Abstract
Podocyte injury is the inciting event in primary glomerulopathies, such as minimal change disease and primary FSGS, and glucocorticoids remain the initial and often, the primary treatment of choice for these glomerulopathies. Because inflammation is not readily apparent in these diseases, understanding the direct effects of glucocorticoids on the podocyte, independent of the immunomodulatory effects, may lead to the identification of targets downstream of glucocorticoids that minimize toxicity without compromising efficacy. Several studies showed that treatment with glucocorticoids restores podocyte differentiation markers and normal ultrastructure and improves cell survival in murine podocytes. We previously determined that Krüppel–like factor 15 (KLF15), a kidney–enriched zinc finger transcription factor, is required for restoring podocyte differentiation markers in mice and human podocytes under cell stress. Here, we show that in vitro treatment with dexamethasone induced a rapid increase of KLF15 expression in human and murine podocytes and enhanced the affinity of glucocorticoid receptor binding to the promoter region of KLF15. In three independent proteinuric murine models, podocyte-specific loss of Klf15 abrogated dexamethasone–induced podocyte recovery. Furthermore, knockdown of KLF15 reduced cell survival and destabilized the actin cytoskeleton in differentiated human podocytes. Conversely, overexpression of KLF15 stabilized the actin cytoskeleton under cell stress in human podocytes. Finally, the level of KLF15 expression in the podocytes and glomeruli from human biopsy specimens correlated with glucocorticoid responsiveness in 35 patients with minimal change disease or primary FSGS. Thus, these studies identify the critical role of KLF15 in mediating the salutary effects of glucocorticoids in the podocyte.
Keywords: podocyte, glucocorticoid, focal segmental glomerulosclerosis, minimal change disease
Podocytes, the visceral glomerular epithelial cells, in normal mature kidneys are regarded as highly differentiated and quiescent cells.1 In many primary glomerular diseases, such as minimal change disease (MCD) and FSGS, podocytes are injured and undergo a major change in phenotype, resulting in a loss of their terminal differentiation markers.2,3 Although the mechanism by which podocyte injury occurs in each of these diseases may differ, the end result is a compromised filtration barrier with ensuing nephrosis.
Human inflammatory diseases are frequently treated with a prolonged course of glucocorticoids (GCs). The immunomodulatory effects of GCs are evident in these proinflammatory states.4,5 However, GCs also exhibit a therapeutic benefit in primary glomerulopathies, such as MCD, where a proinflammatory milieu is not readily apparent. In fact, the initial treatment option for MCD is high–dose GC therapy, and in many instances, alternate immunosuppressive therapy is typically not considered until patients have failed GC therapy.6 Previous studies have described the potential for GCs to have a direct effect on the podocyte by rearrangement of actin cytoskeleton, inhibiting apoptosis and regulating protein trafficking of critical slit diaphragm proteins in murine and human podocytes.7–11 In addition, glucocorticoid receptor (GR) as well as the major components of the GR complex are expressed in human podocytes.8,11 Furthermore, GCs have also been implicated in ameliorating podocyte injury11,12 and improving podocyte survival9 in murine and human cell culture models. Although there is some evidence to suggest that GCs may have an antiapoptotic effect on the podocyte,9–11 the mechanism mediating this process remains largely unexplored.
A comparative promoter analysis of podocyte slit diaphragm molecules revealed that Krüppel-Like Factor (KLF) is one of four common transcriptional binding sites on many podocyte–specific genes.13 KLFs are a subclass of the zinc finger family of DNA binding transcriptional regulators that are involved in a broad range of cellular processes (i.e., cell differentiation, angiogenesis, and erythropoiesis).14 We recently showed that KLF15, a kidney–enriched transcription factor, restores podocyte differentiation markers by transcriptionally regulating podocyte-specific genes, such as nephrin and podocin.15 Because GCs are a major treatment for podocyte injury, we initially reviewed the literature for a possible interaction between GCs and KLF15. Interestingly, a recent promoter analysis in combination with chromatin immunoprecipitation (ChIP) identified putative GR binding sites in the promoter region of KLF15.16 In addition, treatment with GCs increased KLF15 expression in multiple cell types, such as murine embryonic fibroblasts and airway smooth muscle cells.17 Therefore, we hypothesized that GC-induced restoration of podocyte differentiation markers is mediated by KLF15. Here, we show that treatment with GCs induces the expression of KLF15 early in podocytes, suggesting that KLF15 is an early inducible gene. We also observe that a podocyte-specific loss of Klf15 abrogates the beneficial effect of GCs in cell culture as well as in three proteinuric mouse models. In addition, the overexpression of KLF15 in cultured human podocytes prevents the destabilization of the actin cytoskeleton under cell stress. Finally, we show that the podocyte and glomerular expression of KLF15 strongly correlated with GC responsiveness in patients with MCD and primary FSGS.
Results
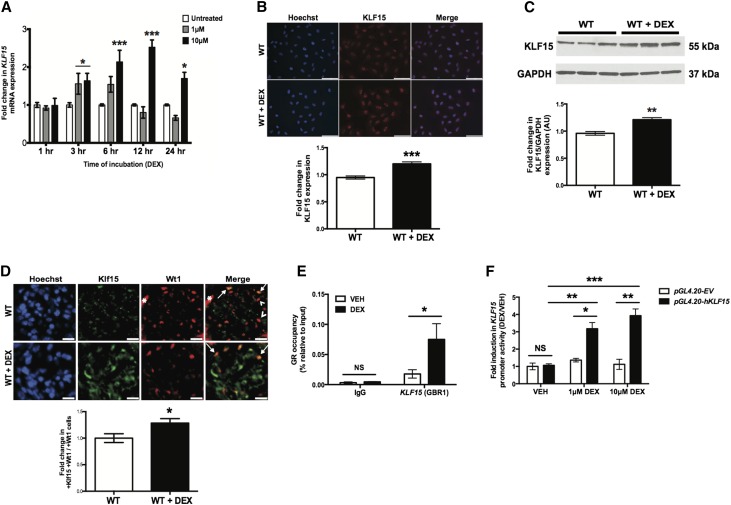
Dexamethasone Induces KLF15 Expression in Podocytes
Because previous studies have shown that KLF15 may mediate the GC-induced differentiation in murine embryonic fibroblasts,16 we initially treated cultured human podocytes with dexamethasone (DEX) and measured KLF15 expression. In comparison with vehicle–treated human podocytes, KLF15 mRNA expression was increased within 3 hours of DEX treatment and peaked at 12 hours (Figure 1A). Immunostaining and Western blot for KLF15 were performed in human podocytes treated with DEX or vehicle for 12 hours and confirmed an increase in KLF15 expression in cells treated with DEX compared with those treated with vehicle (Figure 1, B and C). In addition, we observed that Klf15 expression was increased in podocytes as well as other glomerular cells in mice treated with DEX compared with those treated with vehicle (Figure 1D). To further explore the potential of KLF15 to mediate expression of GC target genes, we performed TRANSFAC promoter analysis18 to identify GC target genes that possess transcriptional binding sites for KLF15. We subsequently performed gene set enrichment analysis on these genes with KLF15 binding sites using Enrichr.19 The NCI-Nature Pathways Gene Set Library in Enrichr revealed a significant increase in the pathways involved in GC signaling (Table 1). On the basis of these findings, we confirmed that GR binding to the promoter region of KLF15 is enhanced in response to DEX treatment in differentiated human podocytes by ChIP followed by real-time PCR (Figure 1E). To define the mechanism by which GR induces KLF15 expression, we transfected human podocytes with reporter construct directed at the KLF15 promoter region (pGL4.20-hKLF15). KLF15 promoter activity was significantly increased in pGL4.20-hKLF15 cells with DEX (1 or 10 μM) treatment compared with vehicle-treated cells and DEX–treated pGL4.20-EV (control) cells (Figure 1F). These findings strongly suggest that KLF15 mediates DEX–induced target gene expression and point to specific genes for additional analysis.
Figure 1.
KLF15 expression is increased with DEX treatment. Cultured human podocytes were initially differentiated for 14 days and subsequently treated with either DEX or vehicle (VEH) for 12 hours. RNA was extracted, and real-time PCR was performed. (A) KLF15 mRNA expression was compared between cultured human podocytes treated with and without DEX (n=6). *P<0.05; ***P<0.001 versus all groups (two–way ANOVA test with Tukey post-test). (B) Immunofluorescence staining for KLF15 with and without DEX for 12 hours is shown. The representative images of six independent experiments are shown in the upper panel. Magnification, ×20. In the lower panel, the intensity of KLF15 expression was quantified (n=6). ***P<0.001 (unpaired t test). (C) Protein was also extracted, and Western blot analysis for Klf15 was performed. The representative blot of three independent experiments is shown. The lower panel shows the quantification of Klf15 by densitometry (n=3). **P<0.01 (Mann–Whitney test). (D) This was confirmed by immunofluorescence using tissue from wild-type (WT) mice treated with and without DEX. The representative pictures of four mice in each group are shown in the upper panel. Arrows show colocalization of Klf15 and Wt1. Arrowheads show a lack of colocalization. Magnification, ×20. *Nonspecific Wt1 staining in the untreated WT mice. In the lower panel, in total, 30 glomeruli per mouse were selected, and quantification of Klf15 staining in the podocytes was determined by the ratio of Klf15+ and Wt1+ cells to Wt1+ cells (n=6). *P<0.05 (unpaired t test). (E) ChIP assay was performed to show the presence of GR binding in the promoter of KLF15 in differentiated human podocytes treated with DEX (10 μM) or VEH for 12 hours. IgG serves as control (n=6). *P<0.05 (Mann–Whitney test). (F) Human podocytes were transfected with reporter construct directed at the KLF15 promoter region (pGL4.20-hKLF15) or empty vector (pGL4.20-EV). Fold induction in KLF15 promoter activity is shown with DEX (1 or 10 μM) treatment compared with VEH-treated cells (n=6). *P<0.05; **P<0.01; ***P<0.001 (two–way ANOVA test with Tukey post-test).
Table 1.
Activation of pathways from gene enrichment analysis of predicted KLF15 binding sites
| Name | P Value | Z Score | Genes |
|---|---|---|---|
| Rapid GC signalinga | 0.02 | −1.32142873 | MAPK11; MAPK9 |
| Glypican 3 networka | 0.02 | −0.929794625 | MAPK9; PTCH1 |
| Hedgehog signaling events mediated by Gli proteinsa | 0.02 | −1.581029069 | PTCH1; SUFU; RBBP7; PRKACA |
| Regulation of nuclear SMAD2/3 signaling | 0.04 | −1.504547684 | SMAD2; SP3; LAMC1; GATA3; RBBP7 |
| GR regulatory network | 0.04 | −1.52849768 | MAPK11; MAPK9; NFATC1; GATA3; PRKACA |
P<0.05 (Fischer exact test). Z score assesses deviation from the expected rank. NCI-Nature Pathways Gene Set Library.
Less than overlap of five genes.
