Abstract
Th1 cells are central pathogenic mediators of crescentic GN (cGN). Mechanisms responsible for Th1 cell downregulation, however, remain widely unknown. Recently, it was proposed that activation of the Th1–characteristic transcription factor T-bet optimizes Foxp3+ regulatory T (Treg) cells to counteract Th1-type inflammation. Because very little is known about the role of T-bet+ Treg1 cells in inflammatory diseases, we studied the function of these cells in the nephrotoxic nephritis (NTN) model of cGN. The percentage of Treg1 cells progressively increased in kidneys of nephritic wild–type mice during the course of NTN, indicating their functional importance. Notably, naïve Foxp3CrexT-betfl/fl mice, lacking Treg1 cells, showed spontaneous skewing toward Th1 immunity. Furthermore, absence of Treg1 cells resulted in aggravated NTN with selectively dysregulated renal and systemic Th1 responses. Detailed analyses of Treg cells from Foxp3CrexT-betfl/fl mice revealed unaltered cytokine production and suppressive capacity. However, in competitive cotransfer experiments, wild–type Treg cells outcompeted T-bet–deficient Treg cells in terms of population expansion and expression levels of Foxp3, indicating that T-bet expression is crucial for general Treg fitness. Additionally, T-bet–deficient Treg cells lacked expression of the Th1–characteristic trafficking receptor CXCR3, which correlated with significant impairment of renal Treg infiltration. In summary, our data indicate a new subtype of Treg cells in cGN. These Treg1 cells are characterized by activation of the transcription factor T-bet, which enhances the overall fitness of these cells and optimizes their capacity to downregulate Th1 responses by inducing chemokine receptor CXCR3 expression.
Keywords: Treg, CXCR3, immunology, Treg1, cytokines, inflammation
Multiple studies from the past have established key pathogenic roles for Th1 and Th17 responses in initiation and perpetuation of crescentic GN (cGN).1–8 These observations have sparked growing interest in mechanisms counter-regulating nephritogenic Th1 and Th17 immunity. To this end, it has recently been proposed that specialized regulatory T (Treg) cell subtypes might exist, which are tailor made to downregulate distinct T helper cell lineages.9–11 Interestingly, these lineage–specific Treg cells seem to achieve their specialization by activating some of the same transcription factors that are needed for programming of their proinflammatory counterparts. Cells specialized at downregulating Th17 responses, for example, coactivate Foxp3 together with the Th17–characteristic transcription factor Stat3. These Treg17 cells effectively control Th17 responses, and their absence leads to selectively overshooting Th17 immunity.12 In a recent study, our group could also prove a protective role for Stat3–dependent Treg17 cells in acute cGN.13 As one main mechanism enabling them to target Th17 responses, we identified expression of the chemokine receptor CCR6. This allows colocalization of Treg17 with Th17 cells, which also highly express the CCR6.14,15 Interestingly, induction of a Treg17 phenotype is specifically achieved by Stat3 activation. In contrast, Foxp3+ cells coexpressing the second Th17 master transcription factor RORγt constitute an independent and bifunctional T cell lineage, which we have recently characterized and termed biTreg cells.16 With respect to regulation of Th1 responses, pioneering studies by Koch et al.17,18 have suggested that Treg cell intrinsic expression of the master Th1 transcription factor T-bet optimizes them to target Th1 cells. Given their specialization for counter-regulation of Th1 responses, we will herein refer to T-bet–expressing Treg cells as Treg1 cells. In analogy to Treg17 cells, T-bet+ Treg1 cells also seem to be equipped to traffic into the same areas as their proinflammatory Th1 counterparts. Close colocalization in inflamed tissues might be achieved by shared expression of the Th1–characteristic chemokine receptor CXCR3,19 which has also been shown by us to mediate renal trafficking of Th1 cells in cGN.20 In line with this hypothesis, various studies have shown coincidental upregulation of CXCR3 and T-bet on Treg cells during Th1-type inflammation caused by infection, cancer, contact hypersensitivity, autoimmunity, or organ transplantation in mice21–24 and humans.25–28 Although all of these studies point toward a role of T-bet in Treg cells for enhancing their immunosuppressive capacity specifically against Th1 responses, a recent study has reported opposing effects. Stimulation of Treg cells with the cytokine IL-12 from subjects with multiple sclerosis resulted in increased T-bet expression. However, in vitro suppressive capacity of these Th1–type T-bet+ Treg cells was significantly reduced.29 The functional role of T-bet activation in Treg cells thus remains elusive. In addition, because Foxp3Cre and T-betfl/fl mice have only recently become available, none of the above studies directly evaluated the role of Treg cell–expressed T-bet but rather, used adoptive transfer models or merely reported associations. To this end, two studies have been published recently during preparation of this manuscript. Yu et al.30 reported that Treg cell–selective loss of T-bet did not result in development of spontaneous autoimmunity in mice. The potential role of T-bet+ Treg cells for downregulation of Th1 responses at steady state or induced Th1–type inflammation, however, was not studied by Yu et al.30 The second manuscript examined the murine EAE model of multiple sclerosis.31 Surprisingly, the authors31 did not find any relevant effects of T-bet deficiency in Treg cells for development and severity of central nervous system inflammation. As in the study by Yu et al,30 effects of T-bet expression in Treg cells on Th1 immunity were not assessed, leaving this aspect unclear.
In summary, many questions regarding T-bet+ Treg cells remain unanswered, including their functional relevance in Th1-mediated inflammation. This study, therefore, aimed to address the following aspects: (1) identify and characterize T-bet+ Treg cells in experimental GN, (2) clarify the role of T-bet for Treg1 cell generation, (3) define the role of Treg1 cells for Th1 homeostasis, (4) study the role of T-bet+ Treg1 cells in experimental GN, and (5) investigate Treg1 cell mechanisms of action.
Results
A Functionally Distinct T-Bet+ Treg Subset Expands during cGN
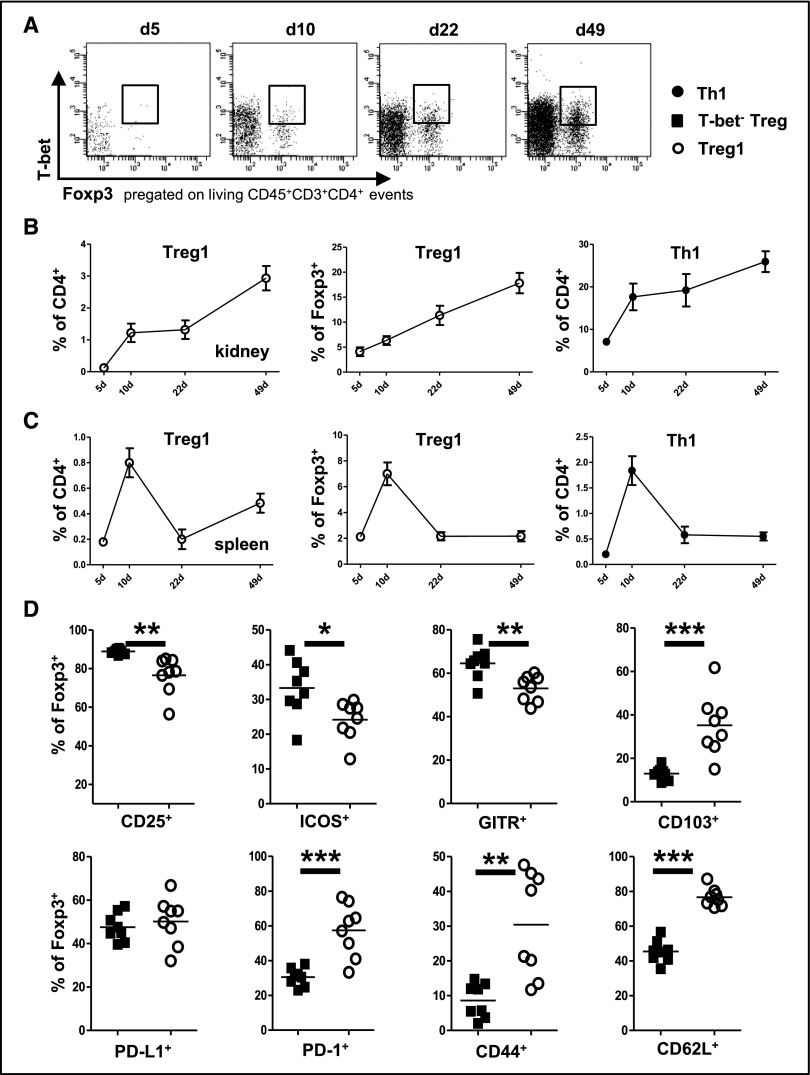
To investigate whether T-bet–expressing Treg1 cells might play a role in cGN, we studied spleens and kidneys of mice at different time points after induction of nephrotoxic nephritis (NTN). Indeed, our analyses showed robust T-bet activation in a subset of Foxp3+ Treg cells in both nephritic kidneys (Figure 1A) and spleens. Interestingly, however, Treg1 cell dynamics showed organ-specific differences. Both renal Treg1 cells (Figure 1B) and T-bet− Treg cells (Supplemental Figure 1A) expanded continuously over the complete observation period up to 49 days after NTN induction. Importantly, however, Treg1 cell percentages steadily increased not only among CD4+ T cells but also, within the Foxp3+ Treg cell population (Figure 1B). The percentages of T-bet− Treg cells among Foxp3+ cells, in contrast, decreased over time (Supplemental Figure 1B). Different from renal Treg cells, splenic Treg1 cells (Figure 1C) and T-bet− Treg cells (Supplemental Figure 1C) expanded to reach a maximum among CD4+ T cells at around 10 days after NTN induction and subsequently retracted. Within the splenic Foxp3+ population, Treg1 percentages also peaked at day 10 (Figure 1C), resulting in consecutively reduced percentages of T-bet− Treg cells at this time point (Supplemental Figure 1D). It is noteworthy that the dynamics of Treg1 cells paralleled those of Th1 cells in both organs (Figure 1, B and C). Detailed analyses of surface molecule expression associated with regulatory function and activation revealed a unique signature on Treg1 cells, highlighting their independent character. Although CD25, ICOS, and GITR were expressed at lower levels, Treg1 cells showed enhanced expression of CD103, PD-1, CD44, and CD62L in comparison with T-bet− Treg cells (Figure 1D).
