Abstract
AKI is histologically characterized by necrotic cell death and inflammation. Diverse pathways of regulated necrosis have been reported to contribute to AKI, but the molecular regulators involved remain unclear. We explored the relative contributions of ferroptosis and necroptosis to folic acid (FA)–induced AKI in mice. FA-AKI in mice associates with lipid peroxidation and downregulation of glutathione metabolism proteins, features that are typical of ferroptotic cell death. We show that ferrostatin-1 (Fer-1), an inhibitor of ferroptosis, preserved renal function and decreased histologic injury, oxidative stress, and tubular cell death in this model. With respect to the immunogenicity of ferroptosis, Fer-1 prevented the upregulation of IL-33, an alarmin linked to necroptosis, and other chemokines and cytokines and prevented macrophage infiltration and Klotho downregulation. In contrast, the pancaspase inhibitor zVAD-fmk did not protect against FA-AKI. Additionally, although FA-AKI resulted in increased protein expression of the necroptosis mediators receptor–interacting protein kinase 3 (RIPK3) and mixed lineage domain–like protein (MLKL), targeting necroptosis with the RIPK1 inhibitor necrostatin-1 or genetic deficiency of RIPK3 or MLKL did not preserve renal function. Indeed, compared with wild-type mice, MLKL knockout mice displayed more severe AKI. However, RIPK3 knockout mice with AKI had less inflammation than their wild-type counterparts, and this effect associated with higher IL-10 concentration and regulatory T cell-to-leukocyte ratio in RIPK3 knockout mice. These data suggest that ferroptosis is the primary cause of FA-AKI and that immunogenicity secondary to ferroptosis may further worsen the damage, although necroptosis-related proteins may have additional roles in AKI.
Keywords: acute renal failure, renal proximal tubule cell, inflammation, cell death, ferroptosis, IL-33
AKI implies that kidney damage results in an acute and usually transient decrease in renal function. The incidence of AKI is increasing as an aging population is subjected to complex medical procedures. Inflammation, parenchymal cell loss, and nephron loss are features of AKI that may eventually lead to tubulointerstitial fibrosis.1 Patients with AKI have a higher risk of developing CKD and ESRD.2 Mortality is as high as 50%, and even a short-timed injury contributes to a persistently higher mortality.1,2 Currently, no satisfactory treatment attenuates AKI or accelerates recovery. Tubular cell death is an early event in AKI that is followed by tubular dedifferentiation, proliferation, and regeneration. This is accompanied by inflammation through release of chemokines from sublethally injured cells and damage–associated molecular patterns (DAMPs) from dying cells.3 Multiple cell death pathways are involved in AKI, but the precise molecular mechanisms have not been completely elucidated; thus, the therapeutic strategy remains unclear.
Regulated necrosis was recently reported to contribute to different forms of tissue injury.4 Unlike apoptosis, regulated necrosis is more immunogenic, because plasma membrane integrity is lost and dying cells release DAMPs, stimulating and amplifying inflammatory responses; in some cases of regulated necrosis (such as pyroptosis or necroptosis), cytokines are actively produced during the process of dying.5 Necroptosis is the best characterized form of regulated necrosis and relies on phosphorylation of receptor–interacting protein kinase 3 (RIPK3) by RIPK1 and subsequent RIPK3–mediated phosphorylation of the pseudokinase mixed lineage kinase domain–like protein (MLKL). High RIPK3 expression may facilitate the aggregation of RIPK3 oligomers to a higher-order structure (referred to as the necrosome) that induces MLKL phosphorylation.6–8 Necrostatin-1 (Nec-1) is an inhibitor of necroptosis that keeps RIPK1 in its inactive state and thereby, prevents necrosome formation. Necroptosis is an important contributor to tissue injury in the heart, skin, and intestine, but its contribution to renal injury is incompletely characterized.4 Necroptosis may represent an important pathway in nephrotoxic AKI induced by cisplatin.9–12
Ferroptosis is an iron–dependent regulated necrosis subroutine characterized by increased lipid peroxidation resulting from lack of activity of the glutathione peroxidase 4 that requires glutathione to function.13–15 The small molecule ferrostatin-1 (Fer-1) reduces lipid oxidation and inhibits ferroptosis. Ferroptosis was initially observed in cancer cells expressing oncogenic RAS, but it may contribute to other diseases, such as Huntington disease and tubular failure.16 In this regard, inducible glutathione peroxidase 4 depletion in mice caused lipid peroxidation and AKI, which were prevented by Fer-1.15 Moreover, most ferroptosis–sensitive tumor cell lines are derived from renal clear cell carcinomas.13 Indeed, in direct comparison, inhibition of ferroptosis was more protective than inhibition of necroptosis in ischemia-reperfusion renal injury.9 Thus, current state of the art may suggest that ferroptosis is involved in ischemia-reperfusion injury, whereas necroptosis relates more to nephrotoxic AKI. However, given the heterogeneity of AKI triggers, this may be a too simplistic view.
We have now explored the relative role of necroptosis and ferroptosis in experimental toxic AKI induced by a folic acid (FA) overdose (FA-AKI). FA-AKI has been described in humans,17 and the experimental model recapitulates all of the major processes in human AKI, including renal cell death, inflammation, and renal cell regeneration. We found kidney lipid peroxidation in FA-AKI, and we show that Fer-1 prevents renal injury, inflammation, and Klotho downregulation, whereas inhibition of necroptosis on a pharmacologic or genetic level or interference with apoptosis did not. We, therefore, identify ferroptosis as the predominant pathway of regulated necrosis in FA-AKI.
