxin A, rhamnolipid, and elastase are the main virulence factors secreted by P. aeruginosa, which play an important role in impeding wound healing and inflammatory reaction. It was reported that exotoxin A-producing strains showed a 20-fold increase in virulence in a murine model compared with exotoxin A-deficient mutants [33]. Rhamnolipid is known for its heat-stable extracellular hemolytic properties [34]. Elastase-producing P. aeruginosa isolates have been shown to significantly degrade human wound fluid as well as human skin proteins ex vivo [9]. Detection of these virulence factors indicated that they could be inhibited by negative pressure. One previous study has found that NPWT could evacuate toxins and exudates with the fluids from the wounds, which is one of its primary mechanisms [27]. However, results in our study might provide another promising explanation for NPWT in removing toxins from the wounds. Biofilm formation was supposed to be the key factor in resulting chronic infection [12, 35]. Our results indicated that P. aeruginosa tended to gather in small aggregates with a few biofilms under negative pressure, as compared to that under atmospheric pressure. In order to further investigate the mechanism of reduction in virulence factors and biofilm formation under negative pressure, the expression of virulence-regulated genes was analyzed. Results showed that these genes were repressed by negative pressure, which supported the observed reduction in virulence factors and biofilm formation of P. aeruginosa. Therefore, the influence of negative pressure on the production of exotoxin A, rhamnolipid, and elastase and biofilm formation might mainly depend on the inhibition of the ToxA, RhlA, LasB, LasI, and RhlI genes. This study has some limitations. First, all detections were carried out at 24 h after interventions. No long-term observation was available because the bacterial growth was inhibited by limited culture medium. Besides, only three virulence factors and five regulatory genes associated with wound infections were investigated in this study. As P. aeruginosa secretes several virulence factors, it is necessary to explore other toxins and regulatory genes in the future.
In conclusion, negative pressure could significantly inhibit the growth of P. aeruginosa. It also led to a decrease in the virulence factor secretion, biofilm formation, and a reduction in the expression level of virulence-regulated genes. This study indicated that a topical negative pressure condition, such as that used in NPWT, has the potential to be a novel anti-infection strategy to prevent and treat wound infections caused by P. aeruginosa.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (no. 81472112).
Competing Interests
The authors declare that they have no conflict of interests.
Authors' Contributions
Guo-Qi Wang, Tong-Tong Li, and Zhi-Rui Li contributed equally to this work.
References
- 1.Holmes C. J., Plichta J. K., Gamelli R. L., Radek K. A. Dynamic role of host stress responses in modulating the cutaneous microbiome: implications for wound healing and infection. Advances in Wound Care. 2015;4(1):24–37. doi: 10.1089/wound.2014.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assadian O., Assadian A., Stadler M., Diab-Elschahawi M., Kramer A. Bacterial growth kinetic without the influence of the immune system using vacuum-assisted closure dressing with and without negative pressure in an in vitro wound model. International Wound Journal. 2010;7(4):283–289. doi: 10.1111/j.1742-481X.2010.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moet G. J., Jones R. N., Biedenbach D. J., Stilwell M. G., Fritsche T. R. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004) Diagnostic Microbiology and Infectious Disease. 2007;57(1):7–13. doi: 10.1016/j.diagmicrobio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Watters C., DeLeon K., Trivedi U., et al. Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Medical Microbiology and Immunology. 2013;202(2):131–141. doi: 10.1007/s00430-012-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seth A. K., Geringer M. R., Galiano R. D., Leung K. P., Mustoe T. A., Hong S. J. Quantitative comparison and analysis of species-specific wound biofilm virulence using an in vivo, rabbit-ear model. Journal of the American College of Surgeons. 