Abstract
Klotho is a type-1 membrane protein predominantly produced in the kidney, the extracellular domain of which is secreted into the systemic circulation. Membranous and secreted Klotho protect organs, including the kidney, but whether and how Klotho directly protects the glomerular filter is unknown. Here, we report that secreted Klotho suppressed transient receptor potential channel 6 (TRPC6)-mediated Ca2+ influx in cultured mouse podocytes by inhibiting phosphoinositide 3-kinase-dependent exocytosis of the channel. Furthermore, soluble Klotho reduced ATP-stimulated actin cytoskeletal remodeling and transepithelial albumin leakage in these cells. Overexpression of TRPC6 by gene delivery in mice induced albuminuria, and exogenous administration of Klotho ameliorated the albuminuria. Notably, immunofluorescence and in situ hybridization revealed Klotho expression in podocytes of mouse and human kidney. Heterozygous Klotho-deficient CKD mice had aggravated albuminuria compared with that in wild-type CKD mice with a similar degree of hypertension and reduced clearance function. Finally, disrupting the integrity of glomerular filter by saline infusion-mediated extracellular fluid volume expansion increased urinary Klotho excretion. These results reveal a potential novel function of Klotho in protecting the glomerular filter, and may offer a new therapeutic strategy for treatment of proteinuria.
Keywords: Klotho, podocyte, TRPC6
Klotho is a type-1 membrane protein produced mainly in the kidney.1 The large extracellular domain of klotho can be cleaved and released into the systemic circulation, urine and cerebrospinal fluid.2 Membranous klotho associates with fibroblast growth factor (FGF) receptors to form coreceptors for the ligand FGF23,3,4 and plays an important role in regulating serum phosphate levels and the total body phosphate store. Klotho-deficient mice die prematurely in large part from hypervitaminosis D and phosphate retention; growth retardation and premature death can be rescued by dietary phosphate and/or vitamin D restriction.5–7
Membranous klotho and secreted soluble klotho have many additional functions such as protection of organs, including the kidney and heart.8–12 Overexpression of the klotho gene reduces renal injury in a mouse model of spontaneous GN (mutation in the Tensin2 gene).8 Also, in vivo klotho gene transfer ameliorates angiotensin II- or hypertension-induced renal damages.11,12 The abundance of klotho in the kidney is decreased in mice and patients with CKD,2,13,14 and levels of circulating soluble klotho are also reduced in CKD.10,13 It has been suggested that klotho deficiency may be a positive feed-forward loop that contributes to CKD progression.13,14 Recent studies have also shown that soluble klotho is cardioprotective and decreases in the levels of soluble klotho may be an important cause of cardiomyopathy in patients with CKD.9,12
The podocyte is a highly specialized cell type in the kidney glomerulus whose dysfunction leads to impairment of glomerular slit diaphragm function and proteinuria.15 Proper organization of the actin cytoskeleton in podocytes is critical for function, and remodeling of the actin cytoskeleton is a key pathologic process for glomerular diseases.15,16 Studies in the past decade have provided important insights on how dysregulation of intracellular Ca2+ ([Ca2+]i) might link derangement of the actin cytoskeleton to podocyte dysfunction and proteinuria.15 The class C transient receptor potential (TRPC) channel family consists of seven members of highly conserved, Ca2+-permeable, nonselective cationic channels. Among them, TRPC5 and TRPC6 are expressed in podocytes and play important roles in regulating Ca2+ homeostasis and actin dynamics in these cells.15,17–19 Human genetics studies have demonstrated that TRPC6 gain-of-function mutations cause hereditary FSGS.20,21 Increased expression and/or activity of TRPC6 have also been implicated in secondary glomerular proteinuric diseases.17,22,23 Animal studies have shown that overactivity of TRPC5 or TRPC6 channels causes actin cytoskeletal rearrangement and proteinuria, and silencing of either channel confers protection.17,18,22 Whether klotho acts on podocytes to protect against glomerular injury and the potential mechanisms are unknown. Having found that klotho downregulates TRPC6,9 here we examine the hypothesis that inhibition of TRPC6-mediated Ca2+ entry in podocytes underlies glomerular protection by klotho.
Results
Klotho Suppresses TRPC6-Mediated Ca2+ Entry in Cultured Mouse Podocytes
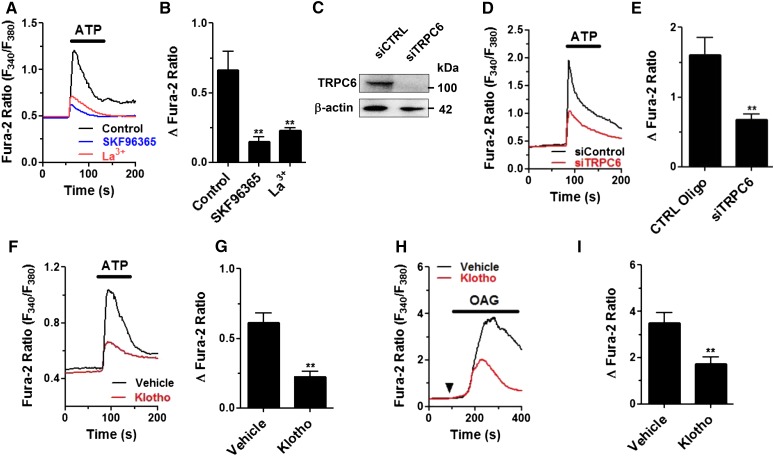
TRPC6, a diacylglycerol-activated channel, plays an important pathogenic role in podocyte injury and glomerular diseases.17,19–21,24 Here, we examined Ca2+ influx via TRPC6 channel in cultured mouse podocytes using ATP as a ligand for Gαq-coupled P2Y receptors known to mediate Ca2+ signaling in podocytes.25–28 Application of ATP caused an increase in [Ca2+]i in podocytes, which was blocked by pretreatment with SKF96365, a nonselective TRPC channel inhibitor (Figure 1, A and B). The increase in [Ca2+]i was completely eliminated by removing extracellular Ca2+ (not shown), indicating the role of Ca2+ entry. TRPC5-mediated Ca2+ entry also plays a pathogenic role in glomerular injury. Lanthanides in micromolar range inhibit TRPC6 but potentiate TRPC5 channel function.29 La3+ (100 μM) markedly inhibited ATP-evoked increases in [Ca2+]i, suggesting that the ATP-induced increase in [Ca2+]i is mediated by TRPC6. In further support, we found that knockdown of endogenous Trpc6 in podocytes by small interference RNA significantly reduced ATP-induced increases in [Ca2+]i compared with cells transfected with control oligonucleotides (Figure 1, C, D, and E).
Figure 1.
