Abstract
Development of CKD secondary to chronic heart failure (CHF), known as cardiorenal syndrome type 2 (CRS2), clinically associates with organ failure and reduced survival. Heart and kidney damage in CRS2 results predominantly from chronic stimulation of G protein–coupled receptors (GPCRs), including adrenergic and endothelin (ET) receptors, after elevated neurohormonal signaling of the sympathetic nervous system and the downstream ET system, respectively. Although we and others have shown that chronic GPCR stimulation and the consequent upregulated interaction between the G–protein βγ–subunit (Gβγ), GPCR-kinase 2, and β-arrestin are central to various cardiovascular diseases, the role of such alterations in kidney diseases remains largely unknown. We investigated the possible salutary effect of renal GPCR–Gβγ inhibition in CKD developed in a clinically relevant murine model of nonischemic hypertrophic CHF, transverse aortic constriction (TAC). By 12 weeks after TAC, mice developed CKD secondary to CHF associated with elevated renal GPCR–Gβγ signaling and ET system expression. Notably, systemic pharmacologic Gβγ inhibition by gallein, which we previously showed alleviates CHF in this model, attenuated these pathologic renal changes. To investigate a direct effect of gallein on the kidney, we used a bilateral ischemia-reperfusion AKI mouse model, in which gallein attenuated renal dysfunction, tissue damage, fibrosis, inflammation, and ET system activation. Furthermore, in vitro studies showed a key role for ET receptor–Gβγ signaling in pathologic fibroblast activation. Overall, our data support a direct role for GPCR-Gβγ in AKI and suggest GPCR-Gβγ inhibition as a novel therapeutic approach for treating CRS2 and AKI.
Keywords: chronic heart failure, chronic kidney disease, acute renal failure, GPCR-g protein signaling, renal fibrosis
Cardiorenal syndrome (CRS) is defined as the pathologic crosstalk between the heart and kidney; it is clinically associated with increased incidence of failure of either or both organs and reduced survival. Worsening renal function coexists with cardiac pathologies in heart failure (HF), where impaired renal function is a strong predictor of mortality in patients with advanced chronic heart failure (CHF) of both ischemic and nonischemic etiologies.1,2 CRS is classified into five types depending on the organ of the initial disease and the type of disease (acute or chronic). CRS2 is characterized as CHF that leads to the development of CKD. CRS2 animal models described in the literature mostly used combined cardiac ischemic injury and renal injury, which generally does not recapitulate clinical settings. Recently, deterioration in renal function and mild focal fibrosis were observed 16 weeks postischemic cardiac injury in a mouse myocardial infarction model.3 Here, we sought to investigate the development of CKD in a nonischemic transverse aortic constriction (TAC) CHF mouse model, possibly identifying a clinically relevant mouse model of CRS2.
Elevated neurohormonal signaling of the sympathetic nervous system (SNS) and its downstream endothelin (ET) system play a driving force in kidney and heart damage in CRS.1,2,4–6 Elevated SNS and ET exert their effects through adrenergic and ET receptors, respectively, which belong to the superfamily of G protein–coupled receptors (GPCRs).1,2,4–6 Chronic agonist stimulation induces GPCR internalization and signal termination, mediated predominantly by the interaction between the G–protein βγ–subunit (Gβγ), the G protein–coupled receptor kinase 2 (GRK2),7–9 and β-arrestin.7–16 This Gβγ-GRK2-β-arrestin signaling pathway is pathologically upregulated in cardiovascular disease,17–21 where we and others have shown that interdicting the Gβγ-GRK2 interaction may be salutary in the onset and progression of HF.10,22–25 However, the role of GPCR-Gβγ signaling in kidney injury remains poorly understood.
Using the murine pressure overload TAC model of HF as well as a direct bilateral ischemia-reperfusion AKI model, we studied the role of GPCR-Gβγ signaling in cardiorenal and renal pathology, respectively. We have recently shown that the novel small molecule Gβγ inhibitor gallein attenuates pathologic Gβγ-GRK2 signaling in the heart and the adrenal gland, thus attenuating HF progression and normalizing SNS overactivity.22 Here, we study the role of GPCR-Gβγ signaling in cardiorenal and renal pathology, thus exploring a possible novel target for CRS2 therapy.
Clinically, renal fibrosis, inflammation, and dysfunction directly worsen HF prognosis and significantly reduce survival.1,2,4–6 Our study identifies the role of GPCR-Gβγ signaling in renal pathologic changes in a murine model of nonischemic CHF as well as in a direct AKI model. Our data suggest GPCR-Gβγ inhibition as a possible novel therapeutic approach to attenuate renal damage and dysfunction in CRS2 and AKI.
Results
CHF Mice Develop CKD 12 Weeks Post-TAC
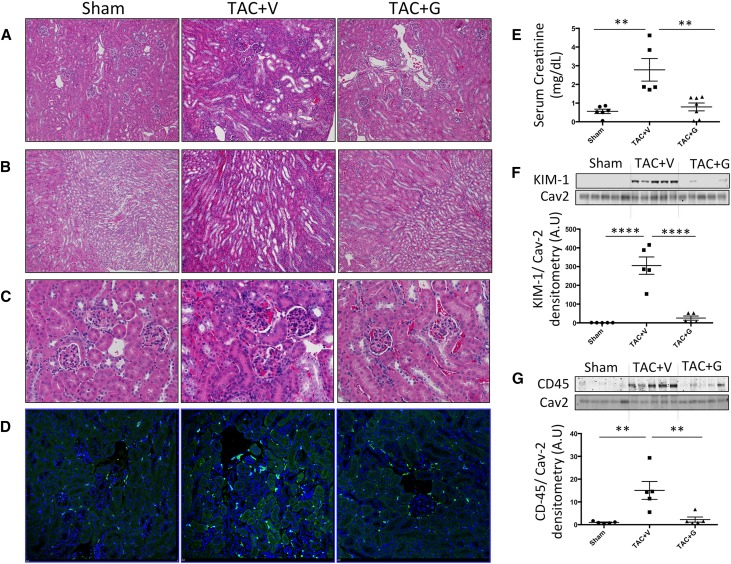
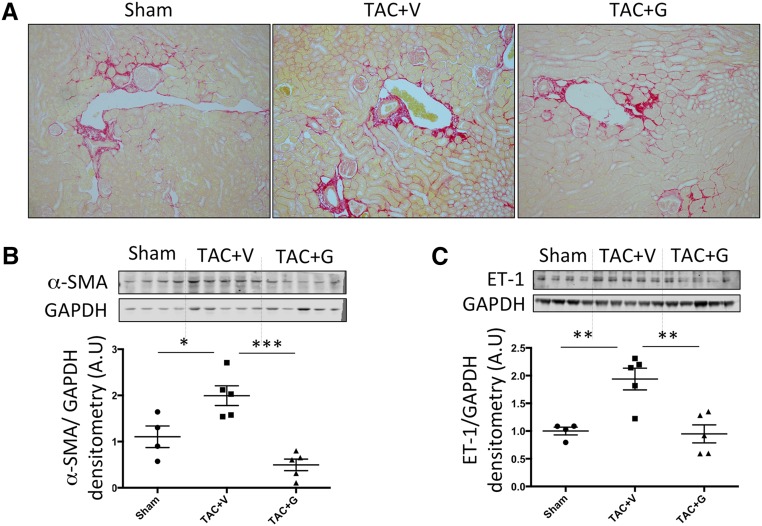
TAC is a nonischemic pressure overload model where mice start developing HF 4–6 weeks post-TAC. At 12 weeks post-TAC, when mice are suffering from end stage nonischemic CHF, we found elevated serum creatinine levels (Figure 1E) and increased kidney injury marker 1 (KIM-1) protein expression (Figure 1F), indicating renal dysfunction and tubular damage, respectively. Histologically, hematoxylin-eosin staining showed focal tubular damage presenting as dilated tubules with simplified epithelia and vacuolized tubules (Figure 1, A and B). Furthermore, there was increased inflammatory cell infiltration in glomerular and tubular regions as shown by hematoxylin-eosin (Figure 1C), CD45 immunofluorescence staining (Figure 1D), and CD45 protein expression (Figure 1G). Picrosirius red staining revealed elevated focal tubulointerstitial and perivascular fibrosis in the kidneys of TAC mice (Figure 2A). Western blotting of whole renal lysates showed elevated protein expression of profibrotic α-smooth muscle actin (α-SMA) (Figure 2B) and ET-1 (Figure 2C), which is indicative of pathologic fibroblast activation and fibrotic signaling.
