Abstract
Observations in patients with ANCA-associated vasculitis suggest that CD8+ T cells participate in disease, but there is no experimental functional evidence of pathologic involvement for these cells. Myeloperoxidase (MPO) is a well defined autoantigen in ANCA-associated vasculitis. Studies in experimental models of anti-MPO GN suggest that, after ANCA–induced neutrophil localization, deposited MPO within glomeruli is recognized by autoreactive T cells that contribute to injury. We tested the hypothesis that CD8+ T cells mediate disease in experimental ANCA–associated vasculitis. CD8+ T cell depletion in the effector phase of disease attenuated injury in murine anti–MPO GN. This protection associated with decreased levels of intrarenal IFN-γ, TNF, and inflammatory chemokines and fewer glomerular macrophages. Moreover, we identified a pathogenic CD8+ T cell MPO epitope (MPO431–439) and found that cotransfer of MPO431–439–specific CD8+ T cell clones exacerbated disease mediated by MPO–specific CD4+ cells in Rag1−/− mice. Transfer of MPO431–439–specific CD8+ cells without CD4+ cells mediated glomerular injury when MPO was planted in glomeruli. These results show a pathogenic role for MPO–specific CD8+ T cells, provide evidence that CD8+ cells are a therapeutic target in ANCA-associated vasculitis, and suggest that a molecular hotspot within the MPO molecule contains important CD8+, CD4+, and B cell epitopes.
Keywords: ANCA, glomerulonephritis, vasculitis, immunology
In ANCA-associated vasculitis (AAV), experimental data indicate that both humoral and cellular arms of the immune system mediate glomerular injury. In experimental models, antimyeloperoxidase (anti-MPO) antibodies activate neutrophils and mediate neutrophil–mediated glomerular injury.1,2 In humans3 and mice,4 this process results in MPO deposition in the glomerulus. Thus, ANCA-activated neutrophils not only induce injury, but also, they deposit the autoantigen MPO within the target tissue, the kidney. Although experimental evidence shows that MPO peptides can be recognized by MPO–specific CD4+ T cells that effect injury,4 several lines of evidence implicate CD8+ cells in AAV. The proportion of activated CD8+ cells is increased in the blood of patients with AAV, including an expanded population of CD8+CD28+CD11b+ cells that can produce IFN-γ.5–7 In the kidney, CD8+ cells are at least as common as CD4+ cells,3,8 and their numbers correlate with lower patient eGFR at presentation.3 Furthermore, an active, nonexhausted CD8+ T cell phenotype is a marker of poor prognosis in AAV, identifying patients who suffer disease relapses.9,10 However, there are no experimental studies confirming an injurious role for CD8+ cells in ANCA-associated GN.
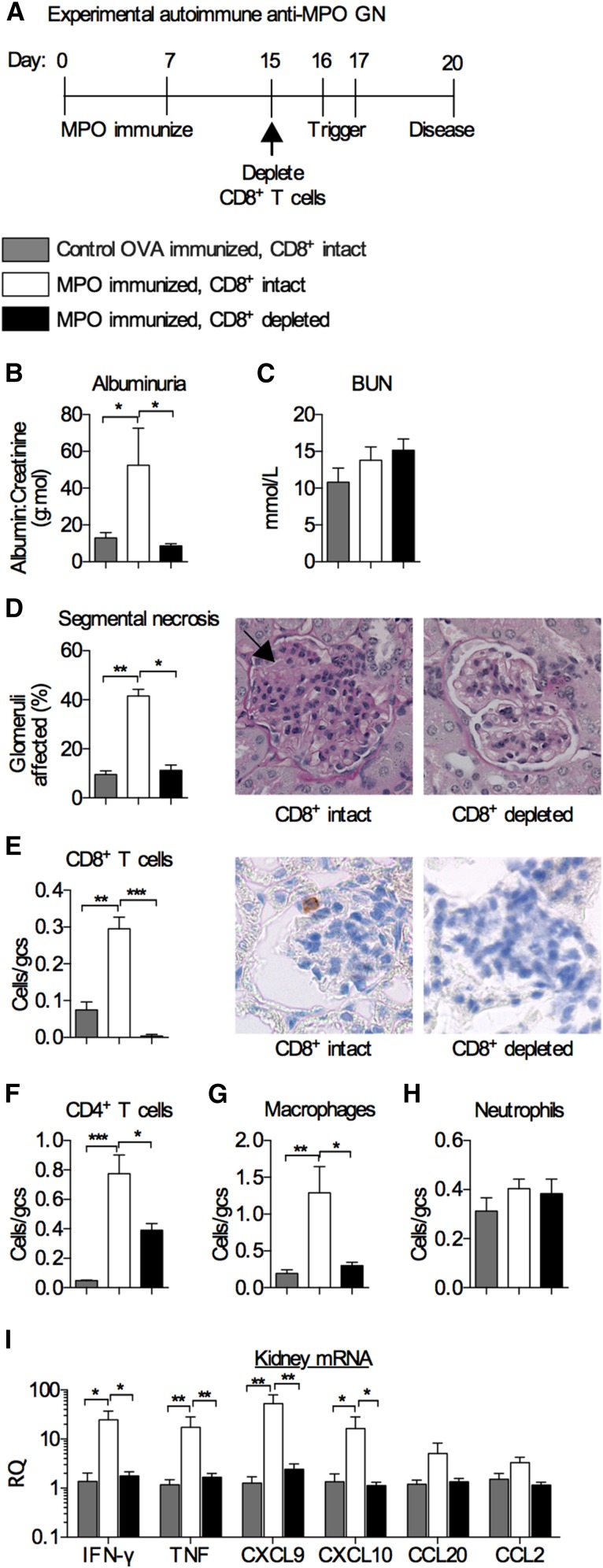
CD8+ T cells recognize peptides presented by MHC class I. They can kill cells via perforin and granzymes and also, secrete cytokines, including IFN-γ and TNF, that have effects on tissue cells and immune cells.11 In addition to their function in host defense, CD8+ cells have been defined as key mediators of autoimmune diseases.12 To determine the role of CD8+ T cells as effectors of glomerular injury in experimental AAV, we depleted CD8+ T cells using an anti-CD8b mAb in experimental murine autoimmune anti–MPO GN. Injury in this model involves inducing anti-MPO autoimmunity in mice. Although anti-MPO antibodies are present in this model, they are insufficient to induce disease, and therefore, disease is triggered by recruiting neutrophils