Soluble klotho inhibits ATP-induced, TRPC6-mediated increases in [Ca2+]i in cultured mouse podocytes. (A) Representative traces showing exposure to ATP (100 μM) caused an increase in [Ca2+]i in cultured mouse podocytes. Preincubation of cells with SKF96365 (50 μM, 1 hour) or La3+ (100 μM, 1 hour) blunted the increases. (B) Summary of results in (A). **denotes P<0.01 versus control (vehicle). (C) Successful knockdown of Trpc6 expression by siRNA (siTRPC6) was evident by Western blot analysis. (D) Representative traces showing ATP-induced increases in [Ca2+]i were reduced in cells transfected with siRNA against Trpc6 versus control oligonucleotides. (E) Summary of results in (D). **denotes P<0.01 versus control oligo (CTRL oligo). (F) Representative traces illustrating effect of pretreatment of purified klotho (500 pM, 1 hour) on ATP-induced increases in [Ca2+]i. (G) Summary of results in (F). **denotes P<0.01 versus vehicle. (H) Representative traces illustrating effect of pretreatment of purified klotho (500 pM, 1 hour) on 1-oleoyl-2-acetyl-sn-glycerol (OAG) (100 μM)–induced increases in [Ca2+]i. (I) Summary of results in (H). **denotes P<0.01 versus vehicle; n=5–7 cells in each group in (B), (E), (G) and (I).
ATP induced a significantly smaller increase in [Ca2+]i in cells continuously incubated with soluble klotho in the extracellular medium compared with vehicle-treated controls (Figure 1, F and G), suggesting that soluble klotho inhibits TRPC6 channel activity in podocytes. The inhibition is not due to disruption of the P2Y receptors or binding of ATP because the effect remained when 1-oleoyl-2-acetyl-sn-glycerol (a membrane-permeable diacylglycerol analog) was used to activate TRPC6 channels (Figure 1, H and I). Similarly, angiotensin II and endothelin-1 caused TRPC6-mediated increases in [Ca2+]i in podocytes, and klotho inhibits the increases (Supplemental Figure 1).
We have found that klotho downregulates TRPC6 in cardiomyocytes by inhibiting PI3K-dependent exocytosis of the channel.9 Here, we report that klotho does not affect intrinsic TRPC6 channel activity (Supplemental Figure 2, Supplemental Material) and that klotho downregulates TRPC6 surface expression in podocytes via PI3K- and Akt-dependent mechanisms similar to cardiomyocytes (Supplemental Figures 3 and 4, Supplemental Material). Assembly of different TRPC family members in multiple combinations when coexpressed in cultured cells may give rise to channels of mixed regulatory and biophysic properties.30 The precise composition of TRPC6-associated channel complexes in podocytes remains elusive. We found that TRPC3 is expressed in mouse podocytes and that TRPC3 and TRPC6 form heteromultimers that can be downregulated by klotho (Supplemental Figures 4 and 5, Supplemental Material).
Klotho Ameliorates ATP-Induced TRPC6-Dependent Actin Cytoskeletal Reorganization and Albumin Leakage in Podocytes
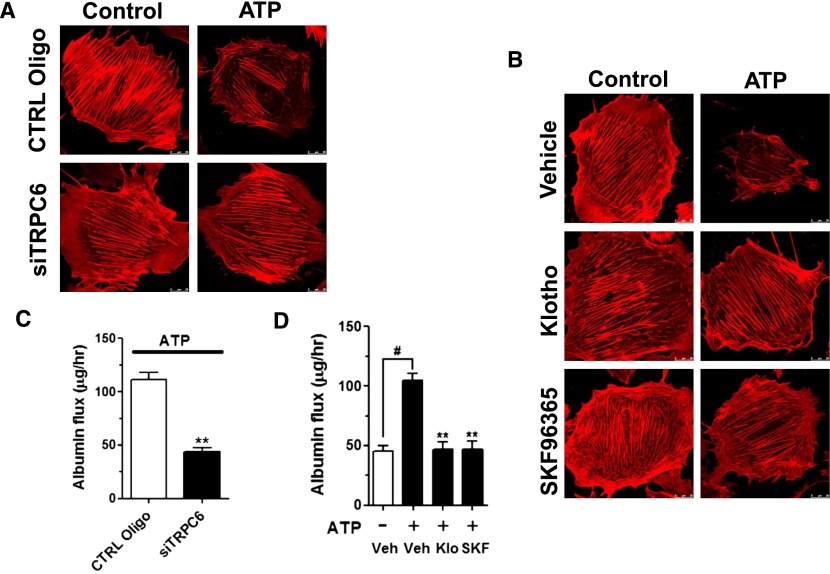
Disturbance of Ca2+ homeostasis in podocytes alters actin dynamics causing podocyte dysfunction and leading to proteinuria. We used phalloidin labeling of actin filaments to probe actin cytoskeletal rearrangement and examine functional relevance of ATP-induced TRPC6-mediated Ca2+ entry in podocytes. ATP treatment caused reorganization of the actin filaments: dissolution of the cytoplasmic radial stress fibers and redistribution as peripheral bundles to cortical/subcortical regions (Figure 2A). Also, ATP stimulated cell contraction, resulting in smaller cell size. The ATP-induced actin rearrangement and cell contraction was abolished by small interfering RNA (siRNA) knockdown of TRPC6 or by SKF96365 (Figure 2, A and B), supporting the notion that TRPC6-mediated Ca2+ overload causes rearrangement of the actin cytoskeleton in podocytes. Next, we found that soluble klotho prevented ATP-induced actin cytoskeleton rearrangement and cell contraction (Figure 2B). Transepithelial albumin flux across a confluent podocyte culture monolayer is an in vitro assay for the formation and function of podocyte foot processes between cells.31 As expected from the results of increased Ca2+ entry and actin cytoskeletal rearrangement, ATP treatment increased transepithelial albumin fluxes (Figure 2C). ATP-stimulated increases in albumin fluxes were abolished by soluble klotho or pretreatment with SKF96365 (Figure 2D). Knockdown of TRPC6 also diminished ATP-stimulated albumin flux (Figure 2C). Thus, soluble klotho reduces albumin permeability caused by TRPC6 activation.
Figure 2.
Klotho suppresses cytoskeletal modeling and transepithelial albumin leak in mouse podocytes. Confluent differentiated mouse podocytes grown on transwell were stimulated by ATP (100 μM, 1 hour) or vehicle (control) and analyzed by phalloidin staining (A and B). (A) Knockdown of Trpc6 prevented actin cytoskeletal remodeling caused by exposure to ATP. Podocytes were transfected with control oligonucleotides (CTRL Oligo) or siRNA for TRPC6 (siTRPC6). (B) Effects of Klotho (500 pM) and SKF96365 (50 μM) on ATP-stimulated actin cytoskeletal remodeling. (C) Effect of Trpc6 knockdown on ATP-stimulated transepithelial albumin permeability in mouse podocytes analyzed as described in Concise Methods. **denotes P<0.01 versus CTRL Oligo. n=6 in each group. (D) Effects of klotho and SKF96365 on albumin permeability in mouse podocytes. Podocytes were stimulated by ATP and with klotho (Klo) or SKF96365 (SKF). #denotes P<0.01 between groups as indicated; **denotes P<0.01 versus +ATP but without klotho or SKF (vehicle); n=6 in each group.