Figure 1.
CRS2 develops in chronic stages of the TAC CHF mouse model. Kidneys from mice 12 weeks post-TAC show focal tubular damage with tubular epithelial simplification and tubular loss in both (A) cortical and (B) medullary regions (hematoxylin-eosin–stained paraffin sections; ×10). (C) Hematoxylin-eosin–stained sections also show increased cellular infiltration in the glomerular and tubular regions (×40). (D) Immunofluorescence staining (×20) and (G) Western blotting analysis for CD45 expression show an increase in kidney inflammation in 12-weeks TAC CHF mice. These histopathologic findings were accompanied by (E) worsening renal function as indicated by the elevated serum creatinine in these mice and (F) renal tissue damage as indicated by the elevated protein expression of KIM-1. All of these pathologic changes are attenuated by gallein treatment. Vehicle or gallein (10 mg/kg per day intraperitoneally) treatment was initiated 4 weeks post-TAC and continued until 12 weeks. Gallein was gradually titrated up to a maximal dose of 10 mg/kg per day and administered by intraperitoneal injection. G, gallein; V, vehicle. **P<0.01 (n=5–7 per group); ****P<0.001 (n=5–7 per group).
Figure 2.
Gallein attenuates renal fibrosis in 12-weeks TAC CHF mice. (A) Picrosirius red staining (×20) shows elevated renal interstitial and perivascular fibrosis (red color) in mice 12 weeks post-TAC, which is attenuated by gallein treatment. Protein expression of (B) the profibrotic marker α-SMA and (C) the fibrotic mediator ET-1 is elevated in the kidneys of CHF mice and attenuated by gallein treatment. Vehicle or gallein (10 mg/kg per day intraperitoneally) treatment was initiated 4 weeks post-TAC and continued until 12 weeks. Gallein was gradually titrated up to a maximal dose of 10 mg/kg per day and administered by intraperitoneal injection. G, gallein; V, vehicle. *P<0.05 (n=4–7 per group); **P<0.01 (n=4–7 per group); ***P<0.001 (n=4–7 per group).
Small Molecule Gβγ Inhibition Attenuates Renal Dysfunction and Tubulointerstitial Damage in a TAC HF Mouse Model
Our recent study22 showed the cardioprotective effects of the small molecule Gβγ inhibitor gallein administered after the development of HF in a TAC HF mouse model, where it reduced mortality, attenuated cardiac hypertrophy and dysfunction, and attenuated adrenal and plasma catecholamine levels. Here, we found that small molecule Gβγ inhibition by gallein initiated 4 weeks post-TAC (after the development of HF) and continued until week 12 (late stages of CHF) attenuated renal dysfunction, tubulointerstitial remodeling, fibrosis, and inflammatory cell infiltration in CHF mice (Figures 1 and 2).
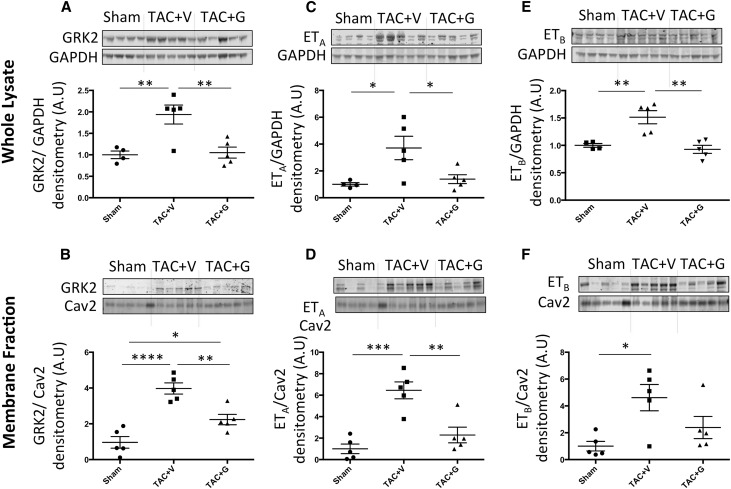
GPCR-Gβγ-GRK2 Signaling Is Elevated in Kidneys of CHF Mice and Attenuated by Gallein
Prior reports from our group and others show that elevated GRK2 protein expression and membrane translocation are key aspects of HF progression.8,9,22–24 GRK2 protein expression and membrane translocation are elevated in kidneys of CHF mice, indicating elevated GPCR-Gβγ-GRK2 signaling. This elevated GRK2 expression was attenuated by gallein treatment (Figure 3, A and B).
Figure 3.
GRK2, ETA, and ETB expression and membrane localization are elevated in the kidneys of TAC mice and attenuated by gallein. Protein expression (A) and membrane translocation (B) of GRK2 are elevated in kidneys of CHF mice and attenuated by gallein treatment. Expression of ET receptors (ETA (C, D) and ETB (E, F)) in the whole renal lysates as well as in the membrane fractions is elevated in CHF mice and attenuated by gallein treatment. Vehicle or gallein (10 mg/kg per day intraperitoneally) treatment was initiated 4 weeks post-TAC and continued until 12 weeks. Gallein was gradually titrated up to a maximal dose of 10 mg/kg per day and administered by intraperitoneal injection. G, gallein; V, vehicle. *P<0.05 (n=4–5 per group); **P<0.01 (n=4–5 per group); ***P<0.001 (n=4–5 per group); ****P<0.001 (n=4–5 per group).