to glomeruli with low–dose sheep anti–mouse glomerular basement membrane (GBM) antibodies (Figure 1A). In these models, recruited neutrophils deposit the autoantigen, MPO, in glomerular capillaries, allowing MPO to be recognized locally by effector T cells.4,13 CD8+ cell depletion efficiency in the circulation was >90% at the time of trigger (Supplemental Figure 1A), and as anticipated, humoral autoimmunity (anti–MPO IgG levels) was unaffected (Supplemental Figure 1B). CD8+ T cell intact mice developed albuminuria and focal proliferative GN, with areas of segmental necrosis. Depletion of CD8+ T cells attenuated albuminuria (Figure 1B), whereas BUN was not elevated in this model (Figure 1C). CD8+ cell depletion also limited histologic injury (Figure 1D). Infiltrating glomerular CD8+ T cells were not present after depletion (Figure 1E), and, glomerular CD4+ T cells and macrophages (but not neutrophils) were also reduced (Figure 1, F–H). Depletion of CD8+ T cells reduced the intrarenal CD8+ T cell cytokines IFN-γ and TNF as well as the IFN-γ–inducible chemokines CXCL9 and CXCL10. There was a trend to decreased CCL20 and CCL2 (Figure 1H), whereas CXCL1 and CCL5 were not increased at this time point in this model (Supplemental Figure 1, C and D). Interstitial cellular infiltrates showed fewer CD8+ T cells, with a trend toward fewer macrophages in CD8+ cell depleted mice (P=0.07), but interstitial CD4+ T cells and neutrophils were not elevated at this stage of disease (Supplemental Figure 1, E–H).
Figure 1.
Depletion of CD8+ T cells attenuates experimental autoimmune anti–MPO GN. (A) Timeline of CD8+ T cell depletion using an anti-CD8b mAb (clone YTS169.4). Disease parameters assessed at day 20 were (B) albuminuria; (C) BUN; (D) glomerular segmental necrosis (representative periodic acid–Schiff–stained micrographs depict segmental necrosis in the CD8+ intact group; arrow); and infiltration of (E) CD8+ T cells (representative DAB Brown–stained micrographs depict an intraglomerular CD8+ T cell in the nondepleted group), (F) CD4+ T cells, (G) macrophages, and (H) neutrophils. (I) Kidney mRNA expression of key CD8+ T cell cytokines IFN-γ and TNF as well as inflammatory chemokines CXCL9, CXCL10, CCL20, and CCL2. All bar graphs represent means±SEMs of n=6 per group. gcs, Glomerular cross-section; RQ, relative quantification. *P<0.05 by ANOVA; **P<0.01 by ANOVA; ***P<0.001 by ANOVA.
When we enumerated the numbers of infiltrating CD8+ T cells and determined the phenotype of the intrarenal CD8+ T cells, we found an increase in the number of intrarenal CD8+ T cells in disease(Supplemental Figure 2A), in particular, CD8+ T cells that made IFN-γ and TNF but not Granzyme B (Supplemental Figure 2B). In the spleen, after MPO or ovalbumin (OVA) immunization, as expected, numbers of CD8+ T cells increased (Supplemental Figure 2C), including increases in the proportions of IFN-γ– and TNF-expressing cells (Supplemental Figure 2D). This increase, compared with that in OVA–immunized control mice, shows that proinflammatory CD8+ T cells infiltrate the kidney. In the context of our previous studies showing that MPO–specific CD4+ T cells can mediate experimental necrotizing GN,4,13 studies implicating signaling through the TCR and the IL-7 receptor in CD8+ cells as predictors of disease relapse in humans,9,10 and human biopsy studies showing significant numbers of intrarenal CD8+ cells at presentation and in relapse,3,8,14 these results support a significant pathogenic role for effector CD8+ T cells.