Klotho Ameliorates TRPC6-Induced Albuminuria in Mice
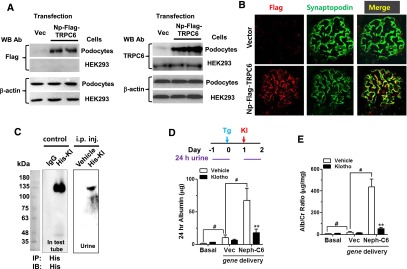
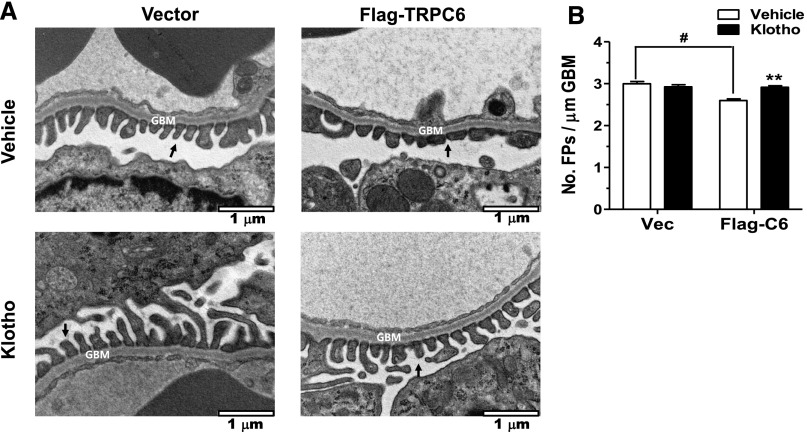
To examine whether soluble klotho protects podocytes in vivo from TRPC6 overexpression, we employed an experimental model of albuminuria induced by podocyte-specific Trpc6 transgene delivered via tail vein injection. Transfection of the plasmid in podocytes, but not HEK cells, produced TRPC6 proteins (Figure 3A). At 48 hours after transgene delivery, recombinant Flag-TRPC6 proteins were detected in extracts from kidney cortex, but not in the liver (Supplemental Figure 6). Colocalization with synaptopodin indicated transgenic TRPC6 protein expression in podocytes (Figure 3B). Recombinant histidine (His)-tagged klotho proteins, administered by intraperitoneal (i.p.) injection 24 hours after tail vein delivery of Trpc6 transgene, could be detected in urine collected between zero and 24 hours after i.p. injection (Figure 3C). Compared with mice that received empty vector, mice that received Trpc6 transgene excreted significantly more albumin in 24-hour urine collected 24–48 hours after gene delivery (Figure 3, D and E), indicating that overexpression of TRPC6 is sufficient to induce albuminuria. Systemic administration of soluble klotho by i.p. injection markedly blunted albuminuria. Transmission electron microscopy showed podocyte foot process fusion in Trpc6 transgenic mice and that klotho administration ameliorated this change (Figure 4, A and B), supporting that Trpc6 transgene expression induces podocyte injury and that klotho beneficially affects podocytes.
Figure 3.
Klotho ameliorates albuminuria in mice induced by podocyte-specific overexpression of Trpc6. (A) Western blot analysis (detected by anti-Flag antibody [left panel] and by anti-TRPC6 antibody [right panel]) of cell lysates from podocytes and HEK cells transfected with podocyte-specific, Flag-tagged TRPC6 (Np-Flag-TRPC6) or empty pCDNA3 vector (Vec). (B) Expression of Flag-TRPC6 protein by immunofluorescence in podocytes (marked by synaptopodin) in mice injected with Trpc6 transgene. (C) Immunoblot analysis of urine samples from mice which received i.p. injection of His-tagged recombinant klotho or vehicle. His-tagged recombinant klotho or vehicle was inoculated in mice i.p. at 24 hours after gene delivery. Twenty-four-hour urine samples were collected from mice by using metabolic cage before and 24 hours after gene delivery. Right panel: His-tagged klotho protein in urine was immunoprecipitated using anti-His antibody followed by immunoblotting using anti-klotho antibody. Left panel: His-tagged klotho or IgG was diluted in PBS and subjected to immunoprecipitation by anti-His antibody as for urine samples. (D and E) Twenty-four-hour urinary albumin excretion (D) and urine albumin-to-creatinine ratio (E) from mice before (basal) and 24–48 hours after in vivo hydrodynamic gene delivery of vector (Vec) or Flag-TRPC6 (Neph-C6) and with i.p. injection of klotho or vehicle. #denotes P<0.01 between indicated groups; **denotes P<0.01 i.p. klotho versus vehicle injection.
Figure 4.
Klotho ameliorates slit diaphragm disruption induced by Trpc6 transgene delivery. Electron microscopic images of glomerular filter of kidneys from mice that received Trpc6 transgene or empty vector delivery and with administration of recombinant klotho or vehicle. (A) Arrow indicates podocyte foot process. Glomerular basement membrane (GBM) is indicated. Compared with in mice which received empty vector (vector), foot processes in mice which received Trpc6 transgene (Flag-TRPC6) are flattened, indicative of fusion. Images at 30,000× magnification. Scale bar is 1 μm. (B) Average number of foot processes per 1 μm length of GBM. Bar represents mean±SEMs of 25–35 images from three to five mice per group. Foot process fusion (flattening) results in fewer per unit length.
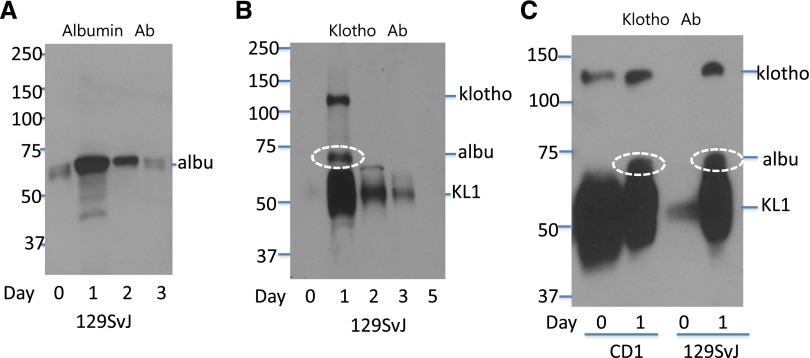
Notably, delivery of empty vector also increased urinary albumin excretion compared with the basal state, and soluble klotho decreased albuminuria in these mice (Figure 3, D and E). The in vivo gene delivery employs injection of a large volume of saline solution (5%–10% of body wt) over a short time to enhance cellular DNA uptake by hydrodynamic force. This causes a large increase of the extracellular fluid (ECF) volume and shear stress to the glomerular filter apparatus. We thus investigated the effect of ECF volume expansion on urinary albumin and klotho excretion. In the basal state, a small amount of albumin was detected in urine from 129SvJ mice (Figure 5A). ECF volume expansion by injecting 10% body wt of saline caused an immediate increase in albumin excretion within 24 hours, which tapered off over the next 48–72 hours. Interestingly, full-length klotho ectodomain and a smaller protein likely representing the KL1 domain fragment were absent in urine at baseline but urinary excretion of both were induced by volume expansion (Figure 5B). Increased luminal fluid flow potentially may stimulate shedding of membranous klotho residing at the apical membrane, which probably will result in a larger increase of full-length klotho ectodomain relative to the KL1 fragment. Yet, we found that urinary KL1 fragment was more abundantly increased by volume expansion relative to the full-length klotho ectodomain, suggesting that enhanced filtration (of smaller size KL1 fragment than full-length ectodomain) may at least play a significant role.