Figure 1.
T-bet+ Treg cells expand during cGN. (A) Representative FACS plots showing renal T-bet+Foxp3+ Treg1 cells at the indicated time points after induction of NTN. (B and C) Quantification of (B) renal and (C) splenic T-bet+Foxp3+ Treg1 and T-bet+Foxp3− Th1 cells as percentages of CD4+ or Foxp3+ cells at the indicated time points after induction of NTN; n≥5 C57BL/6 mice were analyzed at each time point. (D) FACS analysis of surface molecule expression on the indicated splenic Treg cell populations at day 8 after induction of NTN; n=8 C57BL/6 mice were analyzed. Circles in D represent individual animals, and horizontal lines represent mean values. Error bars represent SEM. *P<0.05; **P<0.01; ***P<0.001.
Loss of T-Bet Expression in Treg Cells Results in a Spontaneous Hyper–Th1 Phenotype
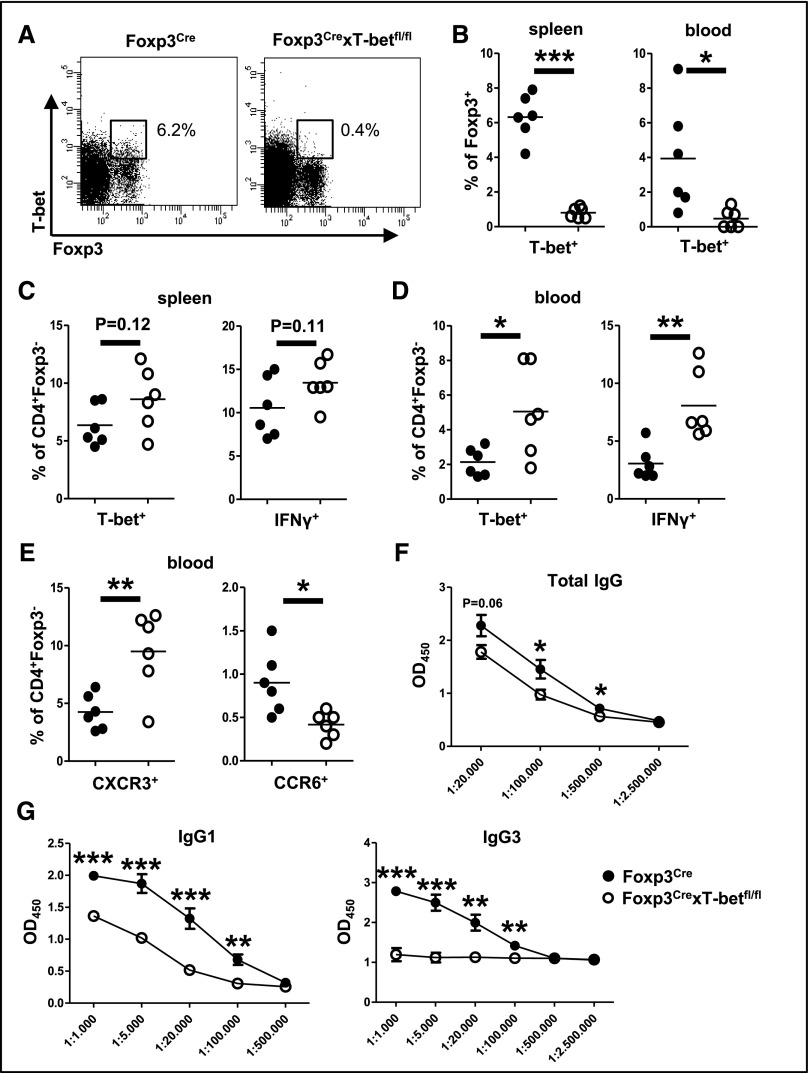
To study the function of T-bet+ Treg1 cells, we generated Foxp3CrexT-betfl/fl mice. FACS (Becton Dickinson, San Diego, CA) analyses confirmed specific absence of T-bet in Foxp3+ Treg cells in both spleens and blood (Figure 2, A and B). Treg1–deficient Foxp3CrexT-betfl/fl mice were viable and fertile, and they did not reveal abnormalities in spleen cell numbers (Supplemental Figure 2A), CD3+, CD4+, or Foxp3+ T cell subset composition in spleens or blood at steady state (Supplemental Figure 2, B and C). Interestingly, however, we found elevated percentages of T-bet+ and IFNγ+ Th1 cells in spleens (Figure 2C) and even more so in the blood (Figure 2D) of Treg1 cell–deficient mice. Th17 responses in contrast were unchanged as shown by similar frequencies of RORγt– and IL-17–expressing Th17 cells (Supplemental Figure 2, D and E). In line with enhanced Th1 responses, expression of the Th1–characteristic chemokine receptor CXCR3 was upregulated on blood T helper cells in Foxp3CrexT-betfl/fl mice, whereas Th17–characteristic CCR6 expression was reduced (Figure 2E). Splenocyte production of various cytokines, including Treg cell–associated IL-10 and TGF-β, was not different between the groups (Supplemental Figure 2F). However, similar to cellular immunity, humoral immune responses were also skewed toward Th1. Levels of total IgG were reduced in Foxp3CrexT-betfl/fl mice (Figure 2F) as a result of drastically impaired production of the Th2–characteristic IgG1 and IgG3 subclasses (Figure 2G), which are known to be suppressed by Th1-derived IFNγ. Th1–dependent IgG2c antibody levels, in contrast, remained unchanged (Supplemental Figure 2G). Importantly, the observed hyper–Th1 phenotype did not result in spontaneous renal pathology as shown by normal renal histology (Supplemental Figure 2, H and I) and absence of albuminuria (Supplemental Figure 2J).
Figure 2.
Spontaneous hyper–Th1 phenotype in Foxp3CrexT-betfl/fl mice. (A) Representative FACS plots of splenic T-bet+Foxp3+ Treg1 cells from naïve mice of the indicated genotypes. (B) Quantification of T-bet+Foxp3+ Treg1 cells in spleens and blood of naïve mice. (C and D) Quantification of (C) splenic and (D) blood T-bet+ or IFNγ+ Foxp3− Th1 cells as indicated. (E) Quantification of chemokine receptor CXCR3 and CCR6 expression on Foxp3− T helper cells in the blood of naïve mice. (F and G) Levels of total IgG, IgG1, and IgG3 subclasses in the serum of naïve mice. ELISA data are shown as OD at 450 nm in serial dilutions as indicated. Numbers in FACS plots represent percentages of Foxp3+ cells; n=6 versus six animals for all analyses. Circles in B–E represent individual animals, and horizontal lines represent mean values. Error bars represent SEM. *P<0.05; **P<0.01; ***P<0.001.