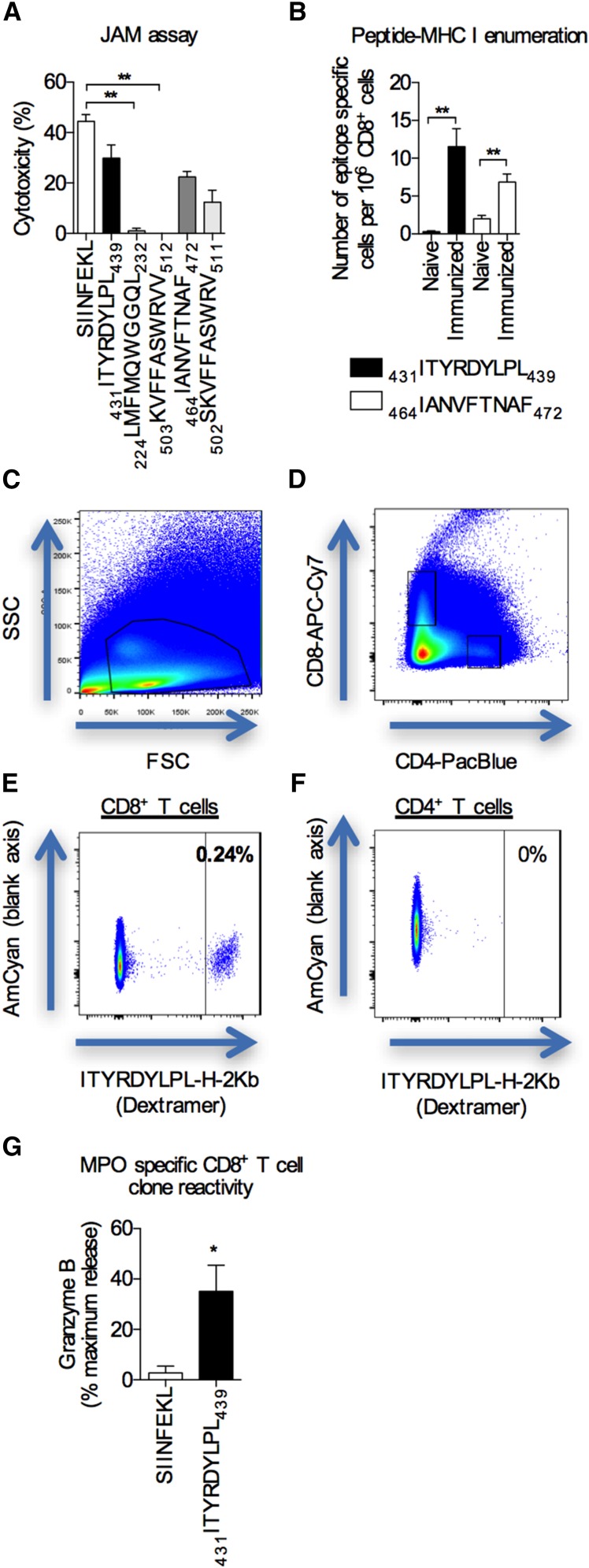
To confirm that MPO–specific CD8+ T cells could mediate glomerular injury, we first identified potential CD8+ T cell epitopes to generate CD8+ clones to test their nephritogenic potential. Using the Immune Epitope Database, we selected five peptides derived from mouse MPO that were predicted in silico to bind to the mouse MHC class I, H-2Kb, that also had the potential to bind to commonly expressed human MHC class I molecules (Supplemental Table 1). To determine the CD8+ T cell–mediated cytotoxicity of these selected epitopes, we performed an in vitro cytotoxicity assay using cells from separate groups of mice immunized with each peptide. A model CD8+ T cell epitope derived from OVA (257SIINFEKL264; subscripts are amino acid positions within the whole protein) served as a positive control. Two of the five selected peptides consistently induced significant cytotoxicity: 431ITYRDYLPL439 and 464IANVFTNAF472 (mouse MPO sequence) (Figure 2A). To determine the immunogenicity of these epitopes in vivo, the expansion of epitope–specific CD8+ T cells was quantified by enumerating the numbers of epitope–specific CD8+ T cells in spleen and lymph nodes of mice using pMHCI dextramers before and after immunization with either 431ITYRDYLPL439 or 464IANVFTNAF472. Both peptides induced expansion of peptide–specific CD8+ T cells in vivo. Mice immunized with 431ITYRDYLPL439 exhibited a 40-fold increase in epitope–specific CD8+ T cells, whereas mice immunized with 464IANVFTNAF472 had a 4-fold increase (Figure 2, B–F). For the 431ITYRDYLPL439 epitope alone, this represents ≥600 cells in secondary lymphoid organs. In autoimmune disease, autoreactive effector T cells localize to sites of inflammation and may represent a significant proportion of the approximately 10,000 excess intrarenal CD8+ cells found in this model (Supplemental Figure 2A).
Figure 2.
Identification of potential MPO CD8+ cell epitopes and selecting MPO–specific CD8+ T cell clones. (A) The potential pathogenicity of MPO CD8+ T cell epitopes (x axis) was determined using a JAM assay using cells from mice immunized with the relevant peptides. The known CD8+ T cell epitope for OVA, SIINFEKL, was used as a positive control. Bar graphs represent the means±SEMs of four independent experiments performed in triplicate. **P<0.01 by one-way ANOVA. (B) The numbers of MPO epitope–specific CD8+ T cells in spleens and lymph nodes from either naïve or immunized (either 431ITYRDYLPL439 or 464IANVFTNAF472) mice were enumerated using peptide-MHC class I dextramers and expressed as numbers of epitope-specific cells per 106 CD8+ T cells. Bar graphs represent the means±SEMs of n=4 per group. **P<0.01 by unpaired t test. (C–F) Representative flow cytometry plots showing the gating strategy used to enumerate MPO epitope–specific CD8+ T cells post-tetramer enrichment. The MFI of the highest CD4+ T cell was used as the extent of the negative control to determine the gate cutoff for epitope–specific CD8+ T cells. In this example, after enrichment, 0.24% is equivalent to 14 epitope-specific cells per 1 million CD8+ T cells. (G) MPO-specific reactivity was measured by pulsing target EL4 cells with the MPO peptide, 431ITYRDYLPL439 (SIINFEKL-pulsed cells were used as a negative control), measuring granzyme B release using a colorimetric granzyme B assay, and expressing the data as percentages of maximum release (Triton X lysed cells). Bar graphs represent the means±SEMs of three independent experiments performed in triplicate. *P<0.01 by unpaired t test.
On the basis of the increased immunogenicity of the 431ITYRDYLPL439 peptide (equivalent to the human sequence 457ITYRDYLPL465), we generated CD8+ T cell clones specific for 431ITYRDYLPL439. To confirm that the generated CD8+ T cell clone was specific for 431ITYRDYLPL439, we performed a granzyme B release assay and showed that coculture of the CD8+ T cell clones induced granzyme B release only when cocultured with its cognate peptide and not in the presence of a control peptide (Figure 2G). Clones were IL-2 dependent and expressed the IL-7Rα (data not shown).