Figure 5.
ECF volume expansion affects urinary excretion of albumin and klotho. (A) ECF volume expansion increased urinary excretion of albumin. At day zero, 2 ml PBS was injected via intravenous route into 129SvJ mice over 30 seconds. Twenty-four-hour urine samples were collected from 24 hours before saline injection to time of injection (day zero), from time of injection to 24 hours after (day one), between 24 and 48 hours after (day two), and between 48 and 72 hours after (day three). Twenty-four-hour urine volume (in ml) was 0.8, 2.4, 1.5, and 0.8 for day zero, one, two, and three, respectively. Five microliters of urine from day zero and the same proportion from each subsequent day were analyzed for the abundance of albumin in Western blot by using anti-albumin antibody. (B) ECF volume expansion induced urinary excretion of klotho. Urine samples as in (A) were analyzed for klotho by using anti-klotho antibody (KM2076). Note that nonspecific staining of albumin by anti-klotho antibody occurs (white oval circle) due to very high abundance of albumin in urine after volume expansion. (C) Urinary excretion of klotho and albumin before and after ECF volume expansion in CD1 and 129SvJ mice. Urine samples were probed by anti-klotho (KM2076) antibody. Note that a longer exposure time was used for autoradiograph shown in (C) compared with that in (B), so that some KL1 fragment can be detected in urine from 129SvJ at baseline.
We next investigated the contribution of genetic heterogeneity to differences in baseline glomerular permeability and in response to volume expansion. CD1 mice, which are more susceptible to kidney injury than 129SvJ mice,32 have more abundant full-length klotho ectodomain and KL1 fragment detectable by anti-klotho antibody in urine at baseline compared with that in 129SvJ (Figure 5C). There was also more urinary albumin excretion at baseline in CD1 than 129SvJ mice (not shown), suggesting that the glomerular filter in CD1 mice may be leakier than in 129SvJ. As in 129SvJ, volume expansion also increased urinary excretion of albumin in CD1 mice, to the extent recognized nonspecifically by anti-klotho antibody (Figure 5C). Thus, urinary klotho excretion is increased when glomerular permeability for albumin is increased either from genetic factors or induced experimentally (see Discussion for implications of the findings).
Klotho Is Expressed in Podocytes of Mouse and Human Kidney
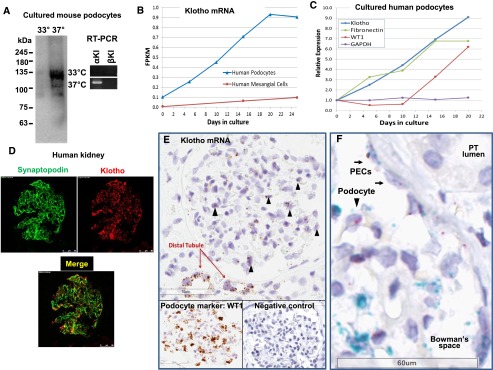
Many studies have reported expression of klotho in renal tubules, more abundantly in the distal convoluted tubules than in other tubular segments.1,33 Whether klotho is expressed in podocytes and/or other cell types in the glomerulus is not known. In immortalized mouse podocytes, we detected expression of klotho (also known as α-klotho) mRNA in differentiated, but not in undifferentiated, cells (Figure 6A). In contrast, β-klotho, encoded by a separate gene and expressed exclusively in the hepatobiliary system,34 was not detected. Similarly, expression of klotho mRNA in human immortalized podocytes was induced and increased progressively during differentiation into mature cells (Figure 6B). In contrast, klotho gene expression was not observed in primary human mesangial cells (Figure 6B). Klotho gene expression in differentiating human podocytes increased coordinately with fibronectin mRNA and preceded expression of the podocyte marker Wilms tumor 1 (WT1) (Figure 6C). Immunofluorescence staining revealed that klotho protein is present in cells within the human glomerulus, including podocytes, as evidenced by colocalization with synaptopodin (Figure 6D). Specificity and validity of the anti-klotho antibody is supported by the finding that the antibody stains glomerular podocytes in kidneys of wild-type (WT) mice but not of homozygous klotho-hypomorphic mice (Supplemental Figure 7). Moreover, Klotho mRNA expression was detected in glomeruli of normal human kidneys by in situ hybridization (ISH) (Figure 6E, Supplemental Figure 8). As expected, klotho mRNA was also detected in distal convoluted tubules. Podocytes are marked by expression of WT1. A negative control probe did not show detectable signals under the same experimental conditions and a probe for WT1 mRNA identified podocytes (Figure 6E, Supplemental Figure 8). Dual ISH using separate probes for WT1 and klotho clearly confirmed expression of klotho mRNA in podocytes (Figure 6F). Interestingly, parietal epithelial cells were also found to coexpress WT1 and klotho. Overall, klotho expression in glomerulus is much less compared with in tubules, perhaps explaining why it was not notable in previous studies.
Figure 6.
Klotho is expressed in podocytes of mouse and human kidney. (A) Expression of klotho in differentiated (37°C), but not in undifferentiated (33°C), cultured mouse podocytes analyzed by Western blot (left panel) and RT-PCR (right) analysis. In RT-PCR, specific primers for α-klotho versus β-klotho were used. (B) Expression of mRNA for klotho in immortalized human podocytes during differentiation into mature cells and in primary human glomerular mesangial cells as determined by RNAseq. At day zero, undifferentiated podocytes were induced to differentiate by switching to the nonpermissive temperature (37°C). (C) Relative expression of klotho and other genes (WT1, podocyte marker; fibronectin, glomerular extracellular matrix and GBM component; GAPDH, housekeeping gene) in immortalized human podocytes during differentiation into mature cells. (D) Expression of klotho in normal human glomerulus revealed by immunofluorescence staining. Expression in podocytes is evidenced by colocalization with synaptopodin. See Supplemental Figure 7 for validation of specificity of anti-Klotho antibody. (E) ISH to detect klotho mRNA in normal human glomerulus. Arrowhead indicates podocytes. Shown is representative of nine studies using tissues from patients who underwent uni-nephrectomy for renal cell carcinoma or from autopsy samples. (F) Klotho expression in podocytes (arrowhead) and parietal epithelial cells (PECs, arrows) revealed by dual ISH. Cell nuclei, purple color; mRNA for WT1 (a podocyte marker), blue color; mRNA for klotho, purple/red color. FPKM, fragments per kilobase of exon per million fragments mapped; GBM, glomerular basement membrane; PT, proximal tubule.