Nephritogenic Renal and Systemic Immunities Are Skewed toward Th1 in Treg1-Deficient Mice
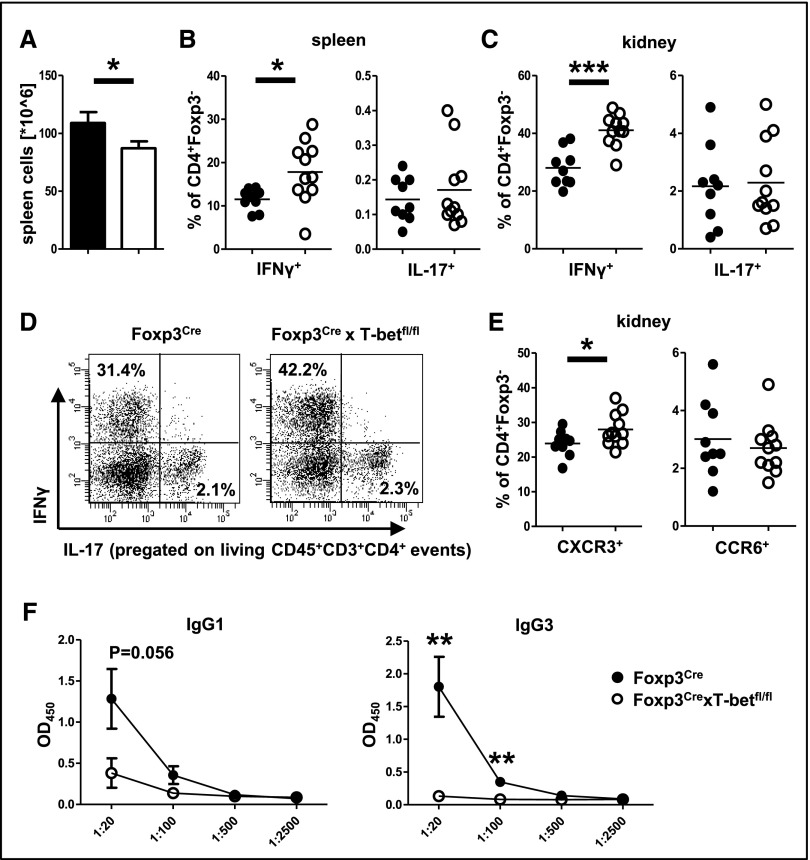
Next, we induced NTN in Foxp3CrexT-betfl/fl and Foxp3Cre control mice. Analyses at day 15 revealed slightly reduced spleen cell numbers in the absence of Treg1 cells (Figure 3A) with unchanged CD3+ and CD4+ T cell percentages (Supplemental Figure 3A). Importantly, both spleens (Figure 3B) and kidneys (Figure 3, C and D) showed significantly enhanced IFNγ+ Th1 cell frequencies, whereas IL-17+ Th17 cells remained unchanged. In line, renal infiltration with T helper cells expressing the Th1–associated chemokine receptor CXCR3 was increased, but percentages of T helper cells expressing the Th17-characteristic CCR6 were unchanged (Figure 3E).
Figure 3.
Skewing of renal and systemic immunity toward Th1 in Treg1 cell–deficient mice. (A) Quantification of spleen cell numbers. (B and C) Quantification of (B) splenic and (C) renal Foxp3− T helper cells expressing IFNγ and IL-17. (D) Representative FACS plots of renal T helper cells expressing the indicated cytokines. (E) Expression of the indicated chemokine receptors on renal Foxp3− T helper cells. Analyses in A–E were performed at day 15 after NTN induction. (F) Serum levels of IgG1 and IgG3 anti–sheep globulin antibodies at day 12 after sheep IgG immunization. ELISA data are shown as OD at 450 nm in serial dilutions as indicated. Numbers in FACS plots represent percentages of CD4+ cells. Nine Foxp3Cre versus 11 Foxp3CrexT-betfl/fl mice were analyzed in A–E, and five Foxp3Cre versus five Foxp3CrexT-betfl/fl mice were analyzed in F. Circles in B, C, and E represent individual animals, and horizontal lines represent mean values. Error bars represent SEM. *P<0.05; **P<0.01; ***P<0.001.
Apart from elevated Th1 responses, systemic cellular immunity was similar between the groups as shown by spleen cell production of various other cytokines (Supplemental Figure 3B). In line with cellular immunity, analysis of sheep globulin–specific antibody production revealed skewing toward Th1-dependent subclasses as evidenced by profoundly impaired production of Th2–associated IgG1 and IgG3 isotypes (Figure 3F). Total IgG, IgG2c, and IgG2b anti–sheep globulin IgG levels remained unchanged (Supplemental Figure 3C).
NTN Is Aggravated in the Absence of Treg1 Cells
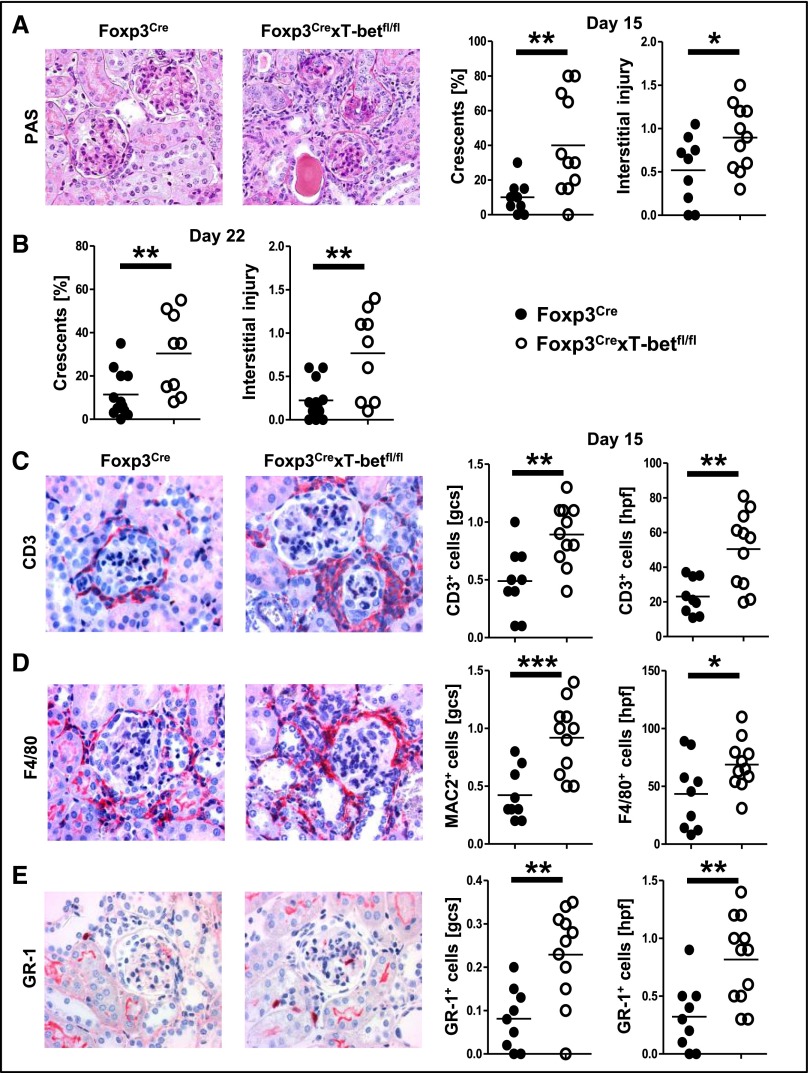
In line with overshooting renal and systemic Th1 responses, tissue damage was significantly aggravated at day 15 after NTN induction in kidneys of Foxp3CrexT-betfl/fl mice, with increased crescent formation and enhanced interstitial injury (Figure 4A). Likewise, renal function was worse in the absence of Treg1 cells as indicated by higher BUN levels (Supplemental Figure 4A). Albuminuria was not significantly different between the groups (Supplemental Figure 4B). Analysis of kidneys at a later time point (22 days after NTN induction) again showed significant aggravation of tissue damage (Figure 4B). In line with enhanced renal injury, analyses of the renal inflammatory cell infiltrate revealed increased numbers of T cells (Figure 4C) and macrophages (Figure 4D) as well as neutrophils (Figure 4E) in both glomeruli and the renal interstitial compartment of Foxp3CrexT-betfl/fl mice at days 15 (Figure 4, C–E) and 22 (Supplemental Figure 4, C–E) after NTN induction.
Figure 4.
NTN is aggravated in the absence of Treg1 cells. (A) Representative photographs of periodic acid–Schiff (PAS) –stained kidney sections and quantification of crescents and interstitial injury at day 15 after NTN. Original magnification, ×200. (B) Quantification of crescents and interstitial injury at day 22 after NTN. (C) Representative photographs and quantification of glomerular and interstitial CD3+ T cells. Original magnification, ×400. (D) Representative photographs of F4/80–stained renal macrophages and quantification of glomerular (MAC-2+) and interstitial (F4/80+) macrophages. Original magnification, ×400. (E) Representative photographs of GR-1–stained kidneys and quantification of glomerular and interstitial GR-1+ neutrophils. Original magnification, ×400. One of two representative sets for A is shown. Nine Foxp3Cre versus 11 Foxp3CrexT-betfl/fl mice were analyzed at day 15, and 12 Foxp3Cre versus nine Foxp3CrexT-betfl/fl mice were analyzed at day 22. Circles represent individual animals, and horizontal lines represent mean values. *P<0.05; **P<0.01; ***P<0.001.