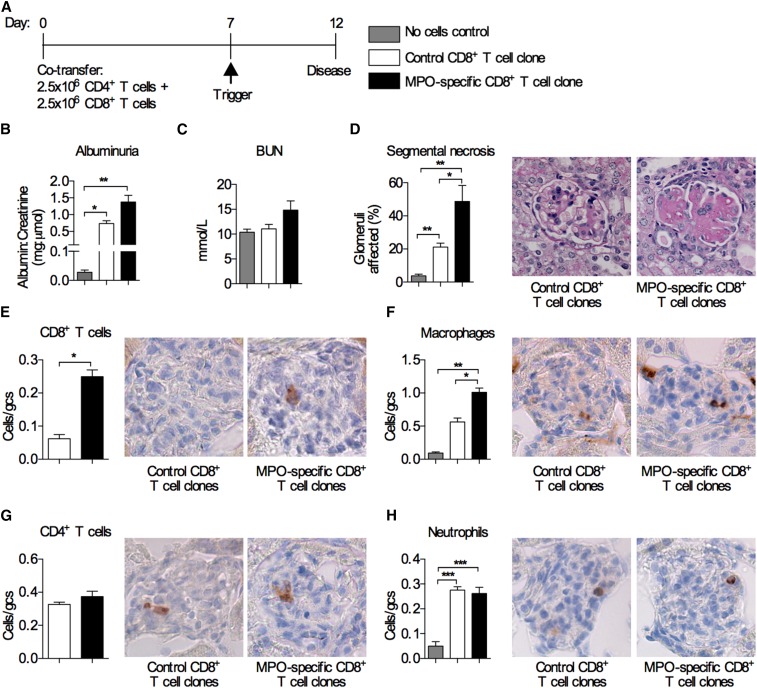
We have previously shown that the transfer of MPO–specific CD4+ T cells into Rag1−/− mice (deficient in both T and B cells) caused focal necrotizing GN after MPO was deposited in glomeruli.4 To determine if MPO–specific CD8+ T cells could augment CD4+ T cell–mediated injury, we cotransferred low doses on the basis of a dose that was one half the number of cells transferred in our previously published models4: 2.5×106 MPO–specific CD8+ T cell clones and 2.5×106 MPO–specific CD4+ T cell clones into Rag1−/− mice. Control mice received either no cells or CD8+ T cell clones specific for 257SIINFEKL264 and MPO–specific CD4+ T cells (Figure 3A). Mice that received the MPO–specific CD8+ T cell clone with CD4+ cells developed more albuminuria and increased proportions of segmental necrosis compared with mice receiving MPO–specific CD4+ cells with control CD8+ T cell clones (Figure 3, B–D). The MPO–specific CD8+ T cell clones’ accumulation within glomeruli (Figure 3E) was accompanied by more glomerular macrophages (Figure 3F). Numbers of glomerular infiltrating CD4+ T cells and neutrophils were similar between the groups (Figure 3, G and H). These results show that MPO–specific CD8+ T cells amplify CD4+ T cell–mediated glomerular injury.
Figure 3.
Cotransfer of MPO–specific CD8+ T cell clones together with MPO–specific CD4+ T cells exacerbates experimental anti–MPO GN. (A) Timeline of MPO–specific CD4+ and CD8+ T cell transfer into Rag1−/− mice and the disease model. Control mice received MPO–specific CD4+ T cells and CD8+ T cell clones specific for OVA 257SIINFEKL264. Another control group received anti-GBM globulin alone without cells. Disease parameters assessed were (B) albuminuria; (C) BUN; (D) glomerular segmental necrosis (periodic acid–Schiff–stained micrographs show segmental necrosis in mice that received the MPO–specific CD8+ T cell clones); and infiltration of (E) CD8+ T cells, (F) macrophages, (G) CD4+ T cells, and (H) neutrophils (DAB Brown–stained micrographs depict illustrative examples of glomerular infiltration of the respective cell type). All bar graphs represent means±SEMs of n=4–6 per group. gcs, Glomerular cross-section. *P<0.05 by ANOVA; **P<0.01 by ANOVA; ***P<0.001 by ANOVA.
