Abstract
Chloride transport by the renal tubule is critical for blood pressure (BP), acid-base, and potassium homeostasis. Chloride uptake from the urinary fluid is mediated by various apical transporters, whereas basolateral chloride exit is thought to be mediated by ClC-Ka/K1 and ClC-Kb/K2, two chloride channels from the ClC family, or by KCl cotransporters from the SLC12 gene family. Nevertheless, the localization and role of ClC-K channels is not fully resolved. Because inactivating mutations in ClC-Kb/K2 cause Bartter syndrome, a disease that mimics the effects of the loop diuretic furosemide, ClC-Kb/K2 is assumed to have a critical role in salt handling by the thick ascending limb. To dissect the role of this channel in detail, we generated a mouse model with a targeted disruption of the murine ortholog ClC-K2. Mutant mice developed a Bartter syndrome phenotype, characterized by renal salt loss, marked hypokalemia, and metabolic alkalosis. Patch-clamp analysis of tubules isolated from knockout (KO) mice suggested that ClC-K2 is the main basolateral chloride channel in the thick ascending limb and in the aldosterone-sensitive distal nephron. Accordingly, ClC-K2 KO mice did not exhibit the natriuretic response to furosemide and exhibited a severely blunted response to thiazide. We conclude that ClC-Kb/K2 is critical for salt absorption not only by the thick ascending limb, but also by the distal convoluted tubule.
Keywords: chloride channels, Bartter-s syndrome, ion transport, transgenic mouse
Bartter syndrome (BS) is a group of renal tubulopathies with autosomal recessive inheritance. First described by Frederic C. Bartter and Pacita Pronove, this syndrome is characterized by marked secondary hyperaldosteronism caused by renal salt-wasting with normal or low BP, hypokalemia, metabolic alkalosis, hypertrophy and hyperplasia of the juxta-glomerular apparatus.1 The syndrome typically presents during the neonatal period and is frequently associated with hypercalciuria and nephrocalcinosis.
The free water clearance and distal fractional chloride reabsorption is low in patients affected by BS. Hence, it has been proposed that the primary pathogenic event leading to the syndrome is impaired NaCl absorption by the thick ascending limb (TAL) of the loop of Henle.2,3 Accordingly, genetic studies revealed that BS is caused by inactivating mutations in one of at least four independent genes (numbered from type 1 to type 4 BS): SLC12A1,4 KCNJ1,5 CLCNKB,6,7 and BSND.8 These genes encode for the Na+/K+/2Cl- cotransporter NKCC2, the potassium channel ROMK, the chloride channel ClC-K2, and Barttin, an essential β-subunit for ClC-K1 and ClC-K2 Cl- channels,9 respectively. These findings suggest a transport model, in which NaCl and K+ are taken up apically via NKCC2. Although K+ has to be recycled across the apical membrane via ROMK to maintain a high luminal K+ concentration that is required for the sustained activity of NKCC2, NaCl leaves the cell basolaterally via the Na+/K+-ATPase and ClC-Kb/Barttin. However, the expression pattern of each ClC-K homolog has not been fully resolved because of the close homology between ClC-K1 and -K2 and the lack of isoform specific antibodies.
Typically, NKCC2 and ROMK inactivation cause a “severe form” of BS, which is characterized by a very early (even antenatal) onset, a marked salt-wasting phenotype, and profound hypokalemia and metabolic alkalosis, as described initially by Bartter and Pronove. This clinical subtype of the syndrome is often associated with polyhydramnios, failure to thrive, and severe hypercalciuria eventually leading to nephrocalcinosis. Because patients excrete high levels of PGE2 with the urine and blockers of PGE2 synthesis significantly alleviate the symptoms, this variant is also called the “hyperprostaglandinuria syndrome”. Mutations in CLCNKB rather cause the “classic” BS, which is diagnosed later in life and characterized by a milder phenotype that lacks both hypercalciuria and nephrocalcinosis. Nevertheless, patients related to CLCNKB show a high phenotypic variability with clinical presentations ranging from very severe salt-losing nephropathy with marked hypokalemia to almost asymptomatic presentation.6 Some patients with CLCNKB mutations exhibit a mild phenotype with moderate salt-wasting, hypocalciuria, and resistance to thiazide diuretics, i.e., typical features of Gitelman syndrome.10 Gitelman syndrome is another salt-losing nephropathy, which is usually caused by inactivating mutations in the gene for the apical NaCl cotransporter NCC (SLC4A3) in the distal convoluted tubule.6,11–15 Finally, mutations in the BSND gene lead to type 4 BS, the most severe form of BS with extreme growth retardation, very severe salt-wasting, and sensorineural deafness.16
Here, we show that disruption of Clcnk2 in the mouse leads to severe BS without hypercalciuria but with elevated levels of PGE2 in the urine. Using two different antibodies in our KO model, we demonstrate that ClC-K1 is expressed in the thin ascending limbs and the medullary TALs (mTALs) of the loop of Henle, whereas ClC-K2 is found in the medullary and cortical portion of the TAL, in the distal convoluted tubule (DCT), and in the basolateral membrane of both α- and β-intercalated cells of the connecting tubule (CNT) and the collecting duct (CD). Unexpectedly, ClC-K2 disruption completely abolished the natriuretic response to both hydrochlorothiazide and furosemide despite the presence of ClC-K1 in the mTAL. In accordance, ClC-K1 activity was barely detectable by patch-clamp experiments. Hence, our data indicate that chloride absorption all along the distal nephron from the TAL to the collecting duct depends on ClC-K2.
Results
Disruption of the Clcnk2 Gene Results in a Severe Phenotype with Early Lethality
To disrupt the Clcnk2 gene in mice, we flanked exons 5–10 with loxP sites by homologous recombination (Supplemental Figure 1A). A KO line was obtained by mating of the floxed line with a cre-Deleter mouse strain17 (Supplemental Figure 1, B–D). Homozygous (Clcnk2−/−) KO mice were born from heterozygous matings at Mendelian ratio. At birth, homozygous KO mice appeared indistinguishable from their wild-type (WT; Clcnk2+/+) and heterozygous (Clcnk2+/−) littermates. Subsequently Clcnk2−/− mice failed to thrive, became hypotrophic (Supplemental Figure 2A), and exhibited early lethality (Supplemental Figure 2B). Compared with controls overall kidney size was reduced (Supplemental Figure 2C). Beginning at 2 weeks of age Clcnk2−/− mice developed hydronephrosis (Supplemental Figure 2D).