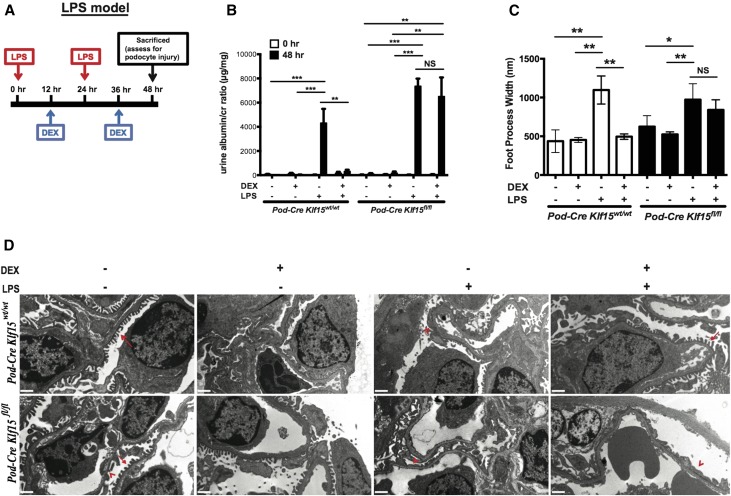
Podocyte-Specific Loss of Klf15 Prevents DEX–Induced Podocyte Recovery in LPS-Treated Mice
To assess whether the loss of Klf15 in podocytes abrogates DEX-induced attenuation of podocyte injury, Klf15 was specifically knocked down in podocytes using the Cre-loxP recombination system. Podocin-Cre mice (C57BL/6) were crossed with Klf15flox/flox (C57BL/6) to generate Podocin-Cre Klf15flox/flox mice (F2). Primary culture of glomerular epithelial cells isolated from Podocin-Cre Klf15+/+ mice and Podocin-Cre Klf15flox/flox mice revealed a significant reduction in Klf15 mRNA and protein expression (Supplemental Figure 1, A–C). These findings were confirmed with colocalization of Klf15 with Wt1, a known podocyte marker (Supplemental Figure 1D). Podocin-Cre Klf15flox/flox mice were viable and fertile, findings consistent with the observations shown in the global Klf15−/− mice.15
To ascertain whether Klf15 is required to attenuate the salutary effects of DEX in podocyte injury, we used the LPS model (Figure 2A). LPS and DEX treatment protocol is described in detail in the Concise Methods. Previous studies have shown that low–dose LPS treatment is a viable model of podocyte injury in mice.15,20–22 We observed that concurrent treatment with DEX and LPS significantly abrogated albuminuria in Podocin-Cre Klf15+/+ at 48 hours (Figure 2B). In comparison, LPS-induced albuminuria in Podocin-Cre Klf15flox/flox was not ameliorated with concurrent DEX administration (Figure 2B). No histologic evidence of glomerulosclerosis, inflammatory exudate in the glomerulus, or interstitial inflammation was observed in the kidneys from either group by periodic acid–Schiff and hematoxylin and eosin (H&E) staining (Supplemental Figure 2). However, treatment with LPS showed histologic evidence of proteinaceous casts with tubular dilation in both wild-type and knockout mice (Supplemental Figure 2A). Also, the Podocin-Cre Klf15+/+ mice concurrently treated with DEX exhibited an improvement in tubular dilation and proteinaceous casts compared with the Podocin-Cre Klf15flox/flox mice concurrently treated with DEX (Supplemental Figure 2A). Furthermore, treatment with DEX restored foot process effacement in Podocin-Cre Klf15+/+ compared with Podocin-Cre Klf15flox/flox mice (Figure 2, C and D).
Figure 2.
LPS–treated Podocin-Cre Klf15flox/flox mice exhibit a lack of recovery in albuminuria and foot process effacement with DEX administration. Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice were concurrently treated with either DEX or vehicle after LPS treatment. Urine was collected weekly, mice were euthanized, and renal cortex was fixed for histology 48 hours post-LPS treatment. (A) Schematic diagram of LPS and DEX treatment protocols. (B) Albuminuria (urine albumin-to-creatinine ratio) was measured (n=6). **P<0.01; ***P<0.001 (two–way ANOVA test with Tukey post-test). (C) Electron microscopy was performed to assess ultrastructural changes in podocyte morphology. Foot process width was quantified by counting the number of slits per length of glomerular basement membrane with ImageJ. *P<0.05; **P<0.01 (Kruskal–Wallis test with Dunn post-test). (D) The representative images from four mice in each group are shown. Red arrows show normal upright foot processes. Red arrowheads show foot process effacement. Magnification, ×10,000.
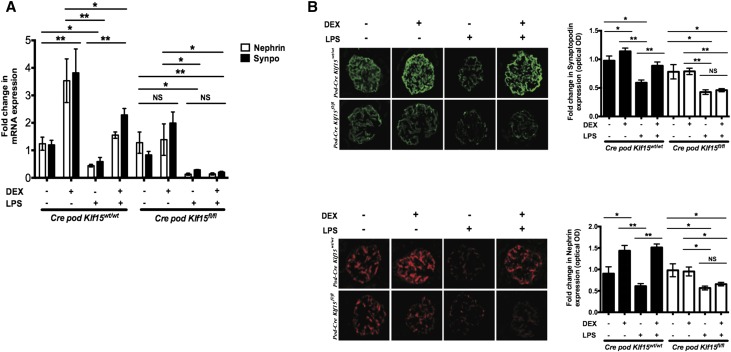
Our previous studies suggest that KLF15 is critical for the restoration of podocyte differentiation markers after treatment with retinoic acid (RA).15 To assess whether the restoration in DEX–mediated podocyte differentiation markers is also mediated by KLF15, we measured Nephrin and Synaptopodin expression in DEX only, in LPS only, and with concurrent LPS and DEX treatment. We observed that Nephrin and Synaptopodin expression remained suppressed in the Podocin-Cre Klf15flox/flox mice compared with in the Podocin-Cre Klf15+/+ mice treated with LPS and DEX concurrently (Figure 3A). Similarly, immunostaining confirmed the lack of restoration in nephrin and synaptopodin expression in LPS–treated Podocin-Cre Klf15flox/flox mice compared with LPS–treated Podocin-Cre Klf15+/+ mice with concurrent DEX administration (Figure 3B). Taken together, these data suggest that Klf15 is required for DEX–mediated podocyte recovery in the setting of glomerular injury.
Figure 3.
LPS–treated Podocin-Cre Klf15flox/flox mice exhibit a lack of restoration of podocyte differentiation markers with DEX administration. Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice were concurrently treated with either DEX or vehicle after LPS treatment. All mice were euthanized, glomeruli were isolated, and RNA were extracted for real-time PCR. (A) Nephrin and Synaptopodin mRNA expression levels are shown relative to untreated mice (n=6). *P<0.05; **P<0.01 (two–way ANOVA test with Tukey post-test). (B) Immunostaining for Synaptopodin (upper panel) and Nephrin (lower panel) was performed in all groups of mice. Representative images from four mice in each group are shown. The glomerular region was selected, and OD was measured and quantified as a relative fold change to untreated mice for Synaptopodin (upper right panel; n=6) and Nephrin (lower right panel; n=6). Magnification, ×20. *P<0.05; **P<0.01 (Kruskal–Wallis test with Dunn post-test).
Pattern of Inflammatory Markers Is Similar between DEX–Treated Podocin-Cre Klf15+/+ and Podocin-Cre Klf15flox/flox Mice
The immunomodulatory effects of GCs are well described.4,5 To determine whether KLF15–mediated DEX–induced podocyte recovery and restoration of podocyte markers is a result of anti-inflammatory effects of DEX, we measured markers of inflammation in Podocin-Cre Klf15+/+ and Podocin-Cre Klf15flox/flox mice treated concurrently with LPS and DEX. Although LPS treatment has previously been described to induce the expression of specific inflammatory cytokines,23,24 studies have not shown histologic evidence of inflammation within the initial 48-hour period post-LPS treatment.25 Similarly, two independent pathologists did not observe any histologic evidence of glomerular or interstitial inflammation in either group of mice by H&E staining (Supplemental Figure 2B). We observed that glomerular-specific expression of Tnfα, Il-6, Il-1β, and Infγ was appropriately increased with LPS treatment (Supplemental Figure 3, A–D). Concurrent treatment with DEX induced a significant reduction in Il-6, with a trend in reduction of Tnfα and Il-1β levels (no statistical significance) in Podocin-Cre Klf15+/+ mice. Similarly, concurrent treatment with DEX induced a significant reduction in Il-6, with a trend in the reduction of Tnfα, Il-1β, and Infγ levels (no statistical significance) in Podocin-Cre Klf15flox/flox mice. Furthermore, no significant changes were observed in the pattern of inflammatory changes between the Podocin-Cre Klf15+/+ and Podocin-Cre Klf15flox/flox mice. In addition, we observed no significant differences in F4/80-positive cells per high power field among all of the groups (Supplemental Figure 3E). Furthermore, the pattern of Gr-1–positive cells was similar between both the Podocin-Cre Klf15+/+ and the Podocin-Cre Klf15flox/flox mice (Supplemental Figure 3F). Combined, these findings suggest that the failure of the LPS–treated Podocin-Cre Klf15flox/flox mice to recover from DEX might be independent of its anti-inflammatory effect.
Loss of Klf15 in Podocytes Prevents DEX-Induced Restoration of Podocyte Differentiation Markers
Podocin-Cre Klf15flox/flox mice exhibited an approximately 50% knockdown of Klf15 in podocytes (Supplemental Figure 1, B and C), which increased the susceptibility to podocyte injury in the LPS proteinuric murine model (Figures 2 and 3). To address whether the efficiency of Klf15 knockdown contributes to podocyte injury and survival, we initially generated human podocytes with stable knockdown for KLF15 using two shRNA constructs, KLF15-shRNA33 and KLF15-shRNA32. Although both constructs achieved significant knockdown in KLF15, the KLF15-shRNA33 cell line exhibited a more robust knockdown in KLF15 compared with KLF15-shRNA32 and EV-shRNA (control) (Supplemental Figure 4A). Because KLF15 expression is reduced in 33°C (permissive) compared with 37°C (nonpermissive) conditions,15 at permissive conditions (33°C), all three cell lines exhibited no significant changes in survival. However, under nonpermissive conditions (37°C), the KLF15-shRNA32 cell line exhibited reduced survival compared with KLF15-shRNA33 and EV-shRNA cell lines at days 4, 7, 10, and 14 of differentiation (Supplemental Figure 4B), suggesting that the efficiency of KLF15 knockdown contributes to cell survival. Subsequently, we sought to determine actin stress fiber formation with KLF15 knockdown in differentiated human podocytes. Because nearly all KLF15-shRNA33 cells died by the 14-day differentiation period in 37°C, we compared actin stress fiber formation between KLF15-shRNA32 and EV-shRNA by staining with conjugated phalloidin-Alexa Fluor 647. These changes in actin cytoskeleton were quantified in a blinded fashion by classifying into the following groups: type A (>90% of cell area filled with thick cables), type B (no thick cables but some cables present), and type C (no cables visible in the central area of the cell). Using this classification, we observed that KLF15-shRNA32 podocytes exhibited a reduction in actin stress fiber formation (fewer type A cables and more types B and C cables) compared with EV-shRNA podocytes (Supplemental Figure 4C). Finally, we also determined that Podocin-Cre Klf15flox/flox mice had slightly fewer podocytes per glomerulus compared with Podocin-Cre Klf15+/+ mice by Wt1 staining (Supplemental Figure 4D).