ET System Is Elevated in Kidneys of CHF Mice and Attenuated by Gallein
Recent reports show a key role for ET signaling in the pathogenesis of renal dysfunction.26–30 ET-1 protein expression is elevated in kidneys of TAC mice (Figure 2C) accompanied by elevated protein expression and membrane localization of ETA and ETB receptors. This elevated expression of the ET system was attenuated by systemic gallein treatment (Figure 3, C–F).
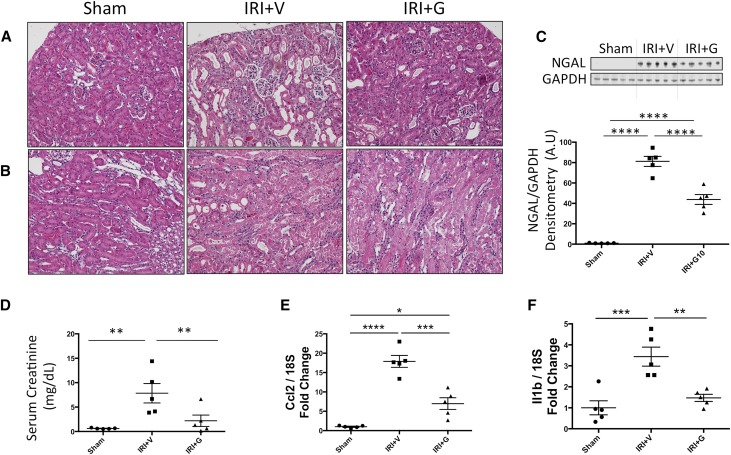
The Small Molecule Gβγ Inhibitor, Gallein, Attenuates Renal Dysfunction and Damage in AKI
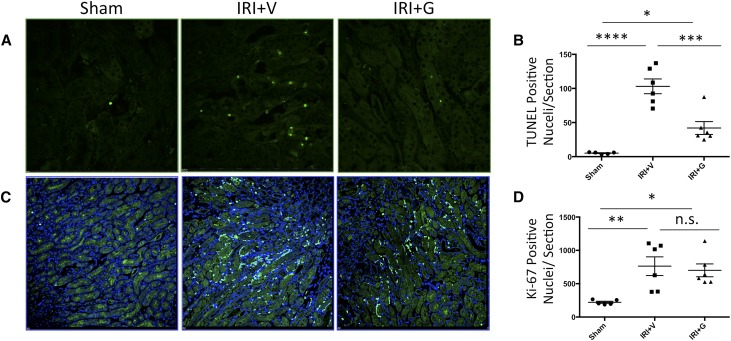
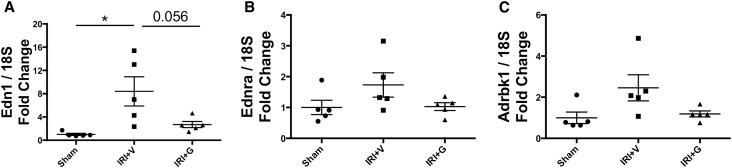
In a bilateral renal ischemia-reperfusion injury (IRI) model of AKI, gallein pretreatment attenuated the elevation in tissue levels of the tubular injury marker NGAL (Figure 4C) and serum creatinine (Figure 4D), indicating attenuated renal tubular damage and dysfunction post-IRI, respectively. Mice pretreated with gallein showed less tubular expansion, tubular epithelial damage, and pathologic cast formation as shown in hematoxylin-eosin–stained kidney sections (Figure 4, A and B). The protective effect of gallein was more pronounced in the renal cortex (Figure 4A). Expression of the inflammatory genes monocyte chemoattractant protein 1 (Ccl2) (Figure 4E) and IL-1β (Il1b) (Figure 4F) was elevated 24 hours post-IRI and attenuated by gallein pretreatment. Furthermore, gallein pretreatment attenuated tubular apoptosis but had no effect on proliferation 24 hours post-IRI as shown by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick–end labeling (TUNEL) staining (Figure 5, A and B) and Ki-67 immunofluorescence (Figure 5, C and D), respectively. Furthermore, gene expression of ET-1 (Edn1) (Figure 6A) and its receptor ETA (Ednra) (Figure 6B) was elevated in AKI and reduced by gallein pretreatment, indicating a role for Gβγ signaling in pathologic ET system elevation in AKI. This was accompanied by elevated gene expression of GRK2 (Adrbk1) (Figure 6C) that was also attenuated by gallein pretreatment, indicating a key role for GPCR-Gβγ-GRK2 signaling in AKI.
Figure 4.
Gβγ inhibition attenuates tubular injury and renal dysfunction 24 hours after AKI. Bilateral renal IRI causes extensive renal tubular damage in AKI mice 24 hours after surgery in both (A) cortical and (B) medullary regions as shown by hematoxylin-eosin staining (×20). (C) Protein expression of the tubular injury marker NGAL is significantly elevated in the kidneys 24 hours post-IRI. (D) Serum creatinine is elevated 24 hours post-IRI, indicating worsened renal function. (E) Monocyte chemoattractant protein 1 (Ccl2) and (F) IL-1β (Il1b) inflammatory gene expressions were significantly elevated in the kidneys of AKI mice. All of these pathologic changes are attenuated by gallein pretreatment. Vehicle or gallein (10 mg/kg per day) was administered 2 days before and on the day of surgery. G, gallein; V, vehicle. *P<0.05 (n=5–7 per group); **P<0.01 (n=5–7 per group); ***P<0.001 (n=5–7 per group); ****P<0.001 (n=5–7 per group).
Figure 5.
GPCR-Gβγ inhibition attenuates renal apoptosis but does not affect proliferation in AKI. Bilateral renal IRI causes a significant elevation in the number of apoptotic nuclei in AKI mice, which is attenuated by gallein pretreatment. (A) TUNEL staining of apoptotic nuclei (green) and (B) quantitative representation of the total number of TUNEL-positive (apoptotic) nuclei per section (×20). In parallel, there is elevation of the proliferation marker Ki-67, which was not significantly changed in the gallein-pretreated group. (C) Immunofluorescence staining (×20) of Ki-67 (green) and nuclei (blue; stained with DAPI). (D) Quantitative representation of the total number of Ki-67–positive (proliferating) nuclei per section. Vehicle or gallein (10 mg/kg per day) was administered 2 days before and on the day of surgery. G, gallein; V, vehicle. *P<0.05 (n=5 per group); **P<0.01 (n=5 per group); ***P<0.001 (n=5 per group); ****P<0.001 (n=5 per group).
Figure 6.
