Abstract
AKI associates with increased long-term risk of mortality, but the prognostic significance of AKI in terms of long-term cardiovascular disease remains unconfirmed. We conducted a systematic review and meta-analysis to assess whether AKI associates with long-term cardiovascular disease. We included cohort studies that examined adults with and without AKI and reported a multivariable-adjusted relative risk (RR) for the association between AKI and cardiovascular mortality, major cardiovascular events, and disease-specific events: congestive heart failure, acute myocardial infarction, and stroke. Twenty-five studies involving 254,408 adults (55,150 with AKI) were included. AKI associated with an 86% and a 38% increased risk of cardiovascular mortality and major cardiovascular events, respectively ([RR 1.86; 95% confidence interval (95% CI), 1.72 to 2.01] and [RR 1.38; 95% CI, 1.23 to 1.55], respectively). For disease-specific events, AKI associated with a 58% increased risk of heart failure (RR 1.58; 95% CI, 1.46 to 1.72) and a 40% increased risk of acute myocardial infarction (RR 1.40; 95% CI, 1.23 to 1.59). The elevated risk of heart failure and acute myocardial infarction persisted in subgroup analyses on the basis of AKI severity and the proportion of adults with baseline ischemic heart disease. Finally, AKI was associated with a 15% increased risk of stroke (RR 1.15; 95% CI, 1.03 to 1.28). In conclusion, AKI associates with an elevated risk of cardiovascular mortality and major cardiovascular events, particularly heart failure and acute myocardial infarction.
Keywords: acute renal failure, cardiovascular disease, Stroke, congestive heart failure, Ischemic Heart Disease
AKI affects patients across multiple clinical settings and is a leading cause of morbidity and mortality.1–7 AKI is also associated with increased hospital length of stay and excess healthcare spending.1 Despite the established clinical importance of AKI, it is undiagnosed in up to 25% of cases in developed countries8 and up to 75% of cases in developing countries.9 Accordingly, guideline committees and public health authorities10,11 have called for more research into the long-term prognostic implications of AKI as a key element of efforts to improve the diagnosis and management of AKI.
While the long-term risk of mortality, CKD, and ESRD in adults with AKI are well established, it remains less clear whether AKI confers an additional long-term risk of cardiovascular disease. Existing studies have provided conflicting results12–14 and prior systematic reviews have pooled unadjusted and adjusted relative risk estimates15; combined estimates for hard endpoints – congestive heart failure (CHF), acute myocardial infarction, and stroke – and soft (revascularization) endpoints15; employed inadequate search strategies for cardiovascular outcomes3; or failed to identify more than two multivariable adjusted studies for acute myocardial infarction or stroke.3 Furthermore, individual studies have been limited to specific patient populations – contrast induced AKI or peri-operative AKI – and it is unclear whether the prognostic importance of AKI varies by clinical factors such as baseline cardiovascular disease. A more comprehensive analysis is therefore needed.
Accordingly, we conducted a systematic review and meta-analysis to determine the long-term risk of cardiovascular mortality and cardiovascular disease associated with AKI. We also examined whether the risk conferred by AKI varied based on the severity of AKI, the recovery of renal function, the clinical setting, and the presence of baseline cardiovascular disease or baseline CKD.
Results
In total, 1192 studies were reviewed and 1034 were excluded in the abstract screen largely because they did not report an association between AKI and an outcome of interest (Supplemental Figure 1). Among 158 full text articles that were reviewed, 133 were further excluded. Accordingly, 25 studies involving 254,408 adults were included in this meta-analysis. Of these individuals, 55,150 adults had AKI (Table 1). With respect to the risk of bias assessment, most studies examined a highly selected group of participants that were not representative of the general population. Accordingly, only one study obtained a full rating (four stars) for cohort selection. Fifteen studies received a full rating for comparability based on their multivariable adjustment and all studies received a full rating for outcome evaluation.
Table 1.