2012;215(3):388–399. doi: 10.1016/j.jamcollsurg.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y., Zhou Q., Wang Y., et al. Negative pressure wound therapy decreases mortality in a murine model of burn-wound sepsis involving pseudomonas aeruginosa infection. PLoS ONE. 2014;9(2) doi: 10.1371/journal.pone.0090494.e90494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heggers J. P., Haydon S., Ko F., Hayward P. G., Carp S., Robson M. C. Pseudomonas aeruginosa exotoxin a: its role in retardation of wound healing: the 1992 lindberg award. Journal of Burn Care and Rehabilitation. 1992;13(5):512–518. doi: 10.1097/00004630-199209000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Jensen P. Ø., Bjarnsholt T., Phipps R., et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology. 2007;153(5):1329–1338. doi: 10.1099/mic.0.2006/003863-0. [DOI] [PubMed] [Google Scholar]
- 9.Schmidtchen A., Holst E., Tapper H., Björck L. Elastase-producing Pseudomonas aeruginosa degrade plasma proteins and extracellular products of human skin and fibroblasts, and inhibit fibroblast growth. Microbial Pathogenesis. 2003;34(1):47–55. doi: 10.1016/s0882-4010(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 10.Pastar I., Nusbaum A. G., Gil J., et al. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0056846.e56846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Gennip M., Christensen L. D., Alhede M., et al. Interactions between polymorphonuclear leukocytes and Pseudomonas aeruginosa biofilms on silicone implants in vivo. Infection and Immunity. 2012;80(8):2601–2607. doi: 10.1128/iai.06215-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao G., Hochwalt P. C., Usui M. L., et al. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair and Regeneration. 2010;18(5):467–477. doi: 10.1111/j.1524-475x.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagami G., Morohoshi T., Ikeda T., et al. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in pressure ulcer infection in rats. Wound Repair and Regeneration. 2011;19(2):214–222. doi: 10.1111/j.1524-475x.2010.00653.x. [DOI] [PubMed] [Google Scholar]
- 14.Blum M. L., Esser M., Richardson M., Paul E., Rosenfeldt F. L. Negative pressure wound therapy reduces deep infection rate in open tibial fractures. Journal of Orthopaedic Trauma. 2012;26(9):499–505. doi: 10.1097/BOT.0b013e31824133e3. [DOI] [PubMed] [Google Scholar]
- 15.Seo S. G., Yeo J. H., Kim J. H., Kim J.-B., Cho T.-J., Lee D. Y. Negative-pressure wound therapy induces endothelial progenitor cell mobilization in diabetic patients with foot infection or skin defects. Experimental and Molecular Medicine. 2013;45(11, article e62) doi: 10.1038/emm.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weed T., Ratliff C., Drake D. B., et al. Quantifying bacterial bioburden during negative pressure wound therapy: Does the Wound VAC Enhance Bacterial Clearance? Annals of Plastic Surgery. 2004;52(3):276–279. doi: 10.1097/01.sap.0000111861.75927.4d. [DOI] [PubMed] [Google Scholar]
- 17.Lalliss S. J., Stinner D. J., Waterman S. M., Branstetter J. G., Masini B. D., Wenke J. C. Negative pressure wound therapy reduces pseudomonas wound contamination more than Staphylococcus aureus . Journal of Orthopaedic Trauma. 2010;24(9):598–602. doi: 10.1097/bot.0b013e3181ec45ba. [DOI] [PubMed] [Google Scholar]
- 18.Krishna S., Miller L. S. Host-pathogen interactions between the skin and Staphylococcus aureus . Current Opinion in Microbiology. 2012;15(1):28–35. doi: 10.1016/j.mib.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z., Yao J.-F., Xu P., et al. Functions and mechanisms of intermittent negative pressure for osteogenesis in human bone marrow mesenchymal stem cells. Molecular Medicine Reports. 2014;9(4):1331–1336. doi: 10.3892/mmr.2014.1968. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y.-G., Yang Z., Zhang H., et al. Effect of negative pressure on human bone marrow mesenchymal stem cells in vitro. Connective Tissue Research. 2010;51(1):14–21. doi: 10.3109/03008200902855891. [DOI] [PubMed] [Google Scholar]