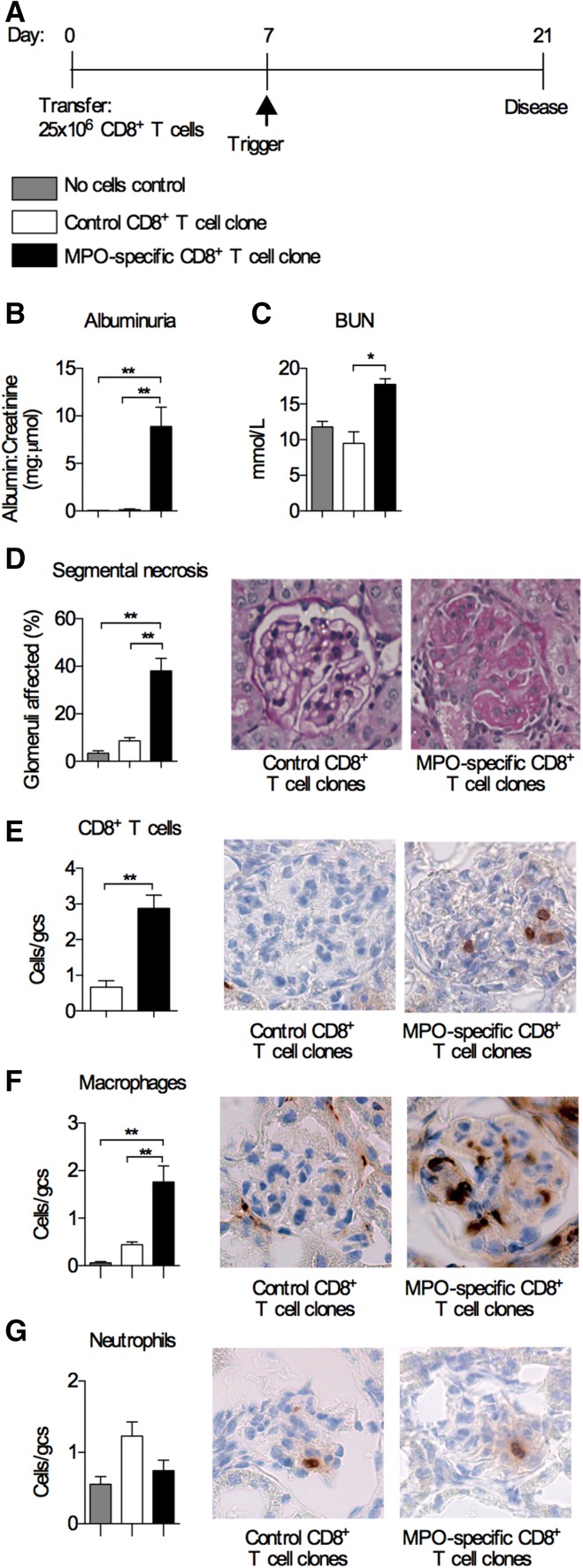
To determine whether effector MPO–specific CD8+ T cells could mediate injury independent of CD4+ effectors, we transferred 25×106 MPO431–439–specific CD8+ T cell clones (the same dose of MPO–specific CD4+ cells was shown to mediate disease4) into Rag1−/− mice, triggered disease 7 days later, and assessed GN 14 days after trigger (Figure 4A). Control Rag1−/− mice received 25×106 CD8+ T cell clones specific for 257SIINFEKL264. Mice that received MPO–specific CD8+ T cells developed markedly elevated albuminuria, BUN, and severe segmental necrotic glomerular lesions (Figure 4, B–D) with more infiltrating CD8+ T cells and macrophages compared with mice receiving control CD8+ T cells (Figure 4, E and F). Neutrophil numbers were similar between groups (Figure 4G). Therefore, even in the absence of CD4+ T cells, effector MPO–specific CD8+ T cells can infiltrate the glomerulus and mediate glomerular injury when MPO is lodged in the glomerulus.
Figure 4.
Transfer of MPO–specific CD8+ T cell clones alone causes anti-MPO GN. (A) Timeline of transfer of MPO–specific CD8+ T cells into Rag1−/− mice and the disease model. Control mice received CD8+ T cells specific for OVA 257SIINFEKL264. Another control group received anti-GBM globulin alone without cells. Disease parameters assessed were (B) albuminuria; (C) BUN; (D) glomerular segmental necrosis (periodic acid–Schiff–stained micrographs show segmental necrosis in mice that received MPO–specific CD8+ T cell clones); and infiltration of (E) CD8+ T cells, (F) macrophages, and (G) neutrophils (DAB Brown–stained micrographs depict illustrative examples of glomerular infiltration of the respective cell type). All bar graphs represent means±SEMs of n=4–6 per group. gcs, Glomerular cross-section. *P<0.05 by ANOVA; **P<0.01 by ANOVA.
In these CD8+ T cell clone transfer experiments, MPO–specific CD8+ T cells accumulate in the kidney when MPO is deposited in glomeruli. Therefore, CD8+ T cells need to recognize MPO to mediate injury in an antigen-specific manner. Although glomerular endothelial cells, a primary tissue cell target in AAV, do not themselves express MPO, several lines of evidence suggest that intrinsic renal cells, including glomerular endothelial cells, can present MPO peptides on their MHC class I derived from MPO released by leukocytes. Several reports have shown that nonleukocyte-associated MPO is present within the lesion of patients with AAV,3,15–17 that endothelial cells themselves can take up MPO,18 and that crosspresentation to CD8+ cells occurs in endothelial cells19 and podocytes.20 In humans, it is likely that MPO-ANCA–mediated neutrophil activation induces glomerular neutrophil recruitment and local release of MPO.
The human disease–associated B cell epitope, 421RKIVGAMVQIITY433 (subscripts indicate mouse amino acid numbering), overlaps with the nephritogenic CD8+ T cell epitope identified in this study, 431ITYRDYLPL439 (bold letters are overlapping sequences).4,21 Furthermore, it is adjacent to the immunodominant murine CD4+ T cell epitope, 409PRWNGEKLYQEARKIVGAMV428.4 This indicates that there may be an immunogenic hotspot within the MPO molecule that could be exploited for peptide–based or antigen–specific tolerogenic therapies. For example, mucosal tolerance using a single peptide spanning the CD4+ and CD8+ T cell epitopes and the B cell epitope could plausibly affect all three key immune cell types in AAV, which has been shown experimentally for anti–MPO CD4+ cells.22 However, we note that our MPO CD8+ cell epitopes are candidate epitopes that serve as proof of concept that MPO–specific CD8+ cells can induce injury, which is shown in our studies.
The results in this study are concordant with observations in other models of autoimmune disease, including diabetes, arthritis, and anti-GBM disease, using depleting anti–CD8 mAbs23–25 as well as experimental autoimmune encephalomyelitis, which showed that the transferred myelin–specific CD8+ T cells induce disease.26 Collectively, these studies support observations in patients with AAV,3,8–10 providing functional evidence that MPO–specific CD8+ T cells are important mediators of focal necrotizing GN.