List of included studies
| Study Author, y | Outcome Reported | AKI Definition | Setting | Subjects (AKI) | CHF (%) | IHD (%) | Stroke (%) | CKD (%) |
|---|---|---|---|---|---|---|---|---|
| Blair, 201120 | MACE | Absolute increase in SCr≥0.3mg/dl | Other | 2021 (279) | 2021 (100) | 1425 (71) | NA | 553 (27) |
| Chawla, 201421 | MACE | AKIN Criteria: Peak SCr during admission at least 1.5–1.9 times higher than baseline or an increase of ≥0.3 mg/dl (stage 1); an increase of at least 2.0–2.9 times baseline (stage 2); or an increase of ≥3.0 times baseline or ≥4.0 mg/dl (stage 3) | Other | 28554 (9633) | 2829 (10) | 28554 (100) | 653 (2) | NA |
| Choi, 201029 | CHF | AKIN Criteria: AKIN 1 – an SCr increase of ≥0.3 mg/dl, or a relative increase of 150%–200%; AKIN 2 – a relative increase of 200%–300%; AKIN 3 – a relative increase >300%, or in individuals with an SCr of ≥4.0 mg/dl, an SCr increase of 0.5 mg/dl from the baseline | Other | 17325 (3060) | 358 (2) | 1186 (7) | NA | 1281 (7) |
| Damman, 200926 | CHF | Absolute increase in SCr>26.5 mmol/L (>0.3 mg/dl) in combination with >25% increase in SCr between two time points | Other | 869 (106) | 869 (100) | 364 (42) | 80 (9) | NA |
| Damman, 201427 | CHF | Absolute increase in SCr of ≥26.5 mmol/L (≥0.3 mg/dl), together with a relative increase in SCr of ≥25% between baseline and 8 wk | Other | 3595 (229) | 3595 (100) | 884 (25) | 321 (9) | NA |
| Gammelager, 201425 | Stroke, IHD, CHF | KDIGO Criteria: Stage 1 – 1.5–1.9 times increase versus baseline or ≥0.3 mg/dl increase within 48 h; Stage 2 – 2.0–2.9 times baseline; ≥3.0 times baseline or increase in SCr to ≥4.0 mg/dl or initiation of renal replacement therapy | Peri-Operative or ICU | 21556 (4792) | 0 | 2620 (12) | 0 | 1903 (9) |
| Giacoppo, 201533 | IHD | Absolute increase of SCr≥0.5 mg/dl or relative increase ≥25% from baseline value within the first 72 h of intervention | CIN | 9512 (1212) | NA | 9512 (100) | 376 (4) | 1702 (18) |
| Goldberg, 200930 | IHD, CHF | Transient mild AKI - In this group, peak SCr during hospital course was ≥0.3–0.49 mg/dl higher than admission SCr, but pre-discharge SCr returned to a level that was <0.3mg/dl higher than admission creatinine; persistent mild AKI– where SCr did not return to <0.3 mg/dl; transient moderate/severe AKI - peak SCr≥0.5mg/dl higher than admission creatinine, but pre-discharge returned <0.5 mg/dl higher than admission SCr; moderate/severe AKI– where SCr did not return to <0.5 mg/dl | CIN | 1957 (294) | 368 (19) | 1957 (100) | NA | 338 (17) |
| Hansen, 201522 | MACE | AKIN Criteria: Stage 1 – Increase in SCr of ≥0.3 mg/dl (26.5 µmol/L) within 48 h OR increase in SCr of 1.5–1.9 times baseline; Stage 2 – Increase in SCr of 2.0–2.9 times baseline; Stage 3 – Increase in SCr of 3.0 times baseline OR increase in SCr to ≥4.0 mg/dl (353.6 µmol/L) and satisfaction of at least stage 1 OR initiation of renal replacement therapy | Peri-Operative or ICU | 4742 (1457) | 717 (15) | 2651 (56) | 282 6) | 823 (17) |
| Holzmann, 201336 | Stroke | Postoperative SCr that was the highest value during the entire postoperative period. Absolute increases in SCr: stage 1, 0.3–0.5 mg/dl (26–44 μmol/L); stage 2, 0.5–1 mg/dl (44–88 μmol/L); and stage 3, ≥1 mg/dl (≥88 μmol/L) | Peri-Operative or ICU | 23584 (2764) | 943 (4) | 23584 (100) | 0 | 4481 (19) |
| James, 201128 | Stroke, IHD, CHF | AKIN Criteria: AKI stage 1, ≥0.3 mg/dl absolute or 1.5- to 2.0-fold relative increase in SCr; AKI stage 2, >2- to 3-fold increase in SCr; AKI stage 3, >3-fold increase in SCr or SCr≥4.0 mg/dl with an acute rise of >0.5 mg/dl | CIN | 14782 (1420) | 2253 (15) | 10617 (72) | 1096 (7) | 3543 (24) |
