Abstract
The cardiac natriuretic peptides (NPs), atrial NP and B-type NP, regulate fluid homeostasis and arterial BP through renal actions involving increased GFR and vascular and tubular effects. Guanylyl cyclase-A (GC-A), the transmembrane cGMP-producing receptor shared by these peptides, is expressed in different renal cell types, including podocytes, where its function is unclear. To study the effects of NPs on podocytes, we generated mice with a podocyte-specific knockout of GC-A (Podo-GC-A KO). Despite the marked reduction of GC-A mRNA in GC-A KO podocytes to 1% of the control level, Podo-GC-A KO mice and control littermates did not differ in BP, GFR, or natriuresis under baseline conditions. Moreover, infusion of synthetic NPs similarly increased the GFR and renal perfusion in both genotypes. Administration of the mineralocorticoid deoxycorticosterone-acetate (DOCA) in combination with high salt intake induced arterial hypertension of similar magnitude in Podo-GC-A KO mice and controls. However, only Podo-GC-A KO mice developed massive albuminuria (controls: 35-fold; KO: 5400-fold versus baseline), hypoalbuminemia, reduced GFR, and marked glomerular damage. Furthermore, DOCA treatment led to decreased expression of the slit diaphragm-associated proteins podocin, nephrin, and synaptopodin and to enhanced transient receptor potential canonical 6 (TRPC6) channel expression and ATP-induced calcium influx in podocytes of Podo-GC-A KO mice. Concomitant treatment of Podo-GC-A KO mice with the TRPC channel blocker SKF96365 markedly ameliorated albuminuria and glomerular damage in response to DOCA. In conclusion, the physiologic effects of NPs on GFR and natriuresis do not involve podocytes. However, NP/GC-A/cGMP signaling protects podocyte integrity under pathologic conditions, most likely by suppression of TRPC channels.
Keywords: cardiac natriuretic peptides, podocyte, albuminuria, progression of renal failure
The glomerular capillaries are covered by podocyte foot processes that interdigitate in a coordinated fashion, leaving a filtration slit between them. The slit is covered by the slit diaphragm, which restricts the passage of macromolecules such as plasma albumin. Due to the critical roles of the podocytes and the slit diaphragm in glomerular ultrafiltration, mutations in slit diaphragm proteins such as podocin,1 nephrin,2 CD2-associated protein3 and transient receptor potential canonical 6 channel (TRPC6)4 result in proteinuria. In addition to genetic diseases affecting the podocytes, several acquired diseases such as diabetic and hypertensive nephropathy affect podocyte integrity and induce proteinuria.
The cardiac natriuretic peptides (NPs), atrial natriuretic peptide (ANP), and B-type natriuretic peptide (BNP) provide an endocrine link between the heart and the kidney.5 Both ANP and BNP activate the same receptor, the membrane-bound guanylyl cyclase-A (GC-A), thereby stimulating cGMP formation.6 GC-A is expressed in various tissues and regulates many body functions. Importantly, the NPs, via GC-A, play a fundamental role in arterial BP homeostasis: they are vasodilators, suppress renin release from the kidney and aldosterone release from the adrenal glands, and they inhibit sympathetic tone.6 In the kidney, GC-A expression has been detected immunohistochemically or functionally in renal vessels, renin-producing juxtaglomerular cells, in cells of proximal tubules, the loop of Henle and collecting ducts7–11 and in mesangial cells and podocytes.7,8,12,13 Cardiac NPs increase the glomerular filtration rate and induce natriuresis and are therefore important regulators of intravascular volume.6 The increase in GFR in response to NPs is mediated by a rise in the glomerular capillary pressure and by increasing the ultrafiltration coefficient.10,14,15 Although the contractile contribution of podocytes to the regulation of glomerular capillary tone is controversially discussed in the literature,16 a functional role of podocytes in the regulation of GFR is well conceivable. In addition to their direct effects on kidney function, NPs exert renoprotective effects. Thus, genetic overexpression of BNP in the liver, which resulted in high circulating BNP levels, or infusion of synthetic ANP ameliorated renal damage in diabetic mice and in immune-mediated renal injury, renal ablation or unilateral ureteral obstruction, as indicated by reduced mesangial expansion, albuminuria and renal fibrosis.17–20 Moreover, short-term infusion of ANP or BNP may preserve kidney function and ameliorate acute kidney failure in patients undergoing cardiovascular surgery (meta-analysis21). Interestingly, perioperative ANP infusion in CKD patients undergoing cardiac surgery reduced serum creatinine concentration during the infusion period and this beneficial effect persisted for up to one year post-surgery.22 Conversely, interruption of NP signaling by genetic deletion of GC-A resulted in mesangial expansion, albuminuria and renal fibrosis, together with increases in proinflammatory cytokine levels, even under baseline conditions.23,24 Treatment of global GC-A knockout mice with aldosterone together with a high salt intake induced massive albuminuria and renal damage.7 Due to the widespread distribution of GC-A and the pleiotropic effects of the cardiac NPs, it is reasonable to assume that the profound renoprotective effects of NPs are related to their beneficial effects on blood pressure and neurohormonal activation, i.e., counterregulation of the sympathetic and the renin-angiotensin-aldosterone systems. However, since previous studies have shown that NPs ameliorated albuminuria, and since GC-A is expressed in podocytes, we hypothesized that cardiac NPs might exhibit a direct protective effect on podocytes. To study this hypothesis, we generated mice with a podocyte specific inactivation of the GC-A gene.
Results
Generation and Validation of Podocyte Specific GC-A Knockout Mice
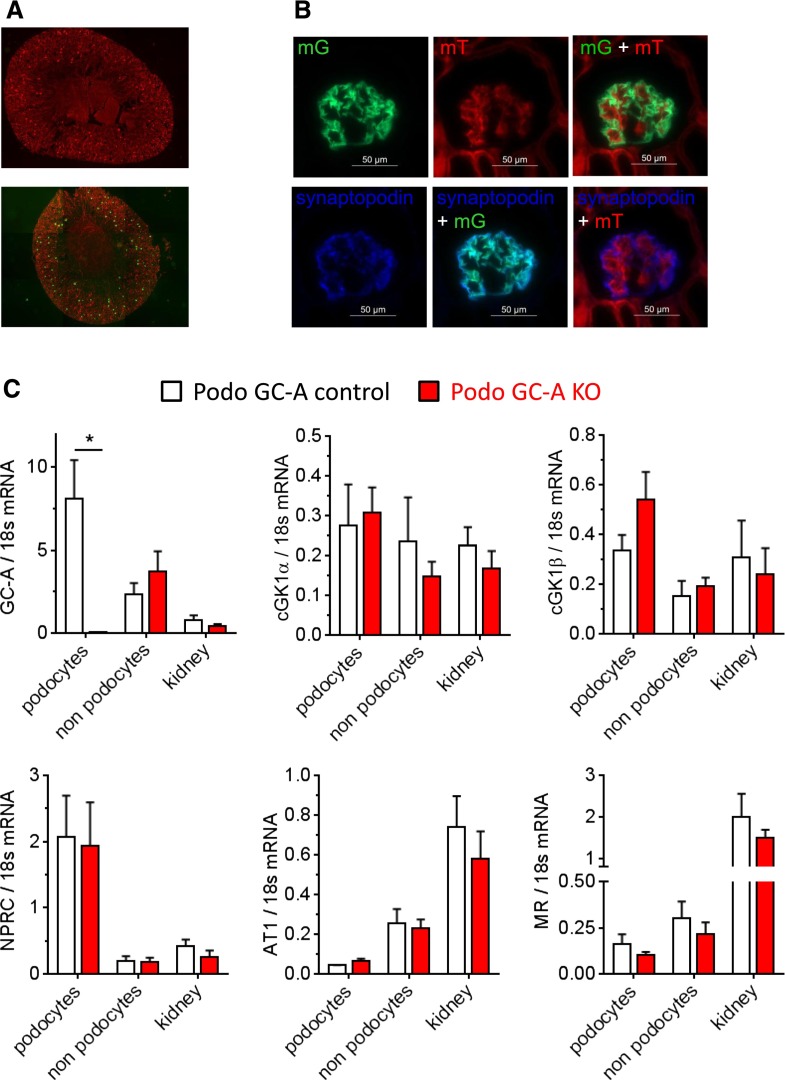
Mice with podocyte-specific ablation of GC-A were generated by crossing a mouse line expressing Cre recombinase under the control of the NPHS2 (podocin) promoter25 with mice carrying floxed alleles of the gene coding for GC-A26 (NPHS2-Cre x GC-Aflox/flox, termed “Podo GC-A KO” throughout this paper). To assess GC-A expression directly in podocytes, Podo GC-A KO mice were crossed with reporter mice,27 and green fluorescent podocytes (Figure 1, A and B) were separated from other glomerular cells by FACS28 (Supplemental Figures 1 and 2). Podocytes of Podo GC-A control mice (NPHS2-Cre x GC-AWT/WT) had higher GC-A mRNA expression levels than other glomerular cells (including mesangial, smooth muscle, and endothelial cells) and nonglomerular kidney tissue. In contrast, GC-A mRNA was virtually absent in podocytes isolated from Podo GC-A KO mice but was not reduced in other renal cell types (Figure 1C) or organs such as the heart, liver, aorta, adrenal glands, or brain from these mice (not shown). The mRNA expression levels of cGMP-dependent protein kinase I α and β, natriuretic peptide receptor-C/NPR-C, angiotensin II receptor AT1, and mineralocorticoid receptors were not different in podocytes from KO and control mice (Figure 1C).
Figure 1.
GC-A is effectively and specifically deleted in podocytes of Podo GC-A KO mice. (A and B) To determine GC-A expression in podocytes, Podo GC-A KO were crossed with reporter mice with global expression of the red fluorescent protein mTomato (mT).27 Upon Cre-mediated recombination, cells stop expressing mT and instead express GFP (mG) and display green fluorescence. (A) Kidney sections of reporter mice without Cre expression (upper panel) or with podocyte-specific Cre expression (Podo-Cre) (lower panel). Green fluorescence indicates successful recombination in glomeruli. (B) Higher magnification of a Podo-Cre positive kidney section reveals that mG expression is detected exclusively in podocytes (costaining with synaptopodin). No overlap of synaptopodin with red fluorescence (mT) is detectable, indicating that all podocytes underwent successful Cre-mediated recombination. (C) After isolation of glomeruli using magnetic beads and subsequent digestion, the glomerular cell suspension was subjected to FACS sorting, separating podocytes (green fluorescence) from glomerular “nonpodocytes” (red fluorescence) (detailed method in Supplemental Figures 1 and 2). Podocytes isolated from Podo GC-A control mice had the highest GC-A mRNA expression levels of all cell/tissue suspensions investigated (podocytes, “nonpodocytes”: glomerular cells without podocytes, “kidney”: kidney homogenate without glomeruli). GC-A expression was virtually absent in Podo GC-A KO podocytes, but was not reduced in other renal cells. The downstream targets of GC-A/cGMP signaling, protein kinase G isoforms 1 α and 1 β, were both expressed in podocytes but were not regulated by GC-A deletion. Likewise, the expression levels of other membrane receptors, such as the natriuretic peptide clearance receptor (NPRC), the angiotensin II AT1 receptors and the mineralocorticoid receptor (MR), were not altered in podocytes of Podo GC-A KO mice.
Physiologic Studies in Podocyte Specific GC-A Knockout Mice
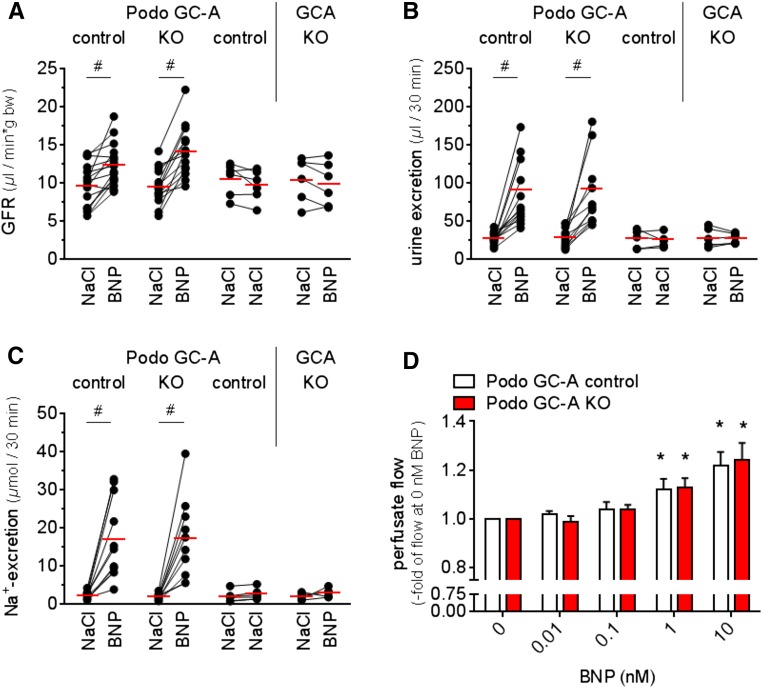
Podo GC-A KO mice were born at the expected ratio and did not show alterations of body weight, arterial blood pressure, GFR, urine and sodium excretion, and plasma volume (Table 1). Infusion of synthetic BNP (1 ng/min per g body wt) markedly stimulated GFR and natriuresis in anesthetized Podo GC-A control and Podo GC-A KO mice to similar extents (Figure 2, A-C), whereas these effects were completely abrogated in mice with global, systemic GC-A deletion (Figure 2, A-C). Finally, ANP (data not shown) and BNP infusions (10 pM–10nM) resulted in similar dose-dependent increases in renal perfusion of isolated perfused kidneys from Podo GC-A KO and control mice (Figure 2D), indicating that GC-A in podocytes does not acutely regulate glomerular hemodynamics. We conclude from these experiments that podocytes do not mediate the physiologic stimulatory effects of NPs on GFR.