- 21.Li T., Wang G., Yin P., et al. Effect of negative pressure on growth, secretion and biofilm formation of Staphylococcus aureus . Antonie van Leeuwenhoek. 2015;108(4):907–917. doi: 10.1007/s10482-015-0545-9. [DOI] [PubMed] [Google Scholar]
- 22.Shigematsu T., Suda N., Okuda K., Fukushima J. Reliable enzyme-linked immunosorbent assay systems for pathogenic factors of Pseudomonas aeruginosa alkaline proteinase, elastase, and exotoxin A: a comparison of methods for labeling detection antibodies with horseradish peroxidase. Microbiology and Immunology. 2007;51(12):1149–1159. doi: 10.1111/j.1348-0421.2007.tb04010.x. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y.-X., Xu Z.-H., Zhang Y.-Q., Tian J., Weng L.-X., Wang L.-H. A new quorum-sensing inhibitor attenuates virulence and decreases antibiotic resistance in Pseudomonas aeruginosa . Journal of Microbiology. 2012;50(6):987–993. doi: 10.1007/s12275-012-2149-7. [DOI] [PubMed] [Google Scholar]
- 24.Pearson J. P., Pesci E. C., Iglewski B. H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. Journal of Bacteriology. 1997;179(18):5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White N. T., Delitto A., Manal T. J., Miller S. The American physical therapy association’s top five choosing wisely recommendations. Physical Therapy. 2015;95(1):9–24. doi: 10.2522/ptj.20140287. [DOI] [PubMed] [Google Scholar]
- 26.Streubel P. N., Stinner D. J., Obremskey W. T. Use of negative-pressure wound therapy in orthopaedic trauma. The Journal of the American Academy of Orthopaedic Surgeons. 2012;20(9):564–574. doi: 10.5435/JAAOS-20-09-564. [DOI] [PubMed] [Google Scholar]
- 27.Huang C., Leavitt T., Bayer L. R., Orgill D. P. Effect of negative pressure wound therapy on wound healing. Current Problems in Surgery. 2014;51(7):301–331. doi: 10.1067/j.cpsurg.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Hsu C.-C., Chow S.-E., Chen C. P.-C., et al. Negative pressure accelerated monolayer keratinocyte healing involves Cdc42 mediated cell podia formation. Journal of Dermatological Science. 2013;70(3):196–203. doi: 10.1016/j.jdermsci.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Hsu C.-C., Tsai W.-C., Chen C. P.-C., Lu Y.-M., Wang J.-S. Effects of negative pressures on epithelial tight junctions and migration in wound healing. American Journal of Physiology—Cell Physiology. 2010;299(2):C528–C534. doi: 10.1152/ajpcell.00504.2009. [DOI] [PubMed] [Google Scholar]
- 30.Castro S. L., Nelman-Gonzalez M., Nickerson C. A., Ott C. M. Induction of attachment-independent biofilm formation and repression of hfq expression by low-fluid-shear culture of Staphylococcus aureus. Applied and Environmental Microbiology. 2011;77(18):6368–6378. doi: 10.1128/AEM.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakimbugwe D., Masschalck B., Atanassova M., Zewdie-Bosüner A., Michiels C. W. Comparison of bactericidal activity of six lysozymes at atmospheric pressure and under high hydrostatic pressure. International Journal of Food Microbiology. 2006;108(3):355–363. doi: 10.1016/j.ijfoodmicro.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Islam N., Kim Y., Ross J. M., Marten M. R. Proteomic analysis of Staphylococcus aureus biofilm cells grown under physiologically relevant fluid shear stress conditions. Proteome Science. 2014;12(1, article 21) doi: 10.1186/1477-5956-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyazaki S., Matsumoto T., Tateda K., Ohno A., Yamaguchi K. Role of exotoxin A in inducing severe Pseudomonas aeruginosa infections in mice. Journal of Medical Microbiology. 1995;43(3):169–175. doi: 10.1099/00222615-43-3-169. [DOI] [PubMed] [Google Scholar]
- 34.Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infection and Immunity. 1980;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seth A. K., Geringer M. R., Gurjala A. N., et al. Treatment of Pseudomonas aeruginosa biofilm–infected wounds with clinical wound care strategies: a quantitative study using an in vivo rabbit ear model. Plastic and Reconstructive Surgery. 2012;129(2):262e–274e. doi: 10.1097/prs.0b013e31823aeb3b. [DOI] [PubMed] [Google Scholar]