| Jose, 200614 | CVD Mortality, IHD, CHF | Increase in SCr>0.3 mg/dl from baseline | Other | 1854 (223) | 108 (6) | 1854 (100) | NA | 610 (33) |
| Kim, 201418 | CVD Mortality, IHD | SCr ≥25% or ≥0.5 mg/dl from baseline within 72 h after contrast exposure | CIN | 297 (55) | NA | 297 (100) | 31 (10) | 297 (100) |
| Lindsay, 200335 | IHD | An increase in the SCr of ≥50% from baseline after the intervention | CIN | 5967 (208) | NA | 5967 (100) | 539 (9) | 0 |
| Metra, 200823 | MACE | A ≥0.3 mg/dl and a≥25% increase in SCr from admission | Other | 318 (107) | 318 (100) | 173 (54) | 22 (7) | NA |
| Mielniczuk, 200913 | CVD Mortality, Stroke, IHD, CHF | RIFLE Criteria: Decrease in eGFR of ≥25% compared with baseline | Other | 3795 (184) | 182 (5) | 3795 (100) | 223 (6) | NA |
| Narula, 201419 | CVD Mortality | Increase in SCr of ≥0.5 mg/dl or a 25% relative rise in creatinine, within 48 h after contrast exposure | CIN | 676 (338) | 75 (11) | 676 (100) | NA | 138 (20) |
| Olsson, 201331 | CHF | Increase in postoperative SCr values: stage 1 – 0.3 to <0.5 mg/dl (26 to <44 μmol/L); stage 2 – 0.5 to <1 mg/dl (44 to <88 μmol/L); and stage 3 - ≥1 mg/dl (≥88 μmol/L) | Peri-Operative or ICU | 24018 (2779) | 0 | 24018 (100) | 1153 (5) | NA |
| Ozrazgat-Baslanti, 201616 | CVD Mortality | KDIGO Criteria: ≥50% or 0.3 mg/dl increase in SCr relative to the reference value and stratified AKI severity based on the maximum change in SCr during hospitalization | Peri-Operative or ICU | 45498 (16854) | 2915 (6) | 2727 (6) | 3731 (8) | 0 |
| Ryden, 201434 | IHD | Postoperative increase in SCr: stage 1 – 0.3–0.5 mg/dl (26–44 μmol/L); stage 2 – >0.5–1 mg/dl (44–88 μmol/L); stage 3 – ≥1.0 mg/dl (≥88 μmol/L) | Peri-Operative or ICU | 27929 (3617) | 1117 (4) | 27929 (100) | 1396 (5) | 5586 (20) |
| Shirakabe, 201212 | CHF | RIFLE Criteria: Class R – increase in SCr ≥ 1.5×baseline; Class I – increase in SCr ≥ 2.0×baseline; Class F – increase in SCr ≥ 3.0×baseline or ≥ 4 mg/dl with an acute rise >0.5 mg/dl | Other | 500 (344) | 500 (100) | 201 (40) | NA | NA |
| Tsai, 201532 | IHD | ICD-9 Code | Peri-Operative or ICU | 2849 (86) | 275 (10) | 281 (10) | 2698 (95) | 164 (6) |
| Uyarel, 200917 | CVD Mortality | An increase in SCr level ≥ 0.5 mg/dl or ≥ 25% from baseline (admission) within 72 h of radiocontrast administration | CIN | 2521 (630) | 154 (6) | 2521 (100) | NA | NA |
| Watabe, 201424 | MACE | A ≥0.5 mg/dl and/or ≥25% absolute increase in SCr from baseline | CIN | 1059 (164) | 151 (14) | 1059 (100) | 90 (8) | 1059 (100) |
| Wu, 201437 | Stroke | RIFLE Criteria: AKI was defined according to the RIFLE criteria | Other | 8630 (4315) | 1011 (12) | 1305 (15) | 0 | 1029 (12) |
MACE, major adverse cardiovascular events; SCr, serum creatinine; NA, not applicable; ICU, intensive care unit; CIN, contract induced nephropathy; CVD, cardiovascular disease.
Cardiovascular Mortality and Major Cardiovascular Events
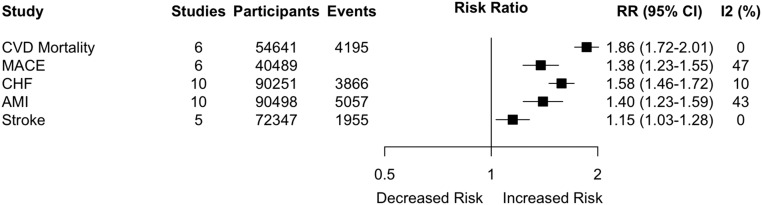
Six studies,13,14,16–19 involving 54,641 participants (18,284 with AKI), examined cardiovascular mortality as an outcome. The median follow-up was 2.6 years (interquartile interval [IQI], 2.0–3.4). The pooled relative risk for patients with AKI versus those without AKI was 1.86 (95% confidence interval [95% CI], 1.72 to 2.01; I2: 0% [Figure 1, Supplemental Figure 2]) and the relative risk did not vary by setting or proportion of adults with baseline CHF or CKD (Supplemental Table 2). There were insufficient studies to examine whether the risk varied by the remaining subgroups of interest.