Table 1.
Baseline characteristics of Podo GC-A control and KO mice
| Parameter | Sex | Podo GC-A control | Podo GC-A KO |
|---|---|---|---|
| Body weight, g | female | 23.4±0.6 (n=8) | 23.3±0.7 (n=8) |
| male | 27.8±0.9 (n=8) | 28.6±0.8 (n=8) | |
| Weight both kidneys, mg | female | 279±13 (n=8) | 284±10 (n=8) |
| male | 356±15 (n=8) | 369±8 (n=8) | |
| Heart weight, mg | female | 100±4 (n=8) | 105±3 (n=8) |
| male | 122±8 (n=8) | 129±5 (n=8) | |
| Systolic BP, mmHg | female | 127±2.1 (n=12) | 128±2.0 (n=11) |
| male | 134±2.1 (n=13) | 140±2.7 (n=13) | |
| Heart rate, 1/min | female | 662±9.1 (n=12) | 665±10.3 (n=11) |
| male | 660±6.8 (n=13) | 651±7.0 (n=13) | |
| GFR, µl/(min × g body wt) | female | 15.1±0.8 (n=8) | 15.4±0.7 (n=8) |
| male | 15.0±0.5 (n=8) | 15.5±0.7 (n=8) | |
| Na+-excretion, µmol/(12h × g body wt) | female | 5.7±0.6 (n=8) | 5.9±1.1 (n=7) |
| male | 5.4±0.7 (n=6) | 5.5±0.9 (n=5) | |
| Plasma volume, % of body wt | female | 4.09±0.25 (n=5) | 3.64±0.17 (n=5) |
| male | 4.28±0.29 (n=5) | 3. 9±0.18 (n=5) | |
| Hematocrit (%) | female | 50.2±0.6 (n=18) | 49.5±0.8 (n=15) |
| male | 49.8±0.4 (n=16) | 49.2±0.7 (n=15) |
Figure 2.
Acute regulation of kidney function by natriuretic peptides is preserved in Podo GC-A KO mice. Infusion of BNP (30-minute control period (NaCl 0.9%) followed by 1 ng/minute per g body wt for 30 minutes) resulted in increases of similar magnitude in glomerular filtration rate (A), urine excretion rate (B), and natriuresis (C) in Podo GC-A control mice and in Podo GC-A KO mice. These effects on kidney function were not observed in response to vehicle infusion (NaCl 0.9%) or in conventional GC-A knockout mice (GC-A KO) with global deletion of GC-A. (D) Infusion of BNP in isolated perfused kidneys of Podo GC-A control and Podo GC-A KO mice induced concentration-dependent increases in renal perfusion, indicating proper renal and glomerular hemodynamics even under the controlled conditions of the isolated perfused kidney model that is devoid of any potential systemic counter-regulations to GC-A deficiency.
Induction of Renal Damage in Podocyte Specific GC-A Knockout Mice by Deoxycorticosterone-Acetate-Salt Treatment for 6 Weeks
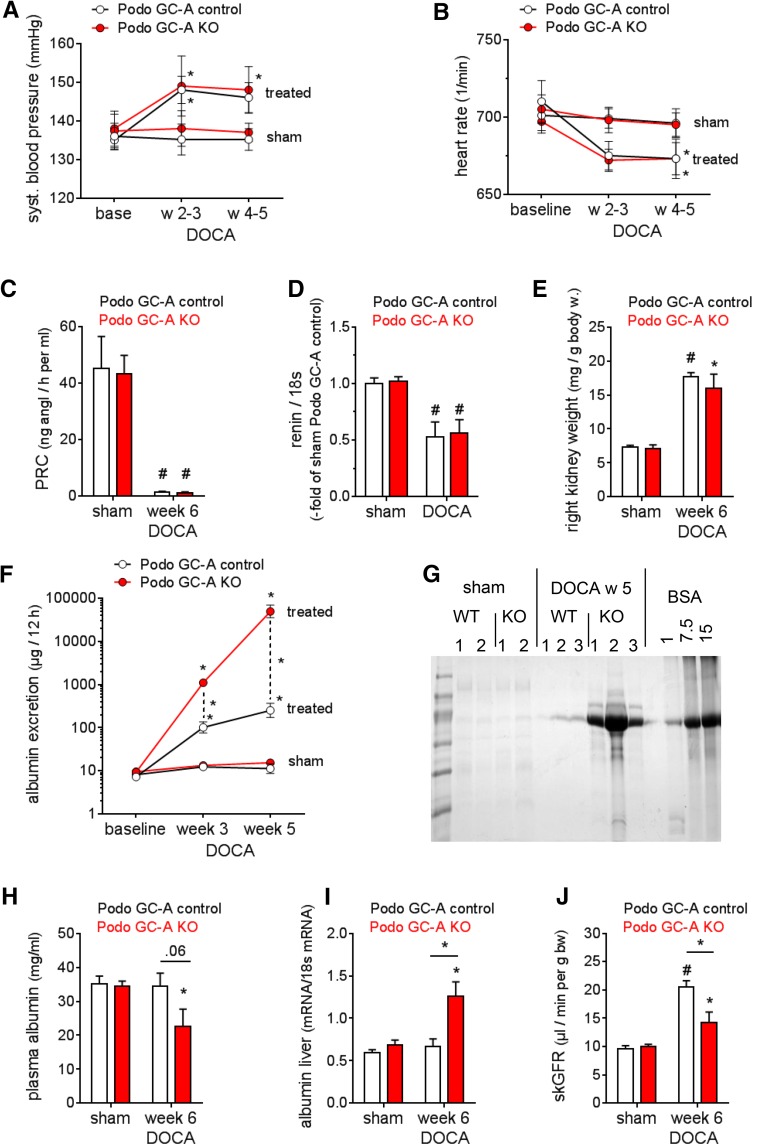
To study whether podocytes are involved in the renoprotective role of NPs under pathologic conditions, we subjected Podo GC-A KO and Podo GC-A control mice to the uninephrectomy/deoxycorticosterone-acetate (DOCA)-salt model, which induces arterial hypertension and podocyte damage.29–31 DOCA treatment over a period of 6 weeks resulted in increases in arterial BP of 10–11 mmHg and concomitant reductions of heart rate in Podo GC-A KO and control mice (Figure 3, A and B). Moreover, DOCA markedly suppressed plasma renin concentration (PRC) and glomerular renin mRNA expression to similar extents in both genotypes (Figure 3, C and D), indicating that the cardiovascular responses to DOCA were similar in both genotypes. Renal hypertrophy in response to DOCA developed to similar degrees in both genotypes compared with sham mice (no uninephrectomy, standard food, no DOCA) (Figure 3E). Urinary albumin excretion was not different between Podo GC-A KO and control mice at baseline (Figure 3F). While moderate albuminuria developed under DOCA treatment in Podo GC-A control mice (35-fold versus baseline), massive albuminuria at nephrotic levels emerged in Podo GC-A KO mice (5400-fold versus baseline) (Figure 3, F and G), resulting in hypoalbuminemia (Figure 3H), and upregulated albumin expression in the liver (Figure 3I). In line with the augmented glomerular damage in the KO mice, the single kidney GFR (skGFR) was significantly lower in DOCA-treated Podo GC-A KO mice compared with Podo GC-A control mice (Figure 3J).
Figure 3.
DOCA-salt treatment results in massive albuminuria in mice with podocyte-specific deletion of GC-A. Mice were subjected to left sided unilateral nephrectomy and were subsequently treated with DOCA and a high salt diet (4% NaCl) (“DOCA”, n=6–7 each genotype). Sham operated mice did not receive unilateral nephrectomy and DOCA treatment and were maintained on standard chow. (A) DOCA induced moderate increases in systolic BP of comparable magnitude in Podo GC-A KO and Podo GC-A control mice. (B) Heart rates were reduced by DOCA to similar levels in both genotypes. (C and D) PRCs and renin mRNA expression levels in isolated glomeruli were markedly downregulated in response to DOCA treatment in Podo GC-A KO and Podo GC-A control mice. (E) Right kidney weights were markedly higher in the DOCA group in both genotypes compared with untreated controls. (F and G) DOCA resulted in massive albuminuria in Podo GC-A KO mice that was 200-fold higher than in Podo GC-A control mice 5 weeks after the start of DOCA treatment. (F) Urine was collected in metabolic cages and albumin concentration in the urine was determined by EIA, (G) analysis of spot urine (1 µl) by gel electrophoresis. (H) Plasma albumin concentration was reduced in DOCA-treated Podo GC-A KO mice compared with untreated sham mice, and (I) albumin mRNA levels in liver tissue were significantly upregulated. (J) The skGFR was determined in conscious mice after 6 weeks of DOCA treatment. Because sham mice had not undergone uninephrectomy and therefore had two kidneys throughout the study, skGFR of sham mice was calculated by dividing GFR by two. The skGFR was significantly increased by DOCA in both genotypes compared with sham. However, the hyperfiltration was attenuated in Podo GC-A KO mice compared with Podo GC-A controls. *P<0.05 versus baseline/sham or versus other genotype as indicated. #P<0.001 versus sham of same genotype.
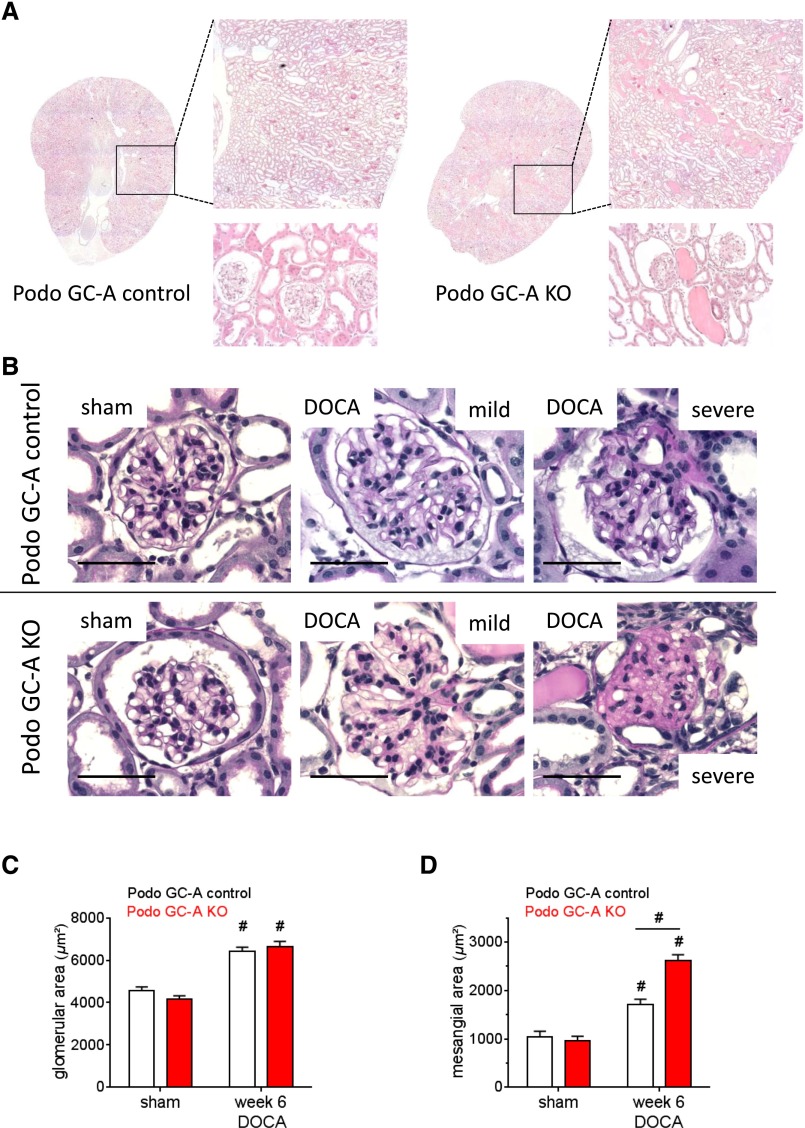
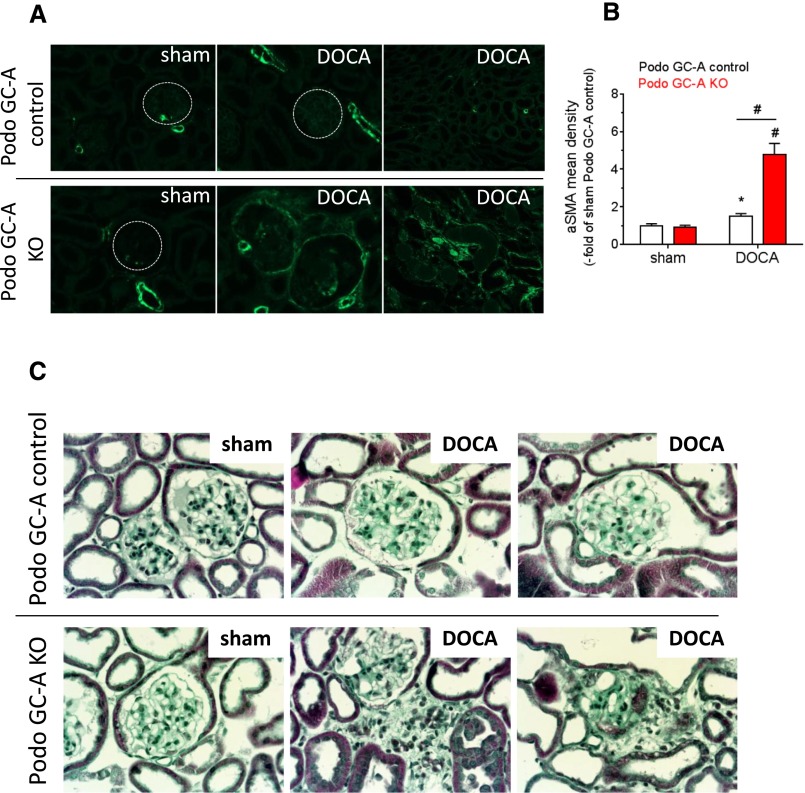
Histologic examination of kidney sections did not reveal any differences between untreated Podo GC-A KO and Podo GC-A control mice (Figures 4–5). In contrast, the kidneys from DOCA-treated Podo GC-A KO mice had massively dilated tubules with segmental accumulations of tubular protein casts, which were not seen in DOCA-treated Podo GC-A control mice (Figure 4A). Glomerular hypertrophy was similar in both genotypes after 6 weeks of DOCA treatment (Figure 4, B and C), whereas mesangial expansion, glomerular adhesions, and glomerulosclerosis were markedly enhanced in Podo GC-A KO mice compared with controls (Figure 4, B and D, Figure 5). Glomerular damage and proteinuria were accompanied by periglomerular and peritubular fibrosis in Podo GC-A KO mice, as demonstrated by enhanced expression of α-smooth muscle actin (α-SMA) (Figure 5, A and B) and by collagen deposition (Figure 5C).
