Abstract
The renal proximal tubule reabsorbs 90% of the filtered glucose load through the Na+-coupled glucose transporter SGLT2, and specific inhibitors of SGLT2 are now available to patients with diabetes to increase urinary glucose excretion. Using expression cloning, we identified an accessory protein, 17 kDa membrane-associated protein (MAP17), that increased SGLT2 activity in RNA-injected Xenopus oocytes by two orders of magnitude. Significant stimulation of SGLT2 activity also occurred in opossum kidney cells cotransfected with SGLT2 and MAP17. Notably, transfection with MAP17 did not change the quantity of SGLT2 protein at the cell surface in either cell type. To confirm the physiologic relevance of the MAP17–SGLT2 interaction, we studied a cohort of 60 individuals with familial renal glucosuria. One patient without any identifiable mutation in the SGLT2 coding gene (SLC5A2) displayed homozygosity for a splicing mutation (c.176+1G>A) in the MAP17 coding gene (PDZK1IP1). In the proximal tubule and in other tissues, MAP17 is known to interact with PDZK1, a scaffolding protein linked to other transporters, including Na+/H+ exchanger 3, and to signaling pathways, such as the A-kinase anchor protein 2/protein kinase A pathway. Thus, these results provide the basis for a more thorough characterization of SGLT2 which would include the possible effects of its inhibition on colocalized renal transporters.
Keywords: cell & transport physiology, diabetes, ion transport, Na transport, glucose reabsorption
Na+/glucose cotransporters employ the Na+ electrochemical gradient to enable glucose uptake against a concentration gradient. The low-affinity Na+/glucose cotransporter SGLT2, a product of the SLC5A2 gene (positioned at 16p11.2), is found almost solely in the apical membranes of renal proximal tubules and reabsorbs over 90% of glucose from the glomerular filtrate.1 Although its cDNA was first cloned in 1992,2 the physiologic role of SGLT2 only became accepted a decade later following identification of SLC5A2 mutations in a large majority of patients presenting with familial renal glucosuria (FRG).3–5 A major reason for this delay was that, unlike the closely related SGLT1, SGLT2 does not express well either in transfected mammalian cells or in Xenopus laevis oocytes injected with SGLT2 mRNA, hindering characterization of this protein.6,7
At least 11 pharmaceutical firms have candidate drugs for inhibiting SGLT2, including three that are presently in clinical use, which should help diabetic patients to control their glycemia by augmenting urinary glucose excretion.5 The drugs are all analogues of phlorizin (Pz), a specific inhibitor for the transporters of the SGLT family. Given the prevalence of type 2 diabetes and the great potential for SGLT2 inhibitors in patients with this metabolic syndrome, it is possible that millions will be taking these drugs in the coming years. Our understanding of SGLT2, and of its inhibitors and their physiologic interactions, would obviously benefit from a robust expression system for this protein.
The SGLT2 protein had been shown to be stable in oocytes and transfected cells even though it exhibited little transport activity.8 This suggested the possibility that a second protein might be required for SGLT2 to function and so we used expression cloning to isolate the putative accessory protein. This protein was identified as MAP17, a 17 kDa subunit with 2 transmembrane segments which had first been cloned in 1995 as a protein whose transcription was upregulated in kidney, colon, breast, and lung cancers.9
Results
Expression Cloning
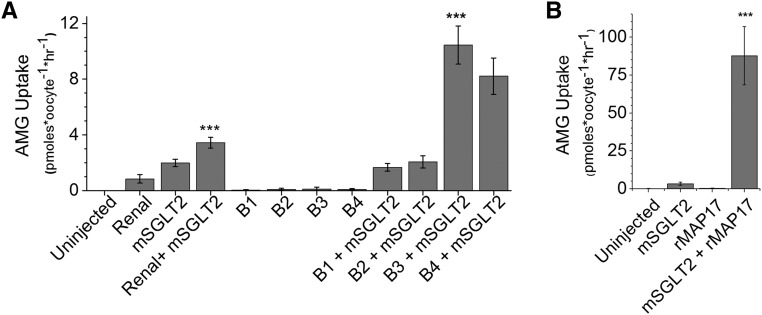
Expression cloning consists of coinjecting Xenopus oocytes with SGLT2 mRNA together with increasingly restricted samples of renal mRNA to ultimately identify a single protein that stimulates SGLT2 activity.10 Expression of either rat renal mRNA or mouse SGLT2 mRNA in oocytes led to enhanced uptake of 14C-labeled α-methyl glucose (AMG, a non-metabolized substrate for SGLT1/SGLT2), while SGLT2 coexpression with renal mRNA caused even greater uptake (Figure 1A). mRNA size-fractionation produced 24 fractions of renal mRNA, and aliquots were combined to create five pooled samples (pools A– E). AMG uptakes with samples from each pool indicated that pool B expressed the factor that affected AMG uptake. Subsequent oocyte injections of aliquots from the four size fractions contained in pool B are shown in Figure 1A. The individual fractions did not induce a significant AMG uptake but coexpression of fractions B3 or B4 with SGLT2 greatly stimulated AMG uptake. Thus, a protein expressed by these size fractions of mRNA (approximately 0.5–1.5 kb) augmented the level of SGLT2 activity.
Figure 1.
Identification of MAP17 as a factor stimulating SGLT2-mediated AMG uptake. (A) The uptake of AMG into Xenopus oocytes expressing murine SGLT2 mRNA (4.6 ng/oocyte) was stimulated by coexpression of rat renal mRNA (46 ng/oocyte) (the four samples at left; mean±SD; n=5–6 oocytes per sample). When the renal mRNA was size-fractionated, no transport activity was associated with expression of fractions B1–B4 (10 ng/oocyte) but these mRNA samples steeply increased mSGLT2 activity when coinjected with mSGLT2 mRNA, proving that the factor responsible acted by augmenting the activity of the mSGLT2 protein. The Renal+mSGLT2 uptake was significantly different (P<0.001, ANOVA/Bonferroni) from the three other samples shown on the left (Uninjected; Renal; mSGLT2). B3+mSGLT2 uptake is significantly different from mSGLT2 uptake (P<0.001). (B) Expression cloning using a cDNA library produced from mRNA fraction B3 produced a single cDNA clone (rMAP17) whose product very significantly augmented murine SGLT2 activity (P<0.001).