Figure 1.
Association between AKI and cardiovascular mortality and cardiovascular events. Horizontal lines indicate 95% CI. Number of events is missing for MACE because it is not reported in all studies. The solid vertical line represents the line of no effect (RR = 1.0), wherein there is no difference in the risk of the outcome of interest between adults with and without AKI. AMI, acute myocardial infarction; CVD, cardiovascular disease; MACE, major adverse cardiovascular events.
Six studies,13,20–24 involving 40,489 participants (11,824 with AKI) examined major cardiovascular events as an outcome. The median follow-up was 1.4 years (IQI, 1.2–1.9). The pooled relative risk was 1.38 (95% CI, 1.23 to 1.55; I2: 47%) (Figure 1, Supplemental Figure 3) and it did not vary by the proportion of adults with baseline CHF, ischemic heart disease (IHD), or stroke (Supplemental Table 3). There were insufficient studies to examine whether the risk varied by the remaining subgroups of interest: AKI severity, recovery of renal function, and clinical setting.
CHF
Ten studies,12–14,25–31 involving 90,251 participants (13,431 with AKI), examined CHF as an outcome. The median follow-up was 2.9 years (IQI, 1.7–3.8). The pooled relative risk of CHF was 1.58 (95% CI, 1.46 to 1.72) (Figure 1, Supplemental Figure 4). Heterogeneity was minimal (I2: 10%). There was also an increase in the relative risk of long-term CHF with increasing severity of AKI (P=0.01 for interaction) (Table 2). In an exploratory analysis that was limited to studies that additionally adjusted for baseline left ventricular ejection fraction, the differential risk for CHF based on AKI severity was maintained (Table 2). Furthermore, the relative risk of CHF was modestly lower in studies in which the majority of adults did not have baseline IHD (1.43; 95% CI, 1.27 to 1.61) compared with studies where a large proportion of adults had baseline IHD (1.71; 95% CI, 1.56 to 1.87; P=0.02) (Table 2). There were no differences based on the clinical setting, the recovery of renal function or the proportion of adults with baseline CHF, stroke, or CKD (Table 2).
Table 2.
Subgroup analysis for the association between AKI and CHF
| Stratification | Number of Studies | Settinga | Relative Risk | 95% CI | I2, % | P Value |
|---|---|---|---|---|---|---|
| CHF – setting | ||||||
| CIN | 2 | - | 1.69 | 1.44 to 1.99 | 0 | 0.43 |
| Peri-operative | 6 | - | 1.57 | 1.23 to 2.01 | 83 | |
| Other | 2 | - | 1.46 | 1.25 to 1.70 | 0 | |
| CHF – recovery | ||||||
| Complete recovery | 3 | CIN (1) | 1.41 | 1.22 to 1.62 | 0 | 0.21 |
| Peri-op (1) | ||||||
| Other (1) | ||||||
| Partial or no recovery | 3 | CIN (1) | 1.65 | 1.35 to 2.03 | 0 | |
| Peri-op (1) | ||||||
| Other (1) | ||||||
| CHF – severity | ||||||
| Mild AKI | 6 | CIN (2) | 1.34 | 1.11 to 1.61 | 61 | 0.01 |
| Peri-op (2) | ||||||
| Other (2) | ||||||
| Moderate to severe AKI | 6 | CIN (2) | 1.94 | 1.62 to 2.31 | 49 | |
| Peri-op (2) | ||||||
| Other (2) | ||||||
| CHF – severity (adjusted for LVEF) | ||||||
| Mild AKI | 3 | CIN (2) | 1.57 | 1.37 to 1.79 | 0 | 0.032 |
| Peri-op (1) | ||||||
| Other (0) | ||||||
| Moderate to severe AKI | 3 | CIN (2) | 1.93 | 1.69 to 2.20 | 0 | |
| Peri-op (1) | ||||||
| Other (0) | ||||||
| CHF – median proportion baseline CHF (min–max) | ||||||
| 0.02 (0–0.06) | 5 | CIN (0) | 1.56 | 1.35 to 1.80 | 44 | 0.91 |
| Peri-op (2) | ||||||
| Other (3) | ||||||
| 1 (0.15–1) | 5 | CIN (2) | 1.58 | 1.38 to 1.80 | 0 | |
| Peri-op (0) | ||||||
| Other (3) | ||||||
| CHF – median proportion baseline IHD (min–max) | ||||||
| 0.25 (0.07–0.42) | 5 | CIN (0) | 1.43 | 1.27 to 1.61 | 0 | 0.020 |
| Peri-op (1) | ||||||
| Other (4) | ||||||
| 1 (0.72–1) | 5 | CIN (2) | 1.71 | 1.56 to 1.87 | 0 | |
| Peri-op (1) | ||||||
| Other (2) | ||||||
| CHF – median proportion baseline stroke (min–max) | ||||||
| 0.05 (0–0.06) | 3 | CIN (0) | 1.55 | 1.25 to 1.92 | 68 | 0.83 |
| Peri-op (2) | ||||||
| Other (1) | ||||||
| 0.09 (0.07–0.09) | 3 | CIN (1) | 1.60 | 1.35 to 1.88 | 0 | |
| Peri-op (0) | ||||||
| Other (2) | ||||||
| CHF – median proportion baseline CKD (min–max) | ||||||
| 0.09 (0.07–0.17) | 3 | CIN (1) | 1.54 | 1.32 to 1.79 | 30 | 0.80 |
| Peri-op (1) | ||||||
| Other (1) | ||||||
| 0.28 (0.24–0.33) | 2 | CIN (1) | 1.59 | 1.32 to 1.91 | 0 | |
| Peri-op (0) | ||||||
| Other (1) |
CIN, contrast induced nephropathy; Peri-op, peri-operative or intensive care unit; LVEF, left ventricular ejection fraction.