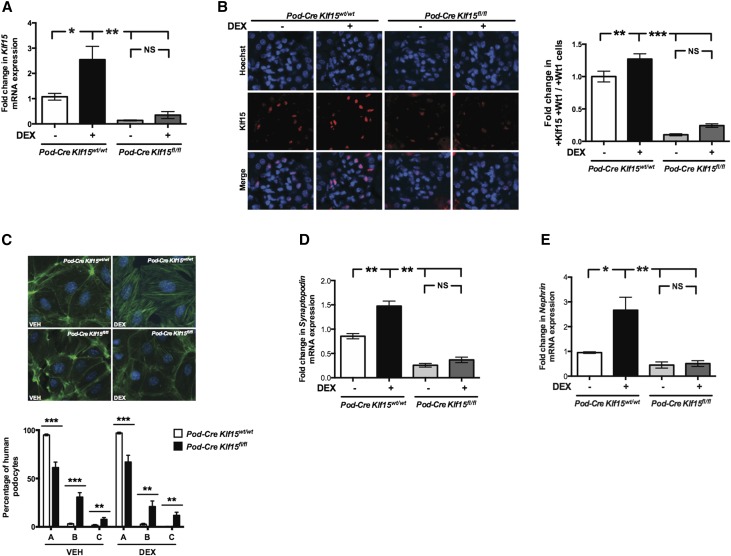
To assess whether Klf15 is essential for GC-mediated restoration of podocyte differentiation markers in primary cell culture, we initially isolated primary podocytes from Podocin-Cre Klf15+/+ and Podocin-Cre Klf15flox/flox mice. Klf15 expression was significantly increased with DEX treatment at 12 hours in Podocin-Cre Klf15+/+ podocytes compared with Podocin-Cre Klf15flox/flox podocytes (Figure 4A). These findings were confirmed by immunofluorescence with quantification for podocyte-specific expression for Klf15 (Figure 4B).
Figure 4.
Podocyte-specific loss of Klf15 abrogates DEX-mediated increase in podocyte differentiation markers. Primary podocytes were isolated from 12-week-old Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice and cultured at 37°C for 1 week. Cells were subsequently treated with DEX or vehicle (VEH) for 12 hours. RNA was extracted, and real-time PCR was performed for (A) Klf15 mRNA expression (n=6). *P<0.05; **P<0.01 (Kruskal–Wallis test with Dunn post-test). (B) Immunostaining for Klf15 was performed. Representative images from three mice in each group are shown (left panel). Quantification for Klf15 expression in podocytes was determined by the ratio of Klf15+ and Wt1+ cells to W11+ cells (n=3). Magnification, ×20. **P<0.01; ***P<0.001 (Kruskal–Wallis test with Dunn post-test). (C) Phalloidin and hoechst staining was performed in isolated primary podocytes. The representative images from three independent experiments are shown. In total, 100 cells were selected in each group, and the cells were classified into type A (>90% of cell area filled with thick cables), type B (no thick cables but some cables present), and type C (no cables visible in the central area of the cell; n=3). **P<0.01; ***P<0.001 (unpaired t test). (D) Nephrin mRNA expression (n=6) and (E) Synaptopodin mRNA expression (n=6). *P<0.05; **P<0.01 (Kruskal–Wallis test with Dunn post-test).
Actin stress fiber formation is a critical feature of differentiated podocytes, and treatment with DEX has been shown to enhance stress fiber formation.12 We observed that Podocin-Cre Klf15flox/flox podocytes exhibited a significant derangement in actin cytoskeleton (fewer type A cables and more types B and C cables) compared with Podocin-Cre Klf15+/+ podocytes with and without DEX treatment (Figure 4C). Furthermore, we showed that Podocin-Cre Klf15+/+ podocytes exhibited a significant increase in Nephrin and Synaptopodin expression with DEX treatment compared with vehicle treatment (Figure 4, D and E). These findings were not observed in Podocin-Cre Klf15flox/flox podocytes (Figure 4, D and E). These data suggest that Klf15 is required for DEX-mediated restoration of podocyte differentiation markers.
Overexpression of KLF15 Prevents Derangement of Actin Cytoskeleton under Cell Stress
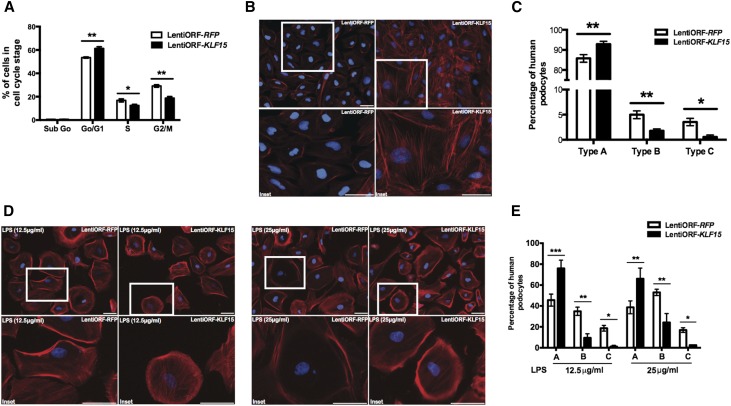
Treatment with DEX increases actin stress fiber formation,12 and we previously showed that overexpression of KLF15 preserves podocyte differentiation markers.15 Because actin stress fiber formation is a critical feature of differentiated podocytes and because KLF15 expression is reduced in 33°C (permissive) compared with 37°C (nonpermissive) conditions,15 we sought to evaluate actin stress fiber formation after overexpression of KLF15 in human podocytes under undifferentiated and permissive conditions. We initially generated human podocytes with stable overexpression of KLF15 (lentiORF-KLF15) (Supplemental Figure 5A). To evaluate the cell cycle stage of cells after KLF15 overexpression, FACS (Becton Dickinson, San Diego, CA) analysis was performed on lentiORF-KLF15 and lentiORF-RFP (control) human podocytes under permissive conditions to determine the percentage of cells in G1/S phase. We observed that lentiORF-KLF15 cells exhibited a significant increase in the percentage of cells in G1/S phase compared with lentiORF-RFP cells (Figure 5A). Furthermore, we determined a significant improvement in actin stress fibers in lentiORF-KLF15 cells compared with lentiORF-RFP cells as defined by an increase in thick, well defined actin stress fibers in these permissive conditions (Figure 5, B and C, Supplemental Figure 5B).
Figure 5.
Overexpression of KLF15 increases the number of cells in G0/G1 phase and actin stress fiber formation. Human podocytes with overexpression for KLF15 (lentiORF-KLF15) and control vector (lentiORF-RFP) were generated. To identify the percentage of cells in G0/G1 phase, confluent lentiORF-KLF15 and lentiORF-RFP human podocytes under permissive conditions (33°C) were harvested for FACS, and cell cycle analysis was performed. (A) Percentages of human podocytes in sub-G0, G0/G1, S, and G2/M phases are shown (n=4). *P<0.05; **P<0.01 (Mann–Whitney test). (B) Phalloidin and hoechst staining was performed in lentiORF-KLF15 and lentiORF-RFP human podocytes under permissive conditions. The representative images from three independent experiments are shown. (C) In total, 150 cells were selected in each group, and the cells were classified into type A (>90% of cell area filled with thick cables), type B (no thick cables but some cables present), or type C (no cables visible in the central area of the cell; n=3). *P<0.05; **P<0.01 (unpaired t test). (D) Next, lentiORF-KLF15 and lentiORF-RFP human podocytes were differentiated at 37°C for 14 days and treated with LPS (12.5 and 25 μg/ml) for 24 hours. Phalloidin and hoechst staining was performed in LPS–treated lentiORF-KLF15 and lentiORF-RFP differentiated human podocytes. The representative images from three independent experiments are shown. (E) In total, 150 cells were selected in each group, and the cells were classified into type A, type B, or type C (n=3). *P<0.05; **P<0.01; ***P<0.001 (unpaired t test).
To evaluate whether the overexpression of KLF15 abrogated the derangement in actin stress fibers observed with LPS treatment in differentiated human podocytes (37°C), we used the LPS dosing conditions (12.5 and 25 μg/ml) known to induce changes in actin cytoskeleton without cell lysis.26 We observed that LPS–treated lentiORF-KLF15 human podocytes exhibited less disruption in actin cytoskeleton than LPS–treated lentiORF-RFP human podocytes at both dosing conditions (Figure 5, D and E). Taken together, these data suggest that the overexpression of KLF15 stabilizes the actin cytoskeleton under cell stress.
Reduced Expression of KLF15 in Podocytes Correlates with Nonresponsiveness to GCs in MCD and FSGS
To ascertain whether the level of KLF15 expression correlates with GC responsiveness in patients with primary glomerulopathies, we collected archived kidney biopsies from patients with MCD and primary FSGS treated with GCs as their initial treatment. We obtained kidney tissue from 16 control subjects (healthy donor nephrectomies) and kidney biopsies from 15 glucocorticoid responders (GC-Rs) and 20 glucocorticoid nonresponders (GC-NRs) with MCD and primary FSGS. Clinical characteristics of the participants at the time of the kidney biopsy are shown in Table 2.
Table 2.