Klotho-Deficient CKD Mice Have Aggravated Albuminuria
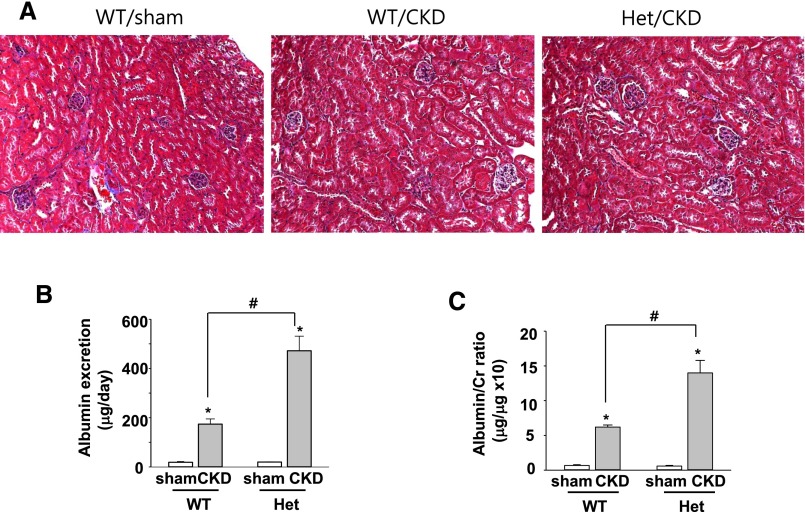
We next examined whether klotho deficiency may alter podocyte function in the basal state or during disease processes. Previous studies have shown that 5/6 nephrectomy for 4–5 weeks in 129SvJ mice caused a modest reduction in the clearance function by approximately 50% and albuminuria.32 The albuminuria is likely due to glomerular hyperfiltration and hemodynamic changes as a part of compensatory responses; no histologic alterations in the glomerulus, tubules, and/or interstitium were observed in the remnant kidney tissues.32 Likewise, we found no detectable histologic differences between the normal kidney of sham-operated mice and remnant kidney tissues from 5/6 nephrectomy (CKD) mice (Figure 7A). Also, no histologic differences between WT and heterozygous (Het) klotho-deficient CKD mice created by 4-week 5/6 nephrectomy. Moreover, we have previously reported that WT and Het klotho-deficient CKD mice exhibited similar degrees of hypertension and reduction in creatinine clearance and several other measured functional parameters, such as hematocrit.12 We further found that baseline albumin excretion was not different between WT and Het sham mice, yet urinary albumin excretion increased in WT CKD mice compared with sham mice, and CKD-induced increases were augmented in Het klotho-deficient mice compared with WT mice (Figure 7, B and C).
Figure 7.
Klotho deficiency aggravates remnant kidney-induced albuminuria without causing detectable histologic changes. (A) Trichrome staining of normal kidney tissue of sham-operated WT mice and remnant kidney tissues from WT and Het klotho-deficient mice received 5/6 nephrectomy (CKD). (B and C) Twenty-four-hour urinary albumin excretion (B) and urine albumin-to-creatinine ratio (C) from WT and Het mice received 5/6 nephrectomy or sham surgery. #denotes P<0.01 between indicated groups; *denotes P<0.01 CKD versus sham; n=6 in each group.
Discussion
Previous studies have shown that klotho protects against several forms of renal injury and progression of kidney disease, and ameliorates proteinuria.11–13,33 Proteinuria is a common consequence of kidney diseases irrespective of the cause of primary injury and considered a marker of severity of disease processes.35,36 Amelioration of proteinuria by klotho does not necessarily reflect a direct cytoprotective effect of klotho on podocytes. Here, we ask the question of whether klotho has a direct protective effect on podocytes and the potential mechanism of protection. Our study reveals the following key findings. First, soluble klotho inhibits TRPC6-dependent Ca2+ entry in cultured podocytes and blunts the associated actin cytoskeletal remodeling. Second, systemic administration of soluble klotho decreases urinary albumin excretion in mice induced by transgenic overexpression of TRPC6 in the kidney. Third, in a remnant kidney model in which albuminuria is induced most likely by glomerular hyperfiltration and without histologic evidence of glomerular and tubulo-interstitial injury, klotho deficiency aggravates the magnitude of albuminuria. These findings provide compelling evidence to support the notion that klotho plays a direct role in protecting podocytes from injury.
Mechanistically, soluble klotho protects podocytes by preventing PI3K and Akt-dependent exocytosis of the channel to surface membrane (Supplemental Figures 3, 4, 5, and 9), reminiscent of klotho protection of cardiac myocytes.9 Wolf et al., reported that soluble klotho interacts with growth factor receptors to inhibit PI3K signaling.37 Increased Ca2+ entry through TRPC6 channels frequently leads to [Ca2+]i overload and disease pathogenesis. This is, in part, due to the fact that the TRPC6 gene contains NFAT-responsive elements in its promoter so that increased Ca2+ entry through TRPC6 leads to a feed-forward amplification by activating the calcineurin-NFAT gene transcription and further TRPC6 expression. In the heart, this calcineurin-NFAT–mediated sustained Ca2+ entry via TRPC6 causes re-expression of fetal genes and cardiac remodeling. In podocytes, it results in rearrangement of the actin cytoskeleton, cellular contraction, and foot process effacement. A recent study suggests that klotho may protect podocyte function by de-repressing Wnt/β-catenin signaling.38 Multiple mechanisms may contribute to podocyte cytoprotection by klotho.
Many growth factors activate PI3K and Akt signaling cascades in podocytes,39 which may maintain a steady-state low level of TRPC6 on the cell surface. Overactivation of growth factor receptors including epithermal growth factor receptor yet promotes glomerular injury and renal failure.40 Chronic stimulation of Gαq-coupled hormonal receptors can cause [Ca2+]i overload in podocytes leading to glomerular injury and proteinuria.17,23,25,26,41 Hence, the TRPC6 channel is one of important mediators for hormone- or growth factor–induced podocyte injury, and klotho protects podocytes by reining in excess Ca2+ entry through TRPC6 (and/or TRPC3/TRPC6 if the heteromultimeric channels are present in human podocytes).
Het klotho-deficient CKD mice created by approximately 4 weeks of 5/6 nephrectomy have worse albuminuria than WT CKD mice despite similar degrees of hypertension and loss of clearance function, and have no detectable histologic difference of remnant kidney tissues. These results indicate that the endogenous level of klotho is important for podocytes in vivo. However, there is no difference in albuminuria between Het klotho-deficient sham-operated and WT sham-operated mice. Thus, klotho deficiency does not cause baseline podocyte dysfunction. The function of klotho is to protect podocytes from injury.
How does klotho reach podocytes to exert protection? Literature so far shows that membranous klotho is expressed in the renal tubules, and that urinary klotho arises from shedding of klotho at the apical membrane of tubules or transcytosis of circulating klotho.1,33,42,43 In this scenario, klotho in the tubular lumen needs to travel back to protect podocytes. Our finding that klotho is expressed in podocytes suggests alternative mechanisms for cytoprotection by klotho in situ or by autocrine or paracrine effects. The effect of klotho on podocytes may be through membranous klotho present on the podocyte cell surface. Or, it may be through the activity of soluble klotho present in Bowman’s space coming from shedding from podocytes.