Figure 4.
DOCA-induced glomerular damage is aggravated in mice with podocyte-specific deletion of GC-A. (A) Histologic sections stained with hematoxylin and eosin show protein casts and dilated tubules in kidneys from Podo GC-A KO mice but not in Podo GC-A control mice treated with DOCA for 6 weeks. (B) Representative views of glomeruli from untreated Podo GC-A KO or Podo GC-A control mice (sham) or mice treated with DOCA for 6 weeks. Kidney sections were stained by PAS, bar represents 50 µm. Compared with sham (B, left panel) glomeruli of DOCA-treated mice of both genotypes (B, middle and right panels) had enlarged cross-sectional areas, indicating glomerular hypertrophy (analysis in C). DOCA treatment induced mesangial expansion in both genotypes. Middle panel of (B) shows an example of a mildly affected glomerulus; right panel shows a representative example of a severely affected glomerulus. Glomerulosclerosis with glomerular adhesions was detected in 25% of all glomeruli in Podo GC-A KO mice but not in control mice, and mesangial expansion (analysis in D) was clearly aggravated in Podo GC-A KO mice compared with Podo GC-A controls. #P<0.001 versus sham of same genotype or versus other genotype at same treatment as indicated.
Figure 5.
DOCA treatment induces periglomerular and peritubular fibrosis in mice with podocyte-specific deletion of GC-A. (A and C) Representative kidney sections from Podo GC-A control mice (upper row) and KO mice (lower row) without treatment (left panels) and with DOCA treatment for 6 weeks. Sections were stained with either an antibody directed against αSMA (panel A) or Masson-Goldner stain (C). (A) To determine the αSMA staining density in glomeruli (results shown in B), blood vessels showing high αSMA expression were omitted from the region of interest. Glomeruli from control mice did not show prominent extravascular αSMA staining, whereas clear αSMA expression was visible in glomeruli (middle panel) and the peritubular interstitium of Podo GC-A KO mice (right panel). Fibrosis was confirmed by Masson-Goldner staining (C), which revealed fibrous tissue in sclerotic glomeruli and in the periglomerular interstitium. #P<0.001 versus sham or versus other genotype at same treatment as indicated. *P<0.05 versus sham.
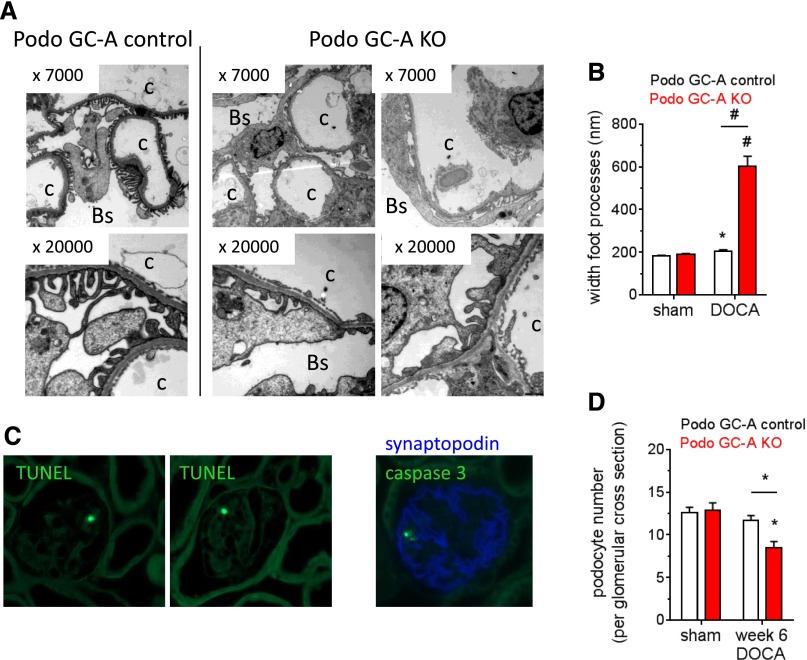
Electronmicroscopy analyses revealed ultrastructural signs of podocyte damage, as podocyte foot process effacement was observed in each of the investigated glomerular capillaries in DOCA-salt-treated Podo GC-A KO mice but not in Podo GC-A control (Figure 6, A and B). Although at a low frequency (approximately 1% of all glomeruli), DOCA-treated Podo GC-A KO but not control glomeruli contained a cell with positive terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) or positive staining for caspase 3, both indicating apoptosis (Figure 6C). Noteworthy, no co-staining with podocyte markers such as podocin (not shown) or synaptopodin (Figure 6C) was detectable in TUNEL or caspase 3 positive cells. The podocyte number per glomerular cross-section (Figure 6D) or per glomerular area (not shown) was significantly reduced in DOCA treated Podo GC-A KO compared with sham or treated Podo GC-A control mice.
Figure 6.
DOCA treatment induces ultrastructural damage in podocytes in Podo GC-A KO mice. (A and B) Electron microscopy showed the normal appearance and only minor widening of podocytes foot processes in Podo GC-A control mice after 6 weeks of DOCA treatment (left panel of A and B). Massive foot process effacement was observed in each of the investigated Podo GC-A KO glomerular capillaries (A, middle and right panels), resulting in a 3-fold increase in foot process width compared with untreated sham or treated Podo GC-A control mice (B). C: capillary lumen; Bs: Bowman’s space. #P<0.001 versus sham or versus other genotype at same treatment as indicated. *P<0.05 versus sham. (C) In Podo GC-A KO mice treated with DOCA for 6 weeks positive TUNEL staining (green fluorescence, left and middle panel) or caspase 3 (green fluorescence, right panel, costaining with synaptopodin in blue) in the periphery of the glomerular tuft is observed in approximately 1% of glomeruli. No TUNEL-positive or caspase 3 positive cells were detected in either untreated sham mice or Podo GC-A control treated with DOCA. D: Podocyte number per glomerular cross-section was significantly reduced in DOCA treated Podo GC-A KO mice. *P<0.05 versus sham.
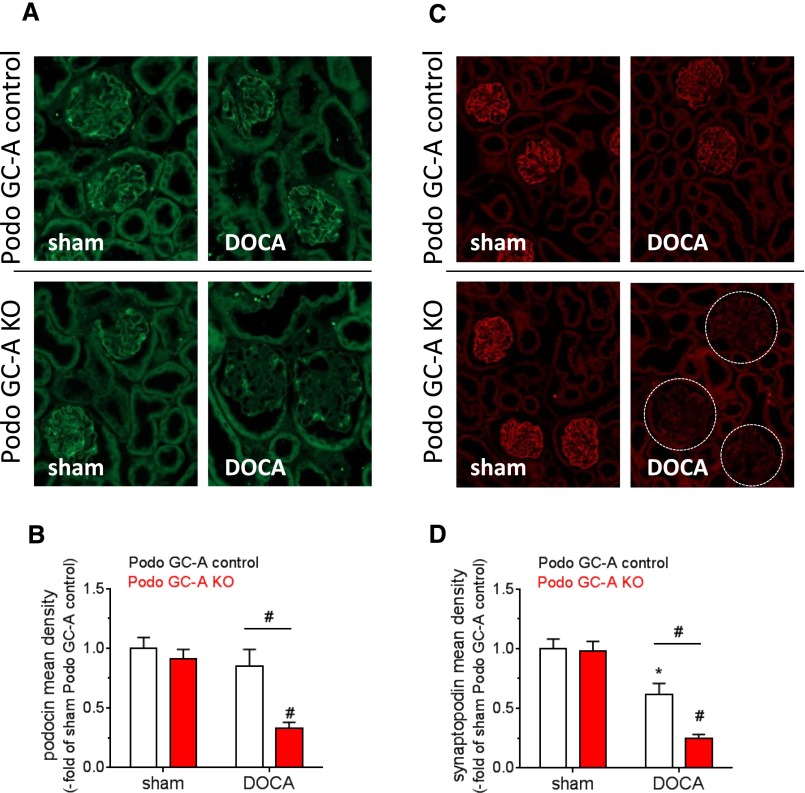
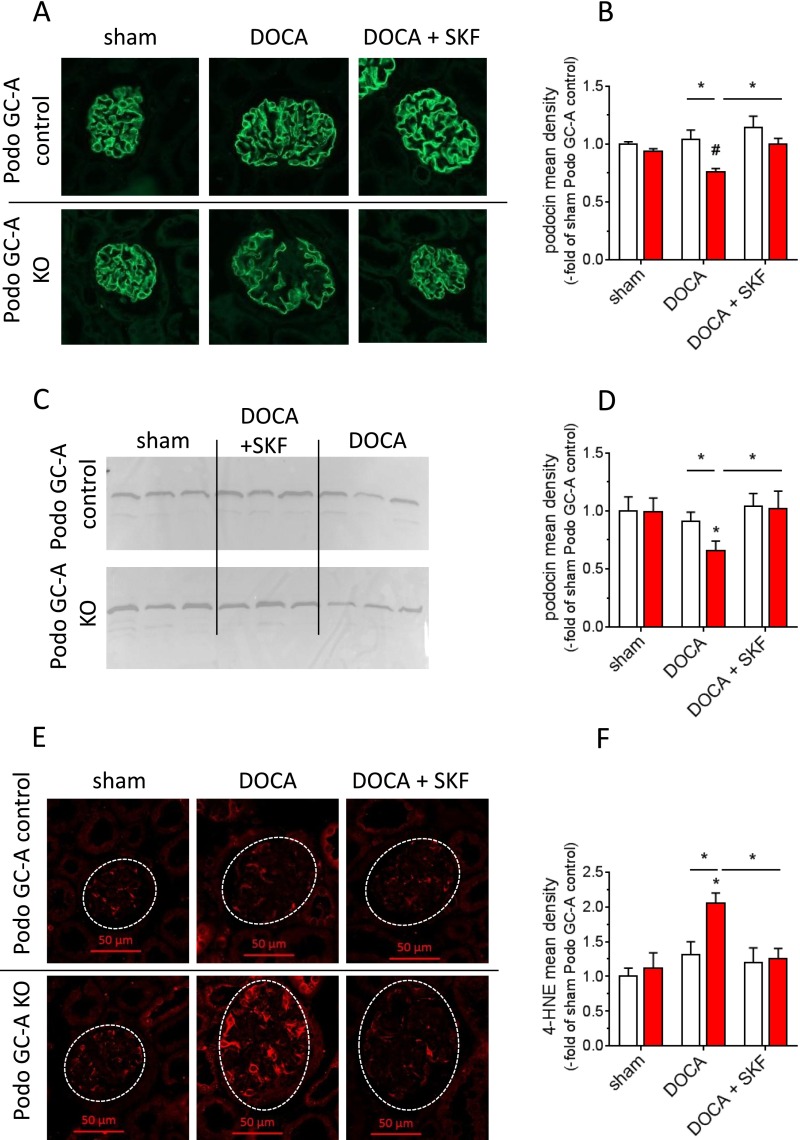
The expression levels of the slit diaphragm proteins podocin (Figure 7, A and B) and nephrin (not shown) were downregulated in DOCA-treated Podo GC-A KO but not Podo GC-A control mice compared with sham mice. The downregulation of synaptopodin, an actin-associated podocyte protein, was aggravated in KO mice compared with Podo GC-A control mice (Figure 7, C and D).