Baseline characteristics
| Characteristics | Controls, n=16 | GC-R, n=15 | GC-NR, n=20 | P Value | P Value | ||
|---|---|---|---|---|---|---|---|
| Controls | GC-R Versus GC-NR | ||||||
| Versus GC-R | Versus GC-NR | ||||||
| Men/women, n/n | 11/5 | 9/6 | 9/11 | 0.90 | |||
| Age at time of biopsy, yr | 63, 53–77 | 23, 8–53 | 13, 9–20 | <0.001 | <0.01 | <0.001 | 0.09 |
| Ethnicity | 0.07 | ||||||
| White | 16 | 8 | 12 | ||||
| Black | — | 3 | 4 | ||||
| Hispanic | — | 3 | 4 | ||||
| Other | — | 1 | 0 | ||||
| MCD/FSGS, n/n | — | 8/7 | 9/11 | 0.74 | |||
| Fibrosis | <0.05 | <0.01 | <0.01 | 0.68 | |||
| None | 16 | 3 | 7 | ||||
| Mild/minimal | — | 9 | 8 | ||||
| Moderate | — | 2 | 2 | ||||
| Severe | — | — | 1 | ||||
| Not reported | — | 1 | 2 | ||||
| CD68 expression | 0.72 | ||||||
| None | 16 | 12 | 16 | ||||
| Mild, <10% | — | 3 | 4 | ||||
| Moderate, >10%–25% | — | — | — | ||||
| Severe, >25% | — | — | — | ||||
| At biopsy | |||||||
| Average serum Cr at bx, mg/dl | 1.00, 0.80–1.10 | 0.89, 0.50–1.44 | 0.70, 0.47–2.00 | 0.49 | |||
| Average eGFR, ml/min per 1.73 m2 | 78, 61–98 | 73, 45–117 | 97, 42–129 | 0.80 | |||
| Urine protein-to-Cr ratio at bx, g/g | — | 4.72, 3.80–7.64 | 4.17, 0.73–10.39 | 0.75 | |||
| 1 yr ±3 mo postbiopsy | |||||||
| Serum Cr, mg/dl | 0.90, 0.79–1.16 | 0.76, 0.59–1.62 | 0.78, 0.50–1.33 | 0.53 | |||
| Average eGFR, ml/min per l.73 m2 | 94, 68–107 | 68, 32–154 | 127, 33–149 | 0.79 | |||
| Urine protein-to-Cr ratio at 1 yr, g/g | — | 0.49, 0.07–1.65 | 2.92, 2.10–6.91 | <0.05 | — | — | 0.01 |
| Concurrent HTN, SBP>I40/90 | 0.77 | ||||||
| Yes | 9 | 10 | 14 | ||||
| No | 7 | 5 | 6 | ||||
| Use of ACEI/ARB | 0.41 | ||||||
| Yes | 6 | 9 | 11 | ||||
| No | 10 | 6 | 9 | ||||
Median, interquartile range (25th to 75th percentile) shown for non-normal distribution of continuous variables (Kruskal–Wallis with Dunn post-test). Frequencies are shown for categorical variables (chi-squared test). —, not available; Cr, creatinine; bx, biopsy; HTN, hypertension; SBP, systolic blood pressure; ACEI, angiotensin–converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
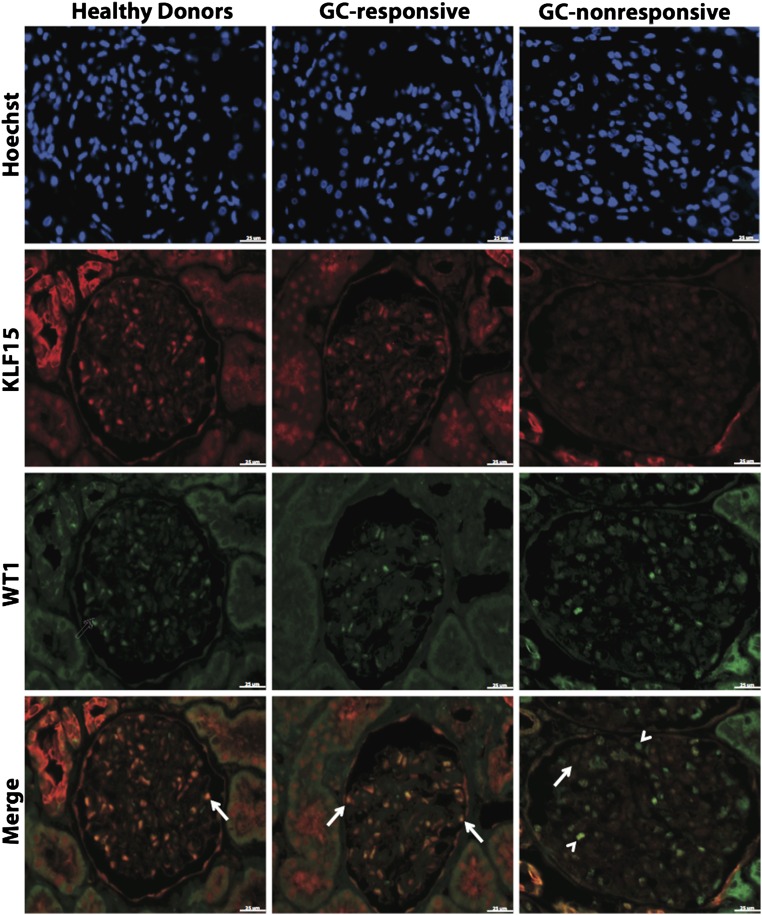
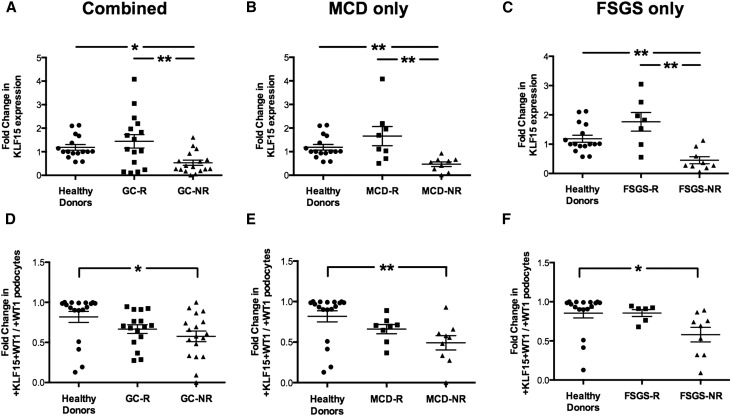
As previously published, immunostaining for KLF15 revealed that KLF15 is expressed in podocytes, endothelial cells, mesangial cells, and parietal epithelial cells in the glomerulus (Figure 6). Furthermore, we observed that the podocyte as well as the glomerular expression of KLF15 by immunostaining were significantly higher in healthy donor nephrectomies and GC-Rs compared with GC-NRs (Figure 6). We confirmed this observation by quantifying the intensity of KLF15 staining in the glomeruli (Figure 7, A–C) and measuring the percentage of podocytes positive for KLF15 (ratio of +WT1 and +KLF15 cells to +WT1 only cells) (Figure 7, D–F). We also provide additional images of KLF15 staining from distinct kidney biopsies representative of the pattern of KLF15 expression in the glomeruli (Supplemental Figure 6). In addition, Nephrin and Synaptopodin expression was reduced in GC-NRs compared with GC-Rs and healthy control subjects (Supplemental Figure 7). Collectively, these data show that a loss of KLF15 expression in podocytes correlates with nonresponsiveness to GCs in MCD and primary FSGS, suggesting a potential role for KLF15 as a prognostic marker for GC responsiveness in the kidney.
Figure 6.
Reduced KLF15 expression in GC-NR MCD and primary FSGS. Immunofluorescence for Hoechst, KLF15, and WT1 was performed in kidney biopsy specimens from 16 healthy donor nephrectomies and 15 GC-R and 20 GC-NR patients. The representative images from each group are shown. Arrows show colocalization of KLF15 and WT1. Arrowheads show a lack of colocalization. Magnification, ×20.
Figure 7.
Reduced podocyte and glomerular expression of KLF15 in GC-NR MCD and primary FSGS. Immunofluorescence for Hoechst, KLF15, and WT1 was performed in kidney biopsy specimens from 16 healthy donor nephrectomies and 15 GC-R and 20 GC-NR patients. Fifteen glomeruli per biopsy specimens were selected, and quantification of KLF15 staining in the glomerulus was performed. (A–C) ImageJ was used to quantify the intensity of KLF15 expression in the glomerulus in all three groups, MCD only, and FSGS only. The glomerular region was selected, and OD was measured and quantified as a relative fold change to healthy subjects. *P<0.05; **P<0.01 (Kruskal–Wallis test with Dunn post-test). (D–F) Quantification of KLF15 staining in the podocytes was determined by the ratio of KLF15+ and WT1+ cells to WT1+ cells. *P<0.05; **P<0.01 (Kruskal–Wallis test with Dunn post-test).
KLF4 Expression Is Not Regulated by DEX
Because recent studies have shown that KLF4, another member of the KLF family, might also play a role in attenuating podocyte injury,27,28 we sought to identify whether KLF4 expression is also regulated by GCs. Unlike KLF15, we observed that KLF4 mRNA and protein expression were not increased with DEX treatment (Supplemental Figure 8, A and B). In addition, KLF4 expression was reduced in the LPS proteinuric mouse model, regardless of DEX treatment (Supplemental Figure 8B). Similarly, glomerular expression of KLF4 was reduced in biopsies from patients with MCD and primary FSGS, regardless of GC responsiveness. Combined, these findings suggest that, unlike KLF15, the expression of KLF4 in the podocytes is not mediated by GCs.
Podocyte-Specific Loss of Klf15 Prevents DEX–Induced Podocyte Recovery in Two Additional Proteinuric Murine Models
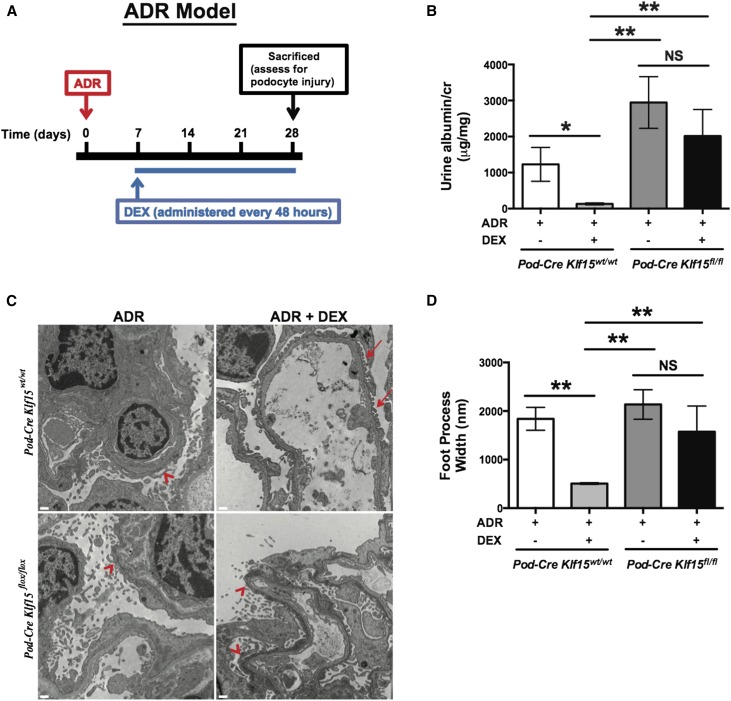
To validate the findings that we observed in the proteinuric murine model of LPS, we performed these studies in two additional independent proteinuric murine models. Although mice on the FVB/n background are resistant to classic FSGS with adriamycin (ADR) treatment,29 previous studies have shown that treatment with ADR at 18 mg/kg induces albuminuria with histologic features consistent with MCD on the FVB/n background.30,31 Podocin-Cre Klf15+/+ and Podocin-Cre Klf15flox/flox mice (backcrossed to the FVB/n background for nine generations) at 12 weeks of age were initially treated with ADR intravenously. At day 7 post-ADR treatment, DEX (2 mg/kg) was initiated intraperitoneally and continued every 48 hours until 4 weeks post-ADR treatment. A schematic of the treatment protocol is provided (Figure 8A), and a detailed description of the protocol is in Concise Methods. We observed that concurrent treatment with DEX significantly abrogated albuminuria in Podocin-Cre Klf15+/+ 4 weeks post-ADR treatment. In comparison, ADR-induced albuminuria in Podocin-Cre Klf15flox/flox was not ameliorated with concurrent DEX administration (Figure 8B). Furthermore, treatment with DEX restored foot process effacement in the ADR–treated Podocin-Cre Klf15+/+ compared with ADR–treated Podocin-Cre Klf15flox/flox mice (Figure 8, C and D).
Figure 8.
ADR–treated Podocin-Cre Klf15flox/flox mice exhibit a lack of recovery in albuminuria and foot process effacement after DEX administration. Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice were initially treated with ADR (18 mg/kg) with subsequent administration of DEX (2 mg/kg every 48 hours) starting on day 7. Urine was collected weekly, mice were euthanized, and renal cortex was fixed for histology 4 weeks post-ADR treatment. (A) Schematic diagram of ADR and DEX treatment protocol. (B) Albuminuria (urine albumin-to-creatinine ratio) was measured (n=6). *P<0.05; **P<0.01 (Kruskal–Wallis test with Dunn post-test). (C) Electron microscopy was performed to assess ultrastructural changes in podocyte morphology. The representative images from four mice in each group are shown. Red arrows show normal upright foot processes. Red arrowheads show foot process effacement. Magnification, ×10,000. (D) Foot process width was quantified by counting the number of slits per length of glomerular basement membrane with ImageJ. **P<0.01 (Kruskal–Wallis test with Dunn post-test).