A cDNA library was constructed from mRNA sample B3 and iterative screenings of pools of plasmids representing ever-smaller numbers of colonies were performed where mRNA was transcribed from the NotI-cut plasmids and coinjected into oocytes with SGLT2 mRNA. Radiolabeled AMG uptake experiments were then performed to identify the pool of plasmids expressing the protein which complemented SGLT2. A plasmid from a single colony was eventually isolated, the transcribed product of which greatly stimulated SGLT2 activity in oocytes (Figure 1B). The cDNA encoded by this clone was fully sequenced and the transcribed protein was identified as MAP17, product of the PDZK1IP1 gene. Similar results were seen when rat SGLT2 mRNA was coinjected with rat MAP17 mRNA (data not shown).
Characterization of Human SGLT2–MAP17 Activity
We obtained human SGLT2 and MAP17 cDNAs by PCR amplification and inserted them into pT7TS to enable transcription of polyA-tailed, capped mRNA. Coexpression with human MAP17 in oocytes greatly stimulated human SGLT2–mediated AMG uptake (150±20 fold for three experiments), confirming that human MAP17 increases SGLT2 activity (Figure 2A). Na+/glucose cotransport generated currents of large amplitude (Figure 2B) which were not observed for control oocytes nor for oocytes solely expressing MAP17 or SGLT2. The cotransport current mediated by human SGLT2 was inhibited by Pz (a specific inhibitor which binds to the glucose binding site) with a Ki of about 30 nM (data not shown). Adding the high affinity inhibitor dapagliflozin at 10 nM in the presence of 2 mM glucose (Figure 2B) inhibited the cotransport current by 90%, consistent with a Ki of 2.5 nM. Coexpression of MAP17 with other polyol-transporting members of the SLC5A family (i.e., SGLT1, SMIT1, SMIT2, SGLT3, and SGLT4) did not cause any significant increase in their transport activities with the exception of SGLT3, whose transport currents were increased by a factor of 2.6±0.8 (data not shown).
Figure 2.
Stimulation of human SGLT2 activity with human MAP17 and MARDI. (A) Coexpression of human MAP17 and human SGLT2 in Xenopus oocytes provided results similar to those seen with rat MAP17 and murine SGLT2 (the hSGLT2+hMAP17 uptake was significantly different [P<0.001; mean±SD; n=5–6 oocytes per sample; ANOVA/Bonferroni] from the three other samples, i.e., uninjected, hSGLT2, and hMAP17 mRNA). (B) SGLT2 activity could also be monitored by electrophysiological measurement of the substrate-induced current passing through the protein under voltage-clamp conditions. All traces employ the same scales (as shown). For the first three traces (all from one oocyte held at −50 mV and expressing both hSGLT2 and hMAP17), the changes in current represent the effect of addition of 2 mM glucose (denoted by gray bars) to the bathing solution in the presence of i) no inhibitor; ii) 10 nM dapagliflozin; iii)100 nM dapagliflozin. The other tracings represent the absence of effect of presenting 2 mM glucose to an uninjected oocyte (iv), and oocytes expressing hMAP17 only (v) or hSGLT2 only (vi). (C) Alignment of hMAP17 and MARDI protein sequences. Sequence identity is indicated by white letters on a black background and the two transmembrane (TM) segments are indicated. (D) Coexpression of MARDI causes augmented SGLT2 activity, though less than that seen with MAP17. A typical experiment is shown, of three experiments, where n=6 oocytes for each sample in each experiment. The stimulatory effect of MARDI on SGLT2 expression was significantly different from the effects seen with oocytes expressing either SGLT2 or MARDI alone (P<0.001, ANOVA/Bonferroni).
Using the default settings for a BLAST search of the human proteome, we have found a single protein (from gene SMIM24) that shows significant sequence similarity to MAP17, which we named MARDI (MAP17-Related Dimer), and which is expressed in the apical membranes of both renal proximal tubules and small intestine (http://www.proteinatlas.org/ENSG00000095932/tissue). When the aligned sequences are examined, it can be seen that the sequence homology ends where the cytoplasmic C-terminal domains commence (Figure 2C). Coexpression of MARDI with SGLT2 showed that it stimulated SGLT2 activity by 18±9 fold, which is quite significant but is also an order of magnitude less than what is seen with MAP17 (Figure 2, A and D).
Expression in Opossum Kidney Cells
To better understand the MAP17–SGLT2 interaction, the two human cDNAs were separately inserted into the vector pcDNA3.1(-) and each received an epitope tag expected to face the extracellular solution when the protein had reached the plasma membrane. The recombinant vectors were used for transient transfection of opossum kidney (OK) cells, which express minimal amounts of endogenous MAP17 mRNA.11 Cotransfection caused a large increase in AMG uptake into the cells (Figure 3A) and a parallel increase in the binding of 14C-labeled Pz (Figure 3B). The transport activity observed with tagged proteins was indistinguishable from that seen with untagged proteins (data not shown).
Figure 3.