Figure 7.
The expression levels of the podocyte proteins podocin and synaptopodin are markedly reduced in Podo GC-A KO mice treated with DOCA. Immunofluorescence staining for podocin (A) and synaptopodin (C) showed a typical podocyte pattern in sham and DOCA-treated Podo GC-A control mice and in sham Podo GC-A KO mice. In contrast, podocin staining was restricted to podocyte cell bodies, and synaptopodin staining was hardly visible in DOCA-treated Podo GC-A KO mice. Analysis of fluorescence density (product of intensity and area) in glomeruli revealed significant downregulation of podocin expression in Podo GC-A KO mice by DOCA treatment (B). Glomerular synaptopodin density was reduced by DOCA in both genotypes, although to lower levels in Podo GC-A KO compared with Podo GC-A control (D). #P<0.001 versus sham or versus other genotype at same treatment as indicated. *P<0.05 versus sham.
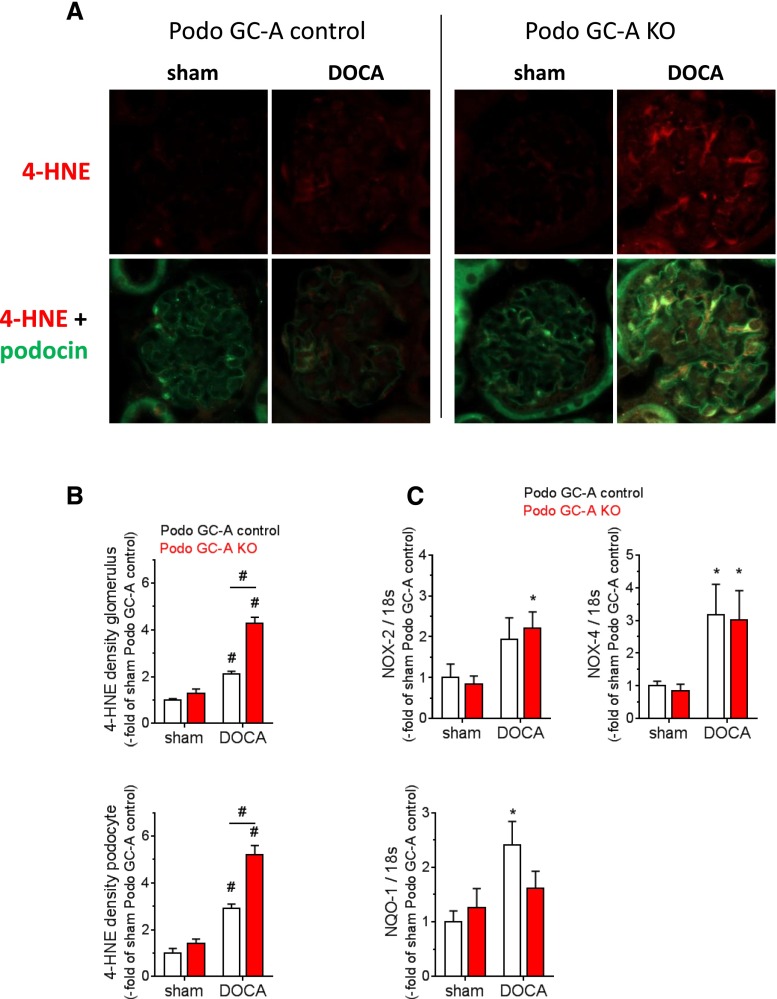
Podocyte damage is associated with oxidative stress in several renal diseases, and activation of mineralocorticoid receptors by aldosterone or DOCA induces formation of reactive oxygen species (ROS) in podocytes.7,32 Staining of 4-hydroxynonenal (4-HNE), a product of lipid peroxidation that not only serves as a surrogate marker for oxidative stress but is also known to induce ROS-dependent apoptosis at least in other cell types,34 indicated enhanced oxidative stress in podocytes of DOCA-treated knockout mice (Figure 8, A and B). In sham animals, only faint 4-HNE staining was detected in glomeruli from Podo GC-A KO and control mice (Figure 8A). DOCA treatment induced 4-HNE formation in mesangial cells and especially in podocytes (costaining with podocin) of both genotypes (Figure 8A). However, the 4-HNE-positive area and the staining intensity were clearly enhanced in Podo GC-A KO glomeruli compared with Podo GC-A control (Figure 8, A and B), indicating augmented oxidative stress in the absence of GC-A in podocytes. In line with enhanced 4-HNE staining DOCA treatment stimulated DOCA treatment stimulated the expression of the NADPH oxidases NOX-2 and NOX-4 to similar levels in isolated glomeruli of both genotypes (Figure 8C). In contrast, the antioxidant enzyme NADPH dehydrogenase, quinone 133 (NQO-1) was significantly upregulated in Podo GC-A control glomeruli but not in glomeruli from Podo GC-A KO mice (Figure 8C); thus, an imbalance in ROS-related enzymes might be present in Podo GC-A KO.
Figure 8.
Oxidative stress is increased in podocytes of Podo GC-A KO mice under DOCA treatment. (A) Kidney sections were stained with an antibody directed against 4-HNE, which is produced by lipid peroxidation and serves as a marker of oxidative stress (red, upper row). For the identification of podocytes, costaining with an antipodocin antibody was performed. Image acquisition parameters for podocin staining had to be adjusted due to different expression intensities between groups (lower row, A). While 4-HNE was hardly visible in sham mice and only faint 4-HNE staining was detected in DOCA-treated Podo GC-A control mice, marked 4-HNE immunofluorescence was evident in the glomeruli of DOCA-treated Podo GC-A KO mice. Enhancement of 4-HNE was not restricted to podocytes but was also visible in both the glomerular mesangium (A) and tubular cells (not shown). Analysis of fluorescence density (product of intensity and area) in both the glomeruli (B, upper panel) and podocytes (B, lower panel) of Podo GC-A control and KO mice. (C) mRNA expression levels of NADPH oxidases NOX-2 and NOX-4 were upregulated by DOCA treatment in the glomeruli of Podo GC-A control and KO glomeruli to similar extents, whereas the expression of the NADPH dehydrogenase NQO-1 was stimulated by DOCA treatment in Podo GC-A control glomeruli but not in Podo GC-A KO glomeruli. #P<0.001 versus sham or versus other genotype at same treatment as indicated. *P<0.05 versus sham.
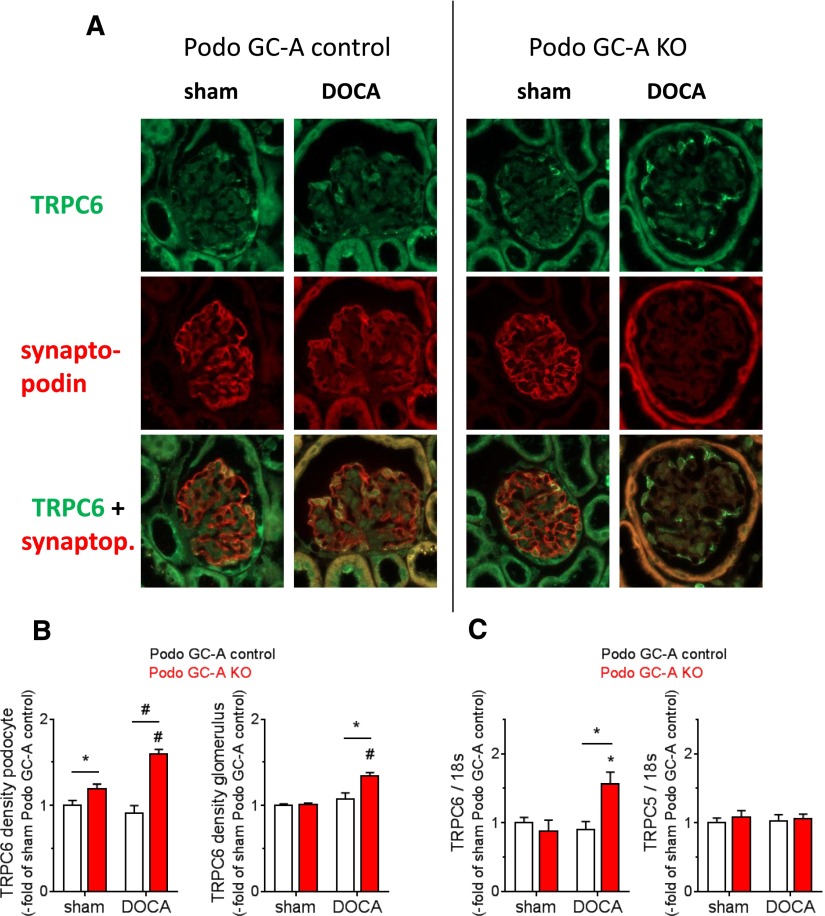
In contrast to the podocyte-specific proteins podocin, nephrin, and synaptopodin, TRPC6 protein expression was enhanced in podocytes from DOCA-treated Podo GC-A KO mice compared with sham or with DOCA-treated Podo GC-A control mice (Figure 9, A and B). Moreover, expression of TRPC6 mRNA but not TRPC5 mRNA was elevated in isolated Podo GC-A KO glomeruli treated with DOCA (Figure 9C).
Figure 9.
Expression of TRPC6 is upregulated in podocytes of DOCA-treated Podo GC-A KO mice. (A) Kidney sections of sham and of DOCA-treated mice were stained with antibodies directed against TRPC6 and against synaptopodin for the identification of podocytes. Image acquisition for TRPC6 was performed at exactly the same adjustments for all treatments and genotypes (upper row). Because synaptopodin expression was reduced in DOCA-treated mice, acquisition settings were adjusted in order to receive a sufficient fluorescence signal (middle row). However, this approach was not fully successful in Podo GC-A KO mice, so that costaining of TRPC6 and synaptopodin was technically hardly achievable in DOCA treated KO mice (lower row). (B) Analysis of TRPC6 fluorescence density in podocytes (left panel) shows higher values in Podo GC-A KO mice compared with Podo GC-A control mice under sham conditions and after 6 weeks of DOCA treatment. When fluorescence density was determined for the entire glomerular area (right panel) no significant difference between Podo GC-A KO and control was detected in sham mice, whereas TRPC6 upregulation was observed in Podo GC-A KO mice after 6 weeks of DOCA treatment (right panel of B). Likewise, TRPC6 mRNA expression was upregulated in glomeruli from DOCA-treated Podo GC-A KO mice (C). #P<0.001 versus sham or versus other genotype at same treatment as indicated. *P<0.05 versus sham or versus other genotype at same treatment as indicated.