Results
Inhibition of Ferroptosis Prevents Renal Injury in FA-AKI
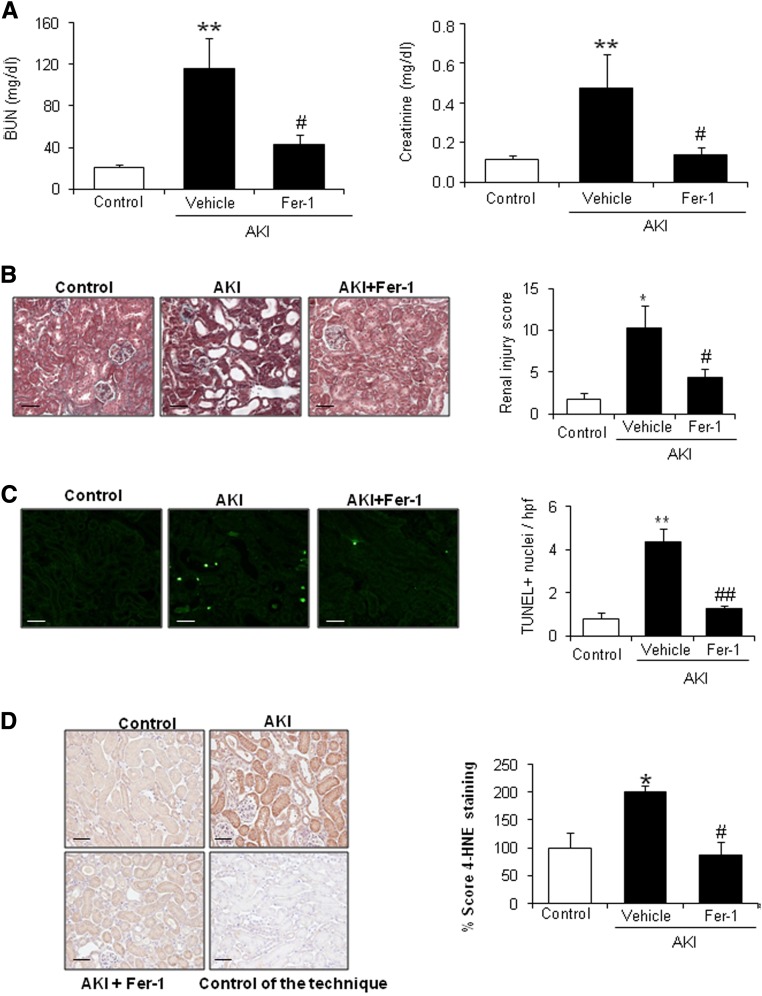
On the basis of the observations that ferroptosis is a major key player in ischemia-reperfusion–induced AKI and that FA-AKI is associated with lipid peroxidation,18 a key feature of ferroptosis, we tested the role of ferroptosis in FA-AKI. Fer-1 pretreatment improved renal function as assessed by plasma BUN and creatinine (Figure 1A) and reduced histologic injury (Figure 1B) and cell death measured by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick–end labeling (TUNEL) (Figure 1C) and lipid peroxidation as assessed by 4-hydroxynonenal levels (Figure 1D). These results suggest that ferroptosis plays a key role in FA-AKI.
Figure 1.
Fer-1 prevented renal injury in FA-induced AKI. C57/BL6 mice were pretreated with Fer-1 for 30 minutes followed by AKI induction by an FA overdose and euthanasia at 48 hours. (A) Renal function was assessed by plasma creatinine and BUN levels. Fer-1 preserved renal function. Mean±SEM of seven mice per group. **P<0.01 versus control; #P<0.04 versus AKI alone. (B) Fer-1 decreased histologic injury in kidneys from mice exposed to FA. Representative image of Masson staining and quantification. Mean±SEM of seven mice per group. Original magnification, ×200. Scale bars, 50 μm. *P<0.02 versus control; #P<0.04 versus AKI alone. (C) Cell death was assessed by TUNEL staining (green). Fer-1 reduced TUNEL+ cells in FA–induced AKI kidneys. Representative image and quantification as mean±SEM of seven mice per group. Original magnification, ×400. Scale bars, 50 μm. **P<0.002 versus control; ##P<0.001 versus AKI alone. (D) 4-Hydroxynonenal (4-HNE) staining in mice with AKI. Fer-1 decreased 4-HNE levels. Mean±SEM of seven animals per group. Original magnification, ×200. Scale bars, 50 μm. *P<0.03 versus control; #P<0.02 versus AKI alone.
Ferroptosis Triggers Inflammation in FA-AKI
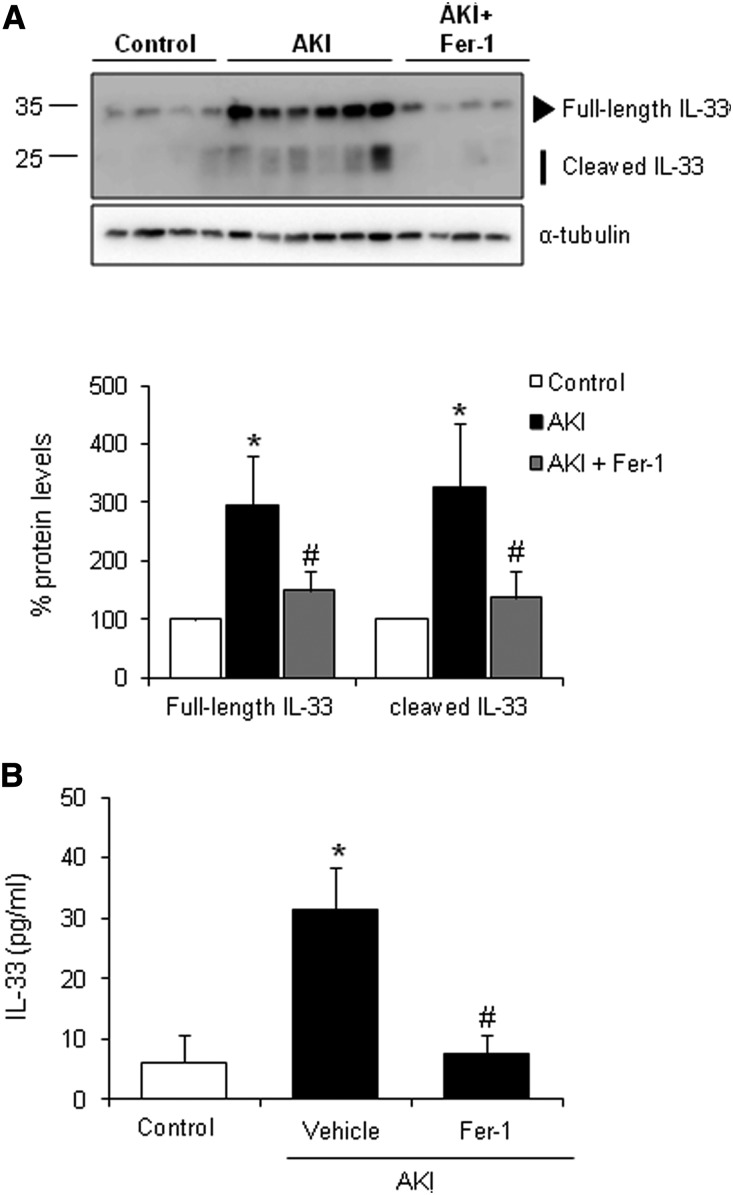
Inflammation plays a key role in amplifying renal injury, and ferroptosis is immunogenic and proinflammatory, because ferroptotic cells release DAMPs and alarmins, which amplify both cell death and inflammation.3 IL-33 is an alarmin released by necroptotic cells that can mediate renal injury,19,20 but a contributing role in ferroptosis has not been suggested. We observed that, in FA-AKI, kidney IL-33 levels are elevated, and there is processing to its inflammatory form and release into plasma (Figure 2). Interestingly, Fer-1 decreased kidney and circulating IL-33 levels in FA-AKI (Figure 2). These data show that IL-33 is not specific for necroptosis but is also released in a non-necroptotic type of regulated necrosis. It remains to be determined if this is a general feature of all pathways of regulated necrosis or if IL-33 release is limited to necroptosis and ferroptosis.
Figure 2.
Fer-1 decreases IL-33 levels and activation in FA-induced AKI. IL-33 protein levels were measured in mice with AKI pretreated or not pretreated with Fer-1. (A) Kidney IL-33 upregulation and processing observed in AKI at 48 hours was prevented with Fer-1 treatment. Representative Western blot and quantification of IL-33 levels. Mean±SEM of seven animals per group. *P<0.01 versus control; #P<0.04 versus AKI. (B) Plasma IL-33 levels assayed by ELISA were incremented in AKI at 48 hours, and this was prevented by Fer-1. Mean±SEM of seven animals per group. *P<0.05 versus control; #P<0.03 versus AKI.