MAP17 activates SGLT2 in transfected OK cells and in oocytes without increasing SGLT2 surface expression. (A) Uptake of radiolabeled AMG into confluent monolayers of OK cells demonstrates that hSGLT2 activity was strongly increased by the presence of hMAP17 (P<0.001) or MAP17Δ where the terminal four amino acids (-STPM) have been removed (P<0.001; mean±SD; n=5 wells per sample; ANOVA/Bonferroni). (B) OK cells expressing both SGLT2 and MAP17 bind more radiolabeled Pz than cells expressing SGLT2 alone (P<0.001; mean±SD; n=5 wells per sample; ANOVA/Bonferroni). Cells expressing SGLT2 alone present a small but significant increase (P<0.05) in Pz binding versus control cells (Ctl). Radiolabeled Pz could be displaced by adding 100 μM cold Pz to the bathing solution. (C) Quantification of superficial immunofluorescent labeling of SGLT2 (flagged with an external F7 epitope) was performed using confluent OK cells fixed with paraformaldehyde. Cells expressing SGLT2 or SGLT2+MAP17 present larger amounts of attached fluorescent antibodies than cells expressing either nothing or MAP17 alone (P<0.01; mean±SD; n=5 wells per sample; ANOVA/Bonferroni). There was no significant difference between SGLT2 and SGLT2+MAP17. (D) OK cells (top panel) were transfected with empty vector (Ctl) or with a vector expressing, F7-hSGLT2 (SGLT2) or F7-hSGLT2 and hMAP17 (SGLT2+MAP17). The amount of primary antibody which had been bound to the cells was observed in a Western blot with a secondary antibody. A similar experiment was performed with plasma membranes isolated from Xenopus oocytes injected with either nothing (Ctl) or mRNAs expressing hSGLT2 or hSGLT2+hMAP17 (n=4). This confirms that the amount of SGLT2 present in the membrane is not increased by the presence of MAP17.
The four C-terminal amino acids of MAP17, i.e., -STPM, comprise a PDZ-binding motif. MAP17 strongly interacts with a scaffolding protein called PDZK1 which contains four distinct PDZ domains.12 To determine whether this interaction is necessary for its stimulation of SGLT2 activity, we measured AMG uptake into OK cells transfected with SGLT2 and either MAP17 or an engineered version of MAP17 lacking the last four amino acids (MAP17-ΔSTPM). The last four amino acids did not affect MAP17’s augmentation of SGLT2 activity (Figure 3A), indicating that this effect does not require interaction with PDZK1.
To distinguish between increased SGLT2 expression at the cell surface versus activation of SGLT2 already present, we fixed transiently-transfected cell cultures with paraformaldehyde and used epitope-tag antibodies along with fluorescent secondary antibodies to fluorescently measure cell surface F7-SGLT2 (F7 tag added to the N terminus of SGLT2). As shown in Figure 3C, MAP17 coexpression caused no change in the amount of SGLT2 measured at the cell surface SGLT2. The data (five separate experiments) was assessed using two-way ANOVA and Bonferroni post-hoc analysis in order to eliminate the inter-experiment variability in background fluorescence. There was a significant increase in fluorescence associated with SGLT2 expression (i.e., SGLT2 and SGLT2+MAP17 were both different from control or MAP17 cells; P<0.01) but there was no significant difference in the amount of fluorescence between SGLT2 and SGLT2+MAP17 cells. To confirm these results, one possibility would be to obtain membrane vesicles from the apical membrane of OK cells and compare SGLT2 abundance with transport activity. Due to the possibility of contamination with intracellular membranes, we choose rather to work with intact OK cells. We exposed unfixed, transfected cells to the murine anti-F7 primary antibody and measured, via a Western blot, the amount of antibody that was attached to the membrane. Under our conditions, control cells bound no detectable primary antibody while similar amounts of primary antibody were found attached to the cells expressing F7-SGLT2 or F7-SGLT2+MAP17 (Figure 3D, top panel). Similar results were found with Xenopus oocytes expressing F7-SGLT2 alone or with MAP17 (Figure 3D, bottom panel).
In agreement with the previous result, equivalent levels of membrane expression of F7-SGLT2 are observed in immunofluorescent staining of OK cells transfected with or without MAP17 (Figure 4, A and B). Thus, the quantity of SGLT2 expressed at the cell membrane is not altered by MAP17 coexpression, but the cotransporter conformation must be changed since the presence of MAP17 significantly increased both Pz binding and AMG transport.
Figure 4.
The presence of MAP17 does not change the expression level of SGLT2. (A) OK cells expressing pcDNA3.1-F7-hSGLT2 have been fixed with and exposed to an anti-F7 antibody and a fluorescent anti-antibody. (B) OK cells expressing a combination of pcDNA3.1-F7-hSGLT2 and pcDNA3.1-hMAP17-HA. Localization of SGLT2 and similar quantities are visible in both cases. The white scale bars indicate distances of 10 µm and the yellow bar indicates the z axis.
