Abstract
Background
Strictures are frequent complications of large endoscopic mucosal resections (EMR) and endoscopic submucosal dissections of the esophagus. Local or systemic steroid therapy has shown promise in the prevention of secondary stenosis. The aim of this study was to evaluate the safety and efficacy of systemic steroid therapy following endoscopic resection of at least hemi-circumferential esophageal mucosa.
Methods
This was a single-center retrospective study in a tertiary center. We evaluated patients who were treated with oral steroids between July 2013 and September 2015, after undergoing a large EMR for Barrett’s esophagus associated with dysplasia or carcinoma. The steroid protocol used was an initial dose of 30 mg prednisolone, tapered over 8 weeks. Exclusion criteria were a previous attempt at radiofrequency ablation or resection.
Results
Thirty-one patients (27 men) were analyzed: 13 with low-grade dysplasia Barrett’s esophagus, 16 with in situ adenocarcinoma, 1 with pT1SM1 adenocarcinoma, and 1 with pT1SM2 adenocarcinoma. Twenty-eight resections (28/31) were completed (R0) in 1-3 sessions (median 2), while 3 resections were R1. The median length of Barrett’s esophagus was C3M5 (range C0M2-C10M11) according to the Prague classification. The median follow up was 10 months (range 4-17), during which 4 patients (13%) developed a secondary stenosis. All stenoses were successfully treated by endoscopic dilation (range 1-4). No complications related to dilation or to the steroid therapy were observed.
Conclusions
Our rate of secondary stricture was lower than expected, given the rates of 17-88% in published studies. Systemic oral steroid therapy seems to be effective in reducing potential esophageal stenosis after EMR. Complementary randomized studies are required to confirm whether systemic steroids are an effective primary prophylaxis for esophageal stenosis.
Keywords: Esophagus, endoscopic mucosal resection, esophageal strictures, oral steroid therapy, Barrett’s esophagus
Introduction
In recent decades, the management of superficial esophageal lesions has evolved significantly with the addition of therapeutic endoscopy, i.e., endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). EMR for Barrett’s esophagus with high-grade dysplasia (HGD) and intramucosal adenocarcinoma (IMC) is performed by multiband or cap mucosectomy, with a success rate of up to 87% for neoplasia [1-4]. Furthermore, after complete endoscopic resection of Barrett’s esophagus with HGD or IMC, the incidence of metachronous neoplasia appears to decrease [5-7]. Complete Barrett’s eradication can be performed by endoscopic resection or radiofrequency ablation, but its effectiveness is limited because of the high risk of iatrogenic stricture, which ranges between 17% and 88% [4,5,8,9]. Treatment of these stenosis requires multiple sessions of dilation, impairing the patient’s quality of life.
Previous studies have shown that either local [10-12] or systemic corticosteroids [13-15] can effectively reduce the need for endoscopic balloon dilation after large ESD. We report our experience from using prophylaxis with oral steroids to prevent iatrogenic stenosis after endoscopic resection of Barrett’s esophagus extending to at least half the circumference. The primary aim of our retrospective study was to determine the rate of stricture under oral steroid prophylaxis after large endoscopic resection. Our secondary aim was to determine the association of this preventive strategy with adverse effects and complications.
Patients and methods
Patients
We retrospectively included all patients who underwent large EMR for Barrett’s esophagus between July 2013 and September 2015 and were treated preventively with oral corticosteroids. Large EMR was defined as resection of more than half of the esophageal mucosa circumference. Indications for EMR were confirmed IMC, HGD, or low-grade dysplasia (LGD) Barrett’s esophagus. Patients were excluded if they received additional radiofrequency treatment, or if they had a known history of psychiatric illness, diabetes or osteoporosis. Oral informed consent to the EMR, steroid therapy, and dilation was obtained from all patients.