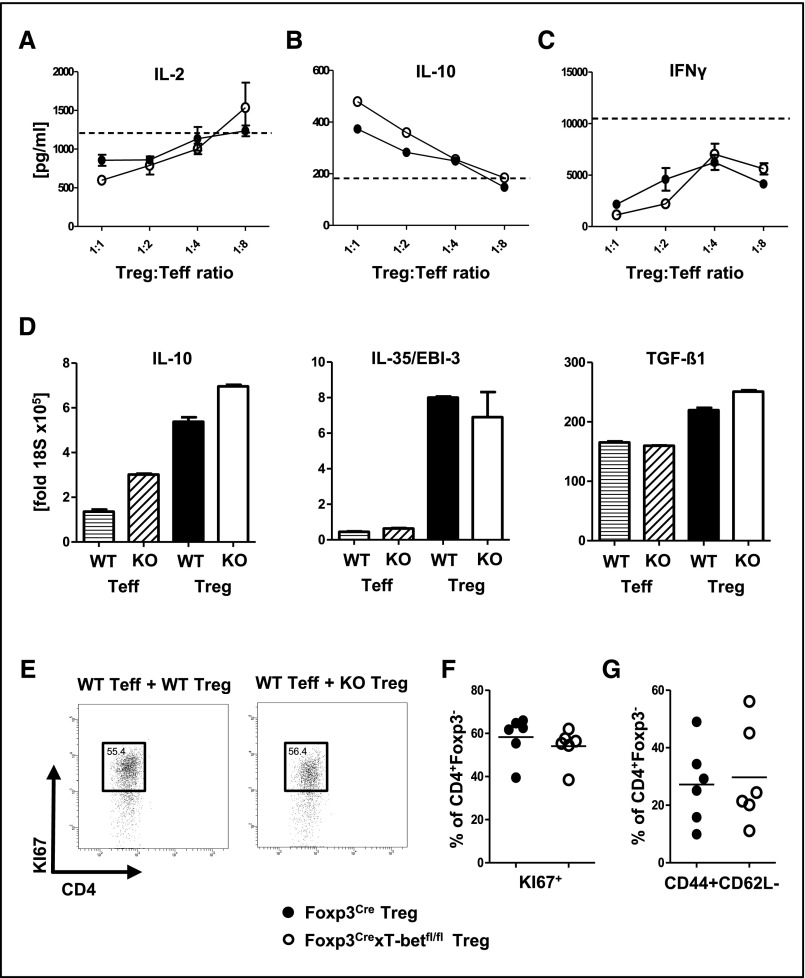
Treg Cell Suppressive Function Is Maintained in the Absence of T-Bet Activation
Next, we wanted to assess whether T-bet deficiency affects Treg cell suppressive functions. We, thus, performed in vitro suppression assays by coculturing effector T cells (Teffs) with Treg cells from Foxp3CrexT-betfl/fl or Foxp3Cre control mice. Our studies showed intact Treg function, including effective dose–dependent suppression of IL-2 production (Figure 5A), as well as induction of IL-10 secretion (Figure 5B). Importantly, also, suppression of IFNγ production remained unaffected by lack of T-bet in Treg cells, indicating unimpaired in vitro potential to suppress Th1 responses (Figure 5C). Furthermore, we isolated Treg cells from spleens of sheep IgG–immunized Foxp3CrexT-betfl/fl or Foxp3Cre control mice and analyzed expression of various Treg cell effector cytokines. No differences were detected with respect to IL-10, IL-35/EBI-3, and TGF-β1 mRNA levels (Figure 5D). In addition, analysis of a broad spectrum of surface markers revealed no major alterations of Treg cells from Foxp3CrexT-betfl/fl mice, with the exception of slightly enhanced GITR expression (Supplemental Figure 5). Finally, we compared the effects of Treg cells on Teff proliferation and activation in vivo. Treg cells from Foxp3Cre wild–type or Foxp3CrexT-betfl/fl mice were cotransferred with wild–type CD4+Foxp3− Teff into Rag1−/− recipients. Spleens of recipient mice were analyzed at day 8 after immunization with the nephritogenic antigen sheep globulin. Analyses revealed, that Treg cell percentages in both groups of recipients were similar. Furthermore, no T-bet expression was found in Treg cells from the knockout recipient group, confirming that no relevant de novo development of Treg cells had occurred (data not shown). Importantly, we found similar proliferation (Figure 5, E and F) and activation (Figure 5G) of Teff in both groups of recipients, which indicates similar suppressive capacity of wild–type and T-bet–deficient Treg cells.
Figure 5.
Intact Treg cell–suppressive function in the absence of T-bet activation. (A–C) In vitro suppression assays were performed by coculturing wild–type CD4+ Teffs with Treg cells from Foxp3CrexT-betfl/fl mice or Foxp3Cre controls at the indicated ratios (n=3 per group). Cytokine levels of (A) IL-2, (B) IL-10, and (C) IFNγ were analyzed in coculture supernatants as indicated. Dotted lines represent Teffs alone without Treg cells (n=3). (D) Quantification of IL-10, IL35/EBI-3, and TGF-β1 mRNA by quantitative RT-PCR from the indicated spleen cell populations FACS sorted at day 12 after immunization with sheep IgG. (E) Representative FACS plots and (F) quantification of KI67+ proliferating CD4+Foxp3− Teffs from spleens of immunized Rag1−/− recipients harboring Treg cells from the indicated mouse strains. (G) FACS analysis of splenic CD4+ Teff activation from immunized Rag1−/− recipients harboring Treg cells of the indicated genotype; n=6 versus six mice were analyzed in E–G. Circles represent individual animals, and horizontal lines represent mean values. Error bars represent SEM. KO, Foxp3CrexT-betfl/fl mice; WT, Foxp3Cre controls.
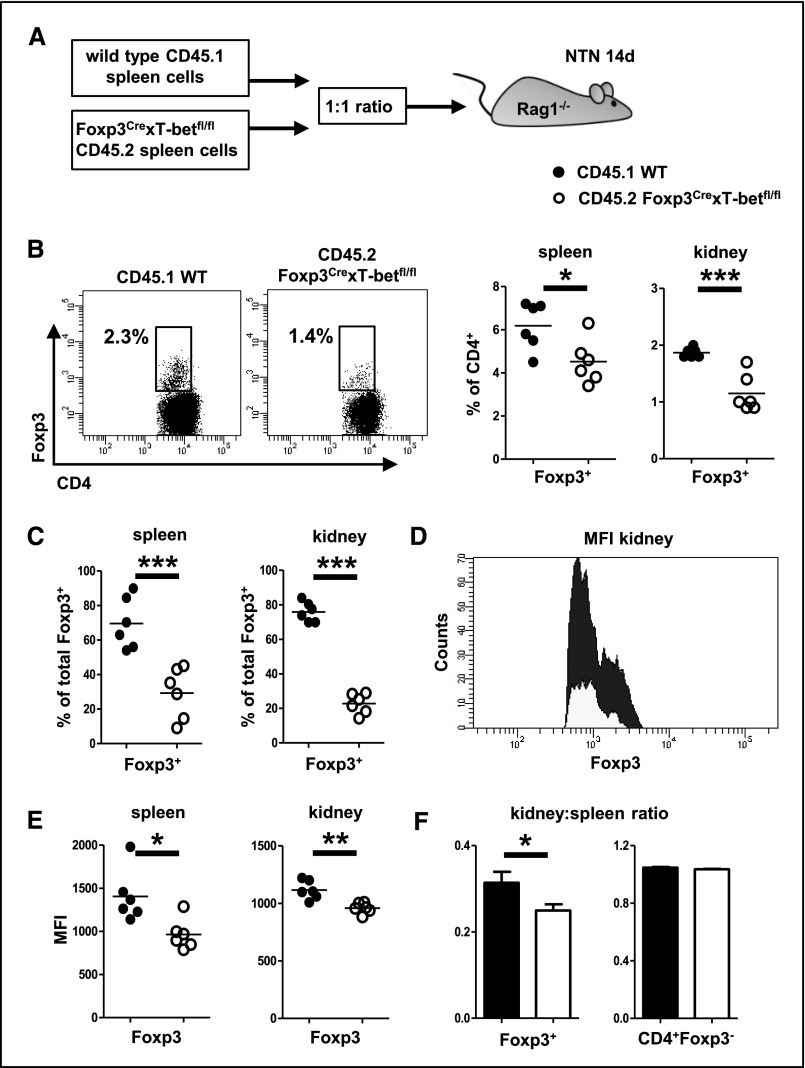
T-Bet Expression in Treg Cells Is Required for Competitive Fitness
Next, we wanted to evaluate whether T-bet expression influences in vivo Treg fitness and thus, performed competitive transfer assays. Spleen cells from wild–type donor mice carrying the congenic marker CD45.1 were mixed at a 1:1 ratio with spleen cells from CD45.2+ Foxp3CrexT-betfl/fl mice and transferred into Rag1−/− recipients. Subsequently, NTN was induced, and Treg cells were analyzed in spleens and kidneys at day 14 (Figure 6A). In both organs, we found that wild–type Treg cells had significantly outcompeted T-bet–deficient Treg cells, because percentages of Treg cells among CD45.1+ wild–type T cells were much higher than Treg cell percentages among CD45.2+ T cells from Foxp3CrexT-betfl/fl mice (Figure 6B). Similarly, percentages of CD45.1+ wild–type Treg cells were significantly higher than those of CD45.2+ T-bet–deficient Treg cells among total Treg cells in both spleens and kidneys (Figure 6C). In line, expression intensity of Foxp3 protein was much lower in Treg cells from Foxp3CrexT-betfl/fl mice, indicating reduced suppressive function (Figure 6, D and E). Finally, we noted decreased kidney-to-spleen ratios of Treg cells from CD45.2 Foxp3CrexT-betfl/fl donor mice, which indicated impaired renal trafficking of T-bet–deficient Treg cells. Ratios of Teffs, as a control, were not different between knockout and wild–type donor populations, confirming that the trafficking defects were Treg cell specific (Figure 6F).