ClC-K1 and ClC-K2 Immunolocalization in the Mouse Kidney
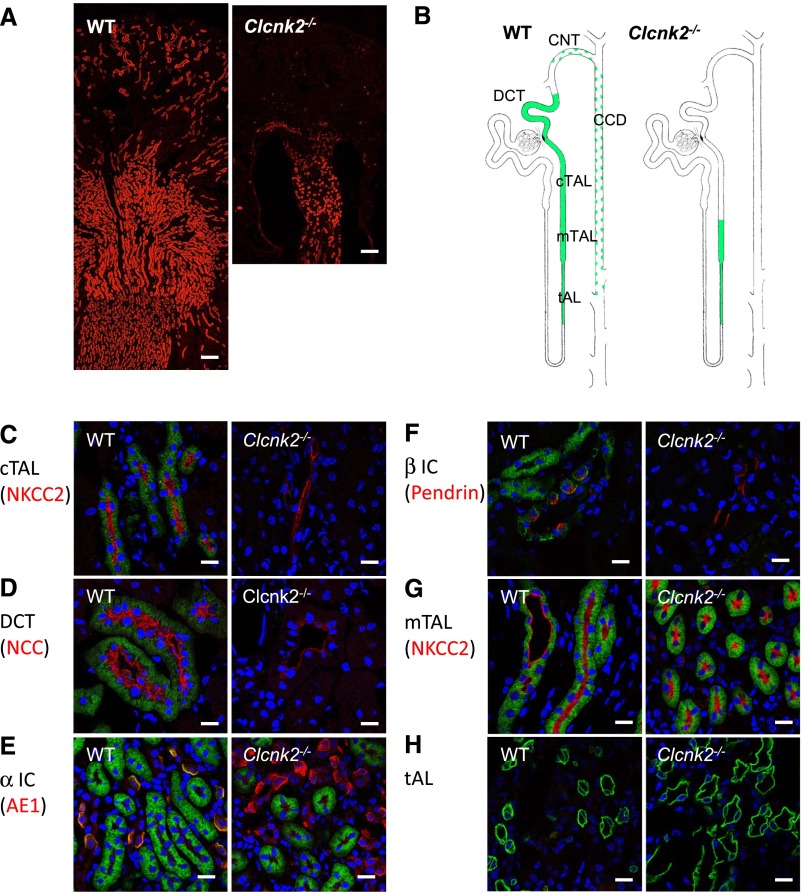
The localization of ClC-K channels was determined with an antibody derived from guinea pig against the synthetic peptide MEELVGLREGSSKKP, which corresponds to the N-terminal end of the ClC-K2 protein. In immunofluorescence studies on Clcnk2+/+ kidney sections the guinea pig antibody reproduced the expression pattern as shown by Kobayashi et al. previously18 (Figure 1, A and B). When the antibody was applied on kidney sections from Clcnk2−/− mice, the signal in the cortical TAL (cTAL), the DCT, the CNT, and ICs was completely abolished (Figure 1, C–F). However, the staining persisted in the mTAL and the tAL (Figure 1, G and H), indicating that ClC-K1 protein is likely to be expressed in the tAL as described previously,18 but is also present in the mTAL. In overexpression studies the guinea pig antibody recognizes both overexpressed ClC-K channels in HEK293 cells by Western blot and immunohistochemistry (Supplemental Figure 3).
Figure 1.
Localization of ClC-K in Clcnk2+/+ and Clcnk2−/− mice. The immunolocalization of ClC-K proteins (red) on kidney sections from WT and Clcnk2−/− mice using our new anti ClC-K antibody (A). Residual staining is detected in the thin ascending limb (tAL) and mTAL in Clcnk2−/− mouse kidney, consistent with a medullary expression of ClC-K1. (B) Schematic representation of the staining along the mouse nephron obtained with this anti ClC-K antibody in WT and Clckn2−/− mice. In the CNT and CCD this antibody also labels intercalated cells as indicated by green dots. Localization of ClC-K staining was ascertained using double labeling of ClC-K1 and 2 in green and different tubular markers in red (C–H): NKCC2 for the cortical (C) and medullary (G) TALs; NCC for the distal convoluted tubules (D); basolateral kidney anion-exchanger 1 (AE1) for α-intercalated cells in the medulla (E); pendrin for β-intercalated cells (F). ClC-K staining is absent in the cortical TAL and the DCT, as well as α- and β-intercalated cells in Clcnk2−/− mice. ClC-K staining is still present in the mTAL and in the tAL in Clcnk2−/− mice. A, scale bar = 200 µm; C, D, E, F, G, and H, scale bar = 25 µm.
In parallel, we tested the rabbit R4 antibody described by Kieferle et al.,19 another ClC-K1/K2 antibody, which is thought to cross react with both homologs, and compared the staining pattern in Clcnk2+/+ and Clcnk2−/− mice. The signal distribution with the R4 antibody19 in kidney sections of Clcnk2+/+ mice was much more restricted compared with the staining reported for rat.20 The basolateral staining in the TAL, DCT, and intercalated cells in the cortex (Supplemental Figure 4, A and B) was consistent with that reported by Kobayashi et al. using their ClC-K1/2 antibody on kidney sections from Clcnk1−/− mice.18 The staining extended to mTALs in the outer medulla but was much less intense than in the cortex. Importantly, the signal detected in the cortex was fully abolished when the R4 antibody was applied on kidney sections from our Clcnk2−/− mice indicating that it is specific for ClC-K2 in the mouse (Supplemental Figure 4, A and B). To explain this finding, we determined the expression of ClC-K1 and ClC-K2 in the mouse kidney by quantitative RT-PCR. We found that ClC-K1 is roughly 25-fold less abundant than ClC-K2 in the adult mouse kidney (Supplemental Figure 5A). The lower expression of ClC-K1 was further corroborated by Western blot analysis of whole kidney lysates obtained from Clcnk2+/+ and Clcnk2−/− mice with the guinea pig and rabbit R4 antibodies, respectively (Supplemental Figure 5, B and C). We conclude that the ClC-K1 expression is below the detection limit in immunofluorescence with the rabbit R4 antibody.