Number of studies is provided in brackets.
Acute Myocardial Infarction
Ten studies,13,14,18,25,28,30,32–35 involving 90,498 participants (12,091 with AKI), examined acute myocardial infarction as an outcome. The median follow-up was 2.3 years (IQI, 1.7–2.9). The pooled relative risk of acute myocardial infarction was 1.40 (95% CI, 1.23 to 1.59) (Figure 1, Supplemental Figure 5). Heterogeneity was moderate (I2: 43%). The relative risk of long-term acute myocardial infarction did not vary by clinical setting (Table 3). Furthermore, there were no differences in the risk of long-term acute myocardial infarction based on AKI recovery nor based on the severity of AKI (P=0.12 and 0.64, respectively) (Table 3). Notably, there was no difference in the relative risk of acute myocardial infarction in subgroup analyses based on the proportion of adults with baseline cardiovascular disease or CKD (Table 3).
Table 3.
Subgroup Analysis for the Association Between Acute Kidney Injury and Acute Myocardial Infarction
| Stratification | Number of Studies | Settinga | Relative Risk | 95% CI | I2, % | P Value |
|---|---|---|---|---|---|---|
| AMI – setting | ||||||
| CIN | 5 | - | 1.43 | 1.18 to 1.75 | 48 | 0.93 |
| Peri-operative | 3 | - | 1.39 | 1.08 to 1.77 | 47 | |
| Other | 2 | - | 1.31 | 0.82 to 2.09 | 55 | |
| AMI – recovery | ||||||
| Complete recovery | 2 | CIN (1) | 1.14 | 0.87 to 1.48 | 0 | 0.12 |
| Peri-op (1) | ||||||
| Other (0) | ||||||
| Partial or no recovery | 2 | CIN (1) | 1.55 | 1.16 to 2.07 | 0 | |
| Peri-op (1) | ||||||
| Other (0) | ||||||
| AMI – severity | ||||||
| Mild AKI | 4 | CIN (2) | 1.45 | 1.28 to 1.65 | 18 | 0.64 |
| Peri-op (2) | ||||||
| Other (0) | ||||||
| Moderate to severe AKI | 4 | CIN (2) | 1.53 | 1.27 to 1.84 | 0 | |
| Peri-op (2) | ||||||
| Other (0) | ||||||
| AMI – median proportion baseline CHF (min–max) | ||||||
| 0.04 (0–0.06) | 4 | CIN (0) | 1.42 | 1.19 to 1.69 | 38 | 0.91 |
| Peri-op (2) | ||||||
| Other (2) | ||||||
| 0.15 (0.10–0.17) | 3 | CIN (2) | 1.40 | 1.17 to 1.67 | 0 | |
| Peri-op (1) | ||||||
| Other (0) | ||||||
| AMI – median proportion baseline IHD (min–max) | ||||||
| 0.12 (0.10–0.72) | 3 | CIN (1) | 1.32 | 1.10 to 1.57 | 2 | 0.44 |
| Peri-op (2) | ||||||
| Other (0) | ||||||
| 1 (1–1) | 7 | CIN (4) | 1.45 | 1.23 to 1.71 | 51 | |
| Peri-op (1) | ||||||
| Other (2) | ||||||
| AMI – median proportion baseline stroke (min–max) | ||||||
| 0.04 (0–0.06) | 4 | CIN (1) | 1.37 | 1.14 to 1.65 | 62 | 0.49 |
| Peri-op (2) | ||||||
| Other (1) | ||||||
| 0.10 (0.07–0.95) | 4 | CIN (3) | 1.59 | 1.08 to 2.34 | 47 | |
| Peri-op (1) | ||||||
| Other (0) | ||||||
| AMI – median proportion baseline CKD (min–max) | ||||||
| 8.8 (0–0.18) | 5 | CIN (3) | 1.32 | 1.09 to 1.61 | 43 | 0.45 |
| Peri-op (2) | ||||||
| Other (0) | ||||||
| 0.28 (0.20–1) | 4 | CIN (2) | 1.47 | 1.23 to 1.75 | 37 | |
| Peri-op (1) | ||||||
| Other (1) |
AMI, acute myocardial infarction; CIN, contrast induced nephropathy; peri-op, peri-operative or intensive care unit.