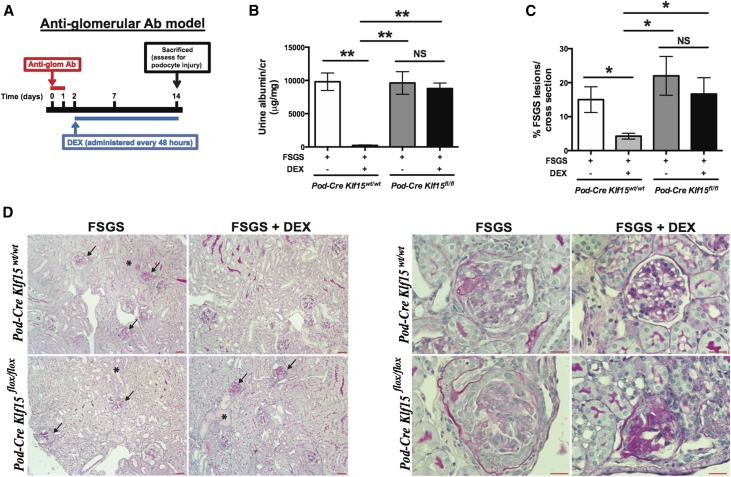
To further assess these findings in a murine model of FSGS, we treated mice with sheep antiglomerular antibody, and administration of the antiglomerular antibody induces albuminuria and histologic features consistent with FSGS within 2 weeks post-treatment.32 Furthermore, concurrent treatment with GCs in this model restores podocyte differentiation markers and ameliorates albuminuria and FSGS without affecting the binding of antiglomerular antibody.32 To show whether the podocyte-specific loss of Klf15 abrogates these DEX–mediated salutary effects on the podocyte, we initially treated 12-week-old Podocin-Cre Klf15+/+ and Podocin-Cre Klf15flox/flox mice (FVB/n background) with antiglomerular antibody. Two days after antiglomerular antibody treatment, DEX (2 mg/kg) was initiated intraperitoneally and continued every 48 hours until day 14. A schematic of the treatment protocol is provided (Figure 9A) with a detailed description of the protocol in Concise Methods. Concurrent treatment with DEX significantly abrogated albuminuria and FSGS in Podocin-Cre Klf15+/+ compared with Podocin-Cre Klf15flox/flox mice (Figure 9, B–D). Collectively, these data suggest that Klf15 is required for DEX–mediated podocyte recovery in the setting of glomerular injury.
Figure 9.
Antiglomerular antibody–treated Podocin-Cre Klf15flox/flox mice exhibit a lack of recovery in albuminuria and FSGS lesions after DEX administration. Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice were initially treated with sheep antiglomerular antibody (5 mg/20 g body wt) with subsequent administration of DEX (2 mg/kg every 48 hours) starting on day 2. Urine was collected weekly, mice were euthanized, and renal cortex was fixed for histology 2 weeks after antiglomerular antibody treatment. (A) Schematic diagram of antiglomerular antibody and DEX treatment protocol. (B) Albuminuria (urine albumin-to-creatinine ratio) was measured (n=4). **P<0.01 (Kruskal–Wallis test with Dunn post-test). (C) All mice were euthanized, and renal cortex was fixed for histology. Periodic acid–Schiff was performed to evaluate for glomerular or tubulointerstitial changes. The percentage of FSGS lesions per cross-section was quantified in a blinded fashion. *P<0.05 (Kruskal–Wallis test with Dunn post-test). (D) The representative images from four mice in each group are shown. Arrows show FSGS lesions. Magnification, ×10 in left panel; ×40 in right panel. *Tubular dilation and proteinaceous casts.
Discussion
GCs are the backbone of therapy in the management of patients with primary glomerulopathies. Despite the wide use of GCs, little is known about the mechanism by which they may regulate the recovery from glomerular injury. Although GCs are presumed to attenuate glomerular disease via the reduction of soluble mediators of disease, recent in vitro studies have shown that GCs may also exert their therapeutic benefits in nephrotic syndrome by direct action on podocytes.9–11 In this study, we identify that KLF15 is required for GC-induced restoration of podocyte differentiation markers after injury. This was shown by the following observations. (1) DEX induces a rapid increase in KLF15 expression in cultured human podocytes and primary mouse podocytes. (2) Podocyte-specific loss of Klf15 abrogated DEX–induced podocyte recovery in three proteinuric murine models. (3) Knockdown of KLF15 destabilized the actin cytoskeleton, whereas overexpression of KLF15 prevented derangement of actin cytoskeleton under cell stress, and (4) GC responsiveness in patients with MCD and primary FSGS correlated with the podocyte and glomerular expression of KLF15.
Molecules mediating the direct salutary effects of GC in the podocyte are largely undefined. A few transcriptional regulators, including WT1 and KLF15, regulate the expression of podocyte differentiation markers.15,33 Here, we identified that GC-induced restoration of podocyte differentiation markers is mediated by KLF15. Furthermore, we showed that Klf15 is required for GC–mediated podocyte recovery in three proteinuric murine models of podocyte injury. In addition, prior studies have shown that putative GC response element sites are present in the first and second intron regions in mouse embryonic fibroblasts and airway smooth muscle cells, respectively.16,34 We confirmed this finding by showing that the affinity for GR binding to KLF15 is enhanced with DEX treatment in cultured human podocytes. These data suggest that KLF15 is a direct target gene of GCs and serves as a mediator for protective effects of GCs in podocytes. However, it remains to be determined whether KLF15 could directly interact with GCs at the promoter sites to regulate GC target genes. In addition, other transcription factors may also mediate the effects of GCs in the podocyte. For instance, GCs were observed to downregulate the expression of GATA-3, a critical transcription factor with multiple putative binding sites in the promoter region of nephrin.11 Interestingly, TRANSFAC promoter analysis predicted eight transcriptional binding sites for KLF15 along the GATA-3 promoter region (Supplemental Material). Furthermore, our prior studies show that KLF15 is a key regulator of RA-induced restoration of podocyte differentiation markers.15 Similar to GCs, review of expression arrays from human podocytes treated with and without RA showed that GATA-3 is a key gene downregulated by RA.15 This suggests that GCs and RA may mediate a complex of downstream targets involved in restoring podocyte differentiation markers after injury that is dependent on KLF15 expression. Future studies are needed to validate the panel of transcripts regulated by the interaction of GC-KLF15 and RA-KLF15 pathways.
Nephrocytes in the Drosophila represent an invertebrate model of kidney podocytes, with expression of genes conserved in human podocytes.35 Recent studies in Drosophila revealed that Klf15 is critical for nephrocyte development and differentiation.36 Combined with our previous work,15 we show here that the KLF15 is required for DEX-induced restoration of podocyte differentiation markers (i.e., expression of nephrin and synaptopodin and actin stress fiber formation). Podocin-Cre Klf15flox/flox mice only exhibited approximately 50% knockdown in KLF15 expression, which likely attributed to less severe podocyte injury in the proteinuric murine models compared with the global Klf15−/− mice.15 In addition, because of a lack of embryonic lethality, we cannot conclude that KLF15 is involved in podocyte differentiation during embryonic development. Although podocyte number is reduced with Klf15 knockdown, we suspect that the lack of embryonic lethality is caused by only a partial knockdown in Klf15 expression in the podocytes. To address this, we showed reduced cell survival and actin stress fiber formation with a more robust knockdown of KLF15 in differentiated human podocytes. Nonetheless, future studies will need to use Crispr/Cas9 technology to design mice with complete podocyte–specific deletion of Klf15 to ascertain whether Klf15 plays an essential role in podocyte differentiation during embryonic development.
Because the anti-inflammatory effects of GCs are well described,4,5 we measured key inflammatory markers expected to increase with LPS treatment.23,24 Not surprisingly, treatment with LPS significantly induced the increase in Tnfα, Il-6, Il-1β, and Infγ in both the Podocin-Cre Klf15+/+ and Podocin-Cre Klf15flox/flox mice. DEX mitigated the expression of these inflammatory markers in the glomerulus independent of podocyte–specific Klf15 expression. Regardless of Klf15 expression in the podocytes, DEX induced the suppression of Il-6, findings consistent with previous studies.11 Similarly, the increase in the number of Gr-1+ cells in the cortex after DEX treatment was not dependent on podocyte–specific Klf15 expression. Taken together with the lack of podocyte recovery in the conditional Klf15 knockout mice after DEX treatment, these findings suggest that the failure of the LPS–treated Podocin-Cre Klf15flox/flox mice to recover from DEX might be independent of its anti-inflammatory effect. In addition, Xing et al.11 showed that, other than DEX-mediated suppression in Il-6 production in the podocyte, no significant changes in cytokine production were observed in a 48-hour period of DEX treatment. Nonetheless, the global loss of Klf15 has previously been shown to activate the NF-ΚB pathway and increase vascular inflammation in murine models of atherosclerosis.37 Consequently, proteinuric murine models need to be performed in global Klf15−/− mice to ascertain whether DEX–induced podocyte recovery is also contingent on its anti-inflammatory effect.
Of particular interest is the observation that the glomerular expression of KLF15 strongly correlates with GC responsiveness in patients with MCD and primary FSGS. We acknowledge that the analyses of our human biopsy data are limited by the small sample size. Larger sample sizes will be required to perform subgroup analyses to determine whether other immunomodulatory agents used in the treatment of GC-NR patients have an association with KLF15 expression as well as control for potential confounders (i.e., age) and test for interactions between GC treatment and other clinical predictors of response. Furthermore, laser capture microdissection of the glomeruli needs to be performed to determine whether KLF15 mRNA expression in the glomeruli also correlates with GC responsiveness. In addition, we will need to rebiopsy GC-Rs and GC-NRs after treatment with GCs to determine whether the failure to recover in GC-NRs is caused by a lack of restoration in KLF15 expression. Although Klf15 expression is reduced in murine models of podocyte dedifferentiation (i.e., HIVAN),15 GC-R did not exhibit a significant decrease in KLF15 expression before treatment, thereby suggesting that these patients may have developed glomerulopathy independent of the KLF15 pathway. In addition, it may be difficult to ascertain whether these changes in Klf15 expression from baseline to disease in mice are generalizable to humans. Consequently, our future studies will need to focus on identifying the mechanisms that may prevent the increase in KLF15 expression in GC-NRs. Furthermore, we have yet to explore whether the expression of KLF15 correlates with the response to other immunomodulatory agents (i.e., cyclosporin, mycophenolate mofetil, cyclophosphamide, and tacrolimus) used in GC-NR MCD and primary FSGS. Consequently, the initiation of these alternate agents in patients with MCD and primary FSGS may also depend on the level of KLF15 expression in the biopsy.