Gallein attenuates elevated gene expression of ET system and GRK2 in AKI. Gene expression of (A) ET-1 (Edn1) and (B) its receptor ETA (Ednra) is elevated in AKI 24 hours after bilateral renal IRI and attenuated by gallein pretreatment. (C) Gene expression of GRK2 (Adrbk1) is elevated in AKI and attenuated by gallein pretreatment. Vehicle or gallein (10 mg/kg per day) was administered 2 days before and on the day of surgery. G, gallein; V, vehicle. *P<0.05 (n=5 per group).
Small Molecule Gβγ Inhibition Attenuates ET Receptor–Mediated Extracellular Signal–Regulated Kinase 1/2 MAPK Activation in Cultured Mouse Embryonic Fibroblasts
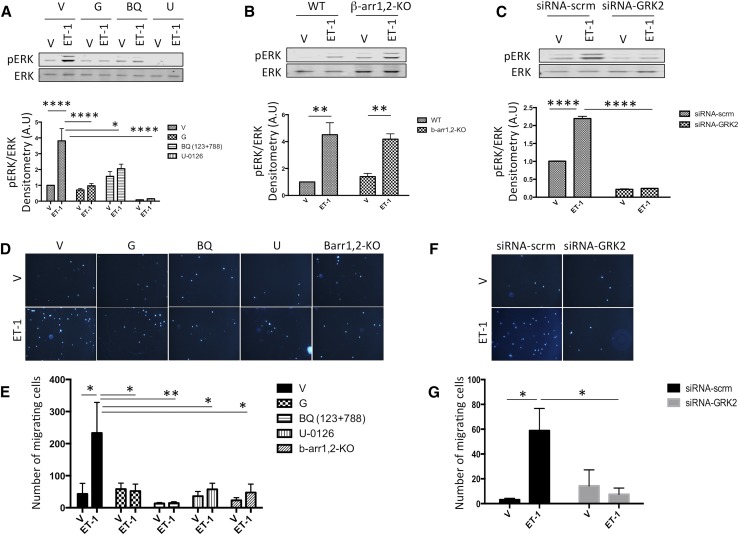
A dose-response study showed that a 100 nM solution of ET-1 induced near–maximum extracellular signal–regulated kinase 1/2 (ERK1/2) phosphorylation in cultured mouse embryonic fibroblasts (MEFs) (Supplemental Figure 1A). Furthermore, a time course study showed that maximum ERK1/2 phosphorylation is attained 15 minutes after ET-1 (100 nM) stimulation (Supplemental Figure 1B). Both gallein pretreatment and knockdown of GRK2 with siRNA attenuated ET-1–mediated ERK1/2 phosphorylation (Figure 7, A and C), indicating a role for Gβγ-GRK2 signaling in ET-1–mediated ERK1/2 phosphorylation in MEF. Interestingly, β-arrestin1,2 knockout had no effect on ET-1–mediated ERK1/2 phosphorylation in MEF (Figure 7B). Finally, combined ETA and ETB antagonism (BQ-123, 10 μM and BQ-788, 10 μM, respectively) and MEK inhibition (U-0126, 10 μM) inhibited ET-1–mediated ERK1/2 phosphorylation in MEF (Figure 7A).
Figure 7.
ET receptor Gβγ signaling mediates in vitro fibroblast activation. (A–C) ET-1–mediated ERK1/2 phosphorylation experiment. Fifteen minutes post–ET-1 stimulation, maximal ERK1/2 phosphorylation is observed, and this effect is inhibited by (A) the small molecule Gβγ inhibitor (gallein; 10 μM) and (B) β-arrestin1,2 knockout (β-arr1,2-KO). (C) GRK2 knockdown by siRNA transfection (siRNA-GRK2) also inhibited ET-1–mediated ERK1/2 phosphorylation relative to the scrambled siRNA transfected group (siRNA-scrm). (D–G) Using the transwell migration assay, MEFs migration was studied 4 hours after incubation with ET-1 (100 nM) in the lower chamber. ET-1 promoted MEF migration, which was inhibited by the small molecule Gβγ inhibitor (gallein, 10 μM), (D and E) β-arrestin1,2 knockout (Barr1,2-KO), and (F and G) siRNA-GRK2. D and F are representative images (×10) of DAPI-stained nuclei (blue) of the MEFs that migrated through the membrane, and E and G are bar graphs showing quantitative analyses of the number of MEFs that migrated through the membrane. ET receptor combined antagonism (ETA antagonist BQ-123 and ETB antagonist BQ-788, 10 μM each) and MAPK inhibition (MEK inhibitor U-0126, 10 μM) blocked the effect of ET-1 on ERK1/2 phosphorylation and MEFs migration. Vehicle or drug treatment was performed before cells were seeded in the upper chamber; n=5 for the ERK1/2 phosphorylation experiment, and n=4 for the transwell migration assay. G, gallein; V, vehicle; WT, wild type. *P<0.05; **P<0.01; ****P<0.001.
In Vitro, ET Receptor Gβγ Signaling Mediates MEF Migration
In a transwell migration assay, ET-1 (100 nM) stimulated cell migration. Gallein (10 μM) pretreatment, GRK2 siRNA knockdown, and β-arrestin1,2 knockout all attenuated cell migration. This indicates a direct role for the Gβγ-GRK2-β-arrestin pathway in ET-1–mediated MEF migration. Combined ETA and ETB antagonism (BQ-123 and BQ-788, 10 μM) and MEK inhibition (U-0126, 10 μM) also attenuated MEF migration (Figure 7, D–G).
Discussion
A CRS2 Model: Nonischemic TAC CHF Mice Develop CKD
CRS2 is a pathologic disorder where chronic dysfunction in the heart induces CKD, which worsens prognosis and reduces survival.1,2,4–6 This study shows that TAC CHF mice chronically develop CKD, suggesting a CRS2 model. To date, the majority of studies investigating cardiorenal pathophysiology use combined ischemic cardiac injury and renal injury, which do not simulate clinical disease progression. Recently, deterioration in renal function and mild focal fibrosis were observed 16 weeks postinjury in a mouse myocardial infarction model.3 Here, we show the development of CKD with deteriorating renal function, tubulointerstitial remodeling, and inflammation in the chronic phase of a nonischemic CHF mouse model (TAC) without surgical induction of kidney injury. Of note, no AKI was detected 24 hours after TAC surgery, and mild renal histopathologic changes and inflammation are observed as early as 7 days post-TAC (Supplemental Figures 2–4).