Functional Role of TRPC6 in the Development of Podocyte Damage in Podocyte Specific GC-A Knockout Mice in Response to DOCA-Salt Treatment for 2 Weeks
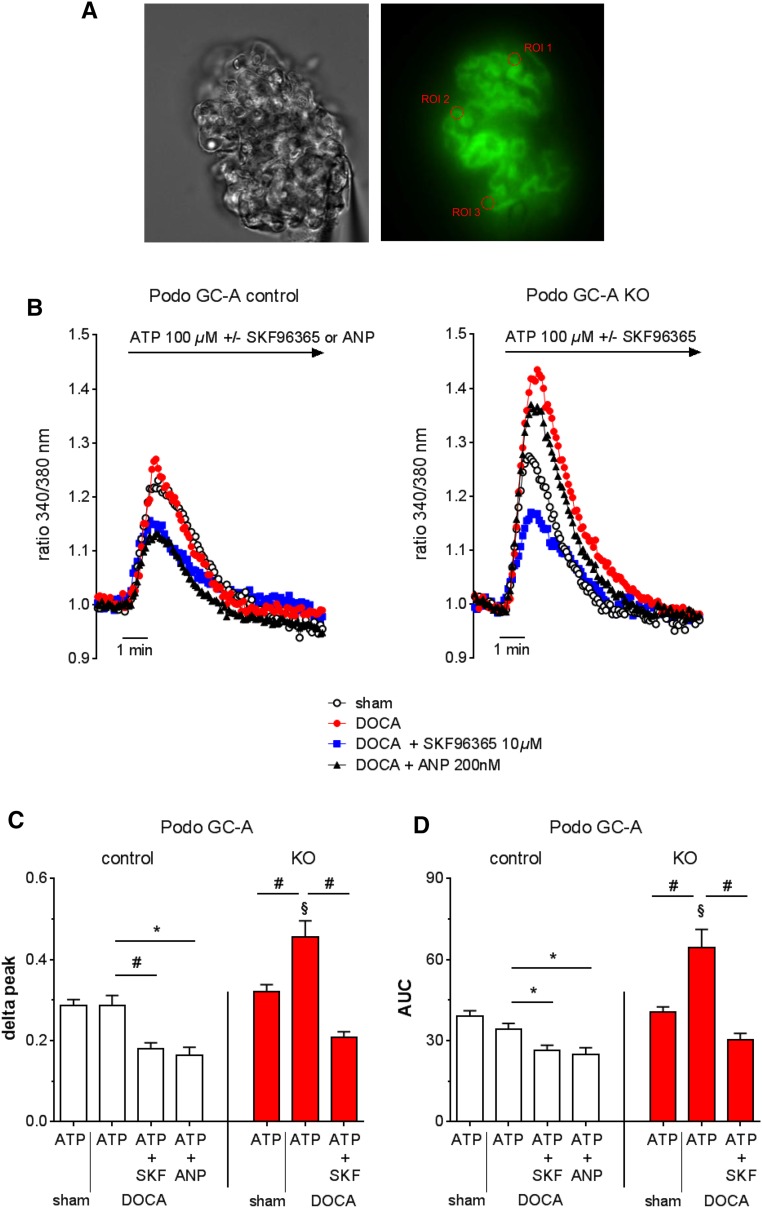
In order to assess the role of TRPC6 channels in the development of podocyte damage and proteinuria in Podo GC-A KO mice, additional experiments were performed in which the mice were treated with DOCA for up to 14 days only. In fact, glomerular TRPC6 function and protein expression was significantly upregulated in DOCA treated Podo GC-A KO but not Podo GC-A control mice (Figure 10, Figure 11, G and H). To assess the functional consequences of TRPC6 upregulation in Podo GC-A KO podocytes, we first determined the cytosolic intracellular calcium concentration ([Ca2+]i) in podocytes of freshly isolated glomeruli from Podo GC-A KO and Podo GC-A control mice (Figure 10). Because ATP elevates podocyte [Ca2+]i35,36 and activates TRPC6 in podocytes37 and because purinergic signaling promotes calcium wave propagation from podocyte to podocyte in response to podocyte damage,38 we used ATP as a stimulus to increase [Ca2+]i. ATP (100 µM) induced [Ca2+]i increases of comparable magnitude in podocytes from sham Podo GC-A KO and Podo GC-A control mice (Figure 10B, white circles). Pretreatment of the mice with DOCA for 9–12 days did not alter the [Ca2+]i response to ATP in podocytes of Podo GC-A control mice, while it markedly enhanced [Ca2+]i in podocytes of KO mice (Figure 10B, red circles). Concomitant application of the TRPC channel blocker SKF96365 (10 µM, blue squares) suppressed the ATP-dependent [Ca2+]i response to comparable levels in both genotypes, compatible with an involvement of TRPC6 in the stimulated calcium response in podocytes of Podo GC-A KO mice. Importantly, ANP (200 nM, black triangles) significantly attenuated the [Ca2+]i response to ATP in podocytes of DOCA treated Podo GC-A control mice (Figure 10). As expected, ANP had no effect on [Ca2+]i in podocytes of Podo GC-A KO mice (Figure 10B).
Figure 10.
Cytosolic calcium influx is enhanced in podocytes from Podo GC-A KO mice. (A) Podo GC-A control and KO mice that had been crossed to a fluorescent Cre-reporter mouse background27 were uninephrectomized and treated with DOCA-salt for 9–12 days. Glomeruli were freshly isolated and loaded with fura-2 acetoxymethyl ester. Podocytes were identified by their green fluorescence, and regions of interest for the determination of fura-2 fluorescence were placed on podocytes. (B-D) As a measure of the cytosolic calcium concentration [Ca2+]i the fura-2 fluorescence emission ratio at 340/380 nm excitation wave length was determined. Calcium influx into podocytes was stimulated by ATP (100 µM) in the absence or the presence of the TRPC channel blocker SKF96365 (10 µM). ATP induced calcium influxes in podocytes of Podo GC-A control and KO mice (B) that had similar peak heights (C) and areas under the curves (D) in untreated control mice (white dots in B). The ATP-induced increase in [Ca2+]i was augmented by DOCA pretreatment (red dots in B) in both genotypes. However, augmentation was pronounced in Podo GC-A KO compared with Podo GC-A controls such that the delta peak (maximum value–mean baseline value, C) and areas under curves (D) were significantly higher in DOCA treated Podo GC-A KO mice. Concomitant application of SKF96365 (begun 5 minutes before beginning of ATP application) significantly attenuated the ATP-induced calcium influx to comparable levels in both genotypes (blue squares in B). Similarly, the increase in [Ca2+]i in response to ATP was inhibited by ANP (200 nM, ANP application was begun 5 minutes before ATP) (black triangles in B) in Podo GC-A control mice. As expected ANP did not alter [Ca2+]i in podocytes from Podo GC-A KO mice (black triangles in B, n=2 glomeruli, no statistics performed). #P<0.001; *P<0.05 as indicated by bar. §P<0.01 versus Podo GC-A control same treatment.
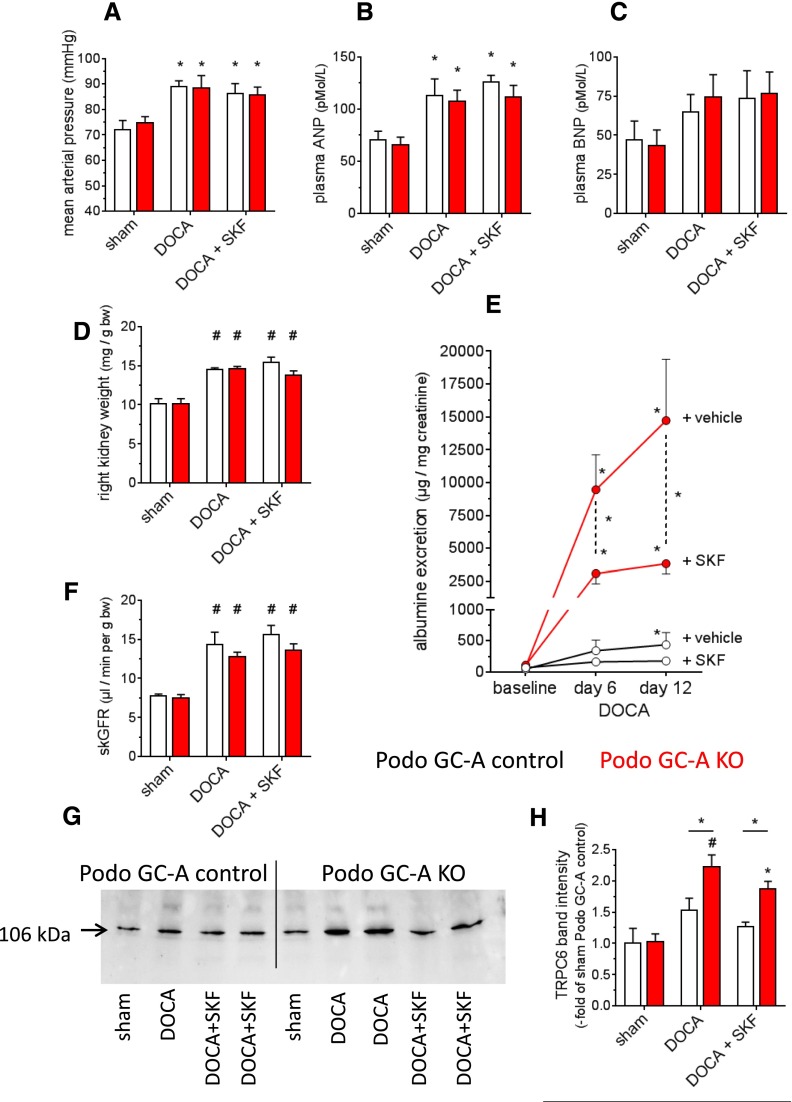
Figure 11.
Albuminuria in response to DOCA salt treatment for 2 weeks is markedly improved by blockade of TRPC channels. Mice were subjected to left sided unilateral nephrectomy and were subsequently treated with DOCA and a high salt diet (4% NaCl) for 14 days (“DOCA”, n=5–7 each genotype). In order to block TRPC channels, one group of mice of each genotype was treated with SKF96365 (10 mg/kg body wt, once daily, i.p.) for the entire experimental period. Sham operated mice did not receive unilateral nephrectomy and DOCA treatment and were maintained on standard chow. (A) DOCA induced moderate increases in systolic BP of comparable magnitude in Podo GC-A KO and Podo GC-A control mice which was not altered by SKF96365. (B) Plasma ANP concentration was elevated in response to DOCA treatment in both genotypes. (C) Plasma BNP concentration tended to be higher in treated mice compared with sham controls; however, this difference did not achieve statistical significance. (D) Right kidney weights were markedly higher in the DOCA group in both genotypes compared with untreated controls independent of SKF96365 treatment. (E) Podo GC-A KO mice developed progressive albuminuria starting 6 days after the start of DOCA treatment. SKF96365 markedly reduced albuminuria. (F) The skGFR was determined in conscious mice after 2 weeks of DOCA treatment. Because sham mice had not undergone uninephrectomy and therefore had two kidneys throughout the study, skGFR of sham mice was calculated by dividing GFR by two. The skGFR was increased by DOCA in both genotypes to similar levels and was not changed by SKF96365. (G and H) Western blot analysis of TRPC6 expression in glomeruli. DOCA resulted in a marked upregulation of TRPC6 expression in glomeruli of Podo GC-A KO mice, which was not significantly reduced by SKF96365 treatment. *P<0.05 versus baseline/sham or as indicated by bar. #P<0.001 versus sham.
Since upregulation of TRPC6 channels resulted in enhanced [Ca2+]i in podocytes of KO mice, we next tested whether blockade of TRPC channels by SKF96365 (10 mg/kg body wt, once daily, i.p.) improves podocyte damage and albuminuria when administered concomitantly with the DOCA treatment in vivo. DOCA treatment for 14 days resulted in arterial hypertension, an increase in plasma ANP concentration, renal hypertrophy, proteinuria and renal hyperfiltration of similar magnitude in both genotypes and none of these parameters were significantly altered by concomitant SKF treatment (Figures 11, A–F). Podo GC-A KO mice developed marked albuminuria already 6 days after the start of DOCA treatment (one hundredfold versus baseline), which was further aggravated after 12 days (170-fold versus baseline, Figure 11E). Application of SKF96365 markedly ameliorated albuminuria (day 6: 28-fold versus baseline; day 12: 35-fold versus baseline) indicating that TRPC channels play a significant role in the disease progression in our model. Podo GC-A control mice developed only a very mild albuminuria during 12 days of DOCA treatment. In parallel to the albuminuria DOCA treated Podo GC-A KO mice showed marked podocyte foot process effacement (Figure 12, A and B), glomerular hypertrophy, and mesangial expansion (Figure 12, C–E). All these indicators of podocyte and glomerular damage were significantly ameliorated by SKF96365. Similarly, downregulation of podocin expression, as determined by immunostaining and Western blot, in response to DOCA was completely prevented by SKF96365 (Figure 13, A–D). Finally, application of DOCA for 14 days resulted in enhanced oxidative stress in podocytes of Podo GC-A KO mice, as indicated by 4-HNE staining and this response was completely abrogated by blockade of TRPC channels using SKF96365 (Figure 13, E and F).