Figure 6.
T-bet expression in Treg cells is required for competitive fitness. (A) CD45.1+ wild–type and CD45.2+ Foxp3CrexT-betfl/fl splenocytes were transferred at a 1:1 ratio into Rag1−/− recipients, and NTN was induced. (B) Representative FACS plots of Foxp3+ Treg cells from wild-type or Foxp3CrexT-betfl/fl donors in kidneys of nephritic Rag1−/− recipients (left panel). Plots are pregated on living CD45.1+ or CD45.2+CD3+ cells as indicated. Numbers in FACS plots indicate percentages of CD4+ events. Quantification of Treg cell percentages among wild–type or Foxp3CrexT-betfl/fl donor CD4+ T cells in spleens and kidneys of nephritic recipient mice (right panel). (C) Quantification of CD45.1 and CD45.2 Treg cell percentages among total Treg cells in spleens and kidney as indicated. (D) Representative FACS plots of Foxp3 mean fluorescence intensity (MFI) in Treg cells from wild-type or Foxp3CrexT-betfl/fl donors in kidneys of nephritic Rag1−/− recipients. Plots are pregated on living CD45.1+ or CD45.2+CD3+CD4+Foxp3+ cells as adequate. (E) Quantification of Foxp3 MFIs in spleens and kidneys. (F) Kidney-to-spleen ratios of Foxp3+ Treg cells (left panel) and CD4+Foxp3− Teffs (right panel); n=6 recipient mice were analyzed in A–F. Circles represent individual animals, and horizontal lines represent mean values. Error bars represent SEM. *P<0.05; **P<0.01; ***P<0.001.
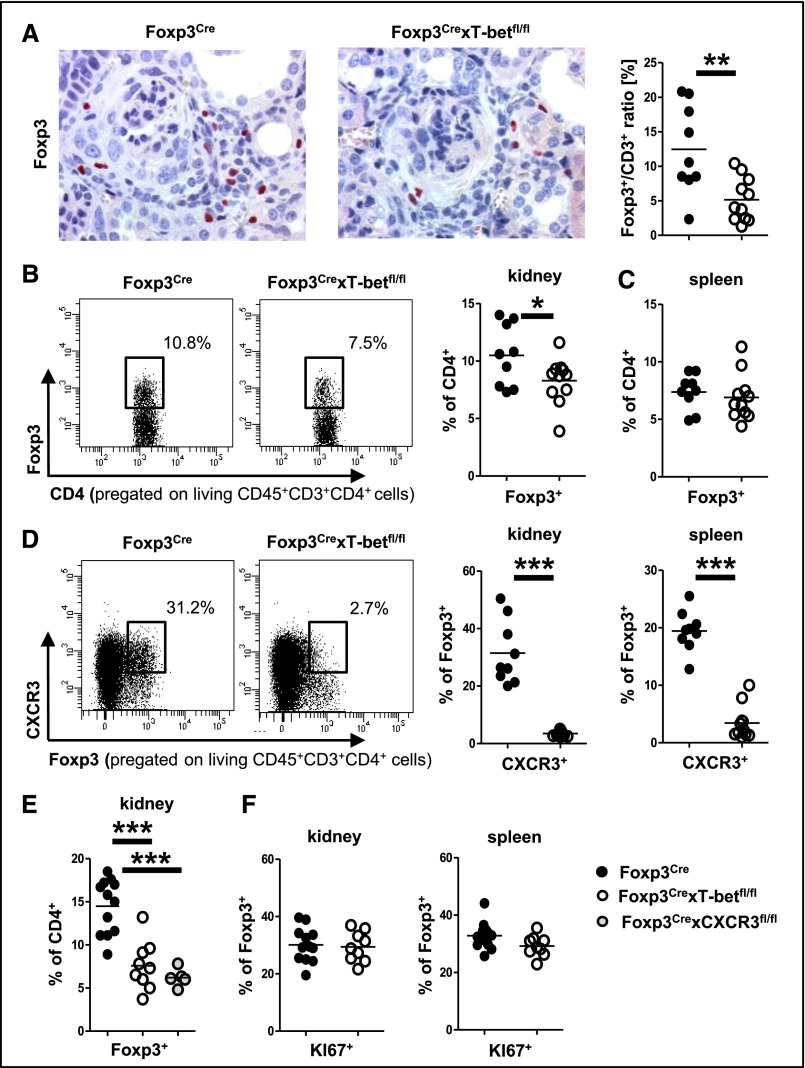
Renal Treg Cell Infiltration Is Impaired in Foxp3CrexT-betfl/fl Mice Because of Absence of CXCR3
We next evaluated whether impairment of Treg cell trafficking might contribute to the observed aggravation of nephritis. To this end, analyses showed reduced frequencies of Treg cells in kidneys from Foxp3CrexT-betfl/fl mice by both immunohistochemistry (Figure 7A) and FACS (Figure 7B). Percentages of Treg cells among spleen cells, in contrast, remained unchanged (Figure 7C).
Figure 7.
Impaired renal Treg cell infiltration in Foxp3CrexT-betfl/fl mice caused by absence of CXCR3. (A) Representative photographs of Foxp3–stained kidney sections and quantification of renal Foxp3-to-CD3 ratios. (B) Representative FACS plots and quantification of renal Foxp3+ Treg cells in mice of the indicated genotypes. Numbers in FACS plots indicate percentages of CD4+ cells. (C) Quantification of splenic Foxp3+ Treg cells by FACS. (D) Representative FACS plots of CXCR3+ renal Treg cells (left panel). Numbers indicate percentages of Foxp3+ events. Quantification of renal and splenic CXCR3+ Treg cells (right panel). Analyses in A–D were performed at day 15 of NTN. (E) Quantification of renal Foxp3+ Treg cells in mice of the indicated genotypes at day 22 after NTN by FACS. (F) Quantification of renal and splenic KI67+ Foxp3+ proliferating Treg cells in mice of the indicated genotypes at day 22 after NTN. Nine Foxp3Cre versus 11 Foxp3CrexT-betfl/fl mice were analyzed in A–D, 12 Foxp3Cre versus nine Foxp3CrexT-betfl/fl versus five Foxp3CrexCXCR3fl/fl mice were analyzed in E, and 12 Foxp3Cre versus nine Foxp3CrexT-betfl/fl were analyzed in F. Circles represent individual animals, and horizontal lines represent mean values. *P<0.05; **P<0.01; ***P<0.001.
Because these findings indicated impaired Treg cell trafficking rather than reduced generation, we analyzed Treg cell chemokine receptor expression. Indeed, we found robust expression of the Th1–characteristic trafficking receptor CXCR3 on Treg cells from nephritic control mice, whereas expression on Treg cells from Foxp3CrexT-betfl/fl mice was completely abrogated in both kidneys and spleens (Figure 7D). Conversely, expression of the Th17–characteristic chemokine receptor CCR6 was strongly upregulated (Supplemental Figure 6A). Expression of the chemokine receptor CCR5 on Treg cells remained unaffected by T-bet deficiency (not shown). To further evaluate whether lack of CXCR3 might, indeed, underlie the observed reduction of renal Treg cells, we studied Foxp3CrexCXCR3fl/fl mice with selective CXCR3 deficiency on Treg cells. In line with our concept, we found that Foxp3CrexCXCR3fl/fl and Foxp3CrexT-betfl/fl mice showed identical reduction of renal Treg cell percentages in comparison with wild-type controls at 22 days after NTN induction (Figure 7E). Treg cell percentages in spleens of both strains were similar to wild-type mice, excluding systemic Treg cell deficiencies as causative for reduced renal Treg cells (Supplemental Figure 6B). Importantly, also, the hyper-Th1 phenotype was almost identical in kidneys of Foxp3CrexCXCR3fl/fl and Foxp3CrexT-betfl/fl mice (Supplemental Figure 6C). This observation strongly supports the hypothesis that Treg1 cells use CXCR3, which is expressed under the control of T-bet, for trafficking into the kidney and control of renal Th1 immunity. Finally, we aimed to determine whether impaired Treg cell proliferation in the absence of T-bet might also contribute to the observed reduction of renal Treg cells. However, our analyses revealed similar renal and splenic Treg cell proliferative activity in Foxp3CrexT-betfl/fl compared with wild-type mice (Figure 7F).