MAP17 Mutation in FRG Patient
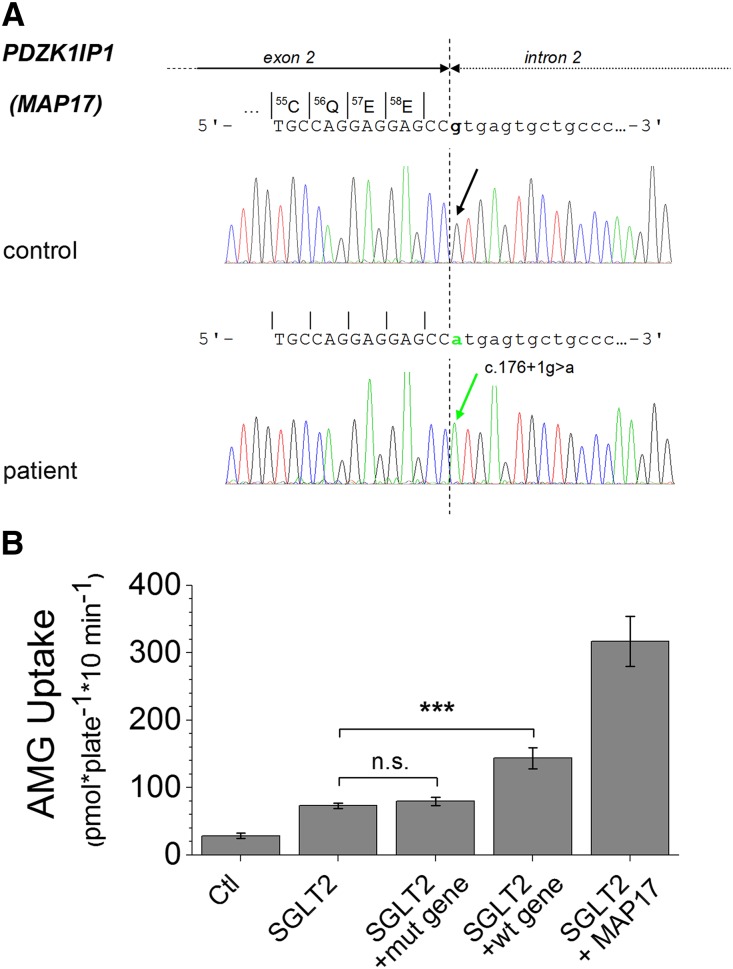
A cohort of 60 individuals with FRG, and some of their family members, was assembled over the past decade.4,13 Of these, 12 were glucosuric without presenting any mutations in SLC5A2 or with mutation on one allele only, a finding not expected to account for the severity of the glucosuria observed. We sequenced the DNA of these 12 patients to determine whether mutations in PDZK1IP1 (locus in 1p13) could explain their glucosuria. For one individual, without any mutation identified in the SLC5A2 gene, homozygosity for a mutation in the PDZK1IP1 gene was found. This patient, the daughter of consanguineous Turkish parents, had reproducible glucosuria in the range of 8–16.7 g/1.73 m2 per day (originally published as case 19–14) and has meanwhile been lost to follow-up. She was homozygous for the mutation c.176+1G>A, affecting the donor splice site of intron 2 of PDZK1IP1. The mutation predicts skipping of exon 2 resulting in a shift of the reading frame (see Figure 5A).
Figure 5.
Detection of a PDZK1IP1 mutation in a patient with renal glucosuria. (A) Schematic presentation of sequencing results of a PCR product containing exon 2 of the PDZK1IP1gene and an adjacent intronic segment in a patient with FRG. The region around c.176 is shown with the boundary between exon 2 and intron 2 indicated by the dotted line. Note homozygosity for a single base exchange at the first position of the consensus sequence of the donor splice site of intron 2 (c.176+1G>A,IVS2+1G>A) in the patient. (B) Uptake of radiolabeled AMG is shown for OK cells that have been transfected with the vector pcDNA3.1(-) itself (Ctl) or with the recombinant vector containing hSGLT2 cDNA, either alone or cotransfected with the same vector expressing the mutated PDZK1IP1 c.176+1G>A gene (mut gene) or the intact PDZK1IP1 gene (wt gene). For comparison, the AMG uptake obtained from cells cotransfected with the recombinant vectors containing hSGLT2 and hMAP17 is also shown. The AMG uptakes in cells expressing SGLT2 + the mutant MAP17 gene was not different from the uptake in cells expressing SGLT2 alone (P>0.05; mean±SD; n=4 wells; and the entire experiment was repeated three times). A significant difference was observed between the cells cotransfected with the hSGLT2 cDNA and the intact human PDZK1IP1 gene (SGLT2 + wt gene) versus the cells cotransfected with the hSGLT2 cDNA alone (P<0.001, ANOVA/Bonferroni). In addition, the control cells and the cells cotransfected with the two cDNAs were significantly different (P<0.001) from all other samples.
To confirm that the PDZK1IP1 mutation was responsible for the glucosuria, we isolated the PDZK1IP1 gene by PCR amplification from normal human genomic DNA. The reaction product was 7.2 kb rather than the 6.2 kb suggested by the human genome GRCh37.p13 Primary Assembly. Sequencing revealed a 1 kb region containing approximately 70 iterations of a 16 bp minisatellite sequence (GGGGGATGGACTCAGT), most of which had been missing from the reference sequence which has a gap replacing some of these iterations. Both the intact gene and the c.176+1G>A mutation (by site-directed mutagenesis) were inserted into pcDNA3.1 and expressed in OK cells. As Figure 5B shows, significant SGLT2 activation was caused by coexpression of the normal PDZK1IP1 gene (P<0.001), but not with the c.176+1G>A mutated gene (P>0.05). Injection of the same recombinant vectors (containing normal or mutated PDZK1IP1) into the nuclei of Xenopus oocytes did not induce measurable transport activity.
Discussion
Expression cloning was used to isolate a cDNA clone for a protein that complemented SGLT2 activity. Utilizing expression of mRNA for SGLT2 ± size-fractionated renal mRNA (Figure 1A) allowed us to identify a specific pool that could enhance the level of activity of SGLT2 without generating any significant glucose transport by itself. Subsequent isolation of a single clone showed conclusively that MAP17 is required for the activity of SGLT2. This was surprising because Blasco et al.,11 using an expression cloning strategy aimed at identifying the renal Na/mannose cotransporter, reported that rat MAP17 could stimulate the endogenous Na/mannose cotransport of the oocyte without any effect on coexpressed rat SGLT1, rat SGLT2, or pig SGLT3. In our hands, the activity levels of both rat and human versions of SGLT2 are similarly augmented by their corresponding MAP17 proteins, results which have consistently been found in several works from different laboratories using different types of vectors in both cell cultures and Xenopus oocytes. After so much time, it is difficult to reasonably speculate on the reason why this interaction was not observed in the previous study. Nevertheless, the glucosuria associated with the c.176+1G>A PDZK1IP1 homozygosity provides conclusive evidence that MAP17 is a necessary activator of SGLT2 in situ.