Our study also suggests potential mechanism of cytoprotection of podocytes by filtered klotho. We find that mice with genetic propensity for albuminuria have increased urinary klotho excretion. Disrupting the glomerular integrity by volume expansion increases urinary excretion of klotho likely by increasing glomerular filtration of circulating klotho. In the experiments examining the effect of recombinant soluble klotho in protecting proteinuria in mice overexpressing TRPC6, klotho was administered during volume-expanded state as a part of the experimental procedure of hydrodynamic gene delivery. The effect of administered klotho thus may reflect filtered klotho acting on podocytes. It is conceivable that filtration of klotho will increase in disease states with increased glomerular filter permeability and systemic administration of klotho may be a treatment for glomerular proteinuria. In summary, our study reveals a potential novel function of klotho in protecting the glomerular filter and offers a potential new therapeutic strategy for treatment of proteinuria.
Concise Methods
Reagents
Unless otherwise noted, all chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant klotho protein was produced and purified as described previously.9,44 For i.p. injection in mice, commercial recombinant His-tagged human klotho was used (5334-KL-025; R&D Systems, Minneapolis, MN).
Knockdown by siRNA
Nontargeting control oligonucleotide (sc37007) and siRNA against mouse TRPC6 (sc42673) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); targeting siRNA for human VAMP2 (M-012498–01–0005) was from Dharmacon (Chicago, IL). Transfection of siRNA oligonucleotide was performed using DharmaFECT-1 siRNA transfection reagent (Thermo Fisher Scientific, Lafayette, CO) per manufacturer’s instructions.
RT-PCR Analysis
Total RNAs were purified from trypsinized pellets of podocyte cell lines (permissive and nonpermissive conditions) using Hybrid-R total RNA purification kit (305–101; GeneAll, Seoul, South Korea). Complementary DNA was synthesized from 1 µg of total RNA by using a ReverTraAce qPCR RT Master Mix with gDNA Remover (FSQ-301; Toyobo, Osaka, Japan). PCR conditions were as follows: 95°C for 5 minutes and 34 cycles of 94°C for 30 seconds, annealing at 58°C for 30 seconds and 72°C for 45 seconds. Primer sequences are in Supplemental Table 1.
Cell Culture, Transfection, and mRNA Analysis by RNAseq
Immortalized mouse podocyte cell line (a kind gift from Dr. Peter Mundel) was cultured and induced for differentiation as previously described.45 Human embryonic kidney 293 (HEK293) cells were cultured and transiently cotransfected with cDNAs as indicated in each experiment as described.9 Transfection was performed using X-tremeGENE HP DNA transfection reagent (Roche, Basel, Switzerland) according to the manufacturer’s instructions. In each experiment, the total amount of DNA for transfection was balanced by using empty vectors.
Conditionally immortalized human podocyte cell line AB 8/13 was differentiated as described46 using tissue culture-treated flasks or wells coated with human collagen IV (Biocoat). Cells expressed highest levels of ‘podocyte-specific’ markers after 15 days of differentiation at the nonpermissive temperature (37°C). Primary human mesangial cells (Cell Systems) were plated on collagen IV-coated wells and cultured as recommended by supplier. When cultures were confluent, cells were washed (DPBS) and lysed by 1 minute of exposure to mirVana lysis buffer (Ambion). Lysed cells were scraped, pipetted up and down, and lysate frozen at –20°C until all samples were collected for processing with the Ambion mirVana miRNA kit. Final RNA was brought up in 100 μl of DEPC-treated water and concentration determined by NanoDrop. Quality was checked by Agilent Bioanalyzer and only RNA preparations with RIN>7 were used for RNAseq with paired-end reads (Covance).
Western Blot and Cell-Surface Biotinylation Assay
Western blotting and cell surface biotinylation assay were performed as described previously.9,47 Primary antibodies used for detection of TRPC6 (ab63038), VAMP2 (ab181869), and β-actin (ab6276) were purchased from Abcam, Inc. (Cambridge, MA); for total Akt, Ser-473 p-Akt, and Thr-308 p-Akt from Cell Signaling Technology (Danvers, MA); for TRPC3 (ACC-016) from Alomone. Antibody against klotho (KM2076) was kindly provided by Dr. Makoto Kuro-o. Horseradish peroxidase–tagged secondary antibodies were purchased from Santa Cruz Biotechnology. All experiments were performed three times with similar results.
Immunoprecipitation
HEK293 cells transiently expressing Flag-tagged TRPC3 and/or GFP-TRPC6 were lysed in Proprep lysis buffer (iNtRON Biotechnology, Sungnam, South Korea) that contained phosphatase inhibitors (PhosSTOP; Roche). Cell lysates were incubated with Flag-tagged agarose beads (Merck GmbH, Darmstadt, Germany) at 4°C for 2 hours and pelleted using a table-top microfuge. Precipitates were analyzed by SDS-PAGE followed by western blot analysis using horseradish peroxidase–tagged anti-flag and anti-GFP antibodies (from Sigma-Aldrich).
Fluorescent Measurement of [Ca2+]i
[Ca2+]i in podocytes was measured using Lambda DG-4 fluorescence measurement system (Sutter Instruments, Novato, CA) as described previously.47,48 Briefly, podocytes were placed on collagen coated-glass coverslips and loaded with fura-2/AM in darkness for 30 minutes at 37°C. After dye loading, cells were washed and transferred to a perfusion chamber on a fluorescence microscope. Cells were constantly superfused (approximately 2 ml/min) by a normal physiologic salt solution that contained (in mM) 135 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 5.5 glucose (pH 7.4). Fura-2 signals were obtained by alternating excitation at 340 or 380 nm, and detecting emission at 510 nm. Data were analyzed using MetaFluor (Sutter Instruments) software. All [Ca2+]i measurements were performed at 37°C using heat controller (Warner Instruments, Hamden, CT).
Electrophysiologic Recording
For recording TRPC6 and TRPC3 currents, HEK293 cells were cotransfected with cDNAs for Flag-TRPC6 or Flag-TRPC3 (0.2 μg each per 35 mm dish) with or without human M3 muscarinic receptor (0.2 μg). TRPC6 and TRPC3 currents were recorded using the whole cell-dialyzed configuration of the patch clamp technique, as described previously.9,47 Whole-cell currents were recorded under voltage-clamp using an Axopatch 200B (Axon Instruments Inc., Foster City, CA) and EPC-9 patch-clamp amplifier (Heka Electronik, Lambrecht, Germany). The patch electrodes were coated with silicone elastomer (Sylgard 184; Dow Corning, Midland, MI), fire polished, and had resistances of 2–3 MΩ when filled with the pipette solution. The cell membrane capacitance and series resistance were compensated (>80%) electronically using an Axopatch-200B or EPC9 amplifier. Data acquisition was performed using ClampX9.2 software (Axon Instruments) or Pulse/Pulsefit (v8.50) software (Heka Electronik). All electrophysiologic recordings were performed at room temperature (approximately 20°C–24°C). The pipette and bath solution contained (in mM): 120 Cs-aspartate (Cs-Asp), 10 CsCl, 1 MgCl2, 2 MgATP, 5 EGTA, 1.5 CaCl2 (free [Ca2+] =70 nM), and 10 HEPES (pH 7.2), and 140 Na-aspartate, 5 KCl, 0.5 EGTA, and 10 HEPES (pH 7.4), respectively.