Treatment protocol
EMR was performed by 4 operators experienced in esophageal EMR and 2 operators with poor experience in esophageal EMR under their direct supervision. Barrett’s esophagus was examined under high-definition white-light endoscopy (EG29-i10, EG-2990Zi, PENTAX MEDICAL©, Tokyo, JAPAN) and, if necessary, by chromoendoscopy with acetic acid. All resections were performed using a multiband mucosectomy technique (Duette; Cook Medical, Winston-Salem NC). If necessary, a submucosal injection of saline mixed with indigo carmine was performed. A microprocessor-controlled electrosurgical generator (ERBE VIO 300; ERBE, Tübingen, Germany) was used. Resection was performed from the palisade vessels of the gastroesophageal junction distally and was extended to remove at least a hemi-circumference of Barrett’s esophagus (Fig. 1). The Prague classification for Barrett’s esophagus [16] was used in all patients and the Paris classification [17] was used for nodular or suspicious lesions. Lesions classified 0-IIc and 0-III by the Paris classification were considered ineligible for EMR. For simplicity, we used the codes R0 for healthy vertical margins and R1 for positive vertical margins (lateral margins could not be evaluated given the large piecemeal EMR).
Figure 1.
Example of large esophageal endoscopic mucosal resection
Oral prednisolone was started 1 day after EMR in a dosage of 30 mg/day. The dose was then gradually tapered in decrements of 5 mg/day every 2 weeks for 1 month, followed by decrements of 5 mg/day every week for the next 4 weeks.
Follow up
Gastroscopy was scheduled at 2, 6, 12, and 24 months after EMR, with biopsies if necessary, and a new EMR session if the initial treatment was incomplete. Any symptomatic esophageal stricture was managed by hydrostatic endoscopic dilations (Hercule; Cook Medical 12 mm/15 mm/18 mm, Winston-Salem, NC). Dilation was performed up to a maximum of 18 mm or until a laceration or tear was seen on direct visualization.
Statistical analysis
Patient demographics and Barrett’s esophagus characteristics were retrieved from a computer database. All data were analyzed retrospectively.
Results
Between July 2013 and September 2015, 31 patients (27 men, 4 women, sex ratio 7:1; mean age 63 years) with biopsy-proven Barrett’s LGD, HGD or IMC met the inclusion criteria. Patients’ characteristics and resection details are shown in Table 1.
Table 1.
Patient demographics and clinical outcome features
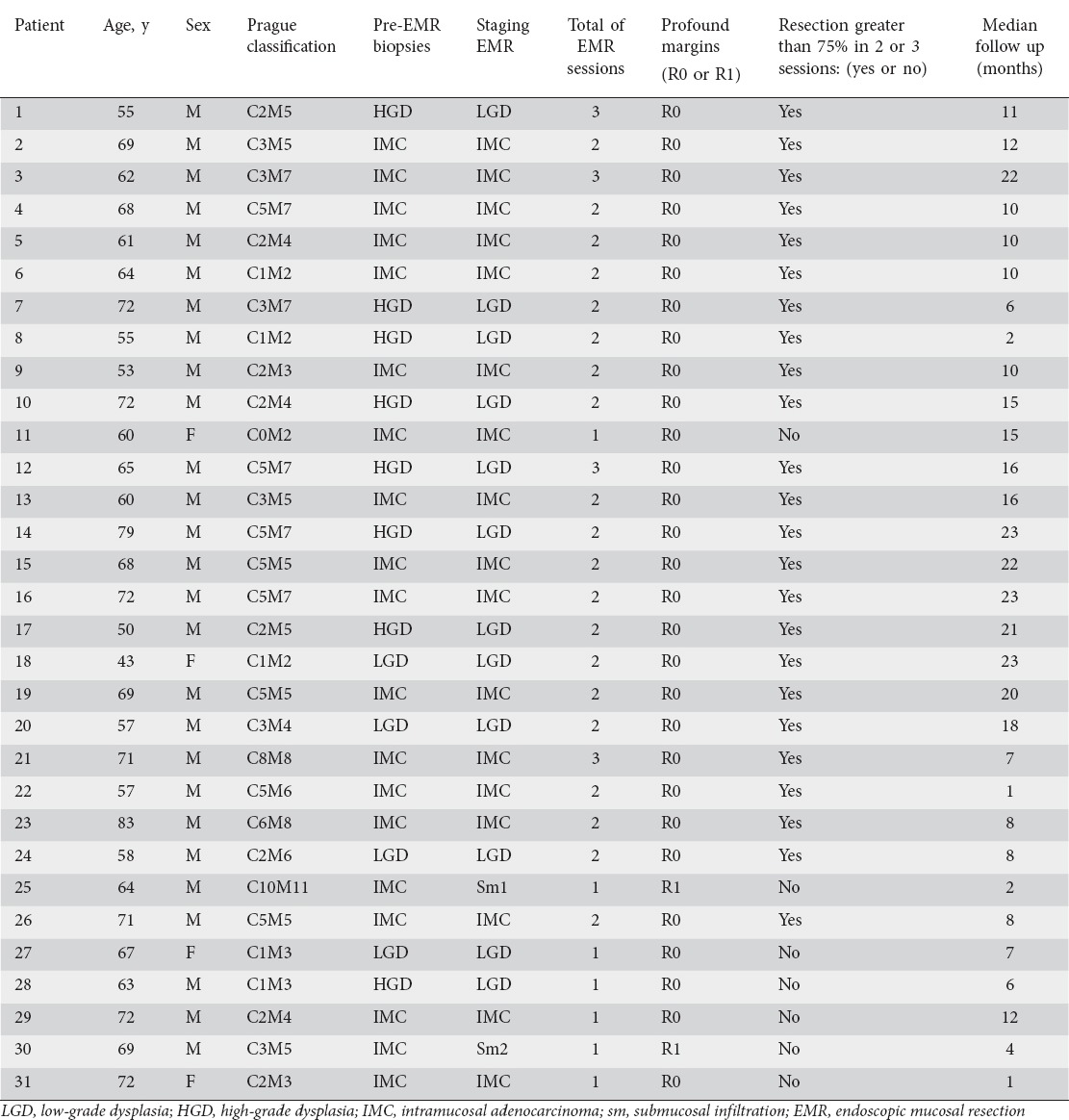