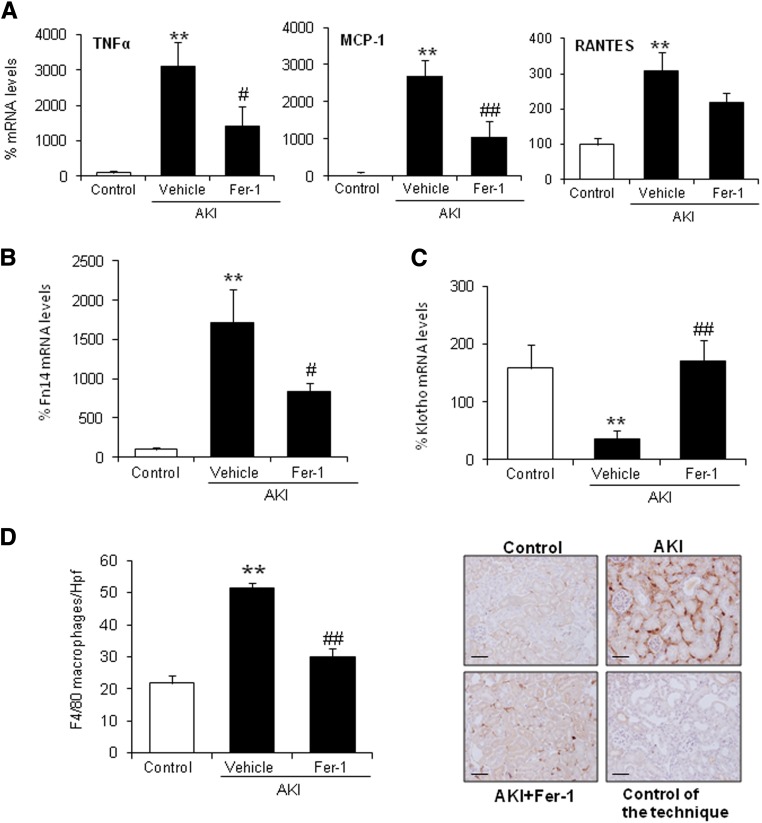
Other than IL-33 processing, we sought to functionally explore the role of ferroptosis-induced inflammation in FA-AKI. Fer-1 partially decreased AKI–associated renal inflammation as shown by the lower kidney expression of proinflammatory cytokines, such as TNFα, MCP-1, and regulated upon activation, normal T cell expressed and secreted (RANTES) (Figure 3A), as well as the TWEAK receptor Fn14 (Figure 3B), a key mediator of AKI.21 Consistent with this, Fer-1 also decreased the number of infiltrating F4/80+ macrophages (Figure 3D), CD3+ T lymphocytes, and myeloperoxidase+ neutrophils (Supplemental Figure 1A). Treg cells are a subtype of T lymphocytes that suppress innate immunity and produce anti-inflammatory cytokines, such as IL-10. Foxp3, a marker for Treg cells, and IL-10 mRNA expressions were increased in kidney tissue in AKI, and this was prevented by Fer-1 (Supplemental Figure 1B). Consistent with the gene expression findings, Fer-1 also decreased the number of kidney–infiltrating Treg cells as assessed by Foxp3 staining (Supplemental Figure 1C). Finally, Fer-1 prevented the decrease in kidney Klotho levels (Figure 3C), an inflammation-dependent phenomenon in AKI.22 This result suggests that ferroptosis may be an early event in AKI that activates an amplification loop of inflammation and cell death. Because tubular cells express Klotho and injured tubular cells are a key source of chemokines and cytokines as well as Fn14 expression, from the observation of milder inflammation and milder Klotho downregulation after Fer-1 treatment, we conclude that the driver of such inflammation is probably tubular necrosis itself. In this regard, Fer-1 seems to decrease all inflammation–associated events, including Treg infiltration, and does not seem to promote Treg–mediated innate immunity suppression.
Figure 3.
Renal inflammation associated with AKI is modulated by Fer-1. (A) Fer-1 prevented the increase in kidney TNFα, MCP-1, and RANTES mRNA levels at 48 hours of AKI. Mean±SEM of seven animals per group. **P<0.002 versus control; #P<0.03 versus AKI alone; ##P<0.003 versus AKI alone. (B and C) Quantification of kidney Fn14 and Klotho mRNA levels. Fer-1 prevented (B) the increase in Fn14 and (C) the decrease in Klotho mRNA expression in AKI at 48 hours. Mean±SEM of seven animals per group. **P<0.003 versus control; #P<0.03 versus AKI alone; ##P<0.003 versus AKI alone. (D) Fer-1 prevented the increase in F4/80–positive interstitial macrophages in AKI kidneys. Mean±SEM of seven animals per group. Original magnification, ×200. Scale bars, 50 μm. **P<0.002 versus control; ##P<0.001 versus AKI alone.