Discussion
The etiology and pathogenesis of most forms of GN are still poorly understood.7,32 As a consequence, GN remains a leading cause for ESRD. Because current therapeutic options are nonspecific and show a high degree of unwanted side effects, the therapy of patients with GN is challenging and often frustrating. Cells of the Th1 response, however, might represent a promising novel therapeutic target. We and others could show their key functions in initiation and progression of renal injury in crescentic forms of GN.1–3,5,8,33 Thus, understanding the mechanisms that downregulate these highly nephritogenic Th1 responses is of great clinical importance. Our study, therefore, aimed to evaluate the new concept of Th1–specific Treg cells. These Treg1 cells have previously been postulated to depend on activation of the transcription factor T-bet, which they share with their proinflammatory Th1 counterpart.17 Because to date, nothing is known about their function in renal inflammation, we studied the NTN model of cGN. Indeed, we found expansion of T-bet+ Treg1 cells in spleens and kidneys within 10 days after induction of NTN. Subsequently, percentages in spleens decreased, whereas the renal Treg1 population steadily increased over time, suggesting preferential accumulation at sites of tissue injury. Interestingly, Treg1 cell dynamics paralleled those of Th1 responses, which is indicative of a role for Treg1 cells in counter-regulation of Th1 immunity. Analysis of Treg1 cell surface molecule expression revealed a distinct phenotype with reduced levels of CD25, ICOS, and GITR, whereas CD103, PD-1, CD44, and CD62L were expressed at much higher levels than on T-bet− Treg cells. Given these findings, which suggested unique and nonredundant functions of Treg1 cells, we next wanted to assess the functional relevance of T-bet expression in Treg cells. We, therefore, generated Foxp3CrexT-betfl/fl mice with a Treg cell–selective T-bet deficiency. In line with a recent report, which was published during preparation of this manuscript, these animals were healthy and fertile, and they showed normal survival.30 However, analysis of immune responses revealed a hitherto unknown phenotype. Although gross leukocyte composition of blood and spleens in naïve mice was unchanged, we found spontaneously overshooting Th1 immunity. In contrast, Th17 responses and production of various Th2– and Treg cell–associated cytokines were not significantly altered. Importantly, the higher systemic numbers of Th1 cells in Foxp3CrexT-betfl/fl mice had a functional effect on another arm of the immune system. Analysis of humoral immunity revealed profoundly reduced levels of Th2–associated IgG1 and IgG3 antibody subclasses, which are known to be suppressed by the Th1 prototype cytokine IFNγ.34 Because we were interested in studying the role of Treg1 cells in GN, we also evaluated the renal phenotype of naïve Foxp3CrexT-betfl/fl mice. Our analyses, however, revealed healthy kidneys with no signs of inflammation or functional impairment. We, thus, went on to study renal and systemic immune responses after induction of NTN. Again, we found selective skewing of immunity toward Th1 in both spleens and kidneys of Foxp3CrexT-betfl/fl mice. In line, antibody levels of the Th2–associated IgG1 and IgG3 subclasses against the nephritogenic antigen, sheep globulin, were significantly reduced. Even more importantly, dysregulation of Th1 responses resulted in enhanced kidney damage as evidenced by increased crescent formation and interstitial injury. Finally, evaluation of the renal inflammatory cell infiltrate showed increased numbers of T cells, macrophages, and neutrophils in kidneys of Treg1 cell–deficient mice. In contrast to aggravation of nephritis in Foxp3CrexT-betfl/fl mice, NTN was previously shown to be alleviated in T-bet pan knockout mice, which not only lack Treg1 cells but also, Th1 responses.3 This observation highlights that, indeed, Th1 cells are the main target of Treg1 cells. To identify the mechanisms underlying enhanced Th1 immunity and subsequent aggravation of cGN in Foxp3CrexT-betfl/fl mice, we analyzed Treg cell–suppressive function. In vitro suppression assays revealed unchanged capacity to reduce IL-2 levels in coculture with Teffs as well as unchanged induction of immunosuppressive IL-10. Importantly, in vitro suppression of IFNγ production also remained intact, indicating that T-bet deficiency does not lead to a per se defective control of Th1 responses. Additionally, quantitation of mRNA expression of various Treg cell–associated effector cytokines showed identical levels of IL-10, IL-35/EBI-3, and TGF-β1 in Treg cells of both groups. Furthermore, analysis of a broad range of surface molecules associated with Treg cell function did not show major differences caused by absence of T-bet. These findings are in line with previous work by McPherson et al.,31 which found unchanged capacity of T-bet–deficient Treg cells to suppress Teff expansion in vitro. To extend these observations, we analyzed in vivo–suppressive Treg cell effects. Indeed, our results showed similar Teff proliferation and activation in the presence of T-bet intact or T-bet–deficient Treg cells. However, alterations in Treg homeostasis and fitness might be masked in our constitutively T-bet–defective Foxp3CrexT-betfl/fl mice because of compensatory mechanisms and lack of competition by wild–type Treg cells. To address this question, competitive transfer assays were performed by coinjecting CD45.2+ spleen cells from Foxp3CrexT-betfl/fl mice with equal numbers of CD45.1+ spleen cells from wild-type controls into lymphopenic Rag1−/− recipients. After induction of NTN, wild–type Treg cells had massively outcompeted their T-bet–deficient counterparts in spleens and nephritic kidneys. Furthermore, expression levels of Foxp3 protein were much lower in transferred knockout Treg cells, indicating reduced regulatory capacity in comparison with wild–type Treg cells. T-bet activation, therefore, enhances population expansion and general Treg cell fitness during inflammation. Additionally, we also found reduced kidney-to-spleen ratios among transferred Treg cells from Foxp3CrexT-betfl/fl mice. This observation indicated impaired competitive renal trafficking of T-bet–deficient Treg cells. Given this observation, we next wanted to study whether defective renal Treg cell recruitment might contribute to the observed Th1–type hyperinflammatory phenotype of Foxp3CrexT-betfl/fl mice. In this respect, multimodal analyses of nephritic kidneys revealed significantly reduced percentages of Treg cells in kidneys of nephritic Foxp3CrexT-betfl/fl mice. In light of unchanged systemic Treg cell percentages, this finding, indeed, indicated impairment of renal trafficking. Because chemokines are the main regulators of directional T cell migration,35,36 we evaluated the Treg cell chemokine receptor profile. In line with several previous reports, including our own report,17,21–28,37 we found robust expression of the Th1–characteristic receptor CXCR3 on splenic and renal Treg cells. Intriguingly, however, CXCR3 expression was completely abrogated on Treg cells from Foxp3CrexT-betfl/fl mice, indicating absolute dependency on T-bet activation. To further investigate if lack of CXCR3 is the main reason for the observed reduction of renal Treg cell percentages, we performed comparisons with mice with Treg cell–selective CXCR3 deficiency. In line with our concept, we found equal impairment of renal Treg cell infiltration and importantly, also, identical enhancement of renal Th1 immunity in Foxp3CrexT-betfl/fl and Foxp3CrexCXCR3fl/fl mice. Furthermore, splenic and renal Treg cell proliferation was not altered by deletion of T-bet. Taken together, these findings indicate that, indeed, impaired trafficking caused by absence of CXCR3 and not diminished proliferative activity underlies the observed reduction in renal Treg cell percentages. Absence of CXCR3 on Treg cells is also likely to impair colocalization with CXCR3+ Th1 cells,37 which could explain the selectively exacerbated Th1 responses in Foxp3CrexT-betfl/fl mice.
In summary, we here provide the first evidence for a role of T-bet+ Treg1 cells in experimental cGN and thus, a model of induced organ inflammation in general. T-bet activation was found to modulate two important Treg cell properties. First, overall Treg cell fitness was significantly enhanced by T-bet expression. Second, T-bet activation induced expression of the chemokine receptor CXCR3. This allows for optimized control of Th1 responses by facilitating Treg1 cell trafficking into areas of Th1-type inflammation. T-bet+ Treg1 cells were, thus, identified as novel regulators of nephritogenic Th1 responses and therefore, warrant exploration as potential therapeutic targets.