The median circumferential extent and maximum Barrett’s mucosa length were respectively 3 cm (range C0-C10) and 5 cm (range M2-M11). Pre-EMR histology showed 19 patients with IMC, 8 with HGD and 4 with LGD. Based on the EMR specimen, definitive histology was LGD in 13 patients, IMC in 16 and submucosal carcinoma in 2 (sm1=1, sm2=1). The resection was complete (R0) in 28 patients, requiring 1-3 sessions (median 2). Both patients with submucosal cancer had positive vertical margins (R1). They underwent surgery and definitive histology was pT1bN0M0.
Oral prednisolone in a dosage of 30 mg/day was started the day after EMR, once the patients were permitted oral intake. All patients included in this study followed the steroid protocol.
Immediate and secondary adverse events were recorded during a median follow up of 10 months (range 1-22). No digestive perforation occurred in any case. Two patients had significant bleeding requiring endoscopic hemostasis. One patient died from a sudden and massive hemorrhage 48 h after the second EMR session.
Four patients (13%) required endoscopic balloon dilation for symptomatic esophageal stricture. The endoscopic dilation was effective in all cases, requiring 1-4 sessions (median 2). The mean dilation diameter was 16.5 mm [15 mm-18 mm]. There were no adverse events related to oral steroids. The study design is shown in Fig. 2.
Figure 2.
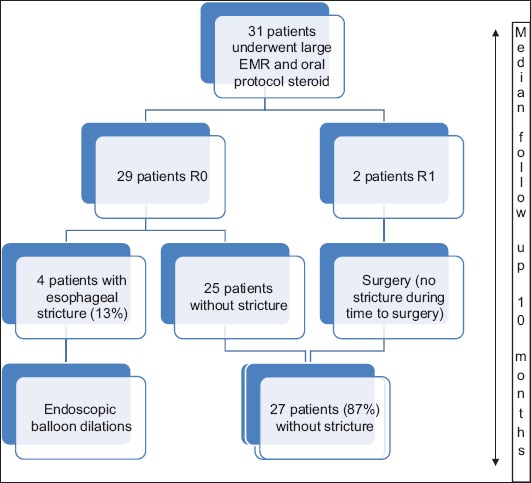
Study design R0, healthy vertical margins; R1, positive vertical margins; EMR, endoscopic mucosal resection
Discussion
Endoscopic resection has recently been accepted as first-line therapy for early esophageal cancers, such as squamous cell carcinoma and dysplasia of Barrett’s esophagus. Secondary esophageal stricture is a common and well known complication after a large resection. It necessitates some iterative endoscopic procedures and hospitalizations, and may delay or compromise treatment if more sessions are needed. Prophylactic self-expandable metal stent insertion has been proposed, but the morbidity rate is excessive [18]. Anti-inflammatory approaches for preventing esophageal strictures after endoscopic resection are based on the concept that subsequent strictures may be suppressed by inhibiting the infiltration of inflammatory cells, the hyperplasia of granulation, and fibrosis. Local injection of triamcinolone seems to be effective, but is of limited use in large circumferential resections [10,11]. Furthermore, it may cause an ulcer at the injection site.