ClC-K2 Is a 10-pS Channel and Constitutes the Predominant Chloride Conductance in the Distal Nephron
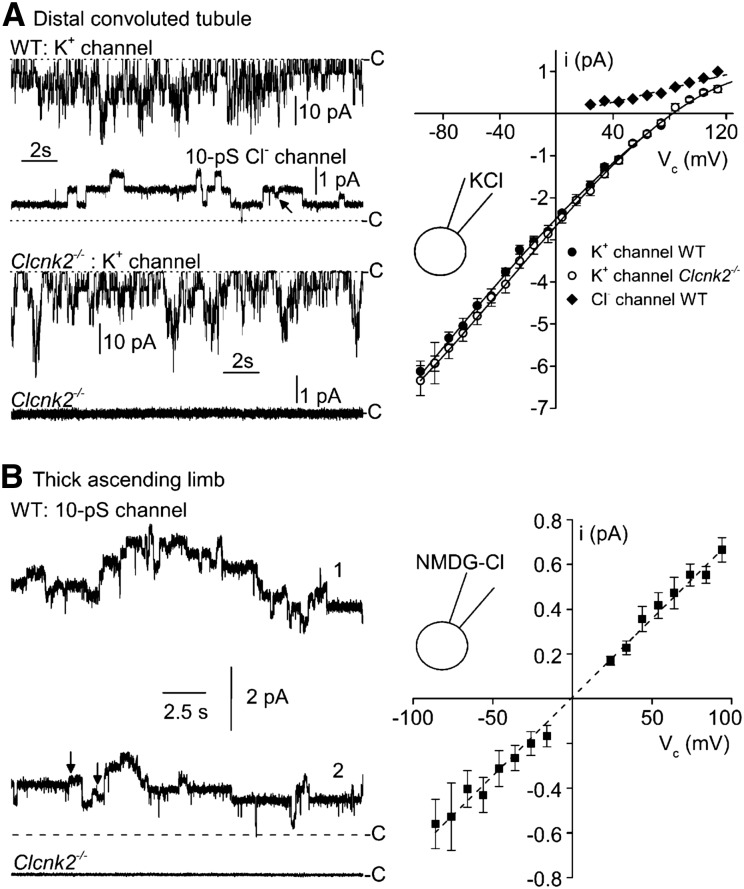
To verify the nature of the different chloride channels along the mouse nephron at the functional level, we performed patch-clamp experiments on several renal segments isolated from Clcnk2+/+ or Clcnk2−/− mice. Previous experiments have shown that the DCT and the intercalated cells of the CNT/cortical collecting duct (CCD) are endowed with a 10-pS channel that we suggested to be mediated by ClC-K2 because of its ion selectivity and regulation.21,22 Additionally, the TAL and late DCT (DCT2) express another 10-pS chloride channel (showing contrasting anion selectivity).23–25 The latter channel was called pseudo-cystic fibrosis transmembrane conductance regulator (CFTR) because of its functional similarities to CFTR.24 In the early DCT (DCT1) and intercalated cells of CNT/CCD only ClC-K2-like 10-pS chloride channels are present.21,26 For this reason, we first searched for Cl- channels in the DCT1 using a K+-rich (145 mM KCl) pipette solution in the cell-attached configuration, in order to record simultaneously K+ channels and Cl- channels. As this part of the renal tubule highly expresses Kir4.1/Kir5.1 potassium channels,27 this allowed us to discard silent patches (i.e., those with no K+ channel activity) as formed by membrane vesicles. We recorded similar K+ channels from Clcnk2+/+ (n=9) and Clcnk2−/− (n=13) DCT1 (Figure 2A). Although all DCT patches from Clcnk2+/+ mice showing K+ channel activity expressed Cl- channels of 9.3±0.5 pS (Figure 2A, Table 1), no 9-pS chloride channels were recorded in DCT patches from Clcnk2−/− mice showing K+-channel activity (Figure 2A, Table 1).
Figure 2.
Channel current recordings in DCT and TAL patches from Clcnk2+/+ and Clcnk2−/− mice in the cell-attached configuration. (A) Distal convoluted tubule. Representative current recordings from membrane patches formed on either a Clcnk2+/+ (WT) or Clcnk2−/− DCT bathed with physiologic saline solution (high-K+ solution in the pipette). The dotted line labeled with C denotes the closed current level. At −76 mV holding potential, the dominating current is due to K+ channel activity (high amplitudes of about 5 pA). At +84 mV holding potential, the activity of the K+ channel is no longer detected because this potential is very close to the reversal potential for K+ currents. It is then possible to record accurately Cl- channels which show considerably lower current amplitudes (about 0.7 pA at this voltage). K+ channels and Cl- channels could be recorded in the same patches from Clcnk2+/+ DCTs (upper panel, left) whereas no Cl- channel activity was recorded from Clcnk2−/− DCTs, even though K+ channel activity was present (lower panel, left). The arrows indicate brief half-openings or closings of Cl- channel activity. Mean single-channel current (i)/voltage (Vc) relationship obtained under the same condition are shown on the right. Each point is the average of 3–9 determinations from nine patches. SEM is shown as error bars when larger than symbols. The K+ channel conductances were similar for both genotypes (gK = 38.8 +/− 1.4 pS for Clcnk2+/+ versus 39.5 +/− 1.7 pS for Clcnk2−/−) as was the number of channels per patch (NK = 5.8 +/− 0.7 for Clcnk2+/+ versus 4.0 +/− 1.1 for Clcnk2−/−). (B) TAL. Left: Representative current recording from a membrane patch formed on Clcnk2+/+ and Clcnk2−/− TAL fragments bathed with physiologic saline solution (NMDGCl solution in the pipette). The dotted line labeled with C denotes the closed current level. Holding potential: 86 mV. Clcnk2+/+: the two traces show the activity of 10-pS Cl- channel under resting conditions (label 1) and when maximal inhibition has been reached (label 2) after superfusing Na-acetate to acidify the intracellular compartment. Clcnk2−/−: no channel activity was recorded having a conductance of 7–10 pS. Right hand side: mean single-channel current (i)/voltage (Vc) relationship for the 10-pS Cl- channel recorded on Clcnk2+/+ TAL fragments. Each point is the average of 3–12 determinations from 14 patches. SEM is shown as error bars when larger than symbols.
Table 1.
Distribution of 10-pS Cl- channels in the DCT and TAL of Clcnk2+/+ and Clcnk2−/− mice
| Part of the Renal Tubule | Status | F | G, pS | NPo |
|---|---|---|---|---|
| DCT [2] | WT (9) | 1 | 9.3±0.5 (9) | 12.0±4.2 (9) |
| DCT [3] | KO (13) | 0 | NA | NA |
| CNT/CCD [4] | WT (24) | 0.70 | 10.9±0.6 (17) | 34.5±9.6 (14) |
| CNT/CCD [3] | KO (10) | 0 | NA | NA |
| TAL [3] | WT (14) | 0.85 | 7.7±0.6 (12) | 13.9±5.5 (10) |
| TAL [2] | KO (13) | 0 | NA | NA |
[n], number of mice used in the experiments; (n), number of measurements. F, frequency for detecting channel activity; G, pS, unit conductance in picosiemens; NPo, channel activity or normalized current due to 10-pS Cl− channels; NA, not available.
For intercalated cells of the CNT and CCD, we used NMDGCl in the pipette to decrease the frequent occurrence of cation channels in these cells. Seventy one percent of the patches formed on the intercalated cells from Clcnk2+/+ mice showed Cl- channels of 10.9±0.6 pS (n=17), whereas no such channels were recorded in the CNT/CCD from Clcnk2−/− mice (n=10) (Figure 2, Table 1).