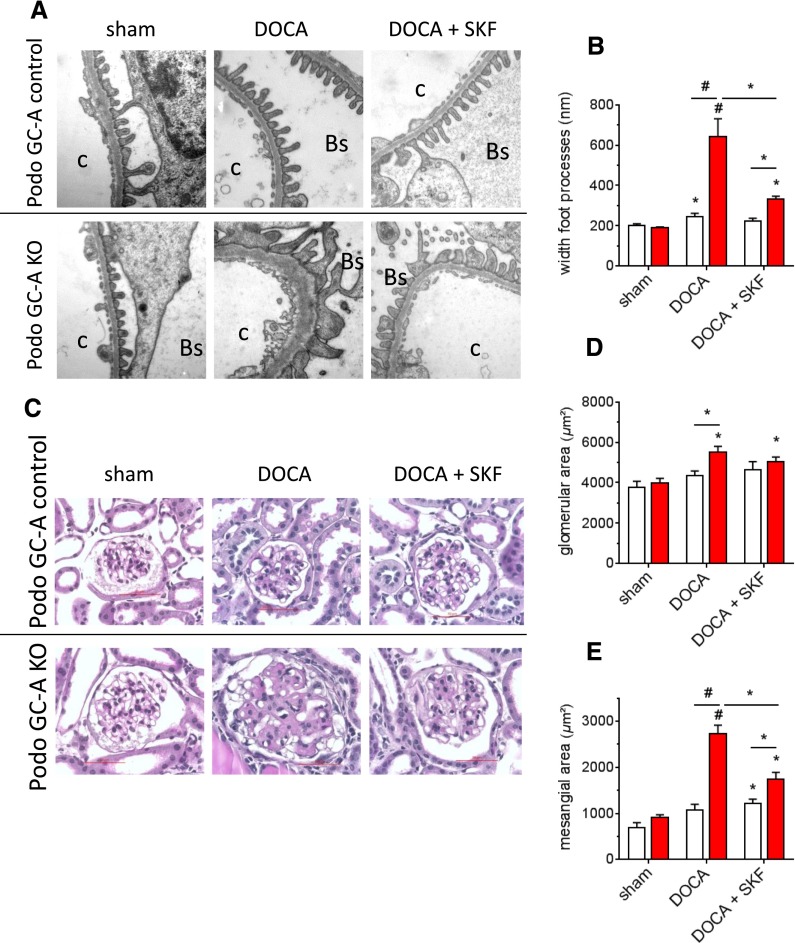
Figure 12.
Blockade of TRPC channels by SKF96365 markedly improves podocyte and glomerular damage in response to DOCA treatment for 2 weeks in Podo GC-A KO mice. (A and B) Electron microscopy showed the normal appearance and only minimal widening of podocytes foot processes in Podo GC-A control mice after 2 weeks of DOCA treatment (upper panel of A and B). Massive foot process effacement was observed already 2 weeks after the start of DOCA treatment in Podo GC-A KO (A lower panel, B). These prominent changes were markedly improved by concomitant treatment with SKF96365 (A lower panel, B). C: capillary lumen; Bs: Bowman’s space. (C) PAS staining revealed glomerular hypertrophy of DOCA treated Podo GC-A KO mice (D). Moreover, DOCA induced a marked mesangial expansion in Podo GC-A KO which was significantly ameliorated by SKF96365 (E). Bar represents 50 µm. *P<0.05 versus sham or as indicated by bar; #P<0.001 versus sham or as indicated by bar.
Figure 13.
Downregulation of podocin and increased oxidative stress in response to 2 weeks of DOCA treatment are prevented by blockade of TRPC channels. (A and B) Immunofluorescence staining for podocin was slightly but significantly reduced after 14 days of DOCA treatment. Downregulation of podocin expression was confirmed by Western blotting (C and D). Treatment of the mice with SKF96365 completely prevented downregulation of podocin expression (A-D). (E) Kidney sections were stained with an antibody directed against 4-HNE, which is produced by lipid peroxidation and serves as a marker of oxidative stress. Prominent 4-HNE immunofluorescence was detected in the glomeruli of DOCA-treated Podo GC-A KO mice compared with sham and Podo GC-A control mice. (E and F) SKF96365 normalized oxidative stress. *P<0.05 versus sham or as indicated by bar. #P<0.001 versus sham or as indicated by bar.
Discussion
The cardiac hormones ANP and BNP play critical roles in the maintenance of body fluid homeostasis and BP control. In the kidney, ANP and BNP do not only induce natriuresis but also result in increases in the GFR. The data of our study clearly indicate that the cardiac natriuretic peptides, although their common receptor GC-A is highly expressed in podocytes, do not exert these classic renal actions via an effect on podocytes. Thus, conscious Podo GC-A KO mice, lacking GC-A exclusively in podocytes, have normal GFR and sodium excretion under control conditions. Moreover, acute infusion of natriuretic peptides in anesthetized Podo GC-A KO mice resulted in prompt increases in GFR and natriuresis which were similar to those of control mice with intact GC-A signaling in podocytes. Finally, even under the controlled conditions of isolated perfused kidneys, renal perfusion was normally enhanced by ANP and BNP. All of the renal effects of natriuretic peptides were completely absent in constitutive GC-A KO mice with global deletion of GC-A, indicating that ANP and BNP regulate kidney function exclusively via activation of GC-A. If not podocytes, which other renal cells are the target of natriuretic peptides? GC-A is expressed along the entire nephron7–11 and ANP can induce natriuresis independent of an increase in GFR.39 Moreover, several functional studies have demonstrated that ANP directly inhibits sodium reabsorption in proximal tubules, the loop of Henle, and collecting ducts (for recent review40). Accordingly, it is plausible that GC-A signaling in podocytes is dispensable for the natriuretic effects of ANP and BNP. The regulation of the GFR by natriuretic peptides appears to be more complex. In general, the GFR is determined by the net filtration pressure and the ultrafiltration coefficient, the latter being the product of the filtration surface and its permeability. GC-A is expressed in all relevant cell types which in principle can contribute to the regulation of GFR (vascular smooth muscle cells of renal arterioles, mesangial cells, and podocytes). The increase in GFR in response to NPs has been attributed to increases in both the glomerular capillary pressure and the ultrafiltration coefficient.10,14,15 Due to their localization at the outer aspect of glomerular capillaries, it has been speculated that podocytes might control glomerular capillary tone and the ultrafiltration coefficient and hereby contribute to the regulation of GFR.16 However, as shown in our study, podocytes do not play a detectable role in the regulation of GFR by natriuretic peptides, so that mesangial cells or vascular smooth muscle cells remain as putative targets of natriuretic peptides in the control of GFR. ANP can stimulate GFR by dilating afferent arterioles in combination with constricting efferent arterioles without affecting the ultrafiltration coefficient.10 Accordingly, activation of GC-A in vascular smooth muscle cells of afferent and efferent arterioles appears to be of major importance in the control of GFR by natriuretic peptides.
Besides the classic renal effects of NPs, they have been shown to exert renoprotective effects, as deletion of their common receptor GC-A enhances, while overexpression or infusion of NPs ameliorates renal damage in different disease models and even clinical trials. Cardiac NPs lower arterial BP, suppress the release of hormones such as angiotensin II and aldosterone and inhibit the sympathetic nervous system, which all exert deleterious effects in renal disease. Accordingly, it has been assumed that NPs ameliorate progression of renal disease mainly by suppression of these disease-promoting factors. The data in the present study show for the first time that the marked renoprotective effects of cardiac natriuretic peptides are directly mediated by the NP/GC-A signaling pathway in podocytes, at least in response to the mineralocorticoid-induced hypertension used in our study. This conclusion is in line with a recent study, in which the effects of mineralocorticoid excess on albuminuria and renal damage were enhanced in conventional GC-A knockout mice with global GC-A deletion compared with wildtype littermates.7 Although the experimental models of mineralocorticoid excess used in both that previous and our study are not identical, it is noteworthy that the degrees of glomerular damage and albuminuria are similar in both conventional GC-A KO mice,7 which are markedly hypertensive, and the mice with podocyte-specific deletion of GC-A used in our study. Thus, it appears that most of the protective renal effects of NPs are directly mediated via the activation of GC-A in podocytes and are not the result of the hemodynamic or neurohumoral responses to NP/GC-A signaling.
Uninephrectomy in combination with DOCA-salt can affect podocytes either indirectly through the accompanying arterial hypertension and the massive glomerular hyperfiltration or directly by the activation of mineralocorticoid receptors in podocytes. The expression of mineralocorticoid receptors in podocytes has been demonstrated in both our present and in previous studies and activation of these receptors in vivo or in cultured podocytes resulted in enhanced oxidative stress.7,32 Moreover, the systemic application of the ROS scavenger tempol ameliorated proteinuria and glomerular damage in DOCA- or aldosterone-treated animals.7,32 Enhanced oxidative stress plays a critical role in disease progression in several renal diseases,41 for instance via the activation of mitogen-activated protein-kinases and the induction of apoptosis.42 In line, podocytes of DOCA-treated Podo GC-A KO mice showed signs of enhanced oxidative stress, indicated by enhanced lipid peroxidation, which was not the case in the kidneys from control mice. Previous studies have shown that ROS are involved in the upregulation of TRPC6 expression in podocytes in response to puromycin aminonucleoside and high glucose43,44 and in the activation of TRPC6 in podocytes by ATP and angiotensin II.37,45 TRPC5 and especially TRPC6 channels have been implicated in the pathogenesis of podocytopathies, and calcium influx into the cytosol of podocytes via these channels has been demonstrated.35,46–50 TRPC6 expression was markedly upregulated in the podocytes and glomeruli of Podo GC-A KO mice in response to DOCA treatment, as was the [Ca2+]i response to ATP in podocytes of DOCA treated Podo GC-A KO mice. Notably, our functional experiments on [Ca2+]i in podocytes were performed 9–12 days after the start of DOCA treatment in an early phase of disease progression, suggesting that upregulation of TRPC6 channels plays a pathogenic role and is not an epiphenomenon of glomerulosclerosis as seen in later disease stages. In contrast to TRPC6, glomerular TRPC5 expression was not altered in either genotype or treatment condition, which is in line with the different regulation of TRPC5 and TRPC6 in podocytes in response to high glucose.48 The enhanced effect of ATP on [Ca2+]i was prevented by the TRPC channel blocker SKF96365, compatible with a functional effect of TRPC6 upregulation on calcium handling in podocytes. This clear-cut conclusion has to be taken with caution, as SKF96365 not only blocks TRPC6 but also other TRPC channels including TRPC5. Nevertheless, our data clearly show that the expression and activity of calcium influx pathways are stimulated by DOCA in the absence of NP/GC-A signaling in podocytes resulting in enhanced [Ca2+]i. Moreover, for the first time, our data show that ANP directly suppresses ATP-induced [Ca2+]i in podocytes. In cardiomyocytes, ANP attenuated cytosolic calcium influx in response to angiotensin II, and cGMP/cGK1 signaling inhibited TRPC6 expression and activity.51,52 Similarly, an inhibitory role of cGMP on TRPC6 activity has recently been proposed in cultured podocytes.53 Enhanced [Ca2+]i has deleterious effects in podocytes through the activation of several downstream signaling pathways, such as activation of calcineurin, degradation of synaptopodin, disorganization of the cytoskeleton, foot process effacement, and apoptosis.50,54,55 Accordingly, maintaining [Ca2+]i in the normal range, which we show can be achieved by NP/GC-A signaling in podocytes by inhibiting TRPC6 expression and agonist-induced calcium influx, is expected to ameliorate podocyte damage. In fact, blockade of TRPC channels using SKF96365 in vivo markedly improved albuminuria, foot process effacement and mesangial expansion in response to DOCA treatment in Podo GC-A KO mice. Moreover, SKF96365 completely prevented downregulation of podocin expression and upregulation of ROS formation in podocytes of Podo GC-A KO mice, indicating that enhanced TRPC6 expression and activity are not the result but the cause of enhanced oxidative stress in podocytes deficient of natriuretic peptide signaling.
Very few glomerular cells of Podo GC-A KO mice showed signs of apoptosis after 6 weeks of treatment with DOCA. These cells were not positive for podocyte markers such as podocin or synaptopodin and thus they are either other glomerular cells such as mesangial cells or they have completely stopped expressing podocyte specific proteins due to apoptosis. Anyway, due to the very low number of apoptotic cells after 6 weeks and the absence of apoptotic cells after 2 weeks of DOCA treatment it appears to be unlikely that apoptosis of podocytes plays a major role in disease progression in our model.
Taken together, the local absence of GC-A in podocytes does not affect renal function under control conditions. However, it results in enhanced ROS formation, upregulation of TRPC6 and calcium influx, foot process effacement, massive albuminuria, and renal fibrosis in response to mineralocorticoid excess. Blockade of TRPC channels markedly improves renal damage indicating their pathophysiological role. Thus, for the first time, this study directly shows that NP/GC-A signaling exerts renoprotection mainly by direct cellular effects in podocytes, preventing podocytes damage and albuminuria.
Concise Methods
Animals:
Sixteen to 24 week old mice were used in this study. Mice with podocyte specific ablation of GC-A were generated by crossing a mouse line expressing a Cre recombinase under the control of the NPHS2 (podocin) promoter25 with mice carrying floxed alleles of the gene coding for GC-A26 (GC-Aflox/flox). To avoid background-related effects, offspring littermates of the resulting heterozygous breeder pairs (NPHS2-Cre x GC-Aflox/WT) were used throughout the study. NPHS2-Cre x GC-AWT/WT mice were considered as controls (Podo GC-A control), NPHS2-Cre x GC-Aflox/flox as knockouts (Podo GC-A KO).
For separation of podocytes from other glomerular cells by FACS and for the identification of podocytes in freshly isolated glomeruli for calcium measurements (see below), NPHS2-Cre x GC-Aflox/WT were crossed with a double fluorescent Cre-reporter mouse (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, mG/mT).27 After further crossing steps, Podo GC-A control and Podo GC-A KO mice with mG/mT expression were received.
All animal experiments were performed according to the guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by local authorities.