Several research teams have studied preventive systemic corticosteroid therapy and have reported promising early results [13-15]. However, high doses of prednisolone have been associated with adverse effects, such as severe infections, peptic ulcers, hyperglycemia, psychiatric symptoms, and osteoporosis [19].
Since the publication of the above results, we have been using a low dose of systemic corticosteroids to reduce the stricture rate post EMR. This study presents the results from clinical practice in our center. This retrospective study provides more support for the use of systemic corticosteroids in this context. Indeed, the stricture rate (13%) was much lower than the 17-88% reported in the literature [4,5,8,9]. We use relatively low doses over a limited period and it seems unlikely that this has an impact in the long term. Moreover, the stricture rate increases drastically when 75% of the circumference is involved [20] and in our study 24/31 patients underwent circumferential resection in 2 to 3 sessions (Table 1). The other patients (7/31) underwent resection in 1 session of esophageal mucosa larger than the half-circumference, but less than 75% of the esophageal lumen (Table 1).
There were 3 early (within 48 h) adverse events (3 hemorrhages and no perforation). Two hemorrhages were treated endoscopically. One patient had a sudden fatal hemorrhagic shock 48 h after the second EMR session. None of the complications were related to steroid therapy. The two patients with submucosal infiltration underwent laparoscopic transhiatal esophago-gastrectomy.
Our study is, of course, limited by being a retrospective analysis and by the relatively small number of patients. Indeed, only 31 patients were able to benefit from the steroid therapy protocol in its entirety. Some patients refused and the institution of the protocol was at the discretion of the operator. Although the treatment duration was short (8 weeks), patients with a psychiatric history, known diabetes or osteoporosis were not treated. Furthermore, we excluded from this analysis patients who underwent radiofrequency ablation after EMR, so as not to add a confounding factor.
The findings of our study are consistent with those reported in the literature, and the side effects appear to be mild, although this will need to be verified by an intention-to-treat study.
In conclusion, esophageal strictures cause dysphagia, which obliges the patient to undergo repeated balloon dilation procedures or implantation of temporary stents. These esophageal strictures decrease the patient’s quality of life and have their own potential complications (perforation, failure). Anti-inflammatory approaches are promising and have been shown to be effective in several studies. However, a multicenter randomized study will be needed to determine the best route of administration and other related factors (local or oral and posology). We believe that several surgical resections related to this complication could be avoided. With developments in the ESD technique the stricture rate may increase [21]. In the future, a tissue-engineering approach could be another line of research, along with cell therapies [22,23]. New biological agents are also promising [24].
Summary Box.