In the cTAL of Clcnk2+/+ and Clcnk2−/− mice (Figure 2B), we consistently recorded channels of 7.7±0.6 pS (12 out of 14 cell-attached patches) from Clcnk2+/+ TALs but not in any of the 13 cell-attached patches from Clcnk2−/− cTALs (see Table 1).
In conclusion, the approximately 10-pS Cl- channel that we recorded in the DCT, TAL, and the intercalated cells of the CNT/CCD is ClC-K2, because this channel is totally absent from the renal tubules of Clcnk2−/− mice. Unexpectedly, we were unable to record pseudo-CFTR channels in Clcnk2−/− TALs. This might be due to low occurrence under our experimental conditions. It could also indicate that this channel is not spontaneously active in cell-attached patches. Because 10-pS chloride channels having ClC-K-like anion selectivity sequence were not reported before, we performed anion substitution experiments in the inside-out configuration to check that the two channels (ClC-K2 and pseudo-CFTR) were present in the cTAL (Supplemental Table 1).
Paulais et al.22 described an approximately 40-pS Cl- channel showing Cl- > Br- > NO3- anion selectivity sequence in the cTAL. This channel is most certainly formed by ClC-K1 because a channel with similar properties was detected when mouse ClC-K1 was expressed in HEK293 cells.28 Because we did not detect ClC-K1-like single channel currents in DCT patches from Clcnk2−/− mice and ClC-K1 was detected at the same low frequency in Clcnk2+/+ and Clcnk2−/− intercalated cells (20% for Clcnk2+/+ versus 10% for Clcnk2−/−) and TALs (14% for Clcnk2+/+ versus 8% for Clcnk2−/−), our results indicate that there is no compensation for ClC-K2 by ClC-K1 (as represented by the 40-pS channel) in the TAL, DCT, and CNT/CCD of ClC-K2 KO mice (Supplemental Table 2).
Clcnk2 Disruption Completely Abolishes Furosemide-Sensitive and Thiazide-Sensitive NaCl Transport
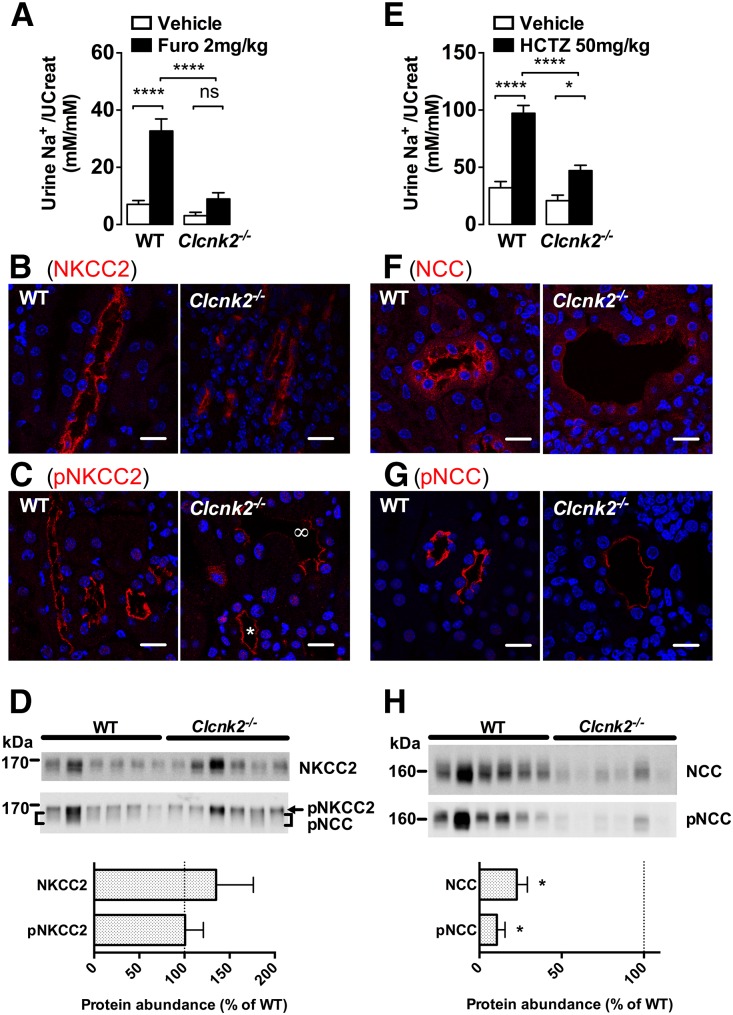
Our preceding experiments support the hypothesis that ClC-K2 is the main basolateral chloride channel all along the distal nephron. We next assessed the impact of Clcnk2 disruption on chloride transport in the distal nephron in vivo. We first tested whether ClC-K2 is the principal basolateral chloride channel in the TAL by assessing the effects of Clcnk2 disruption on the natriuretic response to furosemide, a diuretic that blocks NKCC2 and thereby abolishes NaCl absorption by the TAL. Injection of furosemide (Furo, 2 mg/kg body wt) intraperitoneally elicited a marked increase in urinary sodium excretion in Clcnk2+/+ mice whereas the response was completely abolished in Clcnk2−/− mice (Figure 3A). Furthermore, TALs from Clcnk2−/− mice appeared dilated with flattened epithelial cells when stained with NKCC2 (Figure 3B) and phosphorylated NKCC2 (pNKCC2, Figure 3C). Western blot analysis revealed highly variable NKCC2 and pNKCC2 abundance between individual mice and no significant differences between Clcnk2+/+ and Clcnk2−/− mice (Figure 3D).
Figure 3.
NKCC2 and NCC activities and localization in Clcnk2−/− mice. Both furosemide (Furo, A) and hydrochlorothiazide (HCTZ, E) elicit significant natriuresis in Clcnk2+/+ mice 3 hours after intraperitoneal injection. Furosemide induced natriuresis is abolished in Clcnk2−/− mice and HCTZ natriuretic effects strongly blunted. In the renal cortex of Clcnk2+/+ and Clcnk2−/− mice, both NKCC2 (B) and NCC (F) localize to the apical membrane of the TAL and the DCT. In Clcnk2−/− mice, phospho-NKCC2 signals are reduced in some TAL (∞ in C panel) whereas signals in some TAL are normal (star in C panel) as compared with Clcnk2+/+ mice. NCC signals are also reduced. Decreased signal intensity is also observed for phospho-NCC (G). The DCT in Clcnk2−/− mice has abnormal flattened epithelium and dilatation of the lumen. Western blot for NKCC and pNKCC2 (D) and NCC and pNCC (H) on whole kidney lysates from Clcnk2+/+ and Clcnk2−/− mice. Statistical significance was analyzed by unpaired t test (D, H) or ANOVA with repeated measures (A, E). The effect of the pharmacological treatment reaches significant levels for both HCTZ and furosemide. Bonferroni multiple comparisons are presented in the figure and compare the effect of the treatment for Clcnk2+/+ and Clcnk2−/− mice *P<0.05; ****P<0.001. Furo (Clcnk2+/+, n=9; Clcnk2−/−, n=5); HCTZ (Clcnk2+/+, n=10; Clcnk2−/−, n=8). In panels B, C, F, and G, scale bar = 25 µm.