Targeting Apoptosis or Necroptosis Is Not Protective in FA-AKI
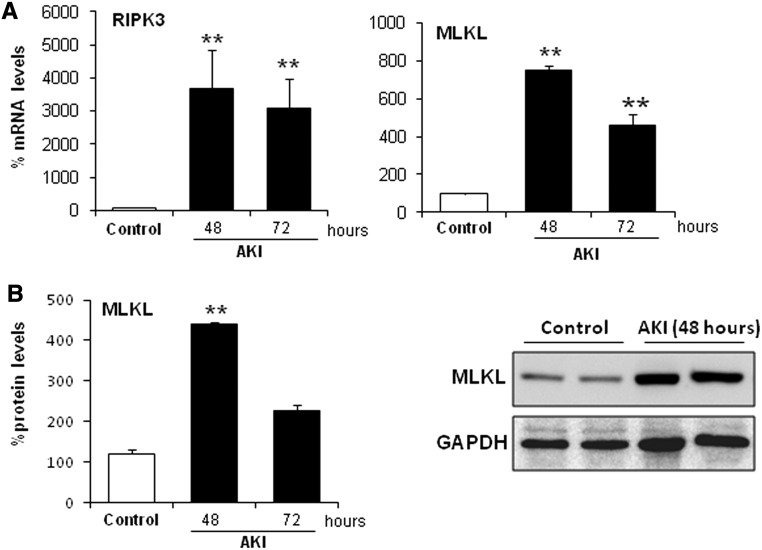
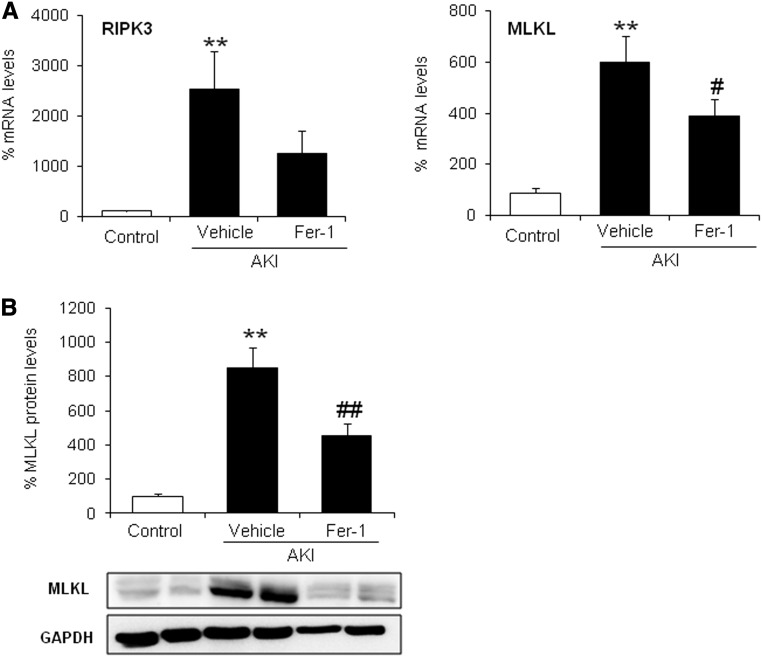
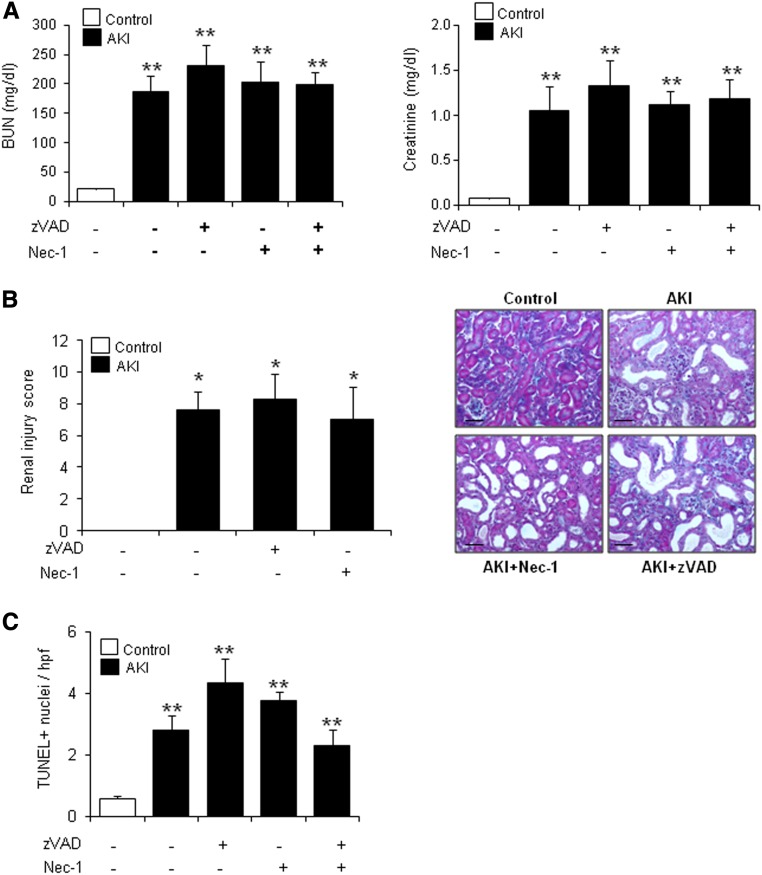
During the course of the FA-AKI, we observed increased protein expression of the key regulators of necroptosis RIPK3 and MLKL (Figure 4). However, necroptosis is activated by phosphorylation of proteins and not activated by their level of expression; therefore, this finding certainly does not reflect the amount of necroptosis that occurs in this model. Ferroptosis might rather increase the susceptibility to necroptosis by amplification of RIPK3 and MLKL. In line with this thought, Fer-1 reduced RIPK3 and MLKL expression (Figure 5). However, although studied in detail in ischemia-reperfusion injury, there is little information on the role of regulated necrosis pathways in nephrotoxic models. We, therefore, explored the contribution of apoptotic and necroptotic cell death pathways in FA-AKI by pretreating with the pancaspase inhibitor zVAD or the RIP1 inhibitor Nec-1, respectively. In line with the high sensitivity to Fer-1 of this AKI model, neither zVAD nor Nec-1 improved renal function (Figure 6A), histologic injury (Figure 6B), or cell death as assessed by TUNEL (Figure 6C). However, cell death pathways could be inter-related, and chemical interference with several pathways may have extra benefit. Thus, we treated mice with zVAD and Nec-1 together, but even this did not result in additional protection (Figure 6, A and C). The lack of protection by zVAD and Nec-1 was not caused by failure to interfere with target molecules. We tested the efficacy of both inhibitors in this in vivo model. We observed that Nec-1 was able to prevent MLKL phosphorylation as assessed by Western blot and that zVAD prevented activation of caspases as assessed by immunohistochemistry of M30 cytodeath that binds to a caspase–generated cytokeratin fragment (Supplemental Figure 2).
Figure 4.
Necroptosis regulatory proteins are upregulated in FA-induced AKI. Kidney RIPK3 and MLKL levels were assessed in mice with AKI over time. (A) RIPK3 and MLKL mRNA expression is increased in a time-dependent fashion during AKI. Mean±SEM of five animals per group. **P<0.001 versus control. (B) Time course of MLKL protein expression in AKI assessed by Western blot. Quantification and representative Western blot. Mean±SEM of five animals per group. **P<0.004 versus control.
Figure 5.
Fer-1 reduces levels of necroptosis regulatory proteins in AKI. (A) Fer-1 prevented the upregulation of kidney RIPK3 and MLKL mRNA expression in AKI. Mean±SEM of seven animals per group. **P<0.003 versus control; #P<0.03 versus AKI alone. (B) Representative Western blot and quantification of kidney MLKL protein. Mean±SEM of seven animals per group. **P<0.002 versus control; ##P<0.003 versus AKI.
Figure 6.
Neither Nec-1 nor zVAD prevents renal injury in FA-induced AKI. C57/BL6 mice were pretreated with zVAD and/or Nec-1 for 30 minutes followed by AKI induction by an FA overdose and euthanasia at 48 hours. (A) Renal function was assessed by plasma creatinine and BUN levels in AKI. Neither zVAD nor Nec-1 preserved renal function. Mean±SEM of seven animals per group. **P<0.003 versus control. (B) Masson staining and quantification of histologic renal damage. Mean±SEM of seven animals per group. Original magnification, ×200. Scale bars, 50 μm. *P<0.04 versus control. (C) Quantification of TUNEL+ cells. Mean±SEM of seven animals per group. **P<0.004 versus control.