The prolonged use of GCs in patients is riddled with adverse effects ranging from weight gain, hyperglycemia, and systemic infections to osteoporotic fractures.38–40 Consequently, targeting molecules downstream of the GC signaling pathway, such as KLF15, specifically in podocytes may minimize systemic toxicity without jeopardizing therapeutic efficacy. Along with our findings, we previously showed that overexpression of KLF15 in cultured human podocytes can prevent the downregulation of podocyte differentiation markers in the setting of injury.15 However, additional studies are required to decipher whether the induction of KLF15 alone can restore podocyte differentiation markers and abrogate injury in murine models of podocyte injury.
To the best of our knowledge, this is the first study to show a novel mediator of GC-induced restoration of podocyte differentiation markers under cell stress. Specifically, our studies suggest that the expression of KLF15 is required for the salutary effects of GCs in the podocyte after injury. In addition, the loss of KLF15 expression in the podocyte strongly correlates with a lack of responsiveness to GCs in human MCD and primary FSGS. Collectively, these findings highlight the critical need to further investigate the potential role of KLF15 as a prognostic marker for GC responsiveness in primary glomerulopathies.
Concise Methods
Cell Culture
Conditionally immortalized human podocytes were a gift from Peter Mundel (Massachusetts General Hospital, Boston, MA). Methods for podocyte cultivation, immortalization, and differentiation were on the basis of previously described protocol.15 These cells proliferate under permissive conditions (γIFN at 33°C) but differentiate under nonpermissive conditions (37°C). To quantify the number of podocytes in cell culture, we followed the manufacturer’s protocol using the Z1 Coulter Particle Counter (Beckman Coulter, Inc., Brea CA). Briefly, cells were trypsinized, washed with 1× PBS, and mixed well before addition of saline in cuvette for cell counting.
Promoter Analyses
Using the TRANSFAC software,18 we scanned the promoters of all mouse genes in the region from −2000 to the transcription start site with the KLF15 position weight matrix provided by the TRANSFAC system. Enrichment analysis was performed using Enrichr, and the Fisher exact test was used to determine the terms that were over-represented among the genes with KLF15 binding sites.19
ChIP Assay
Before performing the ChIP assay, differentiated human podocytes (37°C for 14 days) were treated with and without DEX (10 μM) for 12 hours. The ChIP assay was performed using a kit from Cell Signaling Technology (Danvers, MA) as per the manufacturer’s protocol. Briefly, 3×107 cultured human podocytes were crosslinked with formaldehyde for 10 minutes followed by the addition of 1/20 volume of 2.5 M glycine to quench unreacted formaldehyde. Cells were lysed using a series of non-SDS–containing buffers as per the manufacturer’s protocol. Chromatin extracted from the lysed cells was sonicated using a Misonix 3000 Sonicator with a microtip to generate chromatin fragments of between 300 and 1000 bp. Immunoprecipitation of GR-crosslinked chromatin was carried out using M-280 Dynabeads (Cell Signaling Technology) with rabbit anti–GR (Cell Signaling Technology) antibody. To control for nonspecific IgG binding, sheep anti–rabbit IgG (Cell Signaling Technology) was used. After incubation of chromatin with antibody-coupled Dynabeads, the beads were washed several times, and immunoprecipitated chromatin complexes were eluted from the beads. DNA protein crosslinks were reversed by incubation at 65°C for 6 hours, and then, RNAase A and proteinase K were added sequentially to remove RNA and proteins. Purified DNA was used for the analysis of the KLF15 proximal promoter region by real-time PCR on an ABI PRISM 7900HT using SYBR GreenER qPCR Supermix. PCR primers were derived from previously published sequences (For: 5′-AGTGTCCTCTCTTTAATGCCGGTG-3′; Rev: 5′-ACAGGGTCTTAATGCTGGGCTGAA-3′) for the GRE site in the human KLF15 promoter region (chromosome 3: 126355791–126355900).34 The relative amplification of the promoter sequence of each gene was calculated using the 2−ΔΔCT method, and normalization was performed against the 1:100 diluted input of DNA.
Luciferase Reporter Assay
A 4.3-kb fragment of the human KLF15 promoter upstream of the ATG start codon was amplified by PCR on the RP11–71E19 BAC clone (Roswell Park Division). The amplified fragment contains KpnI/EcoRV overhangs and was subcloned into the KpnI/EcoRV cloning sites of the pGL4.20 firefly luciferase reporter plasmid (Promega, Madison, WI) to generate pGL4.20-hKLF15 (for human KLF15 promoter). After verification of the sequence by restriction and sequencing, the pGL4.20-hKLF15 construct (which contains a puromycin resistance cassette) was transfected into human podocytes followed by selection in puromycin-containing medium for 2 weeks. As previously published,41 we validated this reporter cell line by the stability of luciferase expression over time and measuring the difference in reporter activity between pGL4.20-hKLF15 and pGL4.20-EV (control).
shRNA–Mediated KLF15 Knockdown Using Lentivirus
KLF15 knockdown in human podocytes was performed using the Genecopoeia Lentiviral shRNAmir System with the following constructs: HSH008360–32-LVRU6GP (shRNA32) and HSH008360–33-LVRU6GP (shRNA33). In brief, lentiviral particles were produced by transfecting 293T cells with a combination of lentiviral expression plasmid DNA, pCD/NL-BH ΔΔΔ packaging plasmid, and VSV-G–encoding pLTR-G plasmid. For cell infection, viral supernatants were supplemented with 8 μg/ml polybrene and incubated with cells for a 24-hour period. Cells expressing shRNA were selected with puromycin for 2–3 weeks before use in all studies. GFP expression and Western blot were performed to confirm KLF15 knockdown.
LentiORF-KLF15 Overexpression
LentiORF-KLF15 clone was purchased from Thermo Fisher Scientific (Vernon Hills, IL), and transient KLF15 overexpression was achieved by transfecting human podocytes using Lipofectamine 3000 (Life Technologies, Carlsbad, CA). Cells expressing GFP were selected with blasticidin for 3 days before use in all studies. GFP expression and Western blot were performed to confirm KLF15 overexpression in lentiORF-KLF15 compared with lentiORF-RFP human podocytes.
Flow Cytometry
LentiORF-KLF15 compared with lentiORF-RFP human podocytes were cultured under permissive conditions until 80% confluency. Fixation and preparation for flow cytometry were prepared as in previously described methodology.42 In brief, cells were trypsinized, washed with 1× PBS, and fixed with ice cold 80% ethanol. Subsequently, propidium iodine (400 μg/ml) were added, and cells were incubated for 30 minutes at 37°C. Cell cycle analysis was performed on an FACS Calibur Flow Cytometer at Stony Brook Medicine with data analysis of 10,000 gated events, and data were analyzed using ModFit LT V3.3.11 software. Data are presented as the percentage of cells in the following cell cycle stages: sub-G0/G1, G0/G1, S, and G2/M phases.
Genotyping of Podocin-Cre Klf15flox/flox Mice
Mice with Klf15 targeting vector (C57BL/6) were a gift from Mukesh Jain and generated using the targeting strategy as previously described.37 Klf15flox/flox mice (C57BL/6) were crossed with mice expressing Cre recombinase (cre) under the control of the podocin promoter (B6.Cg-Tg [NPHS2-cre] 295Lbh/J; The Jackson Laboratory, Bar Harbor, ME). After backcrossing, male offspring expressing Cre with two floxed Klf15 alleles were used as the experimental group (Podocin-Cre Klf15flox/flox). Mice with two wild-type alleles and Cre expression were used as controls (Podocin-Cre Klf15+/+). Genotyping by tail prep and PCR were performed at 2 weeks of age as described.43,44
LPS and DEX Treatment of Mice
Baseline urine was collected in the Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice (12 weeks of age; C57BL/6). All mice were administered low-dose LPS (10 μg/g) intraperitoneally at 0 and 24 hours as previously described.25 At the 12-hour time point after the initial LPS injection, mice were administered either vehicle or DEX (1 mg/kg) intraperitoneally. An additional DEX or vehicle injection was administered at the 36-hour time point. Urine was collected at 24-hour increments with 1-ml normal saline boluses intraperitoneally. Mice were euthanized at 48 hours. A schematic of the LPS treatment protocol is provided (Figure 2A).
ADR and DEX Treatment of Mice
Baseline urine was collected in the Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice (12 weeks of age; FVB/n background). All mice were administered ADR (18 mg/kg) intravenously by tail vein injection.31 DEX (2 mg/kg) was administered intraperitoneally starting at day 7 post-ADR treatment and continued every 48 hours. Urine was collected weekly, and all mice were euthanized at 4 weeks post-ADR treatment. Significant podocyte injury has been described typically at 4 weeks post-ADR treatment31; as such, these mice were euthanized, and kidneys were harvested for analysis at 4 weeks post-ADR treatment. The dose and frequency of DEX dosing were extrapolated from patients receiving alternate day GC regimen for the treatment of primary glomerular disease.45 A schematic of the ADR treatment protocol is provided (Figure 8A).
Antiglomerular Antibody and DEX Treatment of Mice
Baseline urine was collected in the Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice (12 weeks of age; FVB/n background). Administration of sheep antiglomerular antibody has been previously described to lead to histologic lesions consistent with FSGS.32 Protocol of administration of sheep antiglomerular antibody and DEX was on the basis of the previously described methods with the following modifications.32,46 All mice were administered sheep antiglomerular antibody (5 mg/20 g body wt) intraperitoneally for 2 consecutive days. Higher doses of sheep antiglomerular antibody contributed to significant mortality in mice on the FVB/n background. DEX (2 mg/kg) was administered intraperitoneally starting at day 3 and continued every 48 hours. Timing of initiating DEX treatment was on the basis of previously described methodology.32 The dose and frequency of DEX dosing were extrapolated from patients receiving alternate day GC regimen for the treatment of primary glomerular disease.45 Urine was collected weekly, and all mice were euthanized at 2 weeks after antiglomerular antibody treatment. A schematic of the antiglomerular antibody treatment protocol is provided (Figure 9A).
Measurement of Urine Albumin and Creatinine
Urine albumin was quantified by ELISA using a kit from Bethyl Laboratory Inc. Urine creatinine levels were measured in the same samples using the QuantiChrom Creatinine Assay Kit (DICT-500; BioAssay Systems) according to the manufacturer’s instructions. The urine albumin excretion rate was expressed as the ratio of albumin to creatinine.
Isolation of Glomeruli from Mice for RNA Extraction
Mouse glomeruli were isolated as described.47 Briefly, mice were perfused with HBSS containing 2.5 mg/ml iron oxide and 1% BSA. At the end of perfusion, kidneys were removed, decapsulated, minced into 1-mm3 pieces, and digested in HBSS containing 1 mg/ml collagenase A and 100 U/ml deoxyribonuclease I. Digested tissue was then passed through a 100-μm cell strainer and collected by centrifugation. The pellet was resuspended in 2 ml HBSS, and glomeruli were collected using a magnet. The purity of glomeruli was verified under microscopy. Total RNA was isolated from kidney glomeruli of mice using the RNeasy Kit (Qiagen, Germantown, MD).