Clcnk2 disruption is expected to blunt the response to thiazides (HCTZ, 50 mg/kg body wt) by blocking NCC-dependent absorption in the DCT and by blocking Pds/Ndcbe-mediated NaCl absorption by β-intercalated cells in the CNT and the CCD.29,30 Accordingly, the thiazide sensitivity was markedly decreased in Clcnk2−/− mice (Figure 3E). Similar to our observations in the TAL, the DCT also showed an abnormal morphology with marked flattening of the cells, tubule dilatation and reduced expression of the apical NaCl-cotransporter NCC (Figure 3F). In contrast to NKCC2 and pNKCC2, NCC and phosphorylated NCC (p-NCC) staining was clearly decreased (Figure 3, F–G). The reduced NCC expression in Clcnk2−/− mice was confirmed by Western blot analysis (Figure 3H). Similarly, we observed an almost complete absence of parvalbumin staining in kidney sections from Clcnk2−/− mice, indicating the atrophy and disappearance of the initial portion of the DCT as has already been described for NCC KO mice31 (Supplemental Figure 6).
Disruption of Clcnk2 in the Mouse Causes a Severe Hyperprostaglandin E Syndrome
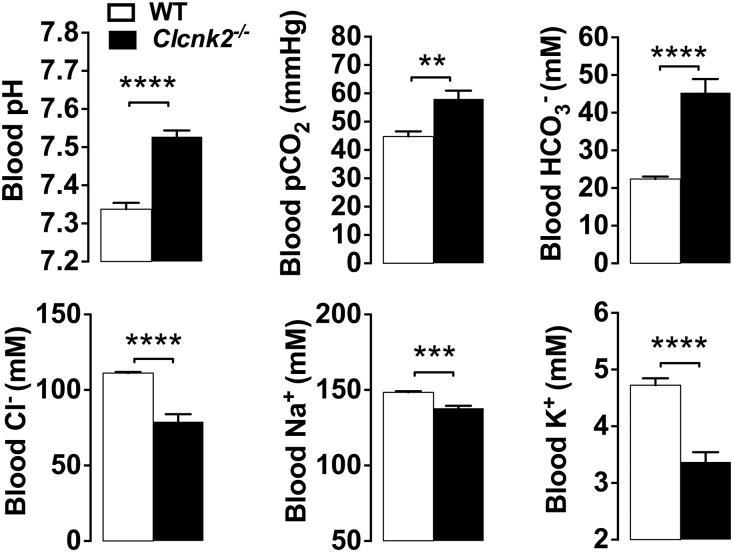
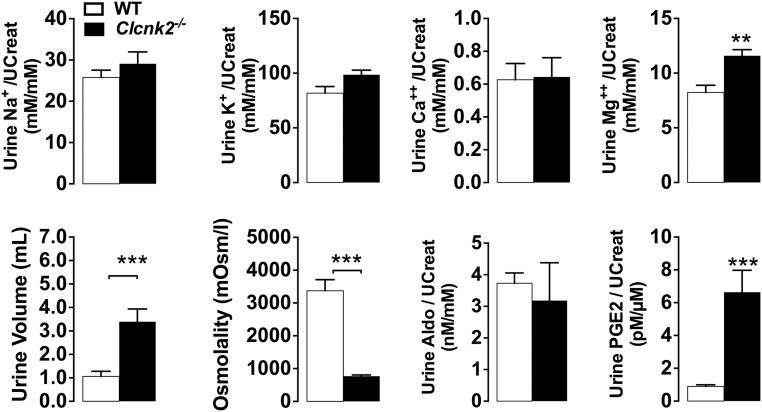
Consistent with the biologic features of severe BS, Clcnk2−/− mice exhibited a very strong metabolic alkalosis with partial respiratory compensation as indicated by a high blood pH and increased [HCO3-] with high pCO2 (Figure 4, Supplemental Table 3). The increase in plasma [HCO3-] was accompanied by a decrease in plasma [Cl-]. Mutant mice also exhibited hypokalemia of (3.34±0.27 mM) compared with Clcnk2+/+ mice (4.62±0.18 mM). Mutant mice suffered from intense polyuria with low urine osmolality, but did not display hypercalciuria as observed in some patients, whereas urinary magnesium excretion was increased (Figure 5).
Figure 4.
Blood parameters in Clcnk2+/+ and Clcnk2−/− mice. Mice were fed a normal salt diet (0.3% Na+). Values are mean±SEM. Statistical significance was analyzed by unpaired t test. **P<0.01; ***P<0.001; ****P<0.001 (Clcnk2+/+, n=7; Clcnk2−/−, n=8).
Figure 5.
Urine measurements in Clcnk2+/+ and Clcnk2−/− mice. Mice were fed a normal salt (0.3% Na+) diet. Excretions of solutes were normalized to urine creatinine concentration. Values are mean±SEM. Statistical significance was analyzed by unpaired t test. **P<0.01; ***P<0.001.
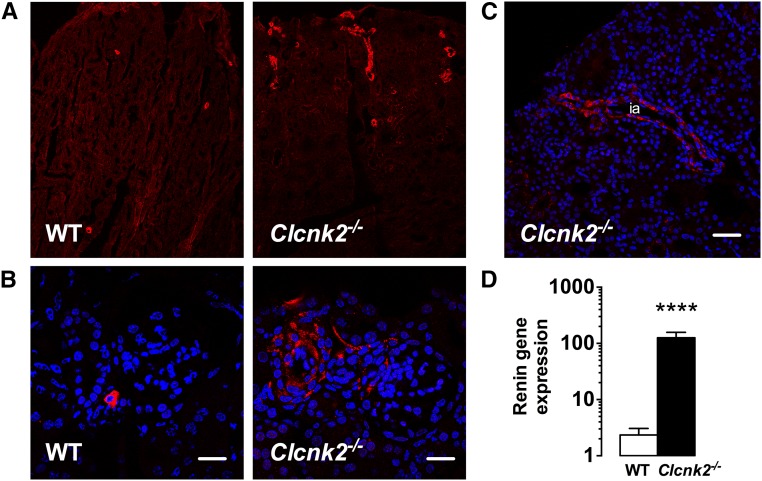
Because of the very severe phenotype we tested whether Clcnk2−/− mice have elevated PGE2 levels in the urine. Indeed, PGE2 excretion in mutant mice was increased approximately eight-fold compared with controls (Figure 5). Clcnk2−/− mice also exhibited a hypertrophy of the juxtaglomerular apparatus and proliferation of renin-secreting cells as described1 (Figure 6, A and B). Renin expressing cells even extended to interlobular arteries (Figure 6C). Accordingly, renin transcript abundance was approximately 50-fold higher in Clcnk2−/− than in Clcnk2+/+ mice (Figure 6D).