Several transport proteins require the presence of a partner protein (often called β subunits) in order to reach the plasma membrane.14–16 Virtually all of these accessory proteins have separate functional activities as well (as does MAP17), and most of the related transporters are found in lipid rafts (as are SGLT1 and SGLT217). While these accessory proteins act by increasing the expression of the transport protein, the case of MAP17 is quite different: it enables the transport activity without changing the amount of transport proteins present at the plasma membrane. In oocytes, the presence of MAP17 must induce a change in the structure of SGLT2 which would then allow glucose transport. In OK cells, even though the stimulatory effect is smaller than in oocytes, MAP17 significantly increases the ability of SGLT2 to transport glucose and to bind Pz. It is possible that a low level of endogenously expressed MAP17 explains the low Na/glucose cotransport activity observed in OK cells after expressing SGLT2 alone. Although other explanations involving indirect signalization pathways are conceivable, our current working hypothesis is that MAP17 activates SGLT2 through direct interaction in the plasma membrane. This hypothesis is consistent with the observation that MARDI, which shares sequence similarities with MAP17 only within the two transmembrane domains, can significantly stimulate SGLT2. The stimulatory effects of MARDI and MAP17 suggest that the interaction with SGLT2 occurs within the membrane plane. Interestingly, the Na/phosphate cotransporter (NaPi-IIa) seems to interact with MAP17 in a similar manner since it has been shown to bind to intact MAP1718 but not to a truncated version of MAP17 which lacked the transmembrane domains.19 The putative interaction of MAP17 and SGLT2 within lipid rafts will need to be directly addressed in future studies.
MAP17 was first identified in 1995 as a protein whose transcription was upregulated in renal (and other) cancers.20–22 Believed to form a dimer linked by Cys bridges,11 immunohistochemical observation detected MAP17 predominantly in the apical membranes of renal proximal tubules.9 Screening of a cDNA library with the carboxyl half of MAP17, using the yeast two-hybrid procedure, identified strong interaction with PDZK1, also known as Na/H exchanger regulatory factor 3(NHERF3).23 PDZK1, with its four PDZ domains, works as a scaffolding protein and has been shown to interact with a number of transport proteins including the cystic fibrosis transmembrane regulator,24 NaPi-IIa,25 the Na proton exchanger (NHE3),26–30 the organic cation transporter, the chloride-formate exchanger and the urate-anion exchanger.31 PDZK1 was also shown to interact with several signaling systems.31,32 Recently, a crystal structure of a protein complex revealed the molecular details of a three-partner interaction involving the fourth PDZ domain of PDZK1, an A-kinase anchoring protein (D-AKAP2), and its attached PKA.33 MAP17, through its interaction with PDZK1, has also been shown to play a role in trafficking plasma membrane proteins18,19; hepatic overexpression of MAP17 in mice caused removal of PDZK1 from the plasma membrane along with the attached high-density lipoprotein receptor SR-B1, leading to increased plasma HDL levels.34
As MAP17 was first cloned on the basis of being overexpressed in tumors, it is not surprising that it has been shown to be an excellent marker for tumorigenesis.35,36 Several studies examining the role of MAP17 in tumorigenesis showed that MAP17 over-expression enhanced tumor cell malignancy by increasing the level of reactive oxygen species.37,38 Very interestingly, these effects can be inhibited by Pz, but the specific Na/glucose cotransporter involved (SGLT1, 2, 3, ...) has not yet been determined.38 Our results provide an additional avenue by which MAP17 may affect reactive oxygen species, via SGLT2. Currently, there is little known about the presence of SGLT2 in tumors, although it has been shown to be significantly overexpressed in metastatic lesions of liver and lymph node39 and functionally expressed in pancreatic and prostate adenocarcinomas.40
MAP17 has been shown to bind to the fourth PDZ domain of PDZK1.27 Direct interaction of SGLT2 with MAP17 would bring the cotransporter into close proximity with other transporters, including NHE3,27 which are known to bind to PDZK1.41 A recent paper reported that activating the Na/glucose cotransporter (presumably SGLT2, which is colocalized with NHE3) by adding 5 mM glucose in the lumen of rat proximal tubules can upregulate the Na/HCO3 transport mediated by NHE3.42 More interestingly, in the total absence of luminal glucose, addition of luminal Pz produced a clear inhibition of NHE3 activity. This unexpected observation could be explained if SGLT2/MAP17 and NHE3 are part of a “signaling platform” held together by the scaffolding protein PDZK1.43,44 Future studies will be needed to establish these interactions within the native environment of a renal proximal tubule. A similar interaction may exist in the jejunum, where SGLT1 activity stimulates NHE3 activity by an unknown mechanism involving Akt and NHERF2.45 Although MAP17 is not required for SGLT1 activity (unlike SGLT2), this does not preclude the possibility of a MAP17–SGLT1 interaction. Supporting the idea of an interaction of MAP17 with other members of the Na+/glucose cotransport family, MAP17 was shown to produce a small but significant increase in SGLT3 activity. In addition, MAP17 and SGLT1 activities appear to be linked in cervical cancers.38 Finally, the demonstration of an interaction between MAP17 and NaPi-IIa using the two-hybrid system25 shows that MAP17 can interact with an even wider variety of transporters.
In summary, we have presented conclusive evidence that MAP17 is required for the normal function of SGLT2 in oocytes and in mammalian cells. This requirement is confirmed by the finding that a mutation in the MAP17 coding gene was found to be associated with a case of familial renal glucosuria, in the absence of SGLT2 mutations. This observation establishes the genetic heterogeneity for this human phenotype. The interaction between SGLT2 and MAP17 suggests that SGLT2 may be working in close proximity with other transporters with which it can establish a local signaling pathway. As millions of diabetic patients are going to use SGLT2 inhibitors as a part of their regular treatment, this study suggests that it would be important to understand the physiologic effects of SGLT2 inhibition not only on glucose transport but also on other renal transport mechanisms operating nearby.
Concise Methods
RNA Preparation
Rat renal RNA was isolated using TriZol reagent (Invitrogen Canada, Burlington, ON, Canada) and mRNA was isolated using oligo-dT chromatography (Invitrogen), following the manufacturer’s instructions.
Sucrose Gradient
A 3.6 ml linear 5%–25% sucrose gradient was prepared in 10 mM Tris, 1 mM EDTA pH 8.0 One hundred and fifty micrograms of mRNA (2 µg/µl) was layered on the gradient and centrifuged for 10 hours × 150,000 g. Twenty-four 0.15 ml fractions were removed and mRNA was recovered by ethanol precipitation.