Concise Methods
Animals
loxP site–flanked Tbx21fl/fl mice38 (referred to as T-betfl/fl) were provided by Steven L. Reiner (Columbia University, New York, NY). CXCR3fl/fl mice were generated as previously published.37 Treg cell–specific deletion of T-bet or CXCR3 was achieved by crossbreeding with mice expressing a yellow fluorescent protein (YFP)-Cre recombinase fusion protein under the control of the Foxp3 locus39 provided by Alexander Y. Rudensky (Memorial Sloan-Kettering Cancer Center, New York, NY). Rag1−/− and CD45.1 mice initially derive from The Jackson Laboratory (Bar Harbor, ME). All mice are on a C57BL/6 background and were bred in our facility under specific pathogen–free conditions.
Animal Experiments and Functional Studies
Naїve mice were analyzed at 11–13 weeks of age. NTN was induced in 8- to 10-wk-old male Foxp3CrexT-betfl/fl, Foxp3CrexCXCR3fl/fl, and Foxp3CrexT-betwt/wt (referred to as Foxp3Cre) littermate controls by intraperitoneal (ip) injection of nephrotoxic sheep serum.40 Organs were harvested at the indicated time points between days 5 and 49 after injection. For immunization studies, mice were ip immunized with 0.5 mg sheep IgG, and organs were harvested at day 11. For comparison of in vivo Treg cell suppression, 4×105 FACS–sorted Treg cells from spleens of Foxp3Cre or Foxp3CrexT-betfl/fl mice were cotransferred together with 1.5×106 CD4+Foxp3− Teffs from spleens of Foxp3Cre mice into two groups of Rag1−/− recipients. Subsequently, recipients were ip immunized with 0.5 mg sheep IgG, and spleens were analyzed at day 8. For competitive transfer assays, splenic leukocytes were isolated from either Foxp3CrexT-betfl/fl (CD45.2+) or wild–type C57BL/6 (CD45.1+) donor mice. Viable cells were counted using a Bio-Rad TC21 Counter (Bio-Rad, Hercules, CA) after trypan blue staining and intravenously injected into Rag1−/− mice in a 1:1 ratio 1 day before induction of NTN. Renal and splenic leukocytes were isolated 14 days after NTN induction as described below and analyzed by FACS.13 Urine samples were collected after housing the mice in metabolic cages. Albuminuria was determined by standard ELISA (Bethyl Laboratories). BUN and urinary creatinine were quantified using standard laboratory methods. Animal experiments were performed according to national and institutional animal care and ethical guidelines and approved by local committees (approval codes 37/11, 73/14, and 07/15).
Morphologic Studies
Crescent formation and glomerular necrosis were determined in a minimum of 50 glomeruli per mouse in 2-μm-thick periodic acid–Schiff–stained kidney sections in a blinded manner. Semiquantitative analysis of tubulointerstitial damage was performed using ten randomly selected cortical areas (×200) as described previously.6 Paraffin-embedded sections were stained with antibodies directed against CD3 (A0452; Dako, Hamburg, Germany), F4/80 (BM8; BMA Biomedicals, Hiddenhausen, Germany), MAC2 (M3/38; Cedarlane Laboratories, Burlington, ON, Canada), GR-1 (NIMP-R14; Hycult Biotech, Uden, The Netherlands), or Foxp3 (FJK-16s; eBiosciences, San Diego, CA) and developed with a polymer–based secondary antibody-alkaline phosphatase kit (POLAP; Zytomed, Berlin, Germany). Fifty glomerular cross-sections and 30 tubulointerstitial high power fields (magnification of ×400) per kidney section were counted in a blinded fashion.13,41
Isolation of Leukocytes from Various Tissues
Spleens were harvested in HBSS and passed through 70-μm nylon meshes. After lysis of erythrocytes with ammonium chloride, cells were washed and passed over 40-μm meshes. Cells were then washed again and counted for either culture or FACS analysis. Kidneys were minced and incubated in digestion medium (RPMI 1640 medium [Gibco, Carlsbad, CA] containing 10% FCS, 1% HEPES, 1% penicillin/streptomycin, 8 μg/ml collagenase D, and 0.4 μg/ml DNase) at 37°C for 40 minutes. Tissues were then dissociated using the gentleMACS Dissociater (Miltenyi Biotec) to get a single-cell suspension and centrifuged at 300×g at 4°C for 8 minutes. To further purify the cells, Percoll gradient (37% Percoll; GE Healthcare, Waukesha, WI) centrifugation was performed at 500×g at room temperature for 20 minutes. Cells were washed and resuspended for staining and FACS analysis. Peripheral blood was drawn into EDTA-coated tubes, and red blood cell lysis was performed. Then, blood cells were washed and prepared for staining.13
Systemic Cellular and Humoral Immune Responses
Splenocytes (4×105 cells per milliliter) were cultured under standard conditions on plates precoated with anti-CD3 mAb (1 μg/ml; BD Biosciences, San Jose, CA), and supernatants were harvested after 72 hours. Commercially available ELISAs were used for detection of IFNγ (BioLegend, San Diego, CA), IL-4 (BioLegend), IL-6 (BioLegend), IL-10 (BioLegend), TGF-β1 (R&D Systems, Minneapolis, MN), and IL-2 (R&D Systems).40 Circulating sheep globulin–specific serum IgG titers were analyzed by ELISA using plates precoated with sIgG.13,41 For analysis of total nonantigen–specific Igs, ELISA plates were precoated with anti–mouse total IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). ELISA plates were incubated with mouse serum, and the following secondary antibodies were used for detection: total IgG (Southern Biotech, Birmingham, AL), IgG1 (Southern Biotech), IgG2b (Invitrogen, Carlsbad, CA), IgG2c (Bethyl, Montgomery, TX), and IgG3 (Jackson ImmunoResearch Laboratories).
Flow Cytometry
Cells were surface stained for 30 minutes at 4°C with fluorochrome-labeled antibodies against CD45, CD45.1 CD45.2, CD4, CD44 (BD Bioscience), CD3, CD25, CD69, CD62L, CCR6, CXCR3, CCR5, ICOS, PD-1, CD103, PD-L1, and GITR (BioLegend) as previously described.13,16 For intracellular and intranuclear staining, samples were processed using a commercial intranuclear staining kit (Foxp3 Staining Kit; eBiosciences). Fluorochrome-labeled antibodies against IL-17 (BioLegend), IFNγ (BioLegend), Foxp3 (eBiosciences), T-bet (eBiosciences), Gata3 (eBiosciences), KI67 (BD Biosciences), and RORγt (BD Biosciences) were used as recently published.13,16 For intracellular cytokine staining, cells were activated with PMA (50 ng/ml; Sigma-Aldrich, St. Louis, MO), ionomycin (1 μg/ml; Calbiochem, San Diego, CA), and brefeldin A (10 µg/ml; Sigma-Aldrich) for 3 hours. LIVE/DEAD Staining (Invitrogen Molecular Probes, Eugene, OR) was used to exclude dead cells during flow cytometry and ensure viability of the cells after the stimulation procedure. Experiments were performed on a BD LSRII Cytometer (Becton Dickinson). FACS sorting was performed from single-cell suspensions enriched for CD4+ T cells by MACS sorting (T Cell Isolation Kit II; Miltenyi Biotec) from spleens of the indicated animal strains by the institutional HEXT FACS Sorting Core Facility using a BD ARIAIII Cytometer (Becton Dickinson) as previously described.13
Treg Cell Suppression Assay
CD4+ spleen cells were enriched by using magnetic–activated cell sorting according to the manufacturer’s protocol (MACS CD4+ T Cell Kit II; Miltenyi Biotec). Treg cells and Teffs were then isolated by FACS sorting (performed on a BD ARIAIII Cytometer; Becton Dickinson). In total, 1×105 CD45+CD4+YFP− Teffs from Foxp3Cre mice were then cultured for 72 hours in anti-CD3 mAb (5 μg/ml; BD Biosciences) precoated 96–well plates either alone or in coculture with CD45+CD4+YFP+ Treg cells from Foxp3Cre or Foxp3CrexT-betfl/fl mice at the indicated ratios. Suppressive capacity was determined by cytokine ELISAs performed from the supernatants as recently published.13,16
Quantitative Real–Time PCR Analyses
Quantitative RT-PCR from RNA derived from FACS–sorted spleen cell populations was performed in a Stepone Plus Detector (Applied Biosystems, Foster City, CA) as described before.42 For detection of IL-10, TGF-β1, and 18S, the sybr green method was used (primer sequences are available on request), whereas detection for EBI-3 was performed using Taqman probes (Applied Biosystems Assay ID: Mm00469294_m1). Samples were run in duplicates and normalized to 18S rRNA.16
Statistical Analyses
Results are expressed as means±SEMs. Groups were compared by t test, or in the case of three groups, ANOVA was used with post hoc analysis according to Tukey. A P value <0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Steven L. Reiner (Columbia University, New York, NY) and Massimo C. Fantini (University of Rome “Tor Vergata,” Rome, Italy) for providing Tbx21fl/fl mice. We also thank M. Schaper and M. Reszka for their excellent technical help.