DOCA + Salt Treatment
All mice subjected to treatment with DOCA received uninephrectomy 2 weeks before the start of treatment. Nephrectomy of the left kidney was performed under aseptic conditions and inhalation anesthesia using isoflurane. After left flank incision, the left kidney was separated from surrounding tissue and the ureter and renal vessels were ligated, sparing the adrenal gland. The kidney was excised, and wound closure by suture (abdominal muscles) and staples (skin) was performed. The kidney was weighed, halved, snap-frozen in liquid nitrogen, and stored at −80°C until further processing. Two weeks after uninephrectomy, a DOCA pellet (50 mg, release 21 days; Innovative Research of America, Sarasota, FL) was implanted subcutaneously (anesthesia with isoflurane) and mice received a food containing 4% sodium chloride (NaCl).
In the first set of experiments, the DOCA pellet was replaced by a new one after 3 weeks and the mice were treated with DOCA for 6 weeks in total. At the end of the experiments, blood samples for the determination of renin and albumin concentration in the plasma were collected by submandibular venipuncture. Thereafter, mice were deeply anesthetized, killed by cervical dislocation and organs were quickly removed, weighed, snap-frozen, and stored at −80°C until further processing. Other groups of mice were used for immunohistochemical or electronmicroscopy studies and for isolation of glomeruli (see below).
In an independent second set of experiments the functional and morphologic changes during disease progression as well as the role of TRPC channels were investigated. Accordingly, the experiments were terminated 14 days after the implantation of the DOCA pellet. The mice were divided into three groups: sham: no uninephrectomy, no DOCA treatment (n=5–6 each genotype); DOCA: uninephrectomy + DOCA salt for 14 days, i.p. injection of 0.9% NaCl once daily (vehicle for SKF96365) (n=6–7 each genotype); DOCA + SKF: DOCA treatment in combination with SKF96365 (Abcam, Inc., Cambridge, UK; 10 mg/kg body wt, dissolved in 0.9% NaCl, once daily, i.p. injection) (n=6–7 each genotype). At the end of the treatment period the mice were anesthetized (isoflurane) and the arterial BP was determined invasively by puncture of the aorta. Thereafter, blood samples were drawn by cardiac puncture and the blood was processed as described previously for the determination of plasma concentration of ANP and BNP.11 Thereafter, the upper pole of the right kidney was removed for isolation of glomeruli by microdissection and the remaining kidney tissue was fixed by perfusion with 4% paraformaldehyde.
Isolation of Glomeruli and Podocytes
Glomeruli were isolated essentially using the magnetic bead method published previously.28,56,57 In brief, mice were anesthetized (12 mg/kg xylazine +80 mg/kg ketamine) and a perfusion cannula was inserted into the abdominal aorta. After ligation of side branches and the aorta proximal to the right renal artery, 15 ml magnetic bead suspension (37°C, 2×106 beads/ml; Invitrogen, Carlsbad, CA) was infused at a pressure of 100 mmHg. After further processing of the kidneys (digestion, cell strainer), the glomeruli were separated from the other kidney tissue by a magnet.28,57 Glomeruli were either snap-frozen and stored at −80°C or they were further processed to receive a single cell suspension, suitable for FACS, as has been described in detail previously.28 For FACS experiments, reporter mice with mG/mT expression were used. On average, 250,000 podocytes were isolated from each mouse and were stored at −80°C until further processing.
In the short term experiments, (14 days DOCA) glomeruli were manually microdissected from the upper pole of the right kidney under a stereomicroscope.
Determination of GFR
Experiments on the acute effects of BNP on GFR were performed in anesthetized mice (isoflurane), using FITC-sinistrin clearance as a measure for GFR. Briefly, FITC-labeled sinistrin was infused via the jugular vein to obtain constant FITC-sinistrin plasma concentration. Urine was collected via a bladder catheter over periods of 30 minutes and FITC-sinistrin clearance was calculated as: urine volume per minute × FITC-sinistrin concentration in urine/FITC-sinistrin concentration in plasma. After a control period of 30 minutes with continuous infusion of 0.9% NaCl (baseline), infusion was either changed to BNP in 0.9% NaCl (1 ng/min per g body wt) or NaCl infusion lacking BNP was continued. Sodium concentration in urine samples was determined by flame photometry (Jenway Ltd., Stone, UK).
The GFR of conscious mice was determined using the clearance kinetics of plasma FITC-labeled sinistrin after a single bolus injection, as described previously.58,59 In brief, FITC-sinistrin (5.6 mg/100 g body wt dissolved in NaCl 0.9%) was injected retrobulbarily under light anesthesia (sevoflurane). Blood samples (approximately 10 μl) were collected by puncturing the tail vein 3, 7, 10, 15, 35, 55, and 75 minutes after injection in conscious mice. Fluorescence was determined in the plasma samples, and the GFR was calculated by the clearance kinetics of FITC-sinistrin using a two-compartment model. The single-kidney GFR in sham mice was calculated by dividing the GFR value of the respective mouse by two. n=5–7 each group and genotype.
Determination of Plasma Volume
Plasma volume was determined in conscious mice by the Evans Blue dilution method as described previously.11 In brief, 30 µl of an Evans Blue solution (5 mg/ml in sterile saline) was injected retrobulbarily under light anesthesia (isoflurane). Blood samples (5 µl) were taken from the tail vein before (baseline) and 10 and 30 minutes after injection of Evans Blue. After centrifugation of the blood, absorbance at 620 nm was determined and the Evans Blue concentration was calculated according to a standard curve. Plasma volume was calculated using a linear regression model.
Determination of Plasma Renin Concentration and Plasma ANP and BNP Concentration
ANP and BNP levels were determined in the plasma of mice treated for 14 days with DOCA using the respective enzyme immunoassays (EIAs) (Phoenix Pharmaceuticals, Burlingame, CA). Briefly, blood was collected by cardiac puncture in tubes containing EDTA and aprotinin, and peptides were extracted by C18-Sep columns. The ANP and BNP EIAs were performed according to the manufacturer’s instructions and described previously.11 The measurements of the PRC in the plasma samples from conscious mice were based on the generation of angiotensin I after the addition of plasma from bilaterally nephrectomized male rats as excess renin substrate. The generated angiotensin I [ng/ml × h–1] was determined by radioimmunoassay (DiaSorin, Dietzenbach Germany) as described previously.11
Isolated Perfused Mouse Kidney
Kidneys from Podo GC-A KO and control mice were perfused ex-situ at a constant perfusion pressure (100 mmHg) as described in detail previously.60 Perfusion medium consisted of a modified Krebs-Henseleit buffer supplemented with bovine serum albumin (6 g/100 ml) and human erythrocytes (10% hematocrit). The renal vein was cannulated and samples of the venous perfusate were collected every 2 minutes for the determination of renal blood flow. Four samples were taken during each experimental period and the last two values were averaged for statistical analysis.
BP Measurement by the Tail Cuff Method
Mice were conditioned by placing them in the holding devices on seven consecutive days before the first measurement was performed. Subsequently, eight BP values per mouse were determined per day and averaged for a total of 5 consecutive days before uninephrectomy (baseline). BP was also determined in weeks 2–3 and 4–5 of DOCA-salt treatment. For statistical analysis, daily BP values of each individual mouse (mean of eight values per mouse) were averaged for weeks 2–3 and 4–5 (n=5 each genotype).
In the short term experiments (14 days DOCA) the arterial BP was determined invasively. Mice were anesthetized (isoflurane), the abdomen was opened by midline incision and the aorta cannulated with a metal cannula (outer diameter 1 mm) (n=5–7 each group).
Urinary Albumin Excretion
In the long term experiments (6 weeks DOCA) mice were housed overnight (12 hours) in metabolic cages and urine was collected (n=6 each group). In the short term experiments (2 weeks DOCA) spot urine was collected after 6 and 12 days of treatment and albumin-to-creatinine ratio was determined. Albumin concentration in the urine and the plasma was determined by a specific mouse albumin EIA according to the manufacturer’s instructions (Dunn Labortechnik, Asbach, Germany). For the direct visualization of proteinuria, urine samples (1 µl) were analyzed on a 15% SDS - polyacrylamide gel together with bovine serum albumin (1, 7, 15 µg) as a standard. Proteins were stained using Coomassie Brilliant Blue.
Histologic Examinations of Kidney Sections
Kidneys were flushed with PBS, fixed by perfusion with 4% paraformaldehyde for 3 minutes (constant pressure 100 mmHg), halved and further stored in 4% paraformaldehyde overnight. Subsequently, samples were dehydrated, embedded in paraffin, and sectioned into 5-µm-thick sections. Sections were stained either with hematoxylin/eosin, periodic acid-Schiff (PAS) reaction or Masson-Goldner-Trichrome using standard protocols and examined by light microscopy (Axiovert 200M; Carl Zeiss, Germany). Cross-sectional and mesangial areas of at least 20 glomeruli per mouse were determined in PAS stained sections using ImageJ software,61 by two independent investigators, who were blinded regarding genotype and treatment (n=4–7 each genotype and treatment).
Electronmicroscopy
Transmission electron microscopy was performed as previously published.57 In brief, kidneys were fixed by paraformaldehyde perfusion (see above) and further fixed by 2% glutaraldehyde overnight. Samples were embedded in Epon and the ultrathin sections were stained with uranyl acetate and lead citrate before visualization by a transmission electron microscope and a cooled charge coupled device (CCD) digital camera (Zeiss EM 902, TRS Tröndle Restlichtverstärkersysteme). Width of foot processes was determined using ImageJ software.61
Immunofluorescence and Apoptosis Staining
Kidneys were fixed by paraformaldehyde perfusion as described above. Cryosections were used for the observation of autofluorescence and costainings in kidneys of mG/mT reporter mice (Figure 1) while all other stainings were performed in paraffin embedded sections (5 µm). The sections were incubated at 4°C overnight with the following primary antibodies: polyclonal goat anti-synaptopodin (sc-21537; Santa Cruz Biotechnology, Santa Cruz, CA), monoclonal mouse anti-αSMA (ab7817; Abcam, Inc.), polyclonal goat anti-podocin (sc-22298; Santa Cruz), polyclonal rabbit anti-TRPC6 (ab62461; Abcam, Inc.), polyclonal goat anti-4-hydroxy-2-nonenal antibody (HNE11-S; Alpha Diagnostic International, San Antonio, TX), polyclonal rabbit anti-caspase 3 antibody (Abcam, Inc.). After washing, incubation with appropriate secondary antibodies was performed for 1 hour: DyLight 488-conjugated AffiniPure donkey anti-mouse IgG (715–485–150; Jackson ImmunoResearch, West Grove, PA), DyLight 488-conjugated AffiniPure donkey anti-goat IgG (705–485–147; Jackson ImmunoResearch), Cy5-conjugated AffiniPure donkey anti-goat IgG (705–175–147; Jackson ImmunoResearch), Alexa Fluor 546-conjugated donkey anti-goat IgG (A11056, molecular probes), Alexa Fluor 488-conjugated AffiniPure donkey anti-rabbit IgG (711–545–152, Jackson ImmunoResearch), TRITC-conjugated AffiniPure donkey anti-rabbit IgG (715–025–152, Jackson ImmunoResearch). Images were collected using an Axiovert 200M fluorescence microscope. Fluorescent area and fluorescence intensity were measured using ImageJ software61 in glomeruli or podocytes as indicated and “mean fluorescence density” as shown in the results was calculated as the product of both parameters.
For the detection of apoptotic cells, TUNEL was performed as described previously.57 Sections were pretreated with proteinase K and then blocked with 5% BSA, 20% FCS, and 1×PBS before the TUNEL reaction mixture (Roche Applied Science) was added for 1 hour at 37°C. Images were collected using an Axiovert 200M fluorescence microscope.
For the determination of the podocyte number kidney sections were stained with synaptopodin together with 4′,6-diamidino-2-phenylindole for the identification of cell nuclei. Nuclei which were definitely located in a synaptopodin positive cell were counted. Analysis was performed as podocytes per glomerular cross-section and per glomerular area.
mRNA Expression by Real-Time PCR
Total RNA was isolated from the glomeruli or podocytes using TRIzol reagent (Life Technologies, Carlsbad, CA). After reverse transcription (Moloney Murine Leukemia Virus reverse transcription, Superscript, Invitrogen), real-time RT-PCR was performed using a LightCycler Instrument (Roche Diagnostics Corporation). Samples in which no reverse transcription had been added, served as negative controls. Amplification products were analyzed by melting curve analysis and the length of the products were controlled by gel electrophoresis.