Phalloidin Staining
Podocytes grown on type-1 collagen (Invitrogen, Carlsbad, CA)-coated coverslips were fixed with 4% paraformaldehyde in PBS for 15 minutes at 37°C, and incubated with Alexa-594-phalloidin (1:40 dilution) (A12381; Molecular Probes-Invitrogen, Carlsbad, CA) for 20 minutes at room temperature under darkness. Fluorescent images were obtained using a laser scanning confocal microscope.
Immunofluorescence
Frozen sections of kidney tissues were fixed in prechilled 100% acetone for 10 minutes at -20°C and incubated with 3% BSA and 3% donkey serum in PBS at room temperature for 1 hour for blocking. Slides were incubated with goat anti-synaptopodin (1:40 dilution; sc21537; Santa Cruz Biotechnology) and rabbit anti–DDDDK-tag antibody (Flag antibody; 1:50 dilution; ab1162; Abcam, Inc.) at 37°C for 1 hour for detection of endogenous synaptopodin and Flag-TRPC6, respectively, and subsequently with anti–goat-Alexa 488 (1:200 dilution; A11001; Molecular Probes-Invitrogen) and donkey anti–rabbit-CyTM3 (1:200 dilution; 711–165–152; Jackson ImmunoResearch Laboratory, West Grove, PA) for 1 hour.
Frozen sections of kidney tissues are from patients with clear cell renal cell carcinoma who underwent radical surgery at the Yonsei University Wonju Severance Christian Hospital, South Korea. The collection of surgical specimens was approved by the institutional review board of Yonsei University Wonju College of Medicine. Adjacent normal kidney tissues were used for immunofluorescence staining as described previously.49 The sections were blocked with 3% BSA, 3% donkey serum in PBS, stained with goat anti-synaptopodin antibody (1:20) for 1 hour at 37°C, followed by rat monoclonal antibody (KM2076) (1:20) for 1 hour at 37°C and overnight at 4°C. Secondary antibodies include Alexa594-conjugated anti-rat antibody and Alexa488-conjugated anti-goat antibody, respectively.
In Vitro Albumin Permeability Assay
Confluent differentiated podocytes grown on transwell (0.4 μm pore; Corning Costar Corporation, Cambridge, MA) were exposed to ATP (100 μM). For measurement of apical to basolateral transepithelial passage of FITC-labeled bovine serum albumin (A9771; Sigma-Aldrich), culture medium in the upper (apical) compartment was replaced by 2 ml of FITC-BSA (250 μg/ml). One hundred microliter samples were drawn from the lower (basolateral) compartment at 0.5, 1, 1.5, 2, 6, 12, and 24 hours. In each sample collection, the lower compartment was replenished with an equal volume of fresh medium. Collected samples were measured for fluorescent signal at 450 nm by fluorescence spectroscopy. Albumin concentration in the samples was calculated using linear regression of a diluted series of the tracer.
In Vivo Gene Delivery of Trpc6
Hydrodynamic gene delivery by tail vein injection was performed using a commercial kit (MIR5340; Mirus Bio LLC, Madison, WI) according to manufacturer’s instructions, with some modifications. Plasmid DNA encoding Flag-tagged human TRPC6 (pNxPRS-hTRPC6 from Dr. Schlondorff) (12 μg) dissolved in 2 ml isotonic saline was injected via tail vein into 5-week-old mice (BKS.Cg-m+/+Leprdb/BomTac). Control animals received empty vector in the same volume. To determine the effect of klotho, mice received 20 μg/kg recombinant klotho (R&D Systems) or vehicle by i.p. injection at 24 hours after gene delivery. For determination of urinary albumin excretion, 24-hour urine was collected from mice kept in metabolic cage individually. Albumin was measured by ELISA specific for mouse albumin (Exocell, Philadelphia, PA). All animal protocols were approved by the Yonsei University Wonju College of Medicine Institutional Animal Care and Use Committee.
ISH
Pieces of kidney tissue from the cortical and medullary regions were collected at the time of surgery from patients who underwent uni-nephrectomy for renal cell carcinoma or from autopsy samples (n=9). Sample tissue from regions histologically unaffected by the tumor were fixed using fresh 10% neutral buffered formalin, then processed and embedded in paraffin. Four micron sections were attached on SuperFrost Plus Slides (Thermo Fisher Scientific, Waltham, MA) and stored at –80°C until used for ISH. Pretreatment and hybridization conditions were as described (RNAscope Sample Preparation Pretreatment Guide for FFPE Tissue; Advanced Cell Diagnostics, Inc.) using manual RNAscope Probes for: human α-klotho, human WT1 (podocyte-selective), human RNA polymerase 2a (positive control), and a standard negative control, DapB (a bacterial gene). Detection was by RNAscope 2.0 HD Detection Kit BROWN (catalog no. 320497) and hematoxylin counterstain provided structural landmarks. Dual ISH detection was performed as described.50 Automated scan of ISH slides used an Aperio ScanScope to capture digital images at magnification ×40.
Transmission Electron Microscopy
Mouse kidney slices were fixed in 2.5% glutaraldehyde for 2 hours at 4°C, washed in 0.1 M PBS (pH 7.4), and then postfixed in 1% osmium tetroxide in the same buffer for 90 minutes. Specimens were dehydrated through a graded series of ethanol, exchanged through propylene oxide and embedded in an Epon mixture. Subsequently, ultrathin sections were obtained by ultramicrotome (Reichert Jung, Vienna, Austria) with a diamond knife. Ultrathin sections were double stained with uranyl acetate and lead citrate and examined using a JEM-1200 EXII transmission electron microscope with image acquisition system (Soft Imaging Systems’ MegaView III) at 80 kV (JEOL, Japan). analySIS 3.0 software (SIS Software GmbH, Leipzig, Germany) was used for data acquisition, processing, measuring, analyzing, and archiving. The transmission electron microscopy images were assessed by a renal pathologist in a blinded fashion. The number of foot processes per glomerular basement membrane length (μm) was calculated using analySIS 3.0 software and results were analyzed by ANOVA, followed by Tukey multiple comparison tests.