Concise Methods
Experimental Autoimmune Anti–MPO–Associated GN
Male C57BL/6 WT mice (8–10 weeks old) were purchased from Monash Animal Services (Melbourne, Victoria, Australia), and Rag1−/− male mice (8–10 weeks old) were purchased from the Walter and Eliza Hall Institute (Melbourne, Victoria, Australia). Studies were approved by the Monash University Animal Welfare Committee. Anti-MPO autoimmunity was induced by subcutaneously injecting C57BL/6 WT mice with murine MPO: first, with 20 μg emulsified in Freund Complete Adjuvant and then, with 10 μg on day 7 in Freund Incomplete Adjuvant. Control mice received an irrelevant antigen, OVA. Disease was triggered by injecting mice intravenously with 1.5 mg sheep anti–mouse GBM globulin on days 16 and 17. Injury was assessed on day 20 (Figure 1A).4,13 To deplete CD8+ T cells, mice were injected with 1 mg anti-CD8b mAb (YTS169.4) 1 day before triggering disease (day 15). Control mice received rat IgG antibodies. The efficiency of depletion was determined by measuring the percentage of CD3+CD8a+ cells in the blood by flow cytometry on day 16. Fluorochrome-conjugated antibodies used were from eBioscience (San Diego, CA): anti–mouse CD3-APC and anti–mouse CD8a-eFluoro780.
To determine the pathogenicity of MPO–specific CD8+ cells, CD8+ cells were transferred into Rag1−/− mice immunized with the cognate peptide. To trigger disease, 1 mg sheep anti–mouse GBM globulin was injected intravenously. In previous studies, CD4+ cells could induce a similar degree of injury when responding to MPO lodged in glomeruli by anti-GBM globulin, MPO peptide–conjugated non–nephritogenic anti–GBM mAbs, or anti-MPO antibodies with LPS.4 Given this equivalence, disease was triggered using sheep anti–mouse GBM globulin. Control mice that did not receive cells were immunized with the MPO peptide.
Assessment of Renal Injury
Urinary albumin was measured by ELISA (Bethyl Laboratories, Montgomery, TX) and expressed as an albumin-to-creatinine ratio.4 BUN was measured by autoanalyzer at Monash Health (Melbourne, Victoria, Australia). Glomerular segmental necrosis was assessed on periodic acid–Schiff–stained, formalin–fixed kidney sections. CD8+ T cells, CD4+ T cells, macrophages, and neutrophils were counted on periodate-lysine paraformaldehyde–fixed kidney sections stained using the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA) and DAB Brown using the following antibodies: anti-mouse CD8a (53–6.72; BioXcell, West Lebanon, NH), anti-mouse CD4 (GK1.5; American Type Culture Collection, Manassas, VA), anti-mouse CD68 (FA/11; gift from Gordon L. Koch, MRC Laboratory of Molecular Biology, Cambridge, United Kingdom), and anti–Gr-1 (RB6–8C5; DNAX, Palo Alto, CA).
To detect intrarenal expression of cytokines and chemokines, RNA was extracted from whole kidney in Trizol (Thermo Fisher Scientific, Vernon Hills, IL ), treated with 1 U DNase I (Thermo Fisher Scientific), primed with random primers (Thermo Fisher Scientific), and reversed transcribed using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Gene-specific amplification was performed using either TaqMan Gene Expression Assays (Thermo Fisher Scientific) or custom oligonucleotide primers designed using the Primer 3 software (Whitehead Institute for Biomedical Research, Cambridge, MA), standardized to 18S, and expressed as relative quantification compared with control mice immunized with OVA using the ΔΔCT method. Serum anti–MPO IgG antibodies were measured by ELISA at a serum dilution of 1:4000.
Enumeration and Characterization of CD8+ T Cells by Flow Cytometry
Cell isolation was performed as previously described.27,28 Kidneys were digested with 5 mg/ml collagenase D (Roche Diagnostics, Indianapolis, IN) and 100 mg/ml DNase I (Roche Diagnostics) in HBBS (Sigma-Aldrich, St. Louis, MO) for 30 minutes at 37°C. Cells were filtered, erythrocytes were lysed, and the CD45+ leukocyte population was isolated using mouse CD45 microbeads (Miltenyi Biotec, San Diego, CA). For IFN-γ and TNF intracellular cytokine staining, CD45+–enriched renal cells were activated by incubation with 50 ng/ml PMA (Sigma-Aldrich), 1 mg/ml ionomycin (Sigma-Aldrich), and 10 mg/ml brefeldin A (Sigma-Aldrich) in RPMI complete medium (Gibco, Carlsbad, CA) for 3 hours at 37°C and 5% CO2. Cells were stained with anti-mouse CD45 (Pacific Blue; 30-F11; BioLegend, San Diego, CA) and anti-mouse CD8 (APC-eFlour780; 53–6.7; eBioscience). Intracellular cytokine staining was performed using the Foxp3/Transcription Factor Fixation/Permeabilization Kit as per the manufacturer’s protocol (eBioscience). Cells were stained with anti–mouse IFN-γ (PE; XMG1.2; BD Biosciences, San Jose, CA), anti-mouse TNF (PE; MP6-XT22; BD Biosciences), and anti–mouse Granzyme B (PerCP-eFluor710; NGZB; eBioscience). Cells were suspended with 10 μl SPHERO AccuCount Particles (1000 beads per microliter; Spherotech, Lake Forest, IL), and 1000 beads were acquired on the FACSCanto II Instrument using FACSDiva software, version 6.1.2 (BD Biosciences). Flow cytometric data were analyzed using FlowJo software, version 8.8.6 (TreeStar, Inc., Ashland, OR).