Oocyte Procedures
The preparation and maintenance of oocytes, as well as RNA injections and electrophysiological measurements, were performed as previously described.46 Oocytes were maintained in Barth solution (in mM: 90 NaCl, 3 KCl, 0.82 MgSO4, 0.74 Ca[NO2]2, 10 HEPES, and adjusted to pH 7.5 with Tris). For electrophysiology and uptake experiments, the same solution was used but with chloride as the sole anion.
Oocyte Uptake Conditions
Uptakes were performed under standard conditions46 where uptake duration was 2 hours and the uptake solution contained 1 mM mannose (to saturate the oocyte high affinity Na/sugar cotransporter), 1 mM galactose (to saturate the renal SGLT1–mediated glucose uptake), and 50 µM 14C-labeled AMG (American Radiolabeled Chemicals, St. Louis, MO).
cDNA Library Preparation
cDNA was directionally synthesized from size-selected mRNA using the Zap cDNA synthesis kit (Stratagene, La Jolla, CA) and inserted into EcoRI, XhoI-cleaved pGem11Zf(+) (Promega, Madison, WI). A library of 15,000 colonies was plated onto nitrocellulose filter-laden Luria-Bertani agar plates with 600 colonies per filter; the colonies were scraped off the filters and plasmids were purified using EZ-10 spin columns (Bio Basic, Markham, ON). After NotI cleavage, capped mRNA was transcribed using the T7 mMessage mMachine kit (Life Technologies, Carlsbad, CA). Subsequent screenings used plates bearing 100 colonies, then single colonies grown in liquid Luria-Bertani medium.
Vector Construction
Human SGLT2, MARDI, and MAP17 were obtained by PCR from renal cortex cDNA and inserted into the vectors pT7TS and pcDNA3.1(-). Epitope-tagged hSGLT2 received the F7 tag47 upstream of human SGLT2, i.e., at the extracellular N-terminus of the protein. Sequences for the primers used are available upon request. For in vitro transcription, the cDNAs in pT7TS were cleaved downstream of the cDNA with SalI, then mRNA was prepared using the cleaved DNA and the T7 mMessage mMachine kit.
Cell Culture
OK cells were cultured in DMEM-F12 media with 10% fetal bovine serum and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin) in 95% air, 5% CO2. Cells were seeded on 24-well plates and transfected with vectors using Lipofectamine 2000 (Invitrogen) on 70%–90% confluent monolayers (0.8 µg per well) in accordance with the manufacturer’s protocol. Experiments were performed after 16–24 hours of incubation.
AMG Uptake and Pz Binding with Cultured Cells
Uptake of radiolabeled AMG and Pz binding was as described earlier48 using 50 µM AMG + 0.5 µCi/ml 14C-labeled substrate in Krebs solution. All uptakes were performed at 37°C for 10 minutes and were stopped by rinsing with ice-cold Krebs (3 × 1 ml/well). Monolayers were dissolved with 2% SDS in 0.2 N NaOH, scintillation cocktail was added, and radioactivity was measured. Binding assays were performed similarly but with 1 µCi/ml 3H-labeled Pz (18 nM) in Krebs solution for 10 minutes at room temperature.
Immunofluorescence
Immunofluorescence detection of F7-SGLT2 was performed on confluent OK cells.49 Twenty-four-well plates were washed with ice-cold PBS+Ca2++Mg2+ (PBS containing 1 mM MgCl2, 0.1 mM CaCl2) then fixed at 4°C for 20 minutes with 4% paraformaldehyde in PBS. Plates were then incubated in 5% BSA in PBS (30 minutes) to block nonspecific sites, exposed to primary antibody (mouse anti-F7 1/1000; Sigma-Aldrich, St. Louis, MO) for 60 minutes in blocking solution at room temperature, rinsed, blocked again, and incubated for 60 minutes with secondary antibody (Alexa Fluor 594 conjugated donkey anti-mouse 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) in the same solution. Plates were washed, then fluorescence was measured using a fluorescence plate reader (Infinite F200 Pro; Tecan). For visualization using an Olympus IX-81 microscope, OK cells were grown on coverslips and were mounted using an anti-quenching agent (Prolong antifade; Invitrogen). The Image-Pro Plus v.5.0 software was used for image deconvolution and Z-stacks.
Detecting Surface-Bound Antibodies
Confluent monolayers of transfected OK cells in 24-well plates were rinsed three times in cold PBS then exposed to the anti-F7 primary antibody (1:100) in PBS (with 5% BSA) for 1.5 hours at 4°C. Cells were then vigorously washed five times with ice-cold PBS. Forty microliters of Laemmli buffer was added to each well; cells were scraped off the plates and sonicated. The homogenates were electrophoresed via SDS-PAGE in 12% polyacrylamide gels, then blotted onto nitrocellulose membranes. Intact Xenopus oocytes were exposed to the primary antibody as explained above but in a Xenopus saline solution. Thirty oocytes were then washed and homogenized in 1 ml of Barth solution followed by 5 minutes of centrifugation at 180 g to remove debris. The supernatant was then centrifuged for 20 minutes at 21,000 g to recover cell membranes. The pellet was resuspended in Laemmli buffer, applied to a lane of the SDS-PAGE gel, and treated as above. Western blotting was performed as previously described49 except that only the secondary antibody was used to probe the blot.
FRG Mutation Analysis
Of 60 FRG patients investigated, eight showed no SLC5A2 mutations and four had mutations on only one allele, despite massive renal glucose excretion. Genomic DNA was extracted from lymphocytes of these 12 patients. PCR products including single exon and adjacent intronic segments of PDZK1IP1 (which encodes the MAP17 protein) were generated with specific primer pairs (available upon request), purified and sequenced with an automated ABI Sequencer. Results were compared with the human PDZK1IP1 Reference Sequence (NM_005764.3).