Figure 6.
Renin is stimulated in Clcnk2−/− mice. Renin immunostaining on kidney sections from Clcnk2+/+ and Clcnk2−/− mice (A). In Clcnk2−/− mice, the strong stimulation of renin is accompanied by a hypertrophy of the juxtaglomerular apparatus and a larger number of renin positive cells (B). Some renin positive cells are even found into the interlobular arteries (C, ia). Renin gene expression was analyzed by quantitative RT-PCR (D) and is strongly stimulated in Clcnk2−/− mice. Values are mean±SEM. Statistical significance was analyzed by unpaired t test. ****P<0.001. B, scale bar = 30 µm; C, scale bar = 60 µm.
Discussion
Here, we report the generation and characterization of a mouse model with a targeted disruption of Clcnk2. KO mice display severe renal salt loss, volume depletion, marked hypokalemia, and metabolic alkalosis. This is surprising because ClC-Kb related patients usually present with a relatively mild phenotype.
From previous work, it was proposed that ClC-K1 can to some extent compensate for the loss of ClC-K2 activity.8,9,22 Because ClC-K1 and ClC-K2 are thought to both be present in the TAL32,33 whereas only ClC-K2 is present in the DCT,21,32 this would explain why some patients with CLCNKB mutations rather present with a phenotype resembling Gitelman syndrome (characterized by impaired thiazide-sensitive absorption of NaCl by DCT cells) although others show Bartter syndrome (characterized by impaired furosemide-sensitive absorption of NaCl by TAL cells). Our patch-clamp analysis on isolated tubules suggests that ClC-K2 corresponds with the 10-pS Cl– channel previously described by us21,34 and mediates most of the basolateral chloride conductance in the TAL and intercalated cells of the CNT/CCD.
In line with previous studies21,34 we found only one dominating 10-pS conductance in WT patches although we noticed some brief half-level openings and closures. This may appear surprising because ClC-K channels are dimers with two independently-gated pores controlled by a common gate. We never observed the characteristic “double-barreled” activity as reported for ClC-0.35 In fact, ClC-K channels do not have the glutamate amino acid in position 166 that is responsible for protopore gating,36 suggesting that ClC-K gating relies mostly on the common slow gate. For this reason, we hypothesize that the 10-pS conductance represents the dimer conductance, which could be proved by mutation of glutamate 166 to create de novo protopore gating, as shown for rat37 and mouse28 ClC-K1. Unfortunately, there has been no successful recording of single-channel ClC-K2 currents until now.38 It is unlikely that the 10-pS conductance is mediated by ClC-K1/ClC-K2 heterodimers, because ClC-K1 has a much larger conductance and is very infrequent in the setting of these experiments. This issue, however, would require direct investigation using ClC-K concateners because there are reports of functional hetero-dimeric ClC channels.39,40
In agreement with previous results ClC-K2 was the only chloride channel detectable at the basolateral surface of DCT cells.31 This nicely fits with our in vivo data that the response to furosemide and to hydrochlorothiazide is blunted in Clcnk2−/− mice. Our results indicate that ClC-K2 ablation is not compensated by ClC-K1 in the TAL and that ClC-K2 is the main pathway for basolateral chloride exit in the TAL, the DCT, and the CNT/CCD.
The less severe phenotype in patients may be explained by some residual ClC-Kb activity whereas our gene targeting strategy leads to a complete ablation of ClC-K2. Though >50 CLCNKB mutations have been found in patients suffering from Bartter syndrome,41 only 20 mutations have been characterized functionally because of the difficulty to obtain robust and reliable expression of ClC-K2 in heterologous systems. We have recently reviewed in-depth the different aspects of this problem,41 and it is noteworthy that among the 20 mutations characterized, only 40% did not show any detectable activity whereas the other 60% exhibited significant chloride currents (> 50% of WT channel activity).41
Interestingly, deletion of ClC-K2 leads to a marked downregulation of NCC. Because our experiments identify ClC-K2 as the main (if not the only) pathway for basolateral chloride exit from DCT cells, whereas NCC is the apical entry pathway, this suggests the presence of a coordinated regulation of apical and basolateral transporters. How renal cells are able to achieve this crosstalk between both membrane domains remains unknown. However, recent studies have highlighted the critical role of intracellular chloride in the control of a cascade of kinases, like for example the with-no-lysine kinase (WNK)-Ste20p-related proline- and alanine-rich kinase (SPAK) pathway that controls NaCl transport by epithelial cells.42,43 For instance, a decrease in [Cl-]i is detected by the chloride-sensing kinase WNKs,43–45 which in turn promotes the activation through an increase in the phosphorylation of the NaCl cotransporter NCC by SPAK.42,46,47 Because ClC-K2 provides the main chloride conductance in DCT cells it is likely that ClC-K2 disruption will lead to significant increase in resting [Cl-]i, which is expected to block WNK activation, and thereby, to decrease NCC phosphorylation and expression which may explain the lack of hypercalciuria in patients and our KO mice. As observed in mice with Gitelman syndrome, Clcnk2−/− mice show a marked atrophy of the DCT1. Thus, the effects of TAL dysfunction on calcium homeostasis, which promotes hypercalciuria as observed in type 1 and type 2 Bartter syndrome, may be compensated by DCT atrophy and dysfunction promoting hypocalciuria as observed in Gitelman syndrome.
Our data further suggest that CLC-K2 mediates basolateral chloride exit in β-intercalated cells. We recently detected the presence of electroneutral thiazide-sensitive NaCl absorption in β-intercalated cells.48 In these cells apical uptake of NaCl is mediated by the parallel operation of pendrin (Slc26a4) and the Na+-driven chloride/bicarbonate exchanger Ndcbe (Slc4a8),48 which is energized by the basolateral V-ATPase.29 Basolateral sodium exit occurs possibly via AE4 (Slc4a9), which according to this model mediates Na-HCO3 cotransport29 and ClC-K2.