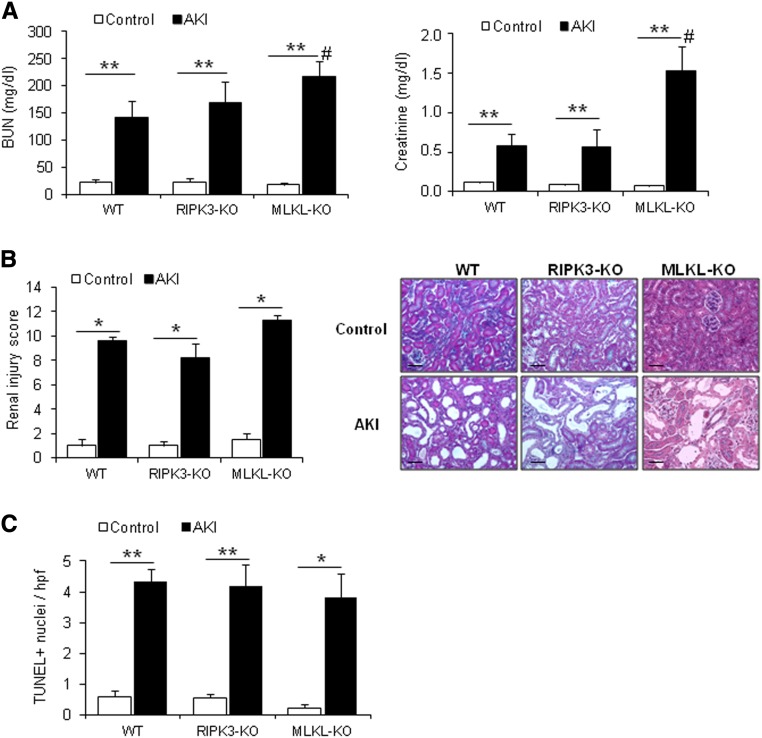
RIPK3 overexpression can sensitize to necroptosis.23 In this regard, necroptosis can be triggered independently of RIPK1. Because in nephrotoxic AKI, RIPK3 and MLKL mRNA and protein expressions were increased (Figure 4), we, therefore, tested the specific role of these proteins in AKI using RIPK3 knockout (KO) mice or MLKL-KO mice. However, the functional analysis of RIPK3-KO and more prominently, MLKL-KO mice revealed a counterintuitive finding. MLKL-KO mice turned out to be hypersensitive to AKI as assessed by renal function parameters (Figure 7A). Worse renal function was associated with more severe histologic damage in MLKL-KO mice (Figure 7B). This finding together with the highly significant upregulation of MLKL after injury (Figure 4) for the first time indicate a role for MLKL in protection from tissue injury or regeneration after specific organ injury, such as FA-AKI.
Figure 7.
RIPK3 and MLKL are required for promoting regeneration after FA-induced AKI. AKI was induced in RIPK3-KO (n=8) and MLKL-KO (n=4–6) mice and their wild-type (WT) littermates (n=7). Mice were euthanized at 48 hours. (A) Renal function was assessed by plasma creatinine and BUN levels. Mean±SEM of four to eight animals per group. **P<0.003 versus control; #P<0.04 versus AKI WT. (B) Masson staining and quantification of histologic renal damage. Mean±SEM of four to eight animals per group. Original magnification, ×200. Scale bars, 50 μm. *P<0.02 versus control. (C) Quantification of TUNEL+ cells. Mean±SEM of four to eight animals per group. *P<0.02 versus control; **P<001 versus control.
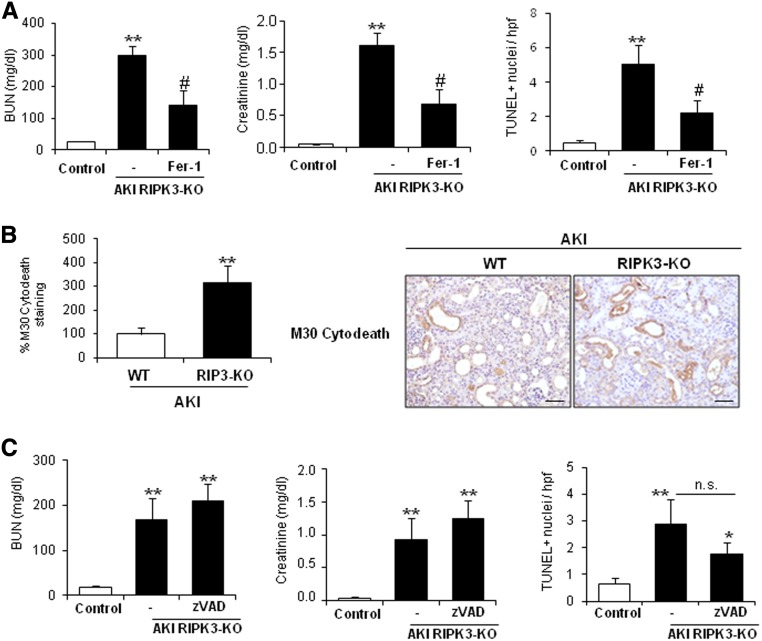
Next, we characterized more in–depth cell death in RIPK3-KO mice. Fer-1 also offered protection in the absence of RIPK3, because it improved renal function and reduced cell death (Figure 8A). RIPK3-KO AKI mice presented increased caspase activation compared with wild–type AKI mice (Figure 8B). However, zVAD did not improve renal injury, despite a nonsignificant trend toward decreasing cell death as assessed by TUNEL (Figure 8C). These results suggest that ferroptosis activation is independent of RIPK3.
Figure 8.
Fer-1, but not zVAD, prevents renal injury in FA-induced AKI in RIPK3-KO mice. (A) RIPK3-KO mice were pretreated with Fer-1 (n=7) or vehicle (n=8) 30 minutes before inducing AKI by an FA overdose and euthanized 48 hours later. Renal function was assessed by plasma creatinine and BUN levels, and cell death was assessed by TUNEL staining. Quantification is expressed as mean±SEM of seven or eight mice per group. **P<0.002 versus control; #P<0.05 versus AKI alone. (B) M30 cytodeath staining to assess caspase activation in RIPK3-KO mice during FA-induced AKI. Representative image and quantification as mean±SEM of seven mice per group. WT, wild type. Original magnification, ×200. Scale bars, 50 μm. **P<0.002 versus WT mice. (C) RIPK3-KO mice were pretreated with zVAD (n=8) or vehicle (n=8) 30 minutes before AKI induction by an FA overdose and euthanized at 48 hours. Renal function was assessed by plasma creatinine and BUN levels, and cell death was assessed by TUNEL staining. Quantification as mean±SEM of eight mice per group. *P<0.02 versus control; **P<0.002 versus control.