What is already known:
Secondary esophageal stricture after large resection is a common and well known complication
Oral or local steroids have shown very promising results in several research teams
The development of new endoscopic resection techniques, such as endoscopic submucosal resection (EMR), will increase the iatrogenic stricture rate
What the new findings are:
This study confirms the potential value of oral steroid therapy in large EMR
This therapy appears to be safe in low doses, without frequent adverse effects
Oral steroid therapy should be considered for any resection involving more than 50% of the circumference
Biography
Paoli-Calmettes Institute, Marseille, France
Footnotes
Conflict of Interest: None
References
- 1.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–1206. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 2.Saligram S, Chennat J, Hu H, Davison JM, Fasanella KE, McGrath K. Endotherapy for superficial adenocarcinoma of the esophagus: an American experience. Gastrointest Endosc. 2013;77:872–876. doi: 10.1016/j.gie.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett’s oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010;59:1169–1177. doi: 10.1136/gut.2010.210229. [DOI] [PubMed] [Google Scholar]
- 4.Lopes CV, Hela M, Pesenti C, et al. Circumferential endoscopic resection of Barrett’s esophagus with high-grade dysplasia or early adenocarcinoma. Surg Endosc. 2007;21:820–824. doi: 10.1007/s00464-006-9187-3. [DOI] [PubMed] [Google Scholar]
- 5.Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma—an American single-center experience. Am J Gastroenterol. 2009;104:2684–2692. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 6.Wani S, Puli SR, Shaheen NJ, et al. Esophageal adenocarcinoma in Barrett’s esophagus after endoscopic ablative therapy: a meta-analysis and systematic review. Am J Gastroenterol. 2009;104:502–513. doi: 10.1038/ajg.2008.31. [DOI] [PubMed] [Google Scholar]
- 7.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 8.van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765–773. doi: 10.1136/gut.2010.229310. [DOI] [PubMed] [Google Scholar]
- 9.Seewald S, Akaraviputh T, Seitz U, et al. Circumferential EMR and complete removal of Barrett’s epithelium: a new approach to management of Barrett’s esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc. 2003;57:854–859. doi: 10.1016/s0016-5107(03)70020-0. [DOI] [PubMed] [Google Scholar]
- 10.Hanaoka N, Ishihara R, Takeuchi Y, et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: a controlled prospective study. Endoscopy. 2012;44:1007–1011. doi: 10.1055/s-0032-1310107. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto S, Kobayashi M, Takeuchi M, Sato Y, Narisawa R, Aoyagi Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1389–1393. doi: 10.1016/j.gie.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 12.Bahin FF, Jayanna M, Williams SJ, Lee EY, Bourke MJ. Efficacy of viscous budesonide slurry for prevention of esophageal stricture formation after complete endoscopic mucosal resection of short-segment Barrett’s neoplasia. Endoscopy. 2016;48:71–74. doi: 10.1055/s-0034-1392603. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2011;73:1115–1121. doi: 10.1016/j.gie.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Sato H, Inoue H, Kobayashi Y, et al. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc. 2013;78:250–257. doi: 10.1016/j.gie.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Isomoto H, Yamaguchi N, Minami H, Nakao K. Management of complications associated with endoscopic submucosal dissection/endoscopic mucosal resection for esophageal cancer. Dig Endosc. 2013;25(Suppl 1):29–38. doi: 10.1111/j.1443-1661.2012.01388.x. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C &M criteria. Gastroenterology. 2006;131:1392–1399. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 17.The Paris endoscopic classification of superficial neoplastic lesions:esophagus, stomach, and colon: November 30 to December 1 2002. Gastrointest Endosc. 2003;58(6 Suppl):S3–S43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 18.Holt BA, Jayasekeran V, Williams SJ, et al. Early metal stent insertion fails to prevent stricturing after single-stage complete Barrett’s excision for high-grade dysplasia and early cancer. Gastrointest Endosc. 2015;81:857–864. doi: 10.1016/j.gie.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Huscher D, Thiele K, Gromnica-Ihle E, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009 Jul;68(7):1119–24. doi: 10.1136/ard.2008.092163. [DOI] [PubMed] [Google Scholar]
- 20.Hanaoka N, Ishihara R, Uedo N, et al. Refractory strictures despite steroid injection after esophageal endoscopic resection. Endosc Int Open. 2016;4:E354–E359. doi: 10.1055/s-0042-100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chevaux JB, Piessevaux H, Jouret-Mourin A, Yeung R, Danse E, Deprez PH. Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett’s neoplasia. Endoscopy. 2015;47:103–112. doi: 10.1055/s-0034-1390982. [DOI] [PubMed] [Google Scholar]
- 22.Takagi R, Yamato M, Murakami D, et al. Fabrication and validation of autologous human oral mucosal epithelial cell sheets to prevent stenosis after esophageal endoscopic submucosal dissection. Pathobiology. 2011;78:311–319. doi: 10.1159/000322575. [DOI] [PubMed] [Google Scholar]
- 23.Ohki T, Yamato M, Ota M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588. doi: 10.1053/j.gastro.2012.04.050. e1-e2. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi Y, Tsuji Y, Ono S, et al. Polyglycolic acid sheets with fibrin glue can prevent esophageal stricture after endoscopic submucosal dissection. Endoscopy. 2015;47:336–340. doi: 10.1055/s-0034-1390787. [DOI] [PubMed] [Google Scholar]