We detected ClC-K2 by immunofluorescence at the basolateral surface of intercalated cells, including α-intercalated cells as described previously.49 In the latter, basolateral chloride exit is critical to recycle the chloride that enters through the basolateral anion exchanger AE1. If this recycling is impaired, AE1 activity is expected to be limited by the resulting rise in [Cl-]i. This will decrease AE1 mediated basolateral bicarbonate extrusion and in turn will block apical proton secretion via the V-ATPase. In agreement with this model, disruption of the basolateral KCl cotransporter KCC4 resulted in distal tubular acidosis in mice.49 Because disruption of ClC-K2 causes a very profound metabolic alkalosis, ClC-K2 appears to be dispensable for renal acidification.
In conclusion, our study and the previous studies from Kobayashi et al.18,32 and from Matsumura et al.18,50 demonstrate that ClC-K1 and ClC-K2 are the two basolateral renal chloride channels with complementary but not redundant roles in the kidney. ClC-K2 is expressed all along the distal nephron where it controls chloride absorption by the TAL, the DCT and the intercalated cells of the CD, whereas ClC-K1 is critical in the thin limb of the loop of Henle where it is required for the normal renal concentrating mechanism.50
Concise Methods
Generation of Clcnk2 Deficient Mice and Balance Studies
The generation of Clcnk2 deficient mice (Clcnk2−/−) is described in detail in the Supplemental Material.
For urine collection, mice were housed individually in metabolic cages (Tecniplast; Buguggiate, Varese, Italy) for a 24-hour period. Retro-orbital blood was sampled from isofluorane-anesthetized mice. Urine and blood were analyzed as described in the Supplemental Material.
Diuretic induced natriuresis was assessed in Clcnk2+/+ and Clcnk2−/− littermates housed in metabolic cages using furosemide (2 mg/kg body wt, Furo) and hydrochlorothiazide (50 mg/kg body wt, HCTZ). After injection, urine was collected for 3 hours and urine sodium output was measured.
Kidney Histology and Immunofluorescence
Kidneys were fixed by heart perfusion of anesthetized mice with a solution of 4% paraformaldehyde in phosphate buffer. Harvested kidneys were washed in ice-cold phosphate buffer before freezing in cold isopentane. The antibodies used for immunohistochemistry are listed in the Supplemental Material. Immunolabeled kidney cryosections were analyzed with an LSM 710 laser scanning microscope (Carl Zeiss GmbH, Jena, Germany).
Patch-Clamp
Renal segments were isolated from the kidneys after collagenase treatment (Worthington CLS II, 300 U/ml) and transferred into a chamber on the stage of an inverted microscope as described previously.20,21,23–27 The tubules were bathed in physiologic saline solution containing (in mM): 140 NaCl, 4.8 KCl, 1 CaCl2, 1.2 MgCl2, 10 glucose, 10 HEPES. The patch pipettes were filled with high-K+ solution (145 mM KCl) or NMDG-solution (145 mM NMDGCl) containing in addition (in mM): 1 CaCl2, 1 MgCl2, 10 HEPES. All solutions were adjusted to pH 7.4. Single-channel currents were recorded from patches of basolateral membranes using the cell-attached and excised, inside-out configurations of the patch-clamp technique. Currents were recorded with List LM-EPC7 or Bio-logic RK 400 patch-clamp amplifiers, and analyzed using Pclamp 10 software (Molecular Devices). Single-channel current recordings were filtered at 700 Hz low-pass (K+ channels) or 300Hz (Cl- channel) by an eight-pole Bessel filter (LPBF-48DG; NPI Electronic) and digitized at a sampling rate of 3 kHz using a Digidata 1200 analog-to-digital converter and Axoscope software (Axon Instruments).
Detailed methods are provided in the Supplemental Material.
Disclosures
None.
Supplementary Material
Acknowledgments
We are indebted to Katrin Schorr (Core Unit Transgenic Animals, Jena) for embryonic stem cell culture and blastocyst injections. We thank Marie Brinckmann and the team of the Jena University Clinic Animal Facility for expert mouse husbandry.
D.E. and J.T. are funded by grant ANR BLANC 14-CE12-0013-01/HYPERSCREEN from l’Agence Nationale de la Recherche (ANR), and by grant ECOS/CONYCIT France-Chile 2014. D.E. is also funded by grant CHLORBLOCK from the Initiative d'excellence Sorbonne Paris Cité. O.A. is funded by Fondation du Rein 2014. The group of C.A.H. is funded by grants of the Interdisziplinäres Zentrum für klinische Forschung Jena to J.C.H., the Else-Kröner-Fresenius foundation to C.A.H. (2014_A126), and the Deutsche Forschungsgemeinschaft to C.A.H. (HU 800/7-1). N.P. was supported by the Fondation du Rein and French Nephrology Society sous égide de la Fondation pour la Recherche Médicale. N.P. is also funded by grant ANR 15-CE14-0024-02/ConTarKiD from ANR.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016010085/-/DCSupplemental.
References
- 1.Bartter FC, Pronove P, Gill JR Jr, MacCardle RC: Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med 33: 811–828, 1962 [DOI] [PubMed] [Google Scholar]
- 2.Baehler RW, Work J, Kotchen TA, McMorrow G, Guthrie G: Studies on the pathogenesis of Bartter’s syndrome. Am J Med 69: 933–938, 1980 [DOI] [PubMed] [Google Scholar]
- 3.Cannon PJ, Leeming JM, Sommers SC, Winters RW, Laragh JH: Juxtaglomerular cell hyperplasia and secondary hyperaldosteronism (Bartter’s syndrome): a re-evaluation of the pathophysiology. Medicine (Baltimore) 47: 107–131, 1968 [DOI] [PubMed] [Google Scholar]
- 4.Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP: Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13: 183–188, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP: Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Konrad M, Vollmer M, Lemmink HH, van den Heuvel LP, Jeck N, Vargas-Poussou R, Lakings A, Ruf R, Deschênes G, Antignac C, Guay-Woodford L, Knoers NV, Seyberth HW, Feldmann D, Hildebrandt F: Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J Am Soc Nephrol 11: 1449–1459, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP: Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat Genet 17: 171–178, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Birkenhäger R, Otto E, Schürmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, Milford DV, Jeck N, Konrad M, Landau D, Knoers NV, Antignac C, Sudbrak R, Kispert A, Hildebrandt F: Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet 29: 310–314, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Estévez R, Boettger T, Stein V, Birkenhäger R, Otto E, Hildebrandt F, Jentsch TJ: Barttin is a Cl- channel beta-subunit crucial for renal Cl- reabsorption and inner ear K+ secretion. Nature 414: 558–561, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Gitelman HJ, Graham JB, Welt LG: A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians 79: 221–235, 1966 [PubMed] [Google Scholar]