Generation of MPO–Specific CD8+ T Cell Clones
Peptides predicted to bind to mouse H2-Kb (Supplemental Table 1) were synthesized at a purity of >95% (Mimotopes, Notting Hill, Victoria, Australia). The ability of the peptides to induce a CD8+ T cell cytotoxic response was determined using a JAM assay.29 Effector T cells were generated by priming C57BL/6 WT mice in vivo by subcutaneous injection in the base of tail with 100 μg peptide emulsified in Freund Incomplete Adjuvant. Ten days after immunization, 25×106 spleen cells were harvested and cultured in vitro with 25×106 irradiated (2000 rads) syngeneic spleen cells in the presence of 50 μg/ml peptide for 5 days at 37°C with 7% CO2. Target cells were generated from B cell blasts by culturing 30×106 spleen cells in 30 ml IMDM containing 50 μg/ml LPS for 48 hours with [3H]-thymidine (5 μg/ml) added during the last 16 hours of culture. Effectors and target cells were cultured in 96–well microtiter plates at effector-to-target ratios between 100:1 and 1:3. Cytotoxic lymphocyte activity was determined by the decrease in counts per minute measured by an automated liquid scintillation counter and expressed as percentage cytotoxicity.29 The result presented in Figure 2A was performed at an effector-to-target ratio of 3:1. The finding that 431ITYRDYLPL439 and 464IANVFTNAF472 had similar cytotoxicity compared with the positive control 257SIINFEKL264 was consistent across all effector-to-target ratios.
Expansion of epitope–specific CD8+ T cells was determined by enumerating the number of epitope-specific cells in naïve mice compared with mice 10 days after immunization with 100 μg peptide emulsified in Freund Complete Adjuvant. Peptide-MHC class 1 dextramers (Immudex, Copenhagen, Denmark) were used to identify CD8+ T cells specific for either 431ITYRDYLPL439 or 464IANVFTNAF472. Spleen and lymph node cells were pooled, stained with peptide-H2-Kb-PE dextramers, enriched using anti–PE magnetic beads,30 and then, stained with anti–mouse CD4-APC (negative control) and anti–mouse CD8-APC-Cy7 antibodies for flow cytometry. The mean fluorescence intensity of the CD4+ T cells was used to determine the cutoff for epitope–specific CD8+ T cells (Figure 2, C–F). The results are expressed as number of epitope specific per 1 million CD8+ T cells.
To generate CD8+ T cell clones,31 C57BL/6 mice were immunized with 100 μg 431ITYRDYLPL439, the CD8+ T cells were isolated from lymph node and spleen cells using magnetic beads (Miltenyi Biotec), and then, they cultured in the presence of 431ITYRDYLPL439 for 3 days. Clonal populations were isolated by repeated micromanipulation and reactivity with 431ITYRDYLPL439 confirmed by measuring IFN-γ in the supernatant. RNA analysis of our selected CD8+ T cell clone showed transcription of IFN-γ, granzyme B, and perforin, consistent with a CD8+ effector T cell phenotype. CD8+ and CD4+ T cell clones were expanded in the presence of cognate peptide (50 μg/ml), irradiated syngeneic spleen cells (2000 rads) as antigen-presenting cells, and 10 ng/ml IL-2 in complete DMEM-20 (Thermo Fisher Scientific). To determine antigen specificity, CD8+ T cell clones were cultured in the presence of target cells (EL4) and media alone, 431ITYRDYLPL439, or SIINFEKL for 4 hours at 37°C. Granzyme B activity was measured spectrophotometrically using N-α-benzyloxycarbonyl-l-lysine thiobenzyl ester and 5,5′-dithio-bis(2-nitrobenzoic acid) and expressed as a percentage of maximum release relative to Triton X lysed cells.32
Statistical Analyses
An unpaired t test was used to analyze the difference between two groups, and a one-way ANOVA was used when there were more than two groups using GraphPad Prism Software (GraphPad Software Inc., San Diego, CA). A P value of <0.05 was considered a statistically significant difference. Data are expressed as means±SEMs.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Claerwen Jones and Prof. Francis Carbone for their advice in the cytotoxic lysis assays.
This work was supported by funding from National Health and Medical Research Council of Australia grant 1084869.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015121356/-/DCSupplemental.