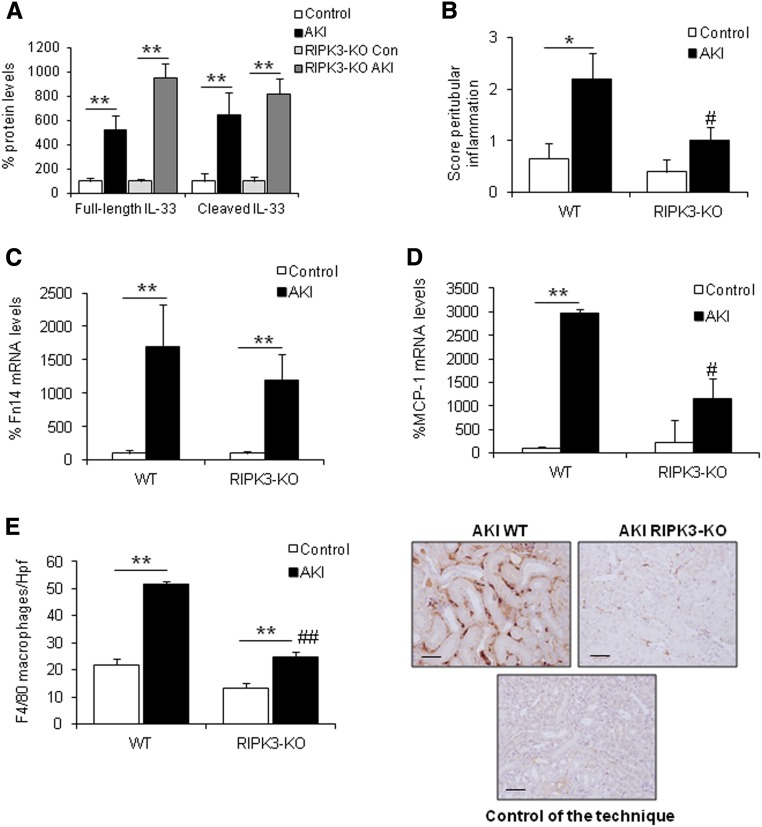
RIPK3 Deficiency Decreases Inflammation in AKI
Finally, we assessed IL-33 protein levels in RIPK3-KO mice to confirm that IL-33 upregulation is a feature of ferroptosis. We observed that IL-33 protein levels were not reduced in RIPK3-KO mice (Figure 9A), whereas these mice presented less inflammation (Figure 9, B–E, Supplemental Figure 3B). Moreover, IL-10 expression is strongly increased in RIPK3-KO mice with AKI but not in MLKL-KO mice compared with wild-type mice (Supplemental Figure 3A). Because IL-33 and IL-10 have anti-inflammatory actions, it is not surprising that MCP-1 levels, peritubular inflammation, and leukocytes infiltration (macrophages, CD3+ T cells, and neutrophils) were strongly reduced in RIPK3-KO mice (Figure 9, B–E, Supplemental Figure 3B). However, Foxp3 mRNA expression and Treg infiltrates, assessed by Foxp3 staining, were similar in RIPK3-KO and wild-type mice (Supplemental Figure 3C). Thus, different leukocyte populations were decreased in RIPK3-KO AKI compared with wild–type AKI mice, but no significant reduction in Tregs was observed, resulting in a higher ratio of Tregs versus other leukocyte populations (Supplemental Figure 3D). Overall, the results are consistent with activation of IL-10–associated anti–inflammatory pathways in RIP3-KO mice, and a role for Tregs is likely, taking into account the parallel effects of the Treg–controlling cytokine IL-33 (Figure 2), whereas zVAD, Nec-1, or MLKL deficiency did not prevent renal inflammation in AKI (Supplemental Figure 4). RIPK3, therefore, seems to be a key regulator in renal inflammation independent of its function on necroptosis.
Figure 9.
RIPK3 deficiency prevented renal inflammation in FA-induced AKI. (A) Kidney IL-33 protein levels were not reduced in RIPK3-KO mice 48 hours after AKI induction. Mean±SEM of seven or eight animals per group. **P<0.004 versus control. (B) Interstitial inflammation was assessed by a peritubular inflammation score, which had a maximum value of three. Data are means±SEM of seven or eight mice per group. *P<0.04 versus control wild type (WT); #P<0.02 versus AKI WT. (C and D) Kidney Fn14 and MCP-1 mRNA levels are lower in RIPK3-KO than in WT mice. Mean of seven or eight animals per group. **P<0.01 versus control WT; #P<0.02 versus AKI WT. (E) RIPK3 deficiency prevented the increase in F4/80–positive interstitial macrophages in AKI kidneys. Quantification as mean±SEM of seven or eight mice per group. Original magnification, ×200. Scale bars, 50 μm. **P<0.001 versus control WT; ##P<0.001 versus AKI WT.
Discussion
The main finding of this study is that ferroptosis plays a key role in nephrotoxic AKI induced by an FA overdose. Reduction of glutathione metabolism and lipid peroxidation, key features of ferroptosis, were noted, and inhibition of ferroptosis with Fer-1 improved renal function and decreased renal injury.
AKI is histologically characterized by tubular cell death and inflammation, but the exact mechanisms of and molecular contributors to cell death are unclear. Recently, regulated necrosis pathways, such as necroptosis and ferroptosis, were proposed to have a key role in acute renal injury. Necroptosis was observed in ischemia-reperfusion injury, cisplatin nephrotoxicity, and rhabdomyolysis- and contrast media–induced AKI.11,24–26 However, necroptosis may not be the main tubular cell death pathway in ischemia-reperfusion injury, where the beneficial effect of necroptosis inhibitors may be because of endothelial protection.9 In fact, ferroptosis seems to be an important death pathway in renal ischemia-reperfusion injury and oxalate nephropathy.9 In accordance with a role of ferroptosis in renal injury in vivo, we observed that, in FA-AKI, Fer-1 improved renal function and decreased lipid peroxidation, tubular injury, and cell death, suggesting that ferroptosis is the main cell death pathway, whereas in other models of renal injury, such as ischemia-reperfusion injury, oxalate nephropathy, and cisplatin, different pathways of cell death, including necroptosis, apoptosis, and ferroptosis, may coexist.9–11,24,27 Although there is evidence that ferroptosis may cause tubular cell death,15 we cannot exclude that Fer-1 is also protecting endothelial cells from death.
Moreover, in FA-AKI, the interference with apoptosis (zVAD) or necroptosis (Nec-1, RIPK3-KO, or MLKL-KO) did not prevent renal injury, although RIPK3 and MLKL protein expressions were upregulated. This suggests that induction of FA-AKI might sensitize the kidney to necroptosis but that necroptosis is not involved in the damage itself in FA-AKI. Unexpectedly, MLKL-KO mice but not RIPK3-KO mice turned out to be more sensitive to FA-AKI compared with wild-type mice. Together with the finding of MLKL upregulation during AKI, this indicates a functional role for MLKL in tissue protection and/or regeneration during FA-AKI in an unexplained manner. To the best of our knowledge, this is the first time that a role for MLKL in tissue protection and/or regeneration has been reported.
These data suggest that, in contrast to other experimental AKI models, where different cell death pathways may initially coexist, ferroptosis seems to be the first cell death pathway to be activated in FA-AKI. Indeed, our results suggest that ferroptosis could be a driver of other pathways of cell death, such as apoptosis or necroptosis, because it promotes inflammatory responses, such as upregulation of cytokines and necroptotic proteins that are quenched by Fer-1.