GPCR-Gβγ Signaling Plays a Key Role in Kidney Injury
CRS is considered a complex molecular and systemic interplay of neurohumoral activation that induces cardiac hypertrophy and both myocardial and renal fibrosis, leading to cardiac and renal dysfunction. Neurohormonal activation, particularly SNS overactivity, plays a key role in the pathologic crosstalk between the heart and the kidney in CRS. Importantly, SNS modulation by renal denervation was recently investigated as a novel therapeutic approach for HF.31 The SNS signals through adrenergic receptors that belong to the GPCR superfamily. Agonist stimulation of the GPCR induces signaling largely through the Gα followed by receptor internalization and signal termination. This internalization occurs because of the recruitment and binding of GRK2 by the free Gβγ to phosphorylate the agonist-occupied receptor.7–16 The phosphorylated GPCR triggers the recruitment of β-arrestins that scaffold the receptor complex and initiate its internalization. Chronic agonist stimulation of the GPCR triggers pathologic GRK2 upregulation and Gβγ-GRK2 overactivity, leading to persistent GPCR phosphorylation and uncoupling of the receptor from its G proteins and resulting in GPCR pathologic signaling. This has been reported in various pathologic conditions, including cardiovascular disease.9–16 However, the role of GPCR-Gβγ signaling in kidney disease remains poorly understood. We and others have shown that interdicting GPCR-Gβγ-GRK2 signaling provides protection in both the onset and the progression of HF.10,22–25 Our recent work22 showed that the small molecule Gβγ inhibitor, gallein, attenuates HF progression by simultaneously inhibiting the pathologically upregulated GPCR-Gβγ signaling in the heart and the adrenal glands. Mechanistically, gallein restores physiologic GPCR signaling in the heart and the adrenal gland and attenuates SNS overactivity by normalizing adrenal secretion of catecholamines into the circulation.
In this study, we report elevated GRK2 protein expression in kidneys of CHF mice, indicating elevated GPCR-Gβγ-GRK2 signaling in these kidneys. Remarkably, systemic small molecule Gβγ inhibition attenuated renal pathologic changes together with the elevated GRK2 expression, suggesting a role for GPCR-Gβγ signaling in kidney injury in CRS2. Importantly, gallein has no direct effect on systemic BP (data not shown); thus, a direct hemodynamic effect of gallein on hypertension in CHF is excluded. However, because gallein is administered systemically, we cannot exclude its effects on normalized SNS activity, improved cardiac function and structure, and hypertension that may play a role in attenuated kidney injury. Thus, we confirmed a direct effect of gallein on kidney injury in an AKI mouse model. We here report, for the first time, elevated GRK2 gene expression in AKI in parallel with tubular damage and elevated inflammatory gene expression. Gβγ inhibition by gallein attenuated the observed inflammation, tubular damage, and subsequent deterioration of renal function in AKI mice.
Renal ET System and Fibrosis in Kidney Injury
Elevated neurohormonal signaling of the SNS and its downstream ET system through adrenergic and ET receptors, respectively, plays a driving force in kidney and heart damage in CRS.8,27,32–35 Both adrenergic and ET receptors belong to the GPCR superfamily and are widely expressed in both the heart and the kidney.8 Numerous preclinical studies have implicated ET-1 in the pathogenesis of CKD27,28 as well as in renal tubulointerstitial damage and fibrosis.29,32,36 Renal fibrosis directly impedes renal function37 and worsens HF in CRS2.35 GRK2-β-arrestin signaling, downstream of the GPCR, plays a key role in cell growth, motility, and fibrosis and activates downstream pathways, such as MAPK, that are involved in pathologic cell proliferation and fibrosis.17–21 However, the role of Gβγ signaling in these processes has not been investigated. We suggest that pathologically elevated renal GPCR-Gβγ-GRK2 signaling, as indicated by the elevated renal GRK2 protein expression and membrane translocation, promotes renal inflammation and fibrosis, thus contributing to HF progression in CRS2. Mechanistically, simultaneous attenuation of SNS overactivity and renal GPCR-Gβγ pathologic signaling by gallein both seem to play a major role in renal protection.
Renal ET-1 production is almost universally increased in kidney disease,29,36 and it is activated downstream of the SNS.32 Evidence exists for renal ET-1 acting as an independent system that plays a major role in renal tissue damage and malfunction, where ETA signaling is believed to be the major mediator of pathologic ET effects and plays a major role in the progression to CKD.26,29,30,36,38 Remarkably, our data show elevated renal ET system in the kidneys of CHF mice; this implies that the ET system plays an important role in kidney injury secondary to CHF. Furthermore, the elevated ET system gene expression as previously reported in AKI30,39 was attenuated by gallein, indicating a direct role for Gβγ signaling in kidney injury in AKI. Our in vitro studies in cultured MEFs showed that ET receptor Gβγ-GRK2 signaling plays a key role in ET-1–mediated fibroblast activation. Altogether, this indicates that GPCR-Gβγ signaling plays a major role in kidney injury in CRS2 and AKI.
Gβγ Plays a Role in ET Receptor–Mediated MAPK Activation and Cell Migration
MAPK signaling in general and ERK1/2 MAPK signaling in particular play a key role in ET-1–induced cell growth and motility,17,18,40 processes that are essential for fibroblast activation and fibrosis.19 ET-1 signaling through ET receptors plays a major role in fibrosis, where ETA-β-arrestin signaling mediates MAPK signaling that promotes proliferation and migration in several cell types.17,18,40 ETB has been thought to be protective in kidney injury27; however, a recent study showed that peptide targeting of the intracellular loop of ETB receptor attenuated its downstream ERK activation in vascular smooth muscle cells.41 Using in vitro cultured MEFs, we showed a direct and upstream role of Gβγ in ET-1–mediated fibroblast ERK1/2 MAPK activation and cell migration. siRNA GRK2 knockdown, MEK inhibition, and ET receptor antagonism also blocked ET-1–mediated ERK1/2 MAPK activation and cell migration. β-Arrestin1,2 knockout in MEFs attenuated cell migration but had no effect on MAPK ERK1/2 activation, which can be explained by the reported differential regulation of GPCR signaling by β-arrestins.17,42 These data show a direct and upstream effect of Gβγ signaling on ET receptor–mediated MAPK ERK1/2 activation and cell migration. Altogether, this indicates that gallein plays a key role in attenuating fibrosis by directly attenuating fibroblast migration and MAPK ERK1/2 activation.
In summary, TAC, in its chronic phase, represents a CRS2 model, where CHF leads to CKD. SNS overactivity plays a major role in the pathologic cardiorenal crosstalk in CHF. This study provides a novel therapeutic approach that simultaneously targets cardiac and renal pathologic GPCR-Gβγ signaling. Current therapies for patients with HF and coexisting renal morbidities target the elevated blood volume and pressure. However, targeting the underlying pathologies, including pathologic GPCR-Gβγ signaling in both the heart and the kidney, represents a promising therapeutic approach for CRS. Furthermore, our study shows that GPCR-Gβγ signaling plays a direct role in AKI and suggests a novel possible therapeutic approach for AKI.