Number of studies is provided in brackets.
Stroke
Five studies,13,25,28,36,37 involving 72,347 participants (13,475 with AKI), examined stroke as an outcome. The median follow-up was 2.7 years (IQI, 2.0–3.4). The pooled relative risk was 1.15 (95% CI, 1.03 to 1.28; I2: 0%) (Figure 1, Supplemental Figure 6). The relative risk of stroke did not vary between subgroups (Supplemental Table 4).
Sensitivity Analyses and Assessment of Heterogeneity
There were minimal differences in the relative risk of long-term cardiovascular disease based on whether studies initiated follow-up after discharge from hospital and thereby excluded inpatient events or based on the duration of follow-up (data not shown). Heterogeneity was limited for all analyses and therefore not explored further. There was no funnel plot asymmetry (Supplemental Figures 7 and 8).
Discussion
This study is a comprehensive summary of the long-term risk of cardiovascular mortality and cardiovascular disease in adults with AKI. There were three major findings. First, we found that AKI was associated with an 86% increased risk of cardiovascular mortality and a 38% increased risk of major cardiovascular events. Second, AKI was associated with a 58% increased risk of CHF and a 40% increased risk of acute myocardial infarction, and the association between AKI and these outcomes persisted robustly in subgroup analyses. Third, AKI was associated with a 15% increased risk of stroke.
Our study is the first comprehensive summary of the long-term risk of cardiovascular mortality and cardiovascular disease in adults with AKI. We noted that AKI was associated with cardiovascular mortality and major cardiovascular events and we also found robust associations for individual disease specific events.
In particular, we found that AKI was associated with a 58% increased risk of CHF. Furthermore, there was an association between the severity of AKI and the risk of CHF and this association persisted in studies that additionally adjusted for left ventricular ejection fraction. Although the risk of CHF was marginally lower in studies with a low proportion of adults with baseline IHD, the relative risks in both subgroups were well above the line of no effect, the threshold for which there may be no difference in the risk of CHF between adults with and without AKI. It is therefore unlikely that the association between AKI and CHF is due to residual confounding. Furthermore, we found that AKI was associated with a 40% increase in the relative risk of acute myocardial infarction. Although the risk of acute myocardial infarction did not increase with AKI severity, it is noteworthy that we combined the moderate and severe AKI categories, which may account for the absence of an association. Finally, the risk of acute myocardial infarction did not vary by the proportion of adults with baseline IHD.
To date, individual studies have focused on specific subgroups of patients (e.g., with established cardiovascular disease),13,28 and it remained unclear whether their findings were generalizable to populations without cardiovascular disease. Our assessment of the consistency of relative risk estimates based on the proportion of adults with baseline cardiovascular disease adds credence to the prognostic importance of AKI in populations with varying levels of cardiovascular risk. AKI may therefore be an important prognostic marker in patients with varying baseline cardiovascular risk.
Finally, with respect to stroke, we found that AKI was associated with a 15% relative risk increase. However, given the small effect size and the proximity of the lower confidence interval to the line of no effect, the relationship between AKI and stroke may also be due to residual confounding.
Taken together, the association between AKI, CHF, and acute myocardial infarction should receive greater attention from clinicians, particularly as small changes in clinical practice can improve outcomes for adults with AKI. For instance, in a retrospective cohort study, investigators demonstrated that AKI was unrecognized in 24% of patients admitted to hospital in a 1 month period.8 The authors also identified important gaps in the management of these patients, including failure to withhold nephrotoxic drugs in 39% of patients.8 With greater awareness and optimal care, the progression of AKI from mild to severe may be preventable, which could reduce long-term cardiovascular morbidity.
Our findings also highlight the need of longitudinal follow-up for adults with AKI. Prior studies have demonstrated decreased mortality in AKI survivors receiving nephrologist follow-up.38 However, it remains unclear what form of clinical care should be provided to these individuals, beyond the management of renal functional loss.38 Our findings highlight the need for screening and management of CHF and acute myocardial infarction in adults with AKI.