Cloning of human PDZK1IP1 Gene
The gene was amplified from human genomic DNA with oligonucleotides GGGGGAATTCCTAGCTCCTCTCCTCCAGGG and AAAAGGATCCACCTAGACACGGTCTGAGCT, then purified, cleaved with BamHI and EcoRI, and inserted into pcDNA3.1(-). The cDNA was sequenced to confirm identity.
Mutating the PDZK1IP1 Gene
Overlap extension was performed using oligonucleotides TAATACGACTCACTATAGGG, AGCACTCATGGCTCCTC, GAGGAGCCATGAGTGCT, and TAGAAGGCACAGTCGAGG. The product was confirmed by sequencing.
Statistical Analyses
All data are expressed as means±SDs. Unless otherwise described, statistical analyses employed one-way ANOVAs with Bonferroni post-hoc analysis.
Study Approval
All Xenopus laevis frog manipulations (anesthesia, surgery, and euthanasia of the frogs after the final collection of oocytes) were performed in accordance with the Canadian guidelines and were approved by the ethics committee of the Université de Montréal (CDEA, protocol #15–042).
Disclosures
None.
Acknowledgments
This work was supported by a Canadian Institute of Health Research grant MOP-10580 (J.Y.L.), a Kidney Foundation of Canada grant (J.Y.L.) and a grant from Nutricia Metabolics, Erlangen, Germany (R.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Sabolic I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H: Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol 302: C1174–C1188, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells RG, Pajor AM, Kanai Y, Turk E, Wright EM, Hediger MA: Cloning of a human kidney cDNA with similarity to the sodium-glucose cotransporter. Am J Physiol 263: F459–F465, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Santer R, Kinner M, Schneppenheim R, Hillebrand G, Kemper M, Ehrich J, Swift P, Skovby F, Schaub J: The molecular basis of renal glucosuria: mutations in the gene for a renal glucose transporter (SGLT2). J Inherit Metab Dis 23[Suppl 1]: 178, 2000 [Google Scholar]
- 4.Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, Brodehl J, Daschner M, Ehrich JH, Kemper M, Li Volti S, Neuhaus T, Skovby F, Swift PG, Schaub J, Klaerke D: Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 14: 2873–2882, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ullman CG, Frigotto L, Cooley RN: In vitro methods for peptide display and their applications. Brief Funct Genomics 10: 125–134, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Hummel CS, Lu C, Loo DD, Hirayama BA, Voss AA, Wright EM: Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol 300: C14–C21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright EM: Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol 280: F10–F18, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Ikari A, Suketa Y: Expression of GFP-tagged low affinity Na+-dependent glucose transporter in Xenopus oocytes and CHO cells. Jpn J Physiol 52: 395–398, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Kocher O, Cheresh P, Lee SW: Identification and partial characterization of a novel membrane-associated protein (MAP17) up-regulated in human carcinomas and modulating cell replication and tumor growth. Am J Pathol 149: 493–500, 1996 [PMC free article] [PubMed] [Google Scholar]
- 10.Hediger MA, Coady MJ, Ikeda TS, Wright EM: Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature 330: 379–381, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Blasco T, Aramayona JJ, Alcalde AI, Catalán J, Sarasa M, Sorribas V: Rat kidney MAP17 induces cotransport of Na-mannose and Na-glucose in Xenopus laevis oocytes. Am J Physiol Renal Physiol 285: F799–F810, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Gisler SM, Kittanakom S, Fuster D, Wong V, Bertic M, Radanovic T, Hall RA, Murer H, Biber J, Markovich D, Moe OW, Stagljar I: Monitoring protein-protein interactions between the mammalian integral membrane transporters and PDZ-interacting partners using a modified split-ubiquitin membrane yeast two-hybrid system. Mol Cell Proteomics 7: 1362–1377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santer R, Calado J: Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol 5: 133–141, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Finch NA, Linser PJ, Ochrietor JD: Hydrophobic interactions stabilize the basigin-MCT1 complex. Protein J 28: 362–368, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Fernández E, Jiménez-Vidal M, Calvo M, Zorzano A, Tebar F, Palacín M, Chillarón J: The structural and functional units of heteromeric amino acid transporters. The heavy subunit rBAT dictates oligomerization of the heteromeric amino acid transporters. J Biol Chem 281: 26552–26561, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Verrey F, Singer D, Ramadan T, Vuille-dit-Bille RN, Mariotta L, Camargo SM: Kidney amino acid transport. Pflugers Arch 458: 53–60, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Lee YJ, Kim MO, Ryu JM, Han HJ: Regulation of SGLT expression and localization through Epac/PKA-dependent caveolin-1 and F-actin activation in renal proximal tubule cells. Biochim Biophys Acta 1823: 971–982, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Pribanic S, Gisler SM, Bacic D, Madjdpour C, Hernando N, Sorribas V, Gantenbein A, Biber J, Murer H: Interactions of MAP17 with the NaPi-IIa/PDZK1 protein complex in renal proximal tubular cells. Am J Physiol Renal Physiol 285: F784–F791, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Lanaspa MA, Giral H, Breusegem SY, Halaihel N, Baile G, Catalán J, Carrodeguas JA, Barry NP, Levi M, Sorribas V: Interaction of MAP17 with NHERF3/4 induces translocation of the renal Na/Pi IIa transporter to the trans-Golgi. Am J Physiol Renal Physiol 292: F230–F242, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kocher O, Cheresh P, Brown LF, Lee SW: Identification of a novel gene, selectively up-regulated in human carcinomas, using the differential display technique. Clin Cancer Res 1: 1209–1215, 1995 [PubMed] [Google Scholar]