- 11.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP: Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Fukuyama S, Hiramatsu M, Akagi M, Higa M, Ohta T: Novel mutations of the chloride channel Kb gene in two Japanese patients clinically diagnosed as Bartter syndrome with hypocalciuria. J Clin Endocrinol Metab 89: 5847–5850, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Jeck N, Konrad M, Peters M, Weber S, Bonzel KE, Seyberth HW: Mutations in the chloride channel gene, CLCNKB, leading to a mixed Bartter-Gitelman phenotype. Pediatr Res 48: 754–758, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Ohkubo K, Matsuzaki T, Yuki M, Yoshida R, Terawaki Y, Maeyama A, Kawashima H, Ono J, Yanase T, Matsunaga A: A novel mutation of CLCNKB in a Japanese patient of Gitelman-like phenotype with diuretic insensitivity to thiazide administration. Meta Gene 2: 342–348, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelikovic I, Szargel R, Hawash A, Labay V, Hatib I, Cohen N, Nakhoul F: A novel mutation in the chloride channel gene, CLCNKB, as a cause of Gitelman and Bartter syndromes. Kidney Int 63: 24–32, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Landau D, Shalev H, Ohaly M, Carmi R: Infantile variant of Bartter syndrome and sensorineural deafness: a new autosomal recessive disorder. Am J Med Genet 59: 454–459, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Schwenk F, Baron U, Rajewsky K: A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23: 5080–5081, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi K, Uchida S, Mizutani S, Sasaki S, Marumo F: Intrarenal and cellular localization of CLC-K2 protein in the mouse kidney. J Am Soc Nephrol 12: 1327–1334, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Kieferle S, Fong P, Bens M, Vandewalle A, Jentsch TJ: Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc Natl Acad Sci U S A 91: 6943–6947, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandewalle A, Cluzeaud F, Bens M, Kieferle S, Steinmeyer K, Jentsch TJ: Localization and induction by dehydration of ClC-K chloride channels in the rat kidney. Am J Physiol 272: F678–F688, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Lourdel S, Paulais M, Marvao P, Nissant A, Teulon J: A chloride channel at the basolateral membrane of the distal-convoluted tubule: a candidate ClC-K channel. J Gen Physiol 121: 287–300, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlingmann KP, Konrad M, Jeck N, Waldegger P, Reinalter SC, Holder M, Seyberth HW, Waldegger S: Salt wasting and deafness resulting from mutations in two chloride channels. N Engl J Med 350: 1314–1319, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Guinamard R, Chraïbi A, Teulon J: A small-conductance Cl- channel in the mouse thick ascending limb that is activated by ATP and protein kinase A. J Physiol 485: 97–112, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marvão P, De Jesus Ferreira MC, Bailly C, Paulais M, Bens M, Guinamard R, Moreau R, Vandewalle A, Teulon J: Cl- absorption across the thick ascending limb is not altered in cystic fibrosis mice. A role for a pseudo-CFTR Cl- channel. J Clin Invest 102: 1986–1993, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teulon J, Lourdel S, Nissant A, Paulais M, Guinamard R, Marvao P, Imbert-Teboul M: Exploration of the basolateral chloride channels in the renal tubule using. Nephron, Physiol 99: 64–68, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Nissant A, Lourdel S, Baillet S, Paulais M, Marvao P, Teulon J, Imbert-Teboul M: Heterogeneous distribution of chloride channels along the distal convoluted tubule probed by single-cell RT-PCR and patch clamp. Am J Physiol Renal Physiol 287: F1233–F1243, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J: An inward rectifier K(+) channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4-Kir5.1 heteromeric channels. J Physiol 538: 391–404, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.L’Hoste S, Diakov A, Andrini O, Genete M, Pinelli L, Grand T, Keck M, Paulais M, Beck L, Korbmacher C, Teulon J, Lourdel S: Characterization of the mouse ClC-K1/Barttin chloride channel. Biochim Biophys Acta 1828: 2399–2409, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hübner CA, Eladari D: Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci U S A 110: 7928–7933, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R: Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, Bloch-Faure M, Hoenderop JG, Shull GE, Meneton P, Kaissling B: Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol 15: 2276–2288, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi K, Uchida S, Okamura HO, Marumo F, Sasaki S: Human CLC-KB gene promoter drives the EGFP expression in the specific distal nephron segments and inner ear. J Am Soc Nephrol 13: 1992–1998, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Paulais M, Teulon J: cAMP-activated chloride channel in the basolateral membrane of the thick ascending limb of the mouse kidney. J Membr Biol 113: 253–260, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Nissant A, Paulais M, Lachheb S, Lourdel S, Teulon J: Similar chloride channels in the connecting tubule and cortical collecting duct of the mouse kidney. Am J Physiol Renal Physiol 290: F1421–F1429, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Miller C, White MM: Dimeric structure of single chloride channels from Torpedo electroplax. Proc Natl Acad Sci U S A 81: 2772–2775, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutzler R, Campbell EB, MacKinnon R: Gating the selectivity filter in ClC chloride channels. Science 300: 108–112, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Fischer M, Janssen AG, Fahlke C: Barttin activates ClC-K channel function by modulating gating. J Am Soc Nephrol 21: 1281–1289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stölting G, Fischer M, Fahlke C: CLC channel function and dysfunction in health and disease. Front Physiol 5: 378, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenz C, Pusch M, Jentsch TJ: Heteromultimeric CLC chloride channels with novel properties. Proc Natl Acad Sci U S A 93: 13362–13366, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stölting G, Fischer M, Fahlke C: ClC-1 and ClC-2 form hetero-dimeric channels with novel protopore functions. Pflugers Arch 466: 2191–2204, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Andrini O, Keck M, Briones R, Lourdel S, Vargas-Poussou R, Teulon J: ClC-K chloride channels: emerging pathophysiology of Bartter syndrome type 3. Am J Physiol Renal Physiol 308: F1324–F1334, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH: Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacheco-Alvarez D, Gamba G: WNK3 is a putative chloride-sensing kinase. Cell Physiol Biochem 28: 1123–1134, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ: Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7: ra41, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pacheco-Alvarez D, Cristóbal PS, Meade P, Moreno E, Vazquez N, Muñoz E, Díaz A, Juárez ME, Giménez I, Gamba G: The Na+:Cl- cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Bazúa-Valenti S, Chávez-Canales M, Rojas-Vega L, González-Rodríguez X, Vázquez N, Rodríguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, García-Valdés J, Hadchouel J, Gamba G: The Effect of WNK4 on the Na+-Cl- Cotransporter Is Modulated by Intracellular Chloride. J Am Soc Nephrol 26: 1781–1786, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D: The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boettger T, Hübner CA, Maier H, Rust MB, Beck FX, Jentsch TJ: Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature 416: 874–878, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Matsumura Y, Uchida S, Kondo Y, Miyazaki H, Ko SB, Hayama A, Morimoto T, Liu W, Arisawa M, Sasaki S, Marumo F: Overt nephrogenic diabetes insipidus in mice lacking the CLC-K1 chloride channel. Nat Genet 21: 95–98, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.