Concise Methods
Animal Experiments
CHF Experiment
Ten-week-old C57/Bl6 wild–type male mice (The Jackson Laboratory, Bar Harbor, ME) were subjected to TAC surgeries and divided into two groups: TAC and vehicle (n=11) and TAC and gallein (n=10). Sham-operated animals (n=6) served as controls. All animal procedures were performed in accordance with the guidelines of the Department of Laboratory Animal Medicine and the University Committee on Animal Resources at Cincinnati Children’s Hospital Medical Center.
Surgery.
TAC was performed as previously described.22,44,45 Briefly, animals were anesthetized with isoflurane, intubated, and ventilated. After thoracotomy, TAC was created by securely placing a ligature around the transverse aorta and a 27-gauge needle, causing complete occlusion of aorta. The needle was then removed, leaving a stenotic aorta.
Treatment.
Mice received a once daily intraperitoneal injection of either vehicle or gallein (10 mg/kg per day). Daily treatment was initiated 4 weeks post-TAC in a dose escalation manner and continued until euthanasia.22
AKI Experiment
To induce AKI, we used a bilateral renal IRI mouse model46; 10-week old C57/Bl6 wild–type male mice (The Jackson Laboratory) were subjected to bilateral IRI surgeries and divided into two groups: IRI and vehicle (n=7) and IRI and gallein (n=7). Sham-operated animals (n=7) served as controls. All animal procedures were performed in accordance with the guidelines of the Department of Laboratory Animal Medicine and the University Committee on Animal Resources at Cincinnati Children’s Hospital Medical Center.
Surgery.
Mice were anesthetized, and an atraumatic (ROBOZ) clip was applied onto the renal artery and vein to induce renal ischemia of both the right and left kidneys for 30 minutes. Successful ischemia was visually confirmed by a gradual uniform darkening of the kidney. Mouse body temperature was maintained at 37°C for the duration of ischemia. Then, the clips were removed, and mice were allowed to recover.
Treatment.
Mice were pretreated with three doses (intraperitoneal injection) of gallein (10 mg/kg per day) or vehicle 2 days before and on the day of surgery. Bilateral 30-minute ischemia followed by reperfusion was performed, mice were euthanized 24 hours post-IRI, and blood and kidneys were collected.
Renal Morphology and Fibrosis
Kidneys were isolated and fixed in 10% formalin solution for 24 hours, and then, they were processed in paraffin blocks. Paraffin sections were used for histologic analyses. Hematoxylin-eosin staining was performed on renal sections to study renal morphology. To assess fibrosis, picrosirius red staining was performed on renal sections, where fibrotic tissue is stained red.
Serum Creatinine
Collected blood was allowed to coagulate and then, centrifuged, and serum was isolated and stored at −80°C until use. Serum creatinine was estimated using the Creatinine Assay Kit from Abcam, Inc. (Cambridge, MA) following the manufacturer’s protocol.
Protein Analyses
Protein Isolation and Fractionation
Isolated kidneys were homogenized in a hypotonic lysis buffer and fractionated into membrane and cytosolic fractions by differential centrifugation as previously described.47
Western Blotting
Western blotting for GRK2, ET-1, ETA, ETB, α-SMA, and NGAL using the total unfractionated lysate was performed with GAPDH as internal standard to determine levels of protein expression. Western blotting for GRK2, ETA, ETB (normalized to caveolin-2 [Cav2]) was performed using membrane fractions to determine their membrane expression. Western blotting for KIM-1 and CD45 (normalized to Cav2) was performed to quantify their protein expression; membrane fractions were used, because both KIM-1 and CD45 are membrane proteins. GRK2 antibody is rabbit IgG from Santa Cruz Biotechnology (Santa Cruz, CA). ETA antibody is sheep IgG from Abcam, Inc., and ET-1, ETB, α-SMA, NGAL, and KIM-1 antibodies are rabbit IgGs from Abcam, Inc. Cav2 antibody is rabbit IgGs from Cell Signaling Technology (Danvers, MA). Additionally, GAPDH antibody is mouse IgG from EMD Millipore, and CD45 antibody is goat IgG from R&D Systems (Minneapolis, MN). Visualization and quantification of target bands were carried out with the LI-COR Odyssey Scanner and software (LI-COR Biosciences, Lincoln, NE) using IRDye secondary antibodies.
Apoptosis
The effect of gallein on the rate of renal apoptosis was determined by TUNEL staining of renal sections using the TUNEL Apoptosis Detection Kit (EMD Millipore) per the manufacturer’s instructions, and apoptotic nuclei were visualized using confocal microscopy.
Immunofluorescence Staining
After euthanasia, kidneys were promptly isolated, fixed in 10% formalin, embedded in paraffin, and sliced into 5-μm sections. After deparaffinization, rehydration, and antigen retrieval, sections were permeabilized, blocked, and incubated with Ki-67 (rabbit IgG; Abcam, Inc.) or CD45 primary antibodies (goat; R&D Systems) followed by Alexa Fluor 488–conjugated secondary antibody (Invitrogen, Carlsbad, CA). Nuclei were stained with DAPI.
Quantitative Real–Time PCR Gene Expression
Gene expression of the inflammatory genes IL-1β and CCL2 as well as GRK2, ET-1, and ETA was quantified by real–time PCR analysis and normalized to the 18S ribosomal subunit as an internal standard, which we have previously described.48–51 mRNA was isolated from heart tissue using the RNeasy Mini Kit (Qiagen, Germantown, MD) per the manufacturer’s instructions.
In Vitro MEFs Experiments
MEFs Culture and ET-1–Mediated ERK1/2 Phosphorylation Experiment
MEFs were cultured in high-glucose DMEM with 10% heat-inactivated FBS, 1% nonessential amino acids, and 1% antibiotic/antimycotic. When cells reached 50% confluency, they were starved in 0.5% FBS in the culture media described above for 48 hours. To assess the role of Gβγ on ET-1–mediated ERK1/2 phosphorylation, cells were pretreated with gallein (10 μM), combined ETA and ETB antagonists (BQ-123 and BQ-788, respectively; 10 μM each), or the MEK inhibitor (U-0126; 10 μM) for 2 hours and then, stimulated with ET-1 (100 nM) for 15 minutes. To assess the role of GRK2 in ET-1–mediated ERK1/2 phosphorylation, GRK2 was knocked down using specific validated siRNA with a control experiment using scrambled siRNA as described below. Finally, to assess the role of β-arrestin1,2 in ET-1–mediated ERK1/2 phosphorylation, β-arrestin1,2 knockout MEFs (isolated from β-arrestin1,2 knockout mice) were used. Cells were washed with ice-cold PBS and harvested in mammalian protein extraction buffer (Fisher Scientific, Waltham, MA) containing a protease/phosphatase inhibitor cocktail. Western blotting for ERK1/2 (p44/42 MAPK) and its phosphorylated form (Thr202/Tyr204) was performed on whole-cell lysate. ERK1/2 antibody is mouse IgG from Cell Signaling Technology, and phosphorylated ERK1/2 antibodies are rabbit IgGs from Cell Signaling Technology.