The following limitations should be considered when interpreting the findings of our study. First, we lacked individual patient data, which prevented us from using a common definition of AKI and from ensuring similar variables were adjusted for in regression models. This is particularly important as the definition of AKI varied between studies and there were insufficient studies to compare the risk estimates based on AKI definition. Thus, the data we report includes pooled populations of patients defined using the Acute Kidney Injury Network (AKIN), RIFLE, Kidney Disease Improving Global Outcomes (KDIGO), and other grading systems and this should be considered when interpreting the findings of our study. Second, the majority of studies included in our analysis did not achieve the full rating for selection in our risk of bias assessment because they examined highly selected populations. Third, due to the small number of studies providing stratified data for our meta-analysis, our assessment of the association between the severity of AKI and cardiovascular outcomes was based on comparisons between mild AKI and the combination of moderate-to-severe AKI, as opposed to the familiar three AKI severity grades defined in the consensus criteria. This may account for the absence of any difference in the risk of cardiovascular disease based on the severity of AKI. Finally, our study can only demonstrate an association between AKI and cardiovascular disease and these associations cannot be deemed as causal in nature. Specifically, it could be that patients who develop AKI have conditions that put them at increased risk for cardiovascular events, rather than being at higher risk because of AKI itself.
Taken together, AKI is associated with an increased relative risk of long-term cardiovascular mortality, major cardiovascular events, and particularly CHF and IHD. In contrast, the association with stroke may be due to residual confounding. Further research is required into interventions to improve long-term outcomes for adults with AKI.
Concise Methods
This study was conducted in accordance with the Meta-Analysis of Observational Studies in Epidemiology guidelines and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.39,40
Data Sources and Searches
We conducted a systematic search of MEDLINE and EMBASE (inception to March 2015). A qualified research librarian developed the search strategy and included but was not limited to search terms such as “acute kidney injury,” “acute renal failure,” “contrast induced nephropathy,” “congestive heart failure,” “myocardial infarction,” and “stroke”. This was supplemented by a review of past meta-analyses,3–5,7,15 a detailed review of references of included studies, and citation tracking with Google Scholar.
Cohort studies that examined adults with and without AKI and reported a measure of relative risk for the association between AKI and cardiovascular disease (see below) were included. Studies were also required to include a minimum of 50 participants with AKI and 50 participants without AKI with at least 6 months mean/median follow-up. Only studies reporting multivariable adjusted estimates were included and at a minimum, studies were required to adjust for age and sex. Unadjusted studies or studies that did not list variables included in their regression model were excluded. No language restrictions were applied.
Data Extraction and Quality Assessment
Two reviewers independently reviewed titles and abstracts to assess studies for their inclusion. Four reviewers independently abstracted data using standardized forms. Where available, we abstracted information on general study characteristics (study name or investigator’s name; recruitment date [mid-point of the recruitment period]; mean follow-up duration; year of publication of the primary findings) and summary information about the studied population that was clinically relevant to the outcomes specified for our study (definition of AKI; number of participants with and without AKI; mean age; number of men; number of adults with baseline CHF, IHD, and stroke). We also extracted information on the following outcomes: cardiovascular mortality, major cardiovascular events (a composite of cardiovascular death, stroke, acute myocardial infarction, and CHF), and disease specific events (CHF, acute myocardial infarction, and stroke).
Relative risk estimates and associated 95% CI for the association between AKI and the aforementioned study outcomes were abstracted. The list of variables included in the multivariable regression model was also abstracted.
A risk of bias assessment was performed using the Newcastle–Ottawa Scale, which assesses studies on three broad domains: the selection of participants for study groups; the comparability of study groups; and the ascertainment of the outcome. A star rating system is used to identify studies that are at low risk of bias and the maximum numbers of stars achievable are: selection – 4 stars; comparability – 2 stars; and outcome (cohort studies only) – 3 stars. We applied strict criteria to assess comparability (of individuals with or without AKI) based on which variables were included in the multivariable models. To receive one star for comparability, studies were required to have adjusted for age, sex, and at least one cardiovascular risk factor (hypertension, diabetes, smoking, cholesterol, and CKD) and a baseline history of cardiovascular disease (stroke or IHD) if applicable. To receive two stars, studies were additionally required to have adjusted for baseline serum creatinine, eGFR, or history of CKD.
Subgroup Analyses, Sensitivity Analyses
We performed four subgroup analyses in which included studies were grouped by the severity of AKI (mild versus moderate-to-severe), the recovery of renal function (complete versus partial or no renal recovery), clinical setting, and baseline cardiovascular disease and CKD. Subgroup analyses were only performed when at least two studies could be obtained for each stratum.