References
- 1.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC: Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huugen D, Xiao H, van Esch A, Falk RJ, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, Jennette JC, Heeringa P: Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: Role of tumor necrosis factor-alpha. Am J Pathol 167: 47–58, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan KM, Lo CY, Summers SA, Elgass KD, McMillan PJ, Longano A, Ford SL, Gan PY, Kerr PG, Kitching AR, Holdsworth SR: Renal participation of myeloperoxidase in antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Kidney Int 88: 1030–1046, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Ooi JD, Chang J, Hickey MJ, Borza DB, Fugger L, Holdsworth SR, Kitching AR: The immunodominant myeloperoxidase T-cell epitope induces local cell-mediated injury in antimyeloperoxidase glomerulonephritis. Proc Natl Acad Sci U S A 109: E2615–E2624, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda M, Tsuru S, Watanabe Y, Kitahara S, Inouye T: Reduced CD4-CD8 T cell ratios in patients with Wegener’s granulomatosis. J Clin Lab Immunol 38: 103–109, 1992 [PubMed] [Google Scholar]
- 6.Schlesier M, Kaspar T, Gutfleisch J, Wolff-Vorbeck G, Peter HH: Activated CD4+ and CD8+ T-cell subsets in Wegener’s granulomatosis. Rheumatol Int 14: 213–219, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Iking-Konert C, Vogl T, Prior B, Wagner C, Sander O, Bleck E, Ostendorf B, Schneider M, Andrassy K, Hansch GM: T lymphocytes in patients with primary vasculitis: Expansion of CD8+ T cells with the propensity to activate polymorphonuclear neutrophils. Rheumatology (Oxford) 47: 609–616, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Berden AE, Kallenberg CG, Savage CO, Yard BA, Abdulahad WH, de Heer E, Bruijn JA, Bajema IM: Cellular immunity in Wegener’s granulomatosis: Characterizing T lymphocytes. Arthritis Rheum 60: 1578–1587, 2009 [DOI] [PubMed] [Google Scholar]
- 9.McKinney EF, Lyons PA, Carr EJ, Hollis JL, Jayne DR, Willcocks LC, Koukoulaki M, Brazma A, Jovanovic V, Kemeny DM, Pollard AJ, Macary PA, Chaudhry AN, Smith KG: A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat Med 16: 586–591, 2010 [DOI] [PMC free article] [PubMed]
- 10.McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG: T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523: 612–616, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakanishi Y, Lu B, Gerard C, Iwasaki A: CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature 462: 510–513, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai S, Shameli A, Santamaria P: CD8+ T cells in type 1 diabetes. Adv Immunol 100: 79–124, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Ruth AJ, Kitching AR, Kwan RY, Odobasic D, Ooi JD, Timoshanko JR, Hickey MJ, Holdsworth SR: Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 17: 1940–1949, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Weidner S, Carl M, Riess R, Rupprecht HD: Histologic analysis of renal leukocyte infiltration in antineutrophil cytoplasmic antibody-associated vasculitis: Importance of monocyte and neutrophil infiltration in tissue damage. Arthritis Rheum 50: 3651–3657, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE: Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15: 623–625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouwer E, Huitema MG, Mulder AH, Heeringa P, van Goor H, Tervaert JW, Weening JJ, Kallenberg CG: Neutrophil activation in vitro and in vivo in Wegener’s granulomatosis. Kidney Int 45: 1120–1131, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Kawashima S, Arimura Y, Sano K, Kudo A, Komagata Y, Kaname S, Kawakami H, Yamada A: Immunopathologic co-localization of MPO, IgG, and C3 in glomeruli in human MPO-ANCA-associated glomerulonephritis. Clin Nephrol 79: 292–301, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Yang JJ, Kettritz R, Falk RJ, Jennette JC, Gaido ML: Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am J Pathol 149: 1617–1626, 1996 [PMC free article] [PubMed] [Google Scholar]
- 19.von Oppen N, Schurich A, Hegenbarth S, Stabenow D, Tolba R, Weiskirchen R, Geerts A, Kolanus W, Knolle P, Diehl L: Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology 49: 1664–1672, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Goldwich A, Burkard M, Olke M, Daniel C, Amann K, Hugo C, Kurts C, Steinkasserer A, Gessner A: Podocytes are nonhematopoietic professional antigen-presenting cells. J Am Soc Nephrol 24: 906–916, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth AJ, Ooi JD, Hess JJ, van Timmeren MM, Berg EA, Poulton CE, McGregor J, Burkart M, Hogan SL, Hu Y, Winnik W, Nachman PH, Stegeman CA, Niles J, Heeringa P, Kitching AR, Holdsworth S, Jennette JC, Preston GA, Falk RJ: Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest 123: 1773–1783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gan PY, Tan DS, Ooi JD, Alikhan MA, Kitching AR, Holdsworth SR: Myeloperoxidase peptide-based nasal tolerance in experimental ANCA-associated GN. J Am Soc Nephrol 27: 385–391, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips JM, Parish NM, Raine T, Bland C, Sawyer Y, De La Pena H, Cooke A: Type 1 diabetes development requires both CD4+ and CD8+ T cells and can be reversed by non-depleting antibodies targeting both T cell populations. Rev Diabet Stud 6: 97–103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raposo BR, Rodrigues-Santos P, Carvalheiro H, Agua-Doce AM, Carvalho L, Pereira da Silva JA, Graca L, Souto-Carneiro MM: Monoclonal anti-CD8 therapy induces disease amelioration in the K/BxN mouse model of spontaneous chronic polyarthritis. Arthritis Rheum 62: 2953–2962, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Reynolds J, Norgan VA, Bhambra U, Smith J, Cook HT, Pusey CD: Anti-CD8 monoclonal antibody therapy is effective in the prevention and treatment of experimental autoimmune glomerulonephritis. J Am Soc Nephrol 13: 359–369, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J: A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med 194: 669–676, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan AJ, Alikhan MA, Odobasic D, Gan PY, Khouri MB, Steinmetz OM, Mansell AS, Kitching AR, Holdsworth SR, Summers SA: Innate IL-17A-producing leukocytes promote acute kidney injury via inflammasome and Toll-like receptor activation. Am J Pathol 184: 1411–1418, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Alikhan MA, Summers SA, Gan PY, Chan AJ, Khouri MB, Ooi JD, Ghali JR, Odobasic D, Hickey MJ, Kitching AR, Holdsworth SR: Endogenous toll-like receptor 9 regulates AKI by promoting regulatory T cell recruitment. J Am Soc Nephrol 27: 706–714, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wonderlich J, Shearer G, Livingstone A, Brooks A: Induction and measurement of cytotoxic T lymphocyte activity. Curr Protoc Immunol 3: 3.11, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK: Tracking epitope-specific T cells. Nat Protoc 4: 565–581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitch FW, Gajewski TF, Hu-Li J: Production of TH1 and TH2 cell lines and clones. Curr Protoc Immunol 3: 3.13, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Taffs R, Sitkovsky M, Takayama H: Granule enzyme exocytosis assay for cytotoxic T lymphocyte activation. Curr Protoc Immunol 3: 3.16, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.