Isolation of Primary Mouse Podocytes
After glomerular isolation (as described above), primary mouse podocytes were isolated as previously described.48,49 In brief, isolated glomeruli were initially cultured on collagen 1–coated culture dishes in RPMI 1640 (Gibco, Carlsbad, CA) containing 10% FBS (Cansera International) supplemented with 1% Insulin-Transferin-Selenium-A liquid media supplement (Life Technologies) and 100 U/ml penicillin. Cultures were incubated in a 37°C humidified incubator. Subculture of primary podocytes was performed after 5 days of culture of isolated glomeruli. Cellular outgrowths were detached with Trypsin EDTA solution (Sigma-Aldrich, St. Louis, MO) and passed through a 40-μm sieve to remove the remaining glomerular cores. The filtered cells were cultured on collagen 1–coated dishes and processed for RNA or protein preparation. The purity of isolation was confirmed by testing for podocyte-specific markers by real-time PCR (Supplemental Figure 1A).
Real-Time PCR
Total RNA was extracted by using TRIzol (Gibco). First-strand cDNA was prepared from total RNA (1.5 μg) using the SuperScript III First-Strand Synthesis Kit (Life Technologies), and cDNA (1 μl) was amplified in triplicate using SYBR GreenER qPCR Supermix on an ABI PRISM 7900HT (Applied Biosystems, Foster City, CA). Primers for human KLF15, mouse Klf15, mouse Nephrin, mouse Podocin, mouse Il-6, mouse Il-1β, mouse Tnfα, and mouse Infγ were designed using NCBI Primer-BLAST and validated for efficiency before application (Supplemental Table 1). Primers for human KLF4 were purchased from SA Biosciences. Light cycler analysis software was used to determine crossing points using the second derivative method. Data were normalized to housekeeping genes (GAPDH) and presented as fold increase compared with RNA isolated from the control group using the 2−ΔΔCT method.
Western Blot
Primary mouse podocytes were lysed with a buffer containing 1% Triton, a protease inhibitor cocktail, and tyrosine and serine-threonine phosphorylation inhibitors. Lysates were subjected to immunoblot analysis using rabbit anti-KLF15 (Genscript) and rabbit anti-GAPDH (MAB374; Millipore).
Immunocytochemistry
Differentiated human podocytes were initially washed with PBS and subsequently fixed with 3.7% formaldehyde in growth medium. Cells were washed and permeabilized with 0.25% Triton X-100. Cells were blocked in 10% normal horse serum and incubated with primary antibody overnight (rabbit anti-KLF15; Genscript). The next day, cells were washed with PBS and incubated in secondary antibody in 10% NHS. Subsequently, cells were washed and incubated with Hoechst (Ana Spec Inc.) before mounting.
To selectively label F actin in fixed human podocytes, we used the Alexa Fluor 647 Phalloidin (Life Technologies). Fixation, permeabilization, and staining with phallodin were performed as per the manufacturer’s protocol (Life Technologies). Quantification and classification of changes in actin cytoskeleton are on the basis of previously published methodology.50 Briefly, phalloidin staining pattern in each cell was classified into the following types: type A (>90% of cell area filled with thick cables), type B (no thick cables but some cables present), and type C (no cables visible in the central area of the cell). Unless specified, 150 cells were quantified in a blinded manner for each group in three independent experiments.
LPS Treatment in Cell Culture
Initially, lentiORF-KLF15 and lentiORF-RFP human podocytes were differentiated at 37°C for 14 days before the start of LPS treatment. LentiORF-KLF15 and lentiORF-RFP human podocytes were treated with LPS or vehicle at two dosing conditions (12.5 and 25 μg/ml) for 24 hours as previously described.26 Subsequently, cells were fixed and stained for F actin as described above.
Light Microscopy
Mice were perfused with HBSS, and the kidneys were fixed in 10% phosphate buffered formalin overnight and switched to 70% ethanol before processing for histology. Kidney tissue was embedded in paraffin by American Histolabs, and 3-μm-thick sections were stained with periodic acid–Schiff and H&E (Sigma-Aldrich).
Transmission Electron Microscopy
Mice were perfused with PBS and then immediately fixed in 2.5% glutaraldehyde for electron microscopy as previously described.43 After embedding of kidney tissues in epoxy resin, ultrathin sections were stained with uranyl acetate and lead citrate, mounted on a copper grid, and photographed under a Hitachi H7650 Microscope (Hitachi, Yokohama, Japan). Briefly, negatives were digitized, and images with a final magnitude of approximately ×5000 and ×10,000 were obtained. Podocyte effacement was quantified as previously described.51
Immunofluorescence and Immunohistochemistry
Specimens were initially baked for 20 minutes in a 55°C to 60°C oven and then processed as previously described.44,52 Briefly, formalin-fixed and paraffin-embedded sections were deparaffinized, and endogenous peroxidase was inactivated with H2O2. All kidney sections from these mice were prepared in identical fashion. Similarly, all paraffin–embedded human kidney biopsy specimens were prepared in identical fashion. Immunofluorescence was performed using polycolonal rabbit anti–KLF15 (Genscript), mouse anti-WT1 (SC-7385; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-Nephrin (NBP1–30130; Novus), rabbit anti–Synaptopodin antibodies (SAB3500585; Sigma-Aldrich), mouse anti–Gr-1 (RB6–8C5; Abd Serotec), and goat anti-KLF4 (AF3158; R&D Systems, Minneapolis, MN). After washing, sections were incubated with a fluorophore–linked secondary antibody (Alexa Fluor 488 anti–rabbit IgG and Alexa Fluor 568 anti–mouse IgG; A10468 and A10494, respectively; Life Technologies). After staining, slides were mounted in Aqua Poly/Mount (Polysciences Inc.) and photographed under a Nikon Eclipse 90i Microscope (Nikon, Tokyo, Japan) with a digital camera.
Immunohistochemistry for F4/80 (rat anti-F4/80; SC-52664; Santa Cruz Biotechnology) and CD68 (mouse monoclonal anti–human CD68; Clone KP1; DAKO) was similarly performed.15 After incubating with secondary antibody (rabbit anti-rat for F4/80 and mouse anti-human for CD68), positive staining was revealed by peroxidase-labeled streptavidin and diaminobenzidine substrate.15
Quantification of Immunostaining
Quantification of KLF15 staining in the podocytes was determined by quantifying the ratio of cells that are +KLF15+WT1+Hoechst to the number of cells that are +WT1+Hoechst staining using ImageJ 1.26t software (National Institutes of Health; rsb.info.nih.gov/ij). F4/80 and Gr-1 staining was quantified by counting the numbers of F4/80+ and Gr-1+ cells per high power field (30–high power field micrographs at a final magnification of approximately ×20 were used). Quantification of CD68 staining was performed semiquantitatively (none, mild/minimal [<10%], moderate [10%–25%], and severe [>25%] of cells in the inflammatory infiltrate positive for CD68) by the pathologist. Quantification of intensity of glomerular KLF15, KLF4, Nephrin, and Synaptopodin staining was performed by measuring area, integrated density, and mean gray value using ImageJ.15
Quantification of podocyte number per glomerulus was determined using Wt1-stained podocytes. After staining for Wt1 and Hoechst by immunofluorescence (as described above), we initially counted the number of cells colocalized for both Wt1+ and Hoechst+ cells. Subsequently, the glomerular area and volume were measured using ImageJ, and the podocyte number per glomerulus was determined using the Weibel method (20).53
Human Kidney Biopsies
We examined 51 archived kidney biopsies in paraffin-embedded blocks (16 healthy donor nephrectomies and 35 MCD and primary FSGS) from 2000 to 2013 at Stony Brook University and Icahn School of Medicine at Mount Sinai. The inclusion criteria were all biopsies from patients diagnosed clinically and subsequently, histologically with MCD or primary FSGS who were treated with GCs for a minimum of a 6-month period subsequent to the biopsy. Exclusion criteria were the following: discontinuation of GC therapy for nonrenal reasons (uncontrolled hyperglycemia, excessive weight gain, infections, osteoporotic fractures, or gastric ulcers), uncontrolled hypertension as defined by systolic BP >160 mmHg at the time of renal biopsy, and/or other known causes of acute kidney disease or CKD, such as diabetic nephropathy, hypertensive nephropathy, pyelonephritis, autosomal dominant polycystic kidney disease, and acute/chronic interstitial nephritis.
Definitions
Response to GC treatment in patients with MCD or primary FSGS was defined as a 50% reduction in proteinuria without an increase in serum creatinine from the time of renal biopsy at 12 months.54–57 Histopathologic examination of renal biopsy specimens was performed with light microscopy, electron microscopy, and immunofluorescence. Diagnosis of MCD and primary FSGS with scoring for chronic tubulointerstitial injury were determined independently by two renal pathologists (M.P.R. and Frederick Miller) at Stony Brook Medicine. Chronic tubulointerstitial injury scores were as follows: none, mild/minimal, moderate, and severe on the basis of the percentage of affected cortical area.45 Hypertension was defined according to the Fourth Report for subjects, the JNC8 for subjects ≤18 years old,58 and the JNC8 for subjects ≥18 years old.59
Calculation of eGFR
For patients ≥18 years of age, eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.60 For patients <18 years of age, eGFR was calculated using the equation by Schwartz et al.61
Statistical Analyses
The t test was used to compare continuous data between two groups, and two-way ANOVA with Tukey post-test was used to compare continuous data between more than two groups. Because we could not assume normality on some of the other datasets with smaller sample sizes, nonparametric statistical tests were performed using the Mann–Whitney test to compare continuous data between two groups and the Kruskal–Wallis test with Dunn post-test to compare continuous data between more than two groups. Chi-squared or Fisher exact test, as appropriate, was used to compare the significance between categorical variables. The exact test used for each experiment is denoted in the figures. Data were expressed as the means±SEMs, means±SDs, medians and interquartile ranges (25th and 75th percentiles), or numbers of patients for categorical variables. All experiments were repeated a minimum of three times, and representative experiments are shown. Statistical significance was considered when P<0.05. All statistical analyses were performed using GraphPad Prism 5.0a (GraphPad Software, La Jolla, CA).
Study Approval
Stony Brook University Animal Institute Committee approved all animal studies, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals was followed strictly. Stony Brook University Institutional Review Board approved the use of deidentified clinical data and the use of archived, deidentified human biopsy specimens for immunostaining.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Anthony N. Gerber for providing the chromatin immunoprecipitation primer sequences for the identification of glucocorticoid response elements on Krüppel–like factor 15.
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants 1K0801DK102519-01 (to S.K.M.) and 1R01DK078897-01 (to J.C.H.), Dialysis Clinic Inc. Paul Teschan Research Grant (to S.K.M.) and grants R01GM098316 (to A. Ma'ayan), R01DK088541 (to A. Ma'ayan), U54HG008230 (to A. Ma'ayan), and U54CA189201 (to A. Ma'ayan), and Chinese 973 Fund grant 2012CB517601 (to J.C.H.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060672/-/DCSupplemental.