- 21.Guijarro MV, Castro ME, Romero L, Moneo V, Carnero A: Large scale genetic screen identifies MAP17 as protein bypassing TNF-induced growth arrest. J Cell Biochem 101: 112–121, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Guijarro MV, Link W, Rosado A, Leal JF, Carnero A: MAP17 inhibits Myc-induced apoptosis through PI3K/AKT pathway activation. Carcinogenesis 28: 2443–2450, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Kocher O, Comella N, Tognazzi K, Brown LF: Identification and partial characterization of PDZK1: a novel protein containing PDZ interaction domains. Lab Invest 78: 117–125, 1998 [PubMed] [Google Scholar]
- 24.Lamprecht G, Seidler U: The emerging role of PDZ adapter proteins for regulation of intestinal ion transport. Am J Physiol Gastrointest Liver Physiol 291: G766–G777, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Gisler SM, Stagljar I, Traebert M, Bacic D, Biber J, Murer H: Interaction of the type IIa Na/Pi cotransporter with PDZ proteins. J Biol Chem 276: 9206–9213, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Donowitz M, Mohan S, Zhu CX, Chen TE, Lin R, Cha B, Zachos NC, Murtazina R, Sarker R, Li X: NHE3 regulatory complexes. J Exp Biol 212: 1638–1646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gisler SM, Pribanic S, Bacic D, Forrer P, Gantenbein A, Sabourin LA, Tsuji A, Zhao ZS, Manser E, Biber J, Murer H: PDZK1: I. a major scaffolder in brush borders of proximal tubular cells. Kidney Int 64: 1733–1745, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Seidler U, Singh AK, Cinar A, Chen M, Hillesheim J, Hogema B, Riederer B: The role of the NHERF family of PDZ scaffolding proteins in the regulation of salt and water transport. Ann N Y Acad Sci 1165: 249–260, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Cinar A, Chen M, Riederer B, Bachmann O, Wiemann M, Manns M, Kocher O, Seidler U: NHE3 inhibition by cAMP and Ca2+ is abolished in PDZ-domain protein PDZK1-deficient murine enterocytes. J Physiol 581: 1235–1246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zachos NC, Tse M, Donowitz M: Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Gisler SM, Madjdpour C, Bacic D, Pribanic S, Taylor SS, Biber J, Murer H: PDZK1: II. an anchoring site for the PKA-binding protein D-AKAP2 in renal proximal tubular cells. Kidney Int 64: 1746–1754, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Kim H, Abd Elmageed ZY, Davis C, El-Bahrawy AH, Naura AS, Ekaidi I, Abdel-Mageed AB, Boulares AH: Correlation between PDZK1, Cdc37, Akt and breast cancer malignancy: the role of PDZK1 in cell growth through Akt stabilization by increasing and interacting with Cdc37. Mol Med 20: 270–279, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarma GN, Moody IS, Ilouz R, Phan RH, Sankaran B, Hall RA, Taylor SS: D-AKAP2:PKA RII:PDZK1 ternary complex structure: Insights from the nucleation of a polyvalent scaffold. Protein Sci 24: 105–116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silver DL, Wang N, Vogel S: Identification of small PDZK1-associated protein, DD96/MAP17, as a regulator of PDZK1 and plasma high density lipoprotein levels. J Biol Chem 278: 28528–28532, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Carnero A: MAP17, a ROS-dependent oncogene. Front Oncol 2: 112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carnero A: MAP17 and the double-edged sword of ROS. Biochim Biophys Acta 1826: 44–52, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Guijarro MV, Leal JF, Blanco-Aparicio C, Alonso S, Fominaya J, Lleonart M, Castellvi J, Ramon y Cajal S, Carnero A: MAP17 enhances the malignant behavior of tumor cells through ROS increase. Carcinogenesis 28: 2096–2104, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Perez M, Praena-Fernandez JM, Felipe-Abrio B, Lopez-Garcia MA, Lucena-Cacace A, Garcia A, Lleonart M, Roncador G, Marin JJ, Carnero A: MAP17 and SGLT1 protein expression levels as prognostic markers for cervical tumor patient survival. PLoS One 8: e56169, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishikawa N, Oguri T, Isobe T, Fujitaka K, Kohno N: SGLT gene expression in primary lung cancers and their metastatic lesions. Jpn J Cancer Res 92: 874–879, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, Moatamed NA, Huang J, Koepsell H, Barrio JR, Wright EM: Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U S A 112: E4111–E4119, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu S, Song E, Tian R, Ma S, Yang T, Mu Y, Li Y, Shao C, Gao S, Gao Y: Systematic analysis of a simple adaptor protein PDZK1: ligand identification, interaction and functional prediction of complex. Cell Physiol Biochem 24: 231–242, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G: Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol 25: 2028–2039, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Claperon A, Mergey M, Fouassier L: Roles of the scaffolding proteins NHERF in liver biology. Clin Res Hepatol Gastroenterol 35: 176–181, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Sugiura T, Shimizu T, Kijima A, Minakata S, Kato Y: PDZ adaptors: their regulation of epithelial transporters and involvement in human diseases. J Pharm Sci 100: 3620–3635, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Lin R, Murtazina R, Cha B, Chakraborty M, Sarker R, Chen TE, Lin Z, Hogema BM, de Jonge HR, Seidler U, Turner JR, Li X, Kovbasnjuk O, Donowitz M: D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology 140: 560–571, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coady MJ, Wallendorff B, Gagnon DG, Lapointe JY: Identification of a novel Na+/myo-inositol cotransporter. J Biol Chem 277: 35219–35224, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Johnson, KY, Liu, L, Vincent, TS: Minimal FLAG sequence useful in the functional epitope tagging of H-Ras. Biotechniques 32: 1270–1280, 2002. [DOI] [PubMed]
- 48.Bissonnette P, Gagné H, Coady MJ, Benabdallah K, Lapointe JY, Berteloot A: Kinetic separation and characterization of three sugar transport modes in Caco-2 cells. Am J Physiol 270: G833–G843, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Bissonnette P, Noël J, Coady MJ, Lapointe JY: Functional expression of tagged human Na+-glucose cotransporter in Xenopus laevis oocytes. J Physiol 520: 359–371, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]