AKI is characterized by an important inflammatory response that amplifies kidney injury. Necrotic cells can amplify inflammation through the release of DAMPs to the extracellular milieu. The term necroinflammation defines this process.28 DAMPs released by necroptotic cells include IL-33, IL-1α, and HMGB1. Full–length IL-33 has proinflammatory activity by itself, but a processed and more active form is released from necroptotic cells.29–31 IL-33 may have a role in renal inflammation, such as was observed in experimental AKI and human renal transplantation.19,20 Our data show that IL-33 processing and release are prevented by Fer-1 but not prevented by RIPK3 deficiency, suggesting for the time that IL-33 release is related with ferroptosis and questioning the role of IL-33 as a specific necroptotic DAMP.31 Fer-1 also decreased other inflammatory markers, such as cytokines and macrophage infiltrates. On the basis of this observation, we hypothesize that in vivo ferroptosis may modulate inflammation through IL-33 activation. However, the exact mechanism of ferroptosis-induced inflammation remains unknown, because an equilibrium between proinflammatory features5 and stabilizing regulatory T cells (see above) seems to regulate the faint balance between systemic and local requirements for inflammatory stimuli. In this sense, less inflammation without improved kidney function was observed in RIPK3-deficient mice, whereas caspase inhibition or the necroptosis inhibitor, Nec-1, was not protective. These findings suggest a role of RIPK3 in renal inflammation independent of cell death and are in line with the recently published regulatory role of RIPK3 in the control of cytokine expression.32,33 Down this line, RIPK3-KO mice showed increased levels of IL-33 in FA-AKI, and it is known that IL-33 can induce a negative feedback loop and repress inflammation.34,35 In this regard, RIPK3-KO mice displayed higher gene expression levels of the anti–inflammatory cytokine IL-10 as well as a higher ratio of Tregs versus other leukocyte populations, suggesting the potential involvement of IL-10 and/or Tregs. Therefore, more studies are necessary to characterize the exact role of RIPK3 in renal inflammation in different models of AKI.
In conclusion, these data show that ferroptosis is a major mechanism of cell death in FA-AKI that promotes renal inflammation. Targeting ferroptosis should be explored as a potential therapeutic strategy to limit kidney injury and inflammation in toxic renal injury. In addition, the mechanisms of novel roles of the kinase RIPK3 in promoting inflammation independently from regulated necrosis and the pseudokinase MLKL in preventing tissue injury or promoting regeneration should be further explored. Mechanistically, it will be interesting to elucidate the functionally relevant targets of RIPK3 in AKI.
Concise Methods
Animal Model
All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory and approved by the animal ethics committee of IIS-Fundacion Jimenez Diaz. FA nephropathy is a classic model of kidney tubulointerstitial injury and inflammation characterized by tubular cell death, interstitial leukocyte infiltration, and subsequent tubular regeneration36 that has been reported in humans.17 C57BL/6 mice (12–14 weeks old; five per experimental group) received a single intraperitoneal injection of FA (Sigma-Aldrich, St. Louis, MO) of 250 mg/kg in 0.3 mol/L sodium bicarbonate or vehicle and were euthanized 48 or 72 hours later. In a second experiment, mice were dosed intraperitoneally with 5 mg/kg Fer-1 (Santa Cruz Biotechnology, Santa Cruz, CA), 1.65 mg/kg Nec-1 (Sigma-Aldrich), 10 mg/kg zVAD (Bachem, Bubendorf, Switzerland), or DMSO (vehicle) 30 minutes before FA injection. Doses were on the basis of previous report.9,24 Mice were euthanized 48 hours later (n=7 per group). The 48-hour time point was chosen, because it coincided with peak renal dysfunction and was not associated with lethality. In a third set of experiments, RIPK3-KO mice (provided by Kim Newton and Vishva Dixit37; Genentech, South San Francisco, CA) and MLKL-KO mice (provided by John Silke and James Murphy to A.L.) on the C57Bl/6 background or wild-type littermates received intraperitoneal FA injection and were killed 48 hours later (n=4–8 per group). In a fourth set of experiments, RIPK3-KO mice were pretreated with zVAD and Fer-1 before induction of AKI and killed 48 hours later (n=7–8 per group). Plasma samples were collected at the time of euthanasia. Kidneys were perfused in situ with cold saline before removal. One kidney was snap frozen in liquid nitrogen for RNA and protein studies, and the other was fixed and paraffin embedded.
TUNEL
TUNEL was performed in 3-μm-thick sections of paraffin-embedded tissue with the In Situ Cell Death Detection Kit Fluorescein (Roche, Basel, Switzerland) according to the manufacturer’s instructions.
RNA Extraction and Real-Time PCR
Total RNA was extracted by the TRI Reagent method (Invitrogen, Carlsbad, CA), and 1 μg RNA was reverse transcribed with the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Quantitative PCR was performed in a 7500 Real Time PCR System with the Prism 7000 System SDS Software using predeveloped primers (Applied Biosystems), and RNA expression of different genes was corrected for GAPDH.36
Western Blot
Cell samples were homogenized in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 0.2% Triton X-100, 0.3% NP-40, 0.1 mM PMSF, and 1 μg/ml pepstatin A) and then separated by 10% SDS-PAGE under reducing conditions. After electrophoresis, samples were transferred to PVDF membranes (EMD Millipore, Billerica, MA), blocked with 5% skimmed milk in PBS/0.5% Tween 20 (vol/vol) for 1 hour, washed with PBS/Tween, and incubated with anti-MLKL (1:1000; reference ab172868; Abcam, Inc., Cambridge, MA), antiphospho-MLKL (1:500; reference ab196436; Abcam, Inc.), and anti–IL-33 (1:1000; reference AF3626; R&D Systems, Minneapolis, MN) diluted in 5% milk PBS/Tween. Blots were washed with PBS/Tween and incubated with the appropriate horseradish peroxidase–conjugated secondary antibody (1:5000; GE Healthcare, Waukesha, WI). After washing with PBS/Tween, blots were developed with the chemiluminescence method (ECL; Fisher Scientific, Waltham, MA) and probed with mouse monoclonal anti–α-tubulin antibody (1:10,000; reference T5168; Sigma-Aldrich) or anti-GAPDH (1:5000; reference mab374; EMD Millipore). Levels of expression were corrected for minor differences in loading.
IL-33 ELISA
Mouse IL-33 ELISA (catalog no. M3300; R&D Systems) was performed according to the manufacturer’s instructions.
Immunohistochemistry
Immunohistochemistry was carried out as described previously in paraffin–embedded tissue sections 5 μm thick.36 Primary antibodies were rat polyclonal anti–F4/80 (1:50; reference MCAP497; Bio-Rad, Hercules, CA), anti–4-hydroxynonenal (1:400; reference ab46545; Abcam, Inc.), polyclonal anti-Foxp3 (1:10; reference 14–5773; eBioscience, San Diego, CA), rabbit polyclonal anti–CD3 (ready to use; DAKO, Glostrup, Denmark), and rabbit polyclonal antimyeloperoxidase (ready to use; DAKO). Sections were counterstained with Carazzi hematoxylin. Negative controls included incubation with a nonspecific Ig of the same isotype as the primary antibody. The total number of F4/80-positive macrophages was quantitated in 15 randomly chosen fields (40×) using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). Samples were examined in a blinded manner.