We performed a time course study and a dose-response study to establish the experimental conditions (concentration and time) for ET-1 stimulation (Supplemental Figure 1).
MEFs siRNA Experiment
To knockdown GRK2, we used a validated GRK2-siRNA (AM51334-id147) from Life Technologies (Carlsbad, CA). Cells were transfected using Lipofectamine RNAiMAX (Life Technologies) following the manufacturer’s reverse transfection protocol and using 20 nM siRNA. Control scrambled siRNA (Life Technologies) was used as a control. A time course study confirming GRK2 knockdown (>80%) until 72 hours was performed (Supplemental Figure 5).
MEFs Transwell Migration Assay
MEFs were cultured to subconfluency, serum starved for 24 hours, trypsinized, and counted, and 104 cells in 100 μl serum-free DMEM were seeded onto the upper surfaces of transwell inserts (8-μm pores; Fisher Scientific). Transwells were placed into wells on a 24-well plate containing vehicle or ET-1 (100 nM; Sigma-Aldrich, St. Louis, MO) in 600 μl medium in the lower chambers. MEFs were left at 37°C in 5% CO2 in humidified air for 4 hours, fixed, and permeabilized in 100% methanol, and nuclei were stained with DAPI. MEFs on the upper surface of the membrane were removed with a cotton swab, and membranes were removed and fixed to glass slides; the numbers of migrated cells on the lower surface of the membrane were counted (×10 objective). To assess the role of ET receptor Gβγ or ERK1/2 in ET-1–stimulated cell migration, MEFs were incubated with the Gβγ inhibitor (gallein, 10 μM), combined ETA and ETB antagonists (BQ-123 and BQ-788, respectively; 10 μM), or the MEK inhibitor (U-0126, 10 μM) for 30 minutes before agonist addition and throughout the assay. To assess the role of GRK2 in ET-1–mediated cell migration, GRK2 was knocked down using specific validated siRNA with a control experiment using scrambled siRNA as described above. Finally, to assess the role of β-arrestin1,2 in ET-1–mediated cell migration, β-arrestin1,2 knockout MEFs (isolated from β-arrestin1,2 knockout mice) were used.
Statistical Analyses
Multiple responses of various physiologic and biochemical assays were analyzed using one-way or repeated measures ANOVA. Post hoc analysis (Bonferroni correction) was performed if statistical significance (P≤0.05) was achieved. All calculations were performed using the GraphPad Prism 6.0 program (GraphPad Software, La Jolla, CA).
Disclosures
None.
Supplementary Material
Acknowledgments
This work was funded by an American Heart Association Postdoctoral Fellowship (to F.A.K.), a Pharmaceutical Research and Manufacturer's of America Foundation Predoctoral Fellowship (to J.G.T.), and National Institute of Health grants P50 DK096418-01 (to P.D.), 1R01 HL091475, 1R01 HL132551, P01HL069779, and 1R01 HL089885 (to B.C.B.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015080852/-/DCSupplemental.
References
- 1.Smilde TD, Hillege HL, Navis G, Boomsma F, de Zeeuw D, van Veldhuisen DJ: Impaired renal function in patients with ischemic and nonischemic chronic heart failure: Association with neurohormonal activation and survival. Am Heart J 148: 165–172, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ: Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102: 203–210, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Lekawanvijit S, Kompa AR, Zhang Y, Wang BH, Kelly DJ, Krum H: Myocardial infarction impairs renal function, induces renal interstitial fibrosis, and increases renal KIM-1 expression: Implications for cardiorenal syndrome. Am J Physiol Heart Circ Physiol 302: H1884–H1893, 2012 [DOI] [PubMed] [Google Scholar]
- 4.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW: Renal insufficiency and heart failure: Prognostic and therapeutic implications from a prospective cohort study. Circulation 109: 1004–1009, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA: Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 351: 1285–1295, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL: Worsening renal function and prognosis in heart failure: Systematic review and meta-analysis. J Card Fail 13: 599–608, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Gärtner F, Seidel T, Schulz U, Gummert J, Milting H: Desensitization and internalization of endothelin receptor A: Impact of G protein-coupled receptor kinase 2 (GRK2)-mediated phosphorylation. J Biol Chem 288: 32138–32148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamal FA, Travers JG, Blaxall BC: G protein-coupled receptor kinases in cardiovascular disease: Why “where” matters. Trends Cardiovasc Med 22: 213–219, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Hata JA, Koch WJ: Phosphorylation of G protein-coupled receptors: GPCR kinases in heart disease. Mol Interv 3: 264–272, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Akhter SA, Eckhart AD, Rockman HA, Shotwell K, Lefkowitz RJ, Koch WJ: In vivo inhibition of elevated myocardial beta-adrenergic receptor kinase activity in hybrid transgenic mice restores normal beta-adrenergic signaling and function. Circulation 100: 648–653, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA: G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci U S A 99: 3872–3877, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kansra V, Hussain T, Lokhandwala MF: Alterations in dopamine DA1 receptor and G proteins in renal proximal tubules of old rats. Am J Physiol 273: F53–F59, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Sanada H, Jose PA, Hazen-Martin D, Yu PY, Xu J, Bruns DE, Phipps J, Carey RM, Felder RA: Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension 33: 1036–1042, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Sidhu A, Vachvanichsanong P, Jose PA, Felder RA: Persistent defective coupling of dopamine-1 receptors to G proteins after solubilization from kidney proximal tubules of hypertensive rats. J Clin Invest 89: 789–793, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uh M, White BH, Sidhu A: Alteration of association of agonist-activated renal D1(A) dopamine receptors with G proteins in proximal tubules of the spontaneously hypertensive rat. J Hypertens 16: 1307–1313, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Ungerer M, Böhm M, Elce JS, Erdmann E, Lohse MJ: Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation 87: 454–463, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Morris GE, Nelson CP, Brighton PJ, Standen NB, Challiss RA, Willets JM: Arrestins 2 and 3 differentially regulate ETA and P2Y2 receptor-mediated cell signaling and migration in arterial smooth muscle. Am J Physiol Cell Physiol 302: C723–C734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris GE, Nelson CP, Standen NB, Challiss RA, Willets JM: Endothelin signalling in arterial smooth muscle is tightly regulated by G protein-coupled receptor kinase 2. Cardiovasc Res 85: 424–433, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao X, Wu G, Gu X, Fu L, Mei C: Kruppel-like factor 15 modulates renal interstitial fibrosis by ERK/MAPK and JNK/MAPK pathways regulation. Kidney Blood Press Res 37: 631–640, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Sun S, Ning X, Zhai Y, Du R, Lu Y, He L, Li R, Wu W, Sun W, Wang H: Egr-1 mediates chronic hypoxia-induced renal interstitial fibrosis via the PKC/ERK pathway. Am J Nephrol 39: 436–448, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Lovgren