This work was supported by Deutsche Forschungsgemeinschaft grants SFB 1192 (to M.A.K. and O.M.S.), STE 1822/2-1 (to O.M.S.), and KFO 228 STE 1822/3-1 (to O.M.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Specialized Regulatory T Cells for Optimal Suppression of T Cell Responses in GN,” on pages 1–2.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015070820/-/DCSupplemental.
References
- 1.Kitching AR, Holdsworth SR, Tipping PG: IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J Am Soc Nephrol 10: 752–759, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Kitching AR, Holdsworth SR, Tipping PG: Crescentic glomerulonephritis--a manifestation of a nephritogenic Th1 response? Histol Histopathol 15: 993–1003, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Phoon RK, Kitching AR, Odobasic D, Jones LK, Semple TJ, Holdsworth SR: T-bet deficiency attenuates renal injury in experimental crescentic glomerulonephritis. J Am Soc Nephrol 19: 477–485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner JE, Paust HJ, Steinmetz OM, Panzer U: The Th17 immune response in renal inflammation. Kidney Int 77: 1070–1075, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Summers SA, Steinmetz OM, Li M, Kausman JY, Semple T, Edgtton KL, Borza DB, Braley H, Holdsworth SR, Kitching AR: Th1 and Th17 cells induce proliferative glomerulonephritis. J Am Soc Nephrol 20: 2518–2524, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinmetz OM, Summers SA, Gan PY, Semple T, Holdsworth SR, Kitching AR: The Th17-defining transcription factor RORγt promotes glomerulonephritis. J Am Soc Nephrol 22: 472–483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couser WG: Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol 23: 381–399, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Kurts C, Panzer U, Anders HJ, Rees AJ: The immune system and kidney disease: Basic concepts and clinical implications. Nat Rev Immunol 13: 738–753, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Feuerer M, Hill JA, Mathis D, Benoist C: Foxp3+ regulatory T cells: Differentiation, specification, subphenotypes. Nat Immunol 10: 689–695, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Campbell DJ, Koch MA: Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol 11: 119–130, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinmetz OM, Turner JE, Panzer U: Staying on top of things right from the start: The obsessive-compulsive disorder of regulatory T cells. J Am Soc Nephrol 21: 6–7, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY: CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326: 986–991, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kluger MA, Luig M, Wegscheid C, Goerke B, Paust HJ, Brix SR, Yan I, Mittrücker HW, Hagl B, Renner ED, Tiegs G, Wiech T, Stahl RA, Panzer U, Steinmetz OM: Stat3 programs Th17-specific regulatory T cells to control GN. J Am Soc Nephrol 25: 1291–1302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G: Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8: 639–646, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Turner JE, Paust HJ, Steinmetz OM, Peters A, Riedel JH, Erhardt A, Wegscheid C, Velden J, Fehr S, Mittrücker HW, Tiegs G, Stahl RA, Panzer U: CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol 21: 974–985, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kluger MA, Meyer MC, Nosko A, Goerke B, Luig M, Wegscheid C, Tiegs G, Stahl RA, Panzer U, Steinmetz OM: RORγt+Foxp3+ cells are an independent bifunctional regulatory T cell lineage and mediate crescentic GN. J Am Soc Nephrol 27: 454–465, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ: The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 10: 595–602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ: T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity 37: 501–510, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groom JR, Luster AD: CXCR3 in T cell function. Exp Cell Res 317: 620–631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panzer U, Steinmetz OM, Paust HJ, Meyer-Schwesinger C, Peters A, Turner JE, Zahner G, Heymann F, Kurts C, Hopfer H, Helmchen U, Haag F, Schneider A, Stahl RA: Chemokine receptor CXCR3 mediates T cell recruitment and tissue injury in nephrotoxic nephritis in mice. J Am Soc Nephrol 18: 2071–2084, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Erhardt A, Wegscheid C, Claass B, Carambia A, Herkel J, Mittrücker HW, Panzer U, Tiegs G: CXCR3 deficiency exacerbates liver disease and abrogates tolerance in a mouse model of immune-mediated hepatitis. J Immunol 186: 5284–5293, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Suga H, Sugaya M, Miyagaki T, Ohmatsu H, Okochi H, Sato S: CXCR3 deficiency prolongs Th1-type contact hypersensitivity. J Immunol 190: 6059–6070, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, Harris TH, Grainger J, Wojno ED, Wagage S, Roos DS, Scott P, Turka LA, Cherry S, Reiner SL, Cua D, Belkaid Y, Elloso MM, Hunter CA: The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 37: 511–523, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HM, Fleige A, Forman R, Cho S, Khan AA, Lin LL, Nguyen DT, O’Hara-Hall A, Yin Z, Hunter CA, Muller W, Lu LF: IFNγ signaling endows DCs with the capacity to control type I inflammation during parasitic infection through promoting T-bet+ regulatory T cells. PLoS Pathog 11: e1004635, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, Hoffmüller U, Baron U, Olek S, Bluestone JA, Brusko TM: Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol 186: 3918–3926, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoerning A, Koss K, Datta D, Boneschansker L, Jones CN, Wong IY, Irimia D, Calzadilla K, Benitez F, Hoyer PF, Harmon WE, Briscoe DM: Subsets of human CD4(+) regulatory T cells express the peripheral homing receptor CXCR3. Eur J Immunol 41: 2291–2302, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K, Valmori D, Ayyoub M: CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res 72: 4351–4360, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Oo YH, Weston CJ, Lalor PF, Curbishley SM, Withers DR, Reynolds GM, Shetty S, Harki J, Shaw JC, Eksteen B, Hubscher SG, Walker LS, Adams DH: Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol 184: 2886–2898, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Dominguez-Villar M, Baecher-Allan CM, Hafler DA: Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med 17: 673–675, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu F, Sharma S, Edwards J, Feigenbaum L, Zhu J: Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat Immunol 16: 197–206, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPherson RC, Turner DG, Mair I, O’Connor RA, Anderton SM: T-bet expression by Foxp3(+) T regulatory cells is not essential for their suppressive function in CNS autoimmune disease or colitis. Front Immunol 6: 69, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Couser WG, Johnson RJ: The etiology of glomerulonephritis: Roles of infection and autoimmunity. Kidney Int 86: 905–914, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Paust HJ, Turner JE, Riedel JH, Disteldorf E, Peters A, Schmidt T, Krebs C, Velden J, Mittrücker HW, Steinmetz OM, Stahl RA, Panzer U: Chemokines play a critical role in the cross-regulation of Th1 and Th17 immune responses in murine crescentic glomerulonephritis. Kidney Int 82: 72–83, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Snapper CM, Paul WE: Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236: 944–947, 1987 [DOI] [PubMed] [Google Scholar]
- 35.Panzer U, Steinmetz OM, Stahl RA, Wolf G: Kidney diseases and chemokines. Curr Drug Targets 7: 65–80, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Bromley SK, Mempel TR, Luster AD: Orchestrating the orchestrators: Chemokines in control of T cell traffic. Nat Immunol 9: 970–980, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Paust HJ, Riedel JH, Krebs CF, Turner JE, Brix SR, Krohn S, Velden J, Wiech T, Kaffke A, Peters A, Bennstein SB, Kapffer S, Meyer-Schwesinger C, Wegscheid C, Tiegs G, Thaiss F, Mittrücker HW, Steinmetz OM, Stahl RA, Panzer U: CXCR3+ regulatory T cells control TH1 responses in crescentic GN [published online ahead of print November 3, 2015]. J Am Soc Nephrol doi:ASN.2015020203 [DOI] [PMC free article] [PubMed]
- 38.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL: Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321: 408–411, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR Jr., Muller W, Rudensky AY: Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28: 546–558, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Luig M, Kluger MA, Goerke B, Meyer M, Nosko A, Yan I, Scheller J, Mittrücker HW, Rose-John S, Stahl RA, Panzer U, Steinmetz OM: Inflammation-induced IL-6 functions as a natural brake on macrophages and limits GN. J Am Soc Nephrol 26: 1597–1607, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kluger MA, Ostmann A, Luig M, Meyer MC, Goerke B, Paust HJ, Meyer-Schwesinger C, Stahl RA, Panzer U, Tiegs G, Steinmetz OM: B-cell-derived IL-10 does not vitally contribute to the clinical course of glomerulonephritis. Eur J Immunol 44: 683–693, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Kluger MA, Zahner G, Paust HJ, Schaper M, Magnus T, Panzer U, Stahl RA: Leukocyte-derived MMP9 is crucial for the recruitment of proinflammatory macrophages in experimental glomerulonephritis. Kidney Int 83: 865–877, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.