Tubular injury was evaluated in Masson section by a pathologist (P.C.-O.) who was blinded to the nature of the samples. Evidence of cell injury (loss of brush border or vacuolization), cell desquamation, and tubular dilation and signs of regeneration were scored on a semiquantitative zero to three scale, and results from each item were added to yield the tubular injury score, which had a maximal value of 18.22
Statistical Analyses
Statistical analysis was performed using the SPSS 11.0 statistical software (IBM SPSS, Chicago, IL). Results are expressed as means±SEM. Significance at the P<0.05 level was assessed by nonparametric Mann–Whitney U tests for two groups and ANOVAs for three or more groups.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by Instituto de Salud Carlos III (ISCIII) and Fondo Europeo de Desarrollo Regional grants: PI13/00047, EUTOX, CP12/03262, CP14/00133, PI15/00298, and PI14/00386; Diabetes Cancer Connect grant PIE13/00051; Sociedad Española de Nefrologia; Fundacion Renal Iñigo Alvarez de Toledo; ISCIII-Redes Temáticas de Investigación Cooperativa en Salud, Red de Investigacion Renal grant RD012/0021; Comunidad de Madrid (CAM) Consorcio de Investigacion en Fracaso Renal Agudo grant S2010/BMD-2378; and Kidney Connect grant FP7-HEALTH-2013-INNOVATION-1-602422 e-PREDICE. Salary support was from Fundacion Conchita Rabago (to D.M.-S.), ISCIII Miguel Servet (to M.D.S.-N. and A.B.S.), and Programa Intensificación Actividad Investigadora (ISCIII/Agencia Laín-Entralgo/CAM; to A.O.). A.L. is supported by German Research Foundation in the Cluster of Excellence “Inflammation at Interfaces” grant EXC306.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015121376/-/DCSupplemental.
References
- 1.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Siew ED, Parr SK, Abdel-Kader K, Eden SK, Peterson JF, Bansal N, Hung AM, Fly J, Speroff T, Ikizler TA, Matheny ME: Predictors of recurrent AKI. J Am Soc Nephrol 27: 1190–1200, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linkermann A, Stockwell BR, Krautwald S, Anders HJ: Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat Rev Immunol 14: 759–767, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Linkermann A, Green DR: Necroptosis. N Engl J Med 370: 455–465, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, Green DR, Oberst A, Albert ML: RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8⁺ T cells. Science 350: 328–334, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK: Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137: 1112–1123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu XN, Yang ZH, Wang XK, Zhang Y, Wan H, Song Y, Chen X, Shao J, Han J: Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ 21: 1709–1720, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X: Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148: 213–227, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Bräsen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S: Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A 111: 16836–16841, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S: Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 110: 12024–12029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z, Wang Y, Huang Z, Ren J, Liu S, Chen X, Han J: A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol 26: 2647–2658, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tristão VR, Pessoa EA, Nakamichi R, Reis LA, Batista MC, Durão Junior MS, Monte JC: Synergistic effect of apoptosis and necroptosis inhibitors in cisplatin-induced nephrotoxicity. Apoptosis 21: 51–59, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR: Regulation of ferroptotic cancer cell death by GPX4. Cell 156: 317–331, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR: Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O’Donnell VB, Kagan VE, Schick JA, Conrad M: Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16: 1180–1191, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR: Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136: 4551–4556, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metz-Kurschel U, Kurschel E, Wagner K, Aulbert E, Graben N, Philipp T: Folate nephropathy occurring during cytotoxic chemotherapy with high-dose folinic acid and 5-fluorouracil. Ren Fail 12: 93–97, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Puri V, Sharma R, Puri S: Folic acid induces acute renal failure (ARF) by enhancing renal prooxidant state. Exp Toxicol Pathol 64: 225–232, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Akcay A, Nguyen Q, He Z, Turkmen K, Won Lee D, Hernando AA, Altmann C, Toker A, Pacic A, Ljubanovic DG, Jani A, Faubel S, Edelstein CL: IL-33 exacerbates acute kidney injury. J Am Soc Nephrol 22: 2057–2067, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thierry A, Giraud S, Robin A, Barra A, Bridoux F, Ameteau V, Hauet T, Girard JP, Touchard G, Gombert JM, Herbelin A: The alarmin concept applied to human renal transplantation: Evidence for a differential implication of HMGB1 and IL-33. PLoS One 9: e88742, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanz AB, Izquierdo MC, Sanchez-Niño MD, Ucero AC, Egido J, Ruiz-Ortega M, Ramos AM, Putterman C, Ortiz A: TWEAK and the progression of renal disease: Clinical translation. Nephrol Dial Transplant 29[Suppl 1]: i54–i62, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno JA, Izquierdo MC, Sanchez-Niño MD, Suárez-Alvarez B, Lopez-Larrea C, Jakubowski A, Blanco J, Ramirez R, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A, Sanz AB: The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol 22: 1315–1325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upton JW, Kaiser WJ, Mocarski ES: Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7: 302–313, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S: Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81: 751–761, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Homsi E, Andreazzi DD, Faria JB, Janino P: TNF-α-mediated cardiorenal injury after rhabdomyolysis in rats. Am J Physiol Renal Physiol 308: F1259–F1267, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Linkermann A, Heller JO, Prókai A, Weinberg JM, De Zen F, Himmerkus N, Szabó AJ, Bräsen JH, Kunzendorf U, Krautwald S: The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice. J Am Soc Nephrol 24: 1545–1557, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulay SR, Desai J, Kumar SV, Eberhard JN, Thomasova D, Romoli S, Grigorescu M, Kulkarni OP, Popper B, Vielhauer V, Zuchtriegel G, Reichel C, Bräsen JH, Romagnani P, Bilyy R, Munoz LE, Herrmann M, Liapis H, Krautwald S, Linkermann A, Anders HJ: Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat Commun 7: 10274, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulay SR, Linkermann A, Anders HJ: Necroinflammation in Kidney Disease. J Am Soc Nephrol 27: 27–39, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaczmarek A, Vandenabeele P, Krysko DV: Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 38: 209–223, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C: IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A 109: 1673–1678, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, Hall C, Spall SK, Phesse TJ, Abud HE, Cengia LH, Corbin J, Mifsud S, Di Rago L, Metcalf D, Ernst M, Dewson G, Roberts AW, Alexander WS, Murphy JM, Ekert PG, Masters SL, Vaux DL, Croker BA, Gerlic M, Silke J: RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157: 1175–1188, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FK: The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity 41: 567–578, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullock AN, Degterev A: Targeting RIPK1,2,3 to combat inflammation. Oncotarget 6: 34057–34058, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pomeshchik Y, Kidin I, Korhonen P, Savchenko E, Jaronen M, Lehtonen S, Wojciechowski S, Kanninen K, Koistinaho J, Malm T: Interleukin-33 treatment reduces secondary injury and improves functional recovery after contusion spinal cord injury. Brain Behav Immun 44: 68–81, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D: TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med 5: 170ra16, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanz AB, Justo P, Sanchez-Niño MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A: The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol 19: 695–703, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton K, Sun X, Dixit VM: Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol 24: 1464–1469, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.