AK, Kovacs JJ, Xie T, Potts EN, Li Y, Foster WM, Liang J, Meltzer EB, Jiang D, Lefkowitz RJ, Noble PW: β-arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Sci Transl Med 3: 74ra23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamal FA, Mickelsen DM, Wegman KM, Travers JG, Moalem J, Hammes SR, Smrcka AV, Blaxall BC: Simultaneous adrenal and cardiac g-protein-coupled receptor-gβγ inhibition halts heart failure progression. J Am Coll Cardiol 63: 2549–2557, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinks H, Boucher M, Gao E, Chuprun JK, Pesant S, Raake PW, Huang ZM, Wang X, Qiu G, Gumpert A, Harris DM, Eckhart AD, Most P, Koch WJ: Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circ Res 107: 1140–1149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan Q, Chen M, Zuo L, Shang X, Huang MZ, Ciccarelli M, Raake P, Brinks H, Chuprun KJ, Dorn GW 2nd, Koch WJ, Gao E: Myocardial ablation of G protein-coupled receptor kinase 2 (GRK2) decreases ischemia/reperfusion injury through an anti-intrinsic apoptotic pathway. PLoS One 8: e66234, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piao L, Fang YH, Parikh KS, Ryan JJ, D’Souza KM, Theccanat T, Toth PT, Pogoriler J, Paul J, Blaxall BC, Akhter SA, Archer SL: GRK2-mediated inhibition of adrenergic and dopaminergic signaling in right ventricular hypertrophy: Therapeutic implications in pulmonary hypertension. Circulation 126: 2859–2869, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andress DL, Coll B, Pritchett Y, Brennan J, Molitch M, Kohan DE: Clinical efficacy of the selective endothelin A receptor antagonist, atrasentan, in patients with diabetes and chronic kidney disease (CKD). Life Sci 91: 739–742, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Dhaun N, Goddard J, Webb DJ: The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol 17: 943–955, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Dhaun N, Webb DJ: The road from AKI to CKD: The role of endothelin. Kidney Int 84: 637–638, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Kohan DE: Endothelins in the normal and diseased kidney. Am J Kidney Dis 29: 2–26, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Zager RA, Johnson AC, Andress D, Becker K: Progressive endothelin-1 gene activation initiates chronic/end-stage renal disease following experimental ischemic/reperfusion injury. Kidney Int 84: 703–712, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Q, Huang H, Wang X, Wang X, Dai Z, Wan P, Guo Z, Yu S, Tang Y, Huang C: Changes of serum neurohormone after renal sympathetic denervation in dogs with pacing-induced heart failure. Int J Clin Exp Med 7: 4024–4030, 2014 [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed MS, Wong CF, Pai P: Cardiorenal syndrome - a new classification and current evidence on its management. Clin Nephrol 74: 245–257, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Jönsson S, Agic MB, Narfström F, Melville JM, Hultström M: Renal neurohormonal regulation in heart failure decompensation. Am J Physiol Regul Integr Comp Physiol 307: R493–R497, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K: Cardiorenal syndrome: Pathophysiology and potential targets for clinical management. Nat Rev Nephrol 9: 99–111, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Cruz DN, Schmidt-Ott KM, Vescovo G, House AA, Kellum JA, Ronco C, McCullough PA: Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: Workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 182: 117–136, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Kohan DE, Pollock DM: Endothelin antagonists for diabetic and non-diabetic chronic kidney disease. Br J Clin Pharmacol 76: 573–579, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eddy AA: Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl (2011) 4: 2–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ, Makino H, Perkovic V, Pritchett Y, Remuzzi G, Tobe SW, Toto R, Viberti G, Parving HH: The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 25: 1083–1093, 2014 [DOI] [PMC free article] [PubMed]
- 39.Forbes JM, Jandeleit-Dahm K, Allen TJ, Hewitson TD, Becker GJ, Jones CL: Endothelin and endothelin A/B receptors are increased after ischaemic acute renal failure. Exp Nephrol 9: 309–316, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Rosanò L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A: Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci U S A 106: 2806–2811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green DS, Rupasinghe C, Warburton R, Wilson JL, Sallum CO, Taylor L, Yatawara A, Mierke D, Polgar P, Hill N: A cell permeable peptide targeting the intracellular loop 2 of endothelin B receptor reduces pulmonary hypertension in a hypoxic rat model. PLoS One 8: e81309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier KE: Arrestins as signaling modulators: The plot thickens: focus on “Arrestins 2 and 3 differentially regulate ETA and P2Y2 receptor-mediated cell signaling and migration in arterial smooth muscle.” Am J Physiol Cell Physiol 302: C721–C722, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Ding B, Abe J, Wei H, Xu H, Che W, Aizawa T, Liu W, Molina CA, Sadoshima J, Blaxall BC, Berk BC, Yan C: A positive feedback loop of phosphodiesterase 3 (PDE3) and inducible cAMP early repressor (ICER) leads to cardiomyocyte apoptosis. Proc Natl Acad Sci U S A 102: 14771–14776, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tachibana H, Naga Prasad SV, Lefkowitz RJ, Koch WJ, Rockman HA: Level of beta-adrenergic receptor kinase 1 inhibition determines degree of cardiac dysfunction after chronic pressure overload-induced heart failure. Circulation 111: 591–597, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Yan C, Ding B, Shishido T, Woo CH, Itoh S, Jeon KI, Liu W, Xu H, McClain C, Molina CA, Blaxall BC, Abe J: Activation of extracellular signal-regulated kinase 5 reduces cardiac apoptosis and dysfunction via inhibition of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop. Circ Res 100: 510–519, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, Vorobiof G, Dunaevsky O, Matavel A, Lopes CM, Smrcka AV, Blaxall BC: Small molecule disruption of G beta gamma signaling inhibits the progression of heart failure. Circ Res 107: 532–539, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blaxall BC, Spang R, Rockman HA, Koch WJ: Differential myocardial gene expression in the development and rescue of murine heart failure. Physiol Genomics 15: 105–114, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Bullard TA, Protack TL, Aguilar F, Bagwe S, Massey HT, Blaxall BC: Identification of Nogo as a novel indicator of heart failure. Physiol Genomics 32: 182–189, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Blaxall BC, Tschannen-Moran BM, Milano CA, Koch WJ: Differential gene expression and genomic patient stratification following left ventricular assist device support. J Am Coll Cardiol 41: 1096–1106, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Pawlinski R, Tencati M, Hampton CR, Shishido T, Bullard TA, Casey LM, Andrade-Gordon P, Kotzsch M, Spring D, Luther T, Abe J, Pohlman TH, Verrier ED, Blaxall BC, Mackman N: Protease-activated receptor-1 contributes to cardiac remodeling and hypertrophy. Circulation 116: 2298–2306, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.