Determination of Cytosolic Calcium Concentration in Podocytes
Podocyte [Ca2+]i was determined in glomeruli from Podo GC-A control and KO mice on the mG/mT reporter background, so that podocytes could be distinguished from other glomerular cells by their green fluorescence. Mice were treated with DOCA-salt as described above for 9–12 days. Glomeruli from untreated mice served as sham controls. Glomeruli were isolated by mincing the kidney tissue and subsequent sieving steps according to the method described by Ilatovskaya in detail previously.46 After isolation, the glomeruli were allowed to settle on glass cover slips and they were incubated with the calcium indicator fura-2 acetoxymethyl ester for 45 minutes. The cover slips were mounted in a superfusion chamber on an inverted microscope (Carl Zeiss, Axio Observer) and the glomerulus was fixed by a holding pipette. After a washing period of approximately 15 minutes, podocytes were identified by bright green fluorescence due to eGFP expression and regions of interest were placed accordingly. As a measure of [Ca2+]i with fura-2, the fluorescence emission ratio at 345 nm/380 nm excitation determined and analyzed using the VisiFluor Ratio Imaging System and VisiView 2.1.4 software package.
Statistics
Values are presented as mean±SEM. The differences between the groups or different time points within a group were analyzed by one-way or two-way ANOVA followed by the Bonferroni post hoc test if necessary. In the isolated perfused kidney experiments, the last 2 values obtained within an experimental period were averaged for statistical analysis. All statistical analyses were performed using GraphPad Prism software. P values <0.05 were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We are very grateful to Jacqueline Igl and Petra Hoffmann (Internal Medicine III, University of Regensburg, Germany) for the excellent support with FACS sorting of podocytes. Moreover, we gratefully acknowledge the expert technical help of Robert Götz, Frieda Webinger and Helga Othmen (Institutes of Physiology and Anatomy, University of Regensburg).
The work was financially supported by the German Research Foundation DFG: SFB699 to F.S. (B3, Z4) and R.W. (Z2), and SFB688 to M.K.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015070731/-/DCSupplemental.
References
- 1.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR: TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H: A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28: 89–94, 1981 [DOI] [PubMed] [Google Scholar]
- 6.Kuhn M: Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res 93: 700–709, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Ogawa Y, Mukoyama M, Yokoi H, Kasahara M, Mori K, Kato Y, Kuwabara T, Imamaki H, Kawanishi T, Koga K, Ishii A, Tokudome T, Kishimoto I, Sugawara A, Nakao K: Natriuretic peptide receptor guanylyl cyclase-A protects podocytes from aldosterone-induced glomerular injury. J Am Soc Nephrol 23: 1198–1209, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritter D, Dean AD, Gluck SL, Greenwald JE: Natriuretic peptide receptors A and B have different cellular distributions in rat kidney. Kidney Int 48: 5758–5766, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Kurtz A, Della Bruna R, Pfeilschifter J, Taugner R, Bauer C: Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc Natl Acad Sci U S A 83: 4769–4773, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn BR, Ichikawa I, Pfeffer JM, Troy JL, Brenner BM: Renal and systemic hemodynamic effects of synthetic atrial natriuretic peptide in the anesthetized rat. Circ Res 59: 237–246, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Demerath T, Staffel J, Schreiber A, Valletta D, Schweda F: Natriuretic peptides buffer renin-dependent hypertension. Am J Physiol Renal Physiol 306: F1489–F1498, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi S, Uchida K, Ito T, Kozuka M, Shimonaka M, Mizuno T, Hirose S: Immunohistochemical localization of atrial natriuretic peptide receptor in bovine kidney and lung. J Histochem Cytochem 37: 1739-1742, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Lewko B, Golos M, Latawiec E, Angielski S, Stepinski J: Regulation of cGMP synthesis in cultured podocytes by vasoactive hormones. J Physiol Pharmacol 57: 599-610, 2006. [PubMed] [Google Scholar]
- 14.Awazu M, Kon V, Harris RC, Imada T, Inagami T, Ichikawa I: Renal sympathetic nerves modulate glomerular ANP receptors and filtration. Am J Physiol 261: F29–F35, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Fried TA, McCoy RN, Osgood RW, Stein JH: Effect of atriopeptin II on determinants of glomerular filtration rate in the in vitro perfused dog glomerulus. Am J Physiol 250: F1119–F1122, 1986 [DOI] [PubMed] [Google Scholar]
- 16.Saleem MA, Zavadil J, Bailly M, McGee K, Witherden IR, Pavenstadt H, Hsu H, Sanday J, Satchell SC, Lennon R, Ni L, Bottinger EP, Mundel P, Mathieson PW: The molecular and functional phenotype of glomerular podocytes reveals key features of contractile smooth muscle cells. Am J Physiol Renal Physiol 295: F959–F970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suganami T, Mukoyama M, Sugawara A, Mori K, Nagae T, Kasahara M, Yahata K, Makino H, Fujinaga Y, Ogawa Y, Tanaka I, Nakao K: Overexpression of brain natriuretic peptide in mice ameliorates immune-mediated renal injury. J Am Soc Nephrol 12: 2652–2663, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Kasahara M, Mukoyama M, Sugawara A, Makino H, Suganami T, Ogawa Y, Nakagawa M, Yahata K, Goto M, Ishibashi R, Tamura N, Tanaka I, Nakao K: Ameliorated glomerular injury in mice overexpressing brain natriuretic peptide with renal ablation. J Am Soc Nephrol 11: 1691–1701, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Makino H, Mukoyama M, Mori K, Suganami T, Kasahara M, Yahata K, Nagae T, Yokoi H, Sawai K, Ogawa Y, Suga S, Yoshimasa Y, Sugawara A, Tanaka I, Nakao K: Transgenic overexpression of brain natriuretic peptide prevents the progression of diabetic nephropathy in mice. Diabetologia 49: 2514–2524, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Nishikimi T, Inaba-Iemura C, Ishimura K, Tadokoro K, Koshikawa S, Ishikawa K, Akimoto K, Hattori Y, Kasai K, Minamino N, Maeda N, Matsuoka H: Natriuretic peptide/natriuretic peptide receptor-A (NPR-A) system has inhibitory effects in renal fibrosis in mice. Regul Pept 154: 44–53, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Mitaka C, Kudo T, Haraguchi G, Tomita M: Cardiovascular and renal effects of carperitide and nesiritide in cardiovascular surgery patients: a systematic review and meta-analysis. Crit Care 15: R258, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sezai A, Hata M, Niino T, Yoshitake I, Unosawa S, Wakui S, Kimura H, Shiono M, Takayama T, Hirayama A: Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP Infusion Therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 58: 897–903, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Das S, Au E, Krazit ST, Pandey KN: Targeted disruption of guanylyl cyclase-A/natriuretic peptide receptor-A gene provokes renal fibrosis and remodeling in null mutant mice: role of proinflammatory cytokines. Endocrinology 151: 5841–5850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das S, Periyasamy R, Pandey KN: Activation of IKK/NF-κB provokes renal inflammatory responses in guanylyl cyclase/natriuretic peptide receptor-A gene-knockout mice. Physiol Genomics 44: 430–442, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M: Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A 99: 7142–7147, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Boerries M, Grahammer F, Eiselein S, Buck M, Meyer C, Goedel M, Bechtel W, Zschiedrich S, Pfeifer D, Laloë D, Arrondel C, Gonçalves S, Krüger M, Harvey SJ, Busch H, Dengjel J, Huber TB: Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int 83: 1052–1064, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Schordan S, Grisk O, Schordan E, Miehe B, Rumpel E, Endlich K, Giebel J, Endlich N: OPN deficiency results in severe glomerulosclerosis in uninephrectomized mice. Am J Physiol Renal Physiol 304: F1458–F1470, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Ji X, Naito Y, Weng H, Endo K, Ma X, Iwai N: P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am J Physiol Renal Physiol 303: F1207–F1215, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Babánková D, Huang J, Swain GM, Wang DH: Deletion of transient receptor potential vanilloid type 1 receptors exaggerates renal damage in deoxycorticosterone acetate-salt hypertension. Hypertension 52: 264–270, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T: Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 49: 355–364, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Dinkova-Kostova AT, Talalay P: NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 501: 116–123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer G, Zarkovic N: Revealing mechanisms of selective, concentration-dependent potentials of 4-hydroxy-2-nonenal to induce apoptosis in cancer cells through inactivation of membrane-associated catalase. Free Radic Biol Med 81: 128–144, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A: Pharmacological characterization of the P2 receptors profile in the podocytes of the freshly isolated rat glomeruli. Am J Physiol Cell Physiol 305: C1050–C1059, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer KG, Saueressig U, Jacobshagen C, Wichelmann A, Pavenstädt H: Extracellular nucleotides regulate cellular functions of podocytes in culture. Am J Physiol Renal Physiol 281: F1075–F1081, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Roshanravan H, Dryer SE: ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species. Am J Physiol Renal Physiol 306: F1088–F1097, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Burford JL, Villanueva K, Lam L, Riquier-Brison A, Hackl MJ, Pippin J, Shankland SJ, Peti-Peterdi J: Intravital imaging of podocyte calcium in glomerular injury and disease. J Clin Invest 124: 2050–2058, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy DR: Effect of synthetic ANP on renal and loop of Henle functions in the young rat. Am J Physiol 251: F220–F225, 1986 [DOI] [PubMed] [Google Scholar]
- 40.Theilig F, Wu Q: ANP-induced signaling cascade and its implications in renal pathophysiology. Am J Physiol Renal Physiol 308: F1047–F1055, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah SV, Baliga R, Rajapurkar M, Fonseca VA: Oxidants in chronic kidney disease. J Am Soc Nephrol 18: 16–28, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Susztak K, Raff AC, Schiffer M, Böttinger EP: Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 43.Wang Z, Wei X, Zhang Y, Ma X, Li B, Zhang S, Du P, Zhang X, Yi F: NADPH oxidase-derived ROS contributes to upregulation of TRPC6 expression in puromycin aminonucleoside-induced podocyte injury. Cell Physiol Biochem 24: 619-626, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Thilo F, Lee M, Xia S, Zakrzewicz A, Tepel M: High glucose modifies transient receptor potential canonical type 6 channels via increased oxidative stress and syndecan-4 in human podocytes. Biochem Biophys Res Commun 450: 312–317, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Anderson M, Roshanravan H, Khine J, Dryer SE: Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J Cell Physiol 229: 434–442, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Ilatovskaya DV, Palygin O, Chubinskiy-Nadezhdin V, Negulyaev YA, Ma R, Birnbaumer L, Staruschenko A: Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int 86: 506–514, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, Ahn W, Wallentin H, Heid H, Hopkins CR, Lindsley CW, Riccio A, Buvall L, Weins A, Greka A: Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest 123: 5298–5309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonneveld R, van der Vlag J, Baltissen MP, Verkaart SA, Wetzels JF, Berden JH, Hoenderop JG, Nijenhuis T: Glucose specifically regulates TRPC6 expression in the podocyte in an AngII-dependent manner. Am J Pathol 184: 1715–1726, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J: Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 18: 29–36, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Greka A, Mundel P: Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J Am Soc Nephrol 22: 1969–1980, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koitabashi N, Aiba T, Hesketh GG, Rowell J, Zhang M, Takimoto E, Tomaselli GF, Kass DA: Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol 48: 713–724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klaiber M, Kruse M, Völker K, Schröter J, Feil R, Freichel M, Gerling A, Feil S, Dietrich A, Londoño JE, Baba HA, Abramowitz J, Birnbaumer L, Penninger JM, Pongs O, Kuhn M: Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2. Basic Res Cardiol 105: 583–595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall G, Rowell J, Farinelli F, Gbadegesin RA, Lavin P, Wu G, Homstad A, Malone A, Lindsey T, Jiang R, Spurney R, Tomaselli GF, Kass DA, Winn MP: Phosphodiesterase 5 inhibition ameliorates angiontensin II-induced podocyte dysmotility via the protein kinase G-mediated downregulation of TRPC6 activity. Am J Physiol Renal Physiol 306: F1442–F1450, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vassiliadis J, Bracken C, Matthews D, O’Brien S, Schiavi S, Wawersik S: Calcium mediates glomerular filtration through calcineurin and mTORC2/Akt signaling. J Am Soc Nephrol 22: 1453–1461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H, Ding J, Fan Q, Liu S: TRPC6 up-regulation in Ang II-induced podocyte apoptosis might result from ERK activation and NF-kappaB translocation. Exp Biol Med (Maywood) 234: 1029–1036, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burghardt T, Kastner J, Suleiman H, Rivera-Milla E, Stepanova N, Lottaz C, Kubitza M, Böger CA, Schmidt S, Gorski M, de Vries U, Schmidt H, Hertting I, Kopp J, Rascle A, Moser M, Heid IM, Warth R, Spang R, Wegener J, Mierke CT, Englert C, Witzgall R: LMX1B is essential for the maintenance of differentiated podocytes in adult kidneys. J Am Soc Nephrol 24: 1830–1848, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD: Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D, Neudecker S, Koenig S, Heinrich R, Hoecklin F, Pill J, Friedemann J, Schweda F, Gretz N, Schock-Kusch D: Transcutaneous measurement of renal function in conscious mice. Am J Physiol Renal Physiol 303: F783–F788, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Schweda F, Wagner C, Krämer BK, Schnermann J, Kurtz A: Preserved macula densa-dependent renin secretion in A1 adenosine receptor knockout mice. Am J Physiol Renal Physiol 284: F770–F777, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Schneider CA, Rasband WS, Eliceiri KW: NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.