General Procedures and Creation of CKD by 5/6 Nephrectomy in Mice
All animal experimentation described here was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals using protocols approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee. The creation of Het klotho-deficient mice on a 129/S1SvImJ background has been described.12 For creation of CKD, a one-step partial nephrectomy was performed as previously described.12 Briefly, after midabdomen incision, the left kidney was exposed with Q-Tips, and the entire upper and lower poles of the left kidney were cauterized, leaving an approximately 5 mm intact segment around the hilum. Thereafter, the right ureter and pedicle were ligated with sutures, and the right kidney was removed. BP was measured using a tail-cuff sphygmomanometer, as previously described.12
Statistical Analyses
Data analyses were performed with the Prism (v6) software (GraphPad Software Inc., San Diego, CA). Statistical comparisons between two groups of data were made using a two-tailed unpaired t test. Multiple comparisons were determined using one-way ANOVA followed by Tukey multiple comparison tests. P values <0.05 and <0.01 were considered significant for single and multiple comparisons, respectively. All data were presented as mean±SEMs.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Shmuel Muallem (the National Institutes of Health [NIH]), Joseph Yuan (University of North Texas), Joo Young Kim (Yonsei University), Johannes Schlondorff (Harvard Medical School), and Peter Mundel (Harvard Medical School) for materials. C.-L.H. holds the Jacob Lemann Professorship in Calcium Transport at the University of Texas Southwestern Medical Center.
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2010-0024789, NRF-2013R1A1A2060764, and NRF-2015R1D1A1A01060454), the Yonsei University Future-leading Initiative of 2014 (2014-22-0127), and by the NIH (DK85726, DK100605, and DK79328).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015080888/-/DCSupplemental.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y: Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M: Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morishita K, Shirai A, Kubota M, Katakura Y, Nabeshima Y, Takeshige K, Kamiya T: The progression of aging in klotho mutant mice can be modified by dietary phosphorus and zinc. J Nutr 131: 3182–3188, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Yoshida T, Fujimori T, Nabeshima Y: Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology 143: 683–689, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Razzaque MS: The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol 5: 611–619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, Fujimori T, Xie P, Kanwar YS: Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A 104: 2331–2336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie J, Cha SK, An SW, Kuro-O M, Birnbaumer L, Huang CL: Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun 3: 1238, 2012. 10.1038/ncomms2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R: In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39: 838–843, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Sun Z: Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 54: 810–817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie J, Yoon J, An SW, Kuro-o M, Huang CL: Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 26: 1150–1160, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuro-o M: Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol 9: 650–660, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Greka A, Mundel P: Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J Am Soc Nephrol 22: 1969–1980, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstädt H, Hsu HH, Schlondorff J, Ramos A, Greka A: Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal 3: ra77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, Ahn W, Wallentin H, Heid H, Hopkins CR, Lindsley CW, Riccio A, Buvall L, Weins A, Greka A: Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest 123: 5298–5309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckel J, Lavin PJ, Finch EA, Mukerji N, Burch J, Gbadegesin R, Wu G, Bowling B, Byrd A, Hall G, Sparks M, Zhang ZS, Homstad A, Barisoni L, Birbaumer L, Rosenberg P, Winn MP: TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol 22: 526–535, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB: A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR: TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer RA, Möller CC, Hamming I, Navis G, Wetzels JF, Berden JH, Reiser J, Faul C, van der Vlag J: Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol 179: 1719–1732, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J: Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 18: 29–36, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Anderson M, Roshanravan H, Khine J, Dryer SE: Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J Cell Physiol 229: 434–442, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Roshanravan H, Dryer SE: ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species. Am J Physiol Renal Physiol 306: F1088–F1097, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burford JL, Villanueva K, Lam L, Riquier-Brison A, Hackl MJ, Pippin J, Shankland SJ, Peti-Peterdi J: Intravital imaging of podocyte calcium in glomerular injury and disease. J Clin Invest 124: 2050–2058, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hohenstein B, Renk S, Lang K, Daniel C, Freund M, Léon C, Amann KU, Gachet C, Hugo CP: P2Y1 gene deficiency protects from renal disease progression and capillary rarefaction during passive crescentic glomerulonephritis. J Am Soc Nephrol 18: 494–505, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A: Pharmacological characterization of the P2 receptors profile in the podocytes of the freshly isolated rat glomeruli. Am J Physiol Cell Physiol 305: C1050–C1059, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung S, Mühle A, Schaefer M, Strotmann R, Schultz G, Plant TD: Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem 278: 3562–3571, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE: Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem 278: 39014–39019, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Macconi D, Abbate M, Morigi M, Angioletti S, Mister M, Buelli S, Bonomelli M, Mundel P, Endlich K, Remuzzi A, Remuzzi G: Permselective dysfunction of podocyte-podocyte contact upon angiotensin II unravels the molecular target for renoprotective intervention. Am J Pathol 168: 1073–1085, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisner C, Faulhaber-Walter R, Wang Y, Leelahavanichkul A, Yuen PST, Mizel D, Star RA, Briggs JP, Levine M, Schnermann J: Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int 77: 519–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW: Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y, Nabeshima Y: Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J Clin Invest 115: 2202–2208, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Patrakka J, Tryggvason K: New insights into the role of podocytes in proteinuria. Nat Rev Nephrol 5: 463–468, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, Rubinek T: Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene 27: 7094–7105, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y: Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 24: 771–785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiser J, Sever S, Faul C: Signal transduction in podocytes--spotlight on receptor tyrosine kinases. Nat Rev Nephrol 10: 104–115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bollée G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M, Schordan E, Sabaa N, Vandermeersch S, Galaup A, Rodenas A, Casal I, Sunnarborg SW, Salant DJ, Kopp JB, Threadgill DW, Quaggin SE, Dussaule JC, Germain S, Mesnard L, Endlich K, Boucheix C, Belenfant X, Callard P, Endlich N, Tharaux PL: Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med 17: 1242–1250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Jirka G, Rosenberg PB, Buckley AF, Gomez JA, Fields TA, Winn MP, Spurney RF: Gq signaling causes glomerular injury by activating TRPC6. J Clin Invest 125: 1913–1926, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, Nakamura K, Iida A, Anazawa H, Koh N, Iwano A, Imura A, Fujimori T, Kuro-o M, Hanai N, Takeshige K, Nabeshima Y: Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun 267: 597–602, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, Ravikumar P, Gillings N, Bian A, Sidhu SS, Kuro-O M, Moe OW: Renal Production, Uptake, and Handling of Circulating αKlotho. J Am Soc Nephrol 27: 79–90, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M: Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286: 8655–8665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das R, Xu S, Quan X, Nguyen TT, Kong ID, Chung CH, Lee EY, Cha SK, Park KS: Upregulation of mitochondrial Nox4 mediates TGF-β-induced apoptosis in cultured mouse podocytes. Am J Physiol Renal Physiol 306: F155–F167, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 47.An SW, Cha SK, Yoon J, Chang S, Ross EM, Huang CL: WNK1 promotes PIP₂ synthesis to coordinate growth factor and GPCR-Gq signaling. Curr Biol 21: 1979–1987, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cha SK, Kim JH, Huang CL: Flow-induced activation of TRPV5 and TRPV6 channels stimulates Ca(2+)-activated K(+) channel causing membrane hyperpolarization. Biochim Biophys Acta 1833: 3046–3053, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JH, Lkhagvadorj S, Lee MR, Hwang KH, Chung HC, Jung JH, Cha SK, Eom M: Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma. Biochem Biophys Res Commun 448: 76–82, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Su N, Wang LC, Wu X, Bui S, Nielsen A, Vo HT, Luo Y, Ma XJ: Dual-color ultrasensitive bright-field RNA in situ hybridization with RNAscope. Methods Mol Biol 1211: 139–149, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.