References
- 1.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Meyrier A: Mechanisms of disease: Focal segmental glomerulosclerosis. Nat Clin Pract Nephrol 1: 44–54, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Barisoni L: Podocyte biology in segmental sclerosis and progressive glomerular injury. Adv Chronic Kidney Dis 19: 76–83, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ: Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin Sci (Lond) 94: 557–572, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Riccardi C, Bruscoli S, Migliorati G: Molecular mechanisms of immunomodulatory activity of glucocorticoids. Pharmacol Res 45: 361–368, 2002 [DOI] [PubMed] [Google Scholar]
- 6.van Husen M, Kemper MJ: New therapies in steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome. Pediatr Nephrol 26: 881–892, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Schönenberger E, Ehrich JH, Haller H, Schiffer M: The podocyte as a direct target of immunosuppressive agents. Nephrol Dial Transplant 26: 18–24, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Guess A, Agrawal S, Wei CC, Ransom RF, Benndorf R, Smoyer WE: Dose- and time-dependent glucocorticoid receptor signaling in podocytes. Am J Physiol Renal Physiol 299: F845–F853, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ: Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: Role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol 16: 2615–2625, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Wada T, Pippin JW, Nangaku M, Shankland SJ: Dexamethasone’s prosurvival benefits in podocytes require extracellular signal-regulated kinase phosphorylation. Nephron, Exp Nephrol 109: e8–e19, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW: Direct effects of dexamethasone on human podocytes. Kidney Int 70: 1038–1045, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Ransom RF, Vega-Warner V, Smoyer WE, Klein J: Differential proteomic analysis of proteins induced by glucocorticoids in cultured murine podocytes. Kidney Int 67: 1275–1285, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Cohen CD, Klingenhoff A, Boucherot A, Nitsche A, Henger A, Brunner B, Schmid H, Merkle M, Saleem MA, Koller KP, Werner T, Gröne HJ, Nelson PJ, Kretzler M: Comparative promoter analysis allows de novo identification of specialized cell junction-associated proteins. Proc Natl Acad Sci U S A 103: 5682–5687, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McConnell BB, Yang VW: Mammalian Krüppel-like factors in health and diseases. Physiol Rev 90: 1337–1381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallipattu SK, Liu R, Zheng F, Narla G, Ma’ayan A, Dikman S, Jain MK, Saleem M, D’Agati V, Klotman P, Chuang PY, He JC: Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J Biol Chem 287: 19122–19135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asada M, Rauch A, Shimizu H, Maruyama H, Miyaki S, Shibamori M, Kawasome H, Ishiyama H, Tuckermann J, Asahara H: DNA binding-dependent glucocorticoid receptor activity promotes adipogenesis via Krüppel-like factor 15 gene expression. Lab Invest 91: 203–215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuno K, Haldar SM, Jeyaraj D, Mailloux CM, Huang X, Panettieri RA Jr., Jain MK, Gerber AN: Expression profiling identifies Klf15 as a glucocorticoid target that regulates airway hyperresponsiveness. Am J Respir Cell Mol Biol 45: 642–649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matys V, Fricke E, Geffers R, Gössling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV, Kloos DU, Land S, Lewicki-Potapov B, Michael H, Münch R, Reuter I, Rotert S, Saxel H, Scheer M, Thiele S, Wingender E: TRANSFAC: Transcriptional regulation, from patterns to profiles. Nucleic Acids Res 31: 374–378, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A: Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14: 128, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P: Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113: 1390–1397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, He L, Takemoto M, Patrakka J, Pikkarainen T, Genové G, Norlin J, Truvé K, Tryggvason K, Betsholtz C: Glomerular transcriptome changes associated with lipopolysaccharide-induced proteinuria. Am J Nephrol 29: 558–570, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Clement LC, Avila-Casado C, Macé C, Soria E, Bakker WW, Kersten S, Chugh SS: Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med 17: 117–122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heemann U, Szabo A, Hamar P, Müller V, Witzke O, Lutz J, Philipp T: Lipopolysaccharide pretreatment protects from renal ischemia/reperfusion injury : Possible connection to an interleukin-6-dependent pathway. Am J Pathol 156: 287–293, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Y, Xie C, Chen J, Zhu J, Zhou H, Thomas J, Zhou XJ, Mohan C: Innate stimuli accentuate end-organ damage by nephrotoxic antibodies via Fc receptor and TLR stimulation and IL-1/TNF-alpha production. J Immunol 176: 632–639, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Reiser J, Mundel P: Danger signaling by glomerular podocytes defines a novel function of inducible B7-1 in the pathogenesis of nephrotic syndrome. J Am Soc Nephrol 15: 2246–2248, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Srivastava T, Sharma M, Yew KH, Sharma R, Duncan RS, Saleem MA, McCarthy ET, Kats A, Cudmore PA, Alon US, Harrison CJ: LPS and PAN-induced podocyte injury in an in vitro model of minimal change disease: Changes in TLR profile. J Cell Commun Signal 7: 49–60, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi K, Sasamura H, Nakamura M, Azegami T, Oguchi H, Sakamaki Y, Itoh H: KLF4-dependent epigenetic remodeling modulates podocyte phenotypes and attenuates proteinuria. J Clin Invest 124: 2523–2537, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi K, Sasamura H, Nakamura M, Sakamaki Y, Azegami T, Oguchi H, Tokuyama H, Wakino S, Hayashi K, Itoh H: Renin-angiotensin blockade resets podocyte epigenome through Kruppel-like Factor 4 and attenuates proteinuria. Kidney Int 88: 745–753, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Hayashi K, Sasamura H, Ishiguro K, Sakamaki Y, Azegami T, Itoh H: Regression of glomerulosclerosis in response to transient treatment with angiotensin II blockers is attenuated by blockade of matrix metalloproteinase-2. Kidney Int 78: 69–78, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Lee VW, Harris DC: Adriamycin nephropathy: A model of focal segmental glomerulosclerosis. Nephrology (Carlton) 16: 30–38, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Pippin JW, Krofft RD, Naito S, Liu ZH, Shankland SJ: Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Renal Physiol 304: F1375–F1389, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White JT, Zhang B, Cerqueira DM, Tran U, Wessely O: Notch signaling, wt1 and foxc2 are key regulators of the podocyte gene regulatory network in Xenopus. Development 137: 1863–1873, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasse SK, Mailloux CM, Barczak AJ, Wang Q, Altonsy MO, Jain MK, Haldar SM, Gerber AN: The glucocorticoid receptor and KLF15 regulate gene expression dynamics and integrate signals through feed-forward circuitry. Mol Cell Biol 33: 2104–2115, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, Ruiz-Gómez M, Skaer H, Denholm B: The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivy JR, Drechsler M, Catterson JH, Bodmer R, Ocorr K, Paululat A, Hartley PS: Klf15 is critical for the development and differentiation of drosophila nephrocytes. PLoS One 10: e0134620, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y, Zhang L, Liao X, Sangwung P, Prosdocimo DA, Zhou G, Votruba AR, Brian L, Han YJ, Gao H, Wang Y, Shimizu K, Weinert-Stein K, Khrestian M, Simon DI, Freedman NJ, Jain MK: Kruppel-like factor 15 is critical for vascular inflammation. J Clin Invest 123: 4232–4241, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE: Glucocorticoid therapy for immune-mediated diseases: Basic and clinical correlates. Ann Intern Med 119: 1198–1208, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Saag KG, Koehnke R, Caldwell JR, Brasington R, Burmeister LF, Zimmerman B, Kohler JA, Furst DE: Low dose long-term corticosteroid therapy in rheumatoid arthritis: An analysis of serious adverse events. Am J Med 96: 115–123, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Da Silva JA, Jacobs JW, Kirwan JR, Boers M, Saag KG, Inês LB, de Koning EJ, Buttgereit F, Cutolo M, Capell H, Rau R, Bijlsma JW: Safety of low dose glucocorticoid treatment in rheumatoid arthritis: Published evidence and prospective trial data. Ann Rheum Dis 65: 285–293, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bialkowska AB, Du Y, Fu H, Yang VW: Identification of novel small-molecule compounds that inhibit the proproliferative Kruppel-like factor 5 in colorectal cancer cells by high-throughput screening. Mol Cancer Ther 8: 563–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He JC, Lu TC, Fleet M, Sunamoto M, Husain M, Fang W, Neves S, Chen Y, Shankland S, Iyengar R, Klotman PE: Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J Am Soc Nephrol 18: 93–102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mallipattu SK, Liu R, Zhong Y, Chen EY, D’Agati V, Kaufman L, Ma’ayan A, Klotman PE, Chuang PY, He JC: Expression of HIV transgene aggravates kidney injury in diabetic mice. Kidney Int 83: 626–634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mallipattu SK, Horne SJ, D’Agati V, Narla G, Liu R, Frohman MA, Dickman K, Chen EY, Ma’ayan A, Bialkowska AB, Ghaleb AM, Nandan MO, Jain MK, Daehn I, Chuang PY, Yang VW, He JC: Krüppel-like factor 6 regulates mitochondrial function in the kidney. J Clin Invest 125: 1347–1361, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, D’Agati V, Appel G: Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2: 445–453, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ: De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol 298: F702–F711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katsuya K, Yaoita E, Yoshida Y, Yamamoto Y, Yamamoto T: An improved method for primary culture of rat podocytes. Kidney Int 69: 2101–2106, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Dai Y, Gu L, Yuan W, Yu Q, Ni Z, Ross MJ, Kaufman L, Xiong H, Salant DJ, He JC, Chuang PY: Podocyte-specific deletion of signal transducer and activator of transcription 3 attenuates nephrotoxic serum-induced glomerulonephritis. Kidney Int 84: 950–961, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian X, Kim JJ, Monkley SM, Gotoh N, Nandez R, Soda K, Inoue K, Balkin DM, Hassan H, Son SH, Lee Y, Moeckel G, Calderwood DA, Holzman LB, Critchley DR, Zent R, Reiser J, Ishibe S: Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Invest 124: 1098–1113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mallipattu SK, Gallagher EJ, LeRoith D, Liu R, Mehrotra A, Horne SJ, Chuang PY, Yang VW, He JC: Diabetic nephropathy in a nonobese mouse model of type 2 diabetes mellitus. Am J Physiol Renal Physiol 306: F1008–F1017, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhong Y, Wu Y, Liu R, Deng Y, Mallipattu SK, Klotman PE, Chuang PY, He JC: Roflumilast enhances the renal protective effects of retinoids in an HIV-1 transgenic mouse model of rapidly progressive renal failure. Kidney Int 81: 856–864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemley KV, Bertram JF, Nicholas SB, White K: Estimation of glomerular podocyte number: A selection of valid methods. J Am Soc Nephrol 24: 1193–1202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyrier A, Noël LH, Auriche P, Callard P; Collaborative Group of the Société de Néphrologie : Long-term renal tolerance of cyclosporin A treatment in adult idiopathic nephrotic syndrome. Kidney Int 45: 1446–1456, 1994 [DOI] [PubMed] [Google Scholar]
- 55.Niaudet P; French Society of Pediatric Nephrology : Treatment of childhood steroid-resistant idiopathic nephrosis with a combination of cyclosporine and prednisone. J Pediatr 125: 981–986, 1994 [DOI] [PubMed] [Google Scholar]
- 56.Lieberman KV, Tejani A: A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol 7: 56–63, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Ponticelli C, Rizzoni G, Edefonti A, Altieri P, Rivolta E, Rinaldi S, Ghio L, Lusvarghi E, Gusmano R, Locatelli F, Pasquali S, Castellani A, Dell Casa-Alberighi O: A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int 43: 1377–1384, 1993 [DOI] [PubMed] [Google Scholar]
- 58.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 59.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr., Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr., Narva AS, Ortiz E: 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.