For the analysis of severity, mild AKI was defined as AKIN 1 according to the AKIN classification,41 or “Risk” according to the RIFLE classification,42 or Stage 1 according to the KDIGO classification. Moderate to severe AKI was defined as either AKIN 2 or 3, RIFLE “Injury” or “Failure,” KDIGO Stage 2 or 3, or AKI requiring dialysis.43 For the analysis of recovery, complete recovery was defined as a return of renal function to below the study-defined threshold for AKI and partial or no recovery was defined as the absence of this level of improvement in kidney function. Finally, with respect to setting, we specifically examined contrast-induced AKI and AKI in the peri-operative or ICU setting. For the subgroup analysis by baseline cardiovascular disease, we calculated the proportion of adults with a past history of CHF, IHD, stroke, and CKD at cohort entry and separated studies into halves based on this proportion. For studies which were limited to adults with acute myocardial infarction or adults receiving percutaneous coronary interventions or coronary artery bypass grafting, all study participants were identified as having baseline IHD. We compared the prognostic importance of AKI in the two groups.
Finally, we performed two sensitivity analyses for the overall pooled estimates for each outcome. First, studies were grouped based on whether follow-up for cardiovascular events commenced immediately after entry into the cohort or after a period of time (e.g., after hospital discharge). Second, studies were divided into halves based on average length of follow-up.
Data Synthesis, Analysis, and Planned Assessment of Heterogeneity
Overall summary estimates for each of the aforementioned outcomes were computed using a random effects meta-analysis. Where studies reported multiple relative risk estimates for specific subgroups without reporting an overall summary estimate, the subgroup estimates were pooled using fixed effects meta-analysis to ensure a single relative risk was obtained for each study prior to the overall analysis. Heterogeneity was assessed using the I2 statistic. Results are presented graphically using forest plots.
In accordance with Cochrane collaboration guidelines, when at least ten studies were included in meta-analysis we performed assessment for publication bias by visual inspection of funnel plots, supported by Egger tests.44 Where an asymmetric funnel plot was identified, the Copas selection model was used to account for small study effect bias.45
When high heterogeneity was observed (I2>60%), we planned to assess whether heterogeneity was reduced by subgroup analyses and in sensitivity analyses. If heterogeneity was not reduced to acceptable levels (I2<60%), we additionally assessed if age accounted for heterogeneity by performing meta-regression based on the average age (categorical variable divided into halves). All analyses were performed using R Statistical Software (Version 3.0).
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016010105/-/DCSupplemental.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Coca SG, Bauling P, Schifftner T, Howard CS, Teitelbaum I, Parikh CR: Contribution of acute kidney injury toward morbidity and mortality in burns: a contemporary analysis. Am J Kidney Dis 49: 517–523, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corredor C, Thomson R, Al-Subaie N: Long-Term Consequences of Acute Kidney Injury After Cardiac Surgery: A Systematic Review and Meta-Analysis. J Cardiothorac Vasc Anesth 30: 69–75, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Pickering JW, James MT, Palmer SC: Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 65: 283–293, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Gargiulo G, Sannino A, Capodanno D, Perrino C, Capranzano P, Barbanti M, Stabile E, Trimarco B, Tamburino C, Esposito G: Impact of postoperative acute kidney injury on clinical outcomes after transcatheter aortic valve implantation: A meta-analysis of 5,971 patients, Catheter Cardiovasc Interv 86: 518–527, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Aitken E, Carruthers C, Gall L, Kerr L, Geddes C, Kingsmore D: Acute kidney injury: outcomes and quality of care. QJM 106: 323–332, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, He Q, Chen J, Chen M, Liu X, Zhu Z, Yang L, Lian X, Ding F, Li Y, Wang H, Wang J, Wang R, Mei C, Xu J, Li R, Cao J, Zhang L, Wang Y, Xu J, Bao B, Liu B, Chen H, Li S, Zha Y, Luo Q, Chen D, Shen Y, Liao Y, Zhang Z, Wang X, Zhang K, Liu L, Mao P, Guo C, Li J, Wang Z, Bai S, Shi S, Wang Y, Wang J, Liu Z, Wang F, Huang D, Wang S, Ge S, Shen Q, Zhang P, Wu L, Pan M, Zou X, Zhu P, Zhao J, Zhou M, Yang L, Hu W, Wang J, Liu B, Zhang T, Han J, Wen T, Zhao M, Wang H; ISN AKF 0by25 China Consortiums : Acute kidney injury in China: a cross-sectional survey. Lancet 386: 1465–1471, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Ftouh S, Thomas M; Acute Kidney Injury Guideline Development Group : Acute kidney injury: summary of NICE guidance. BMJ 347: f4930, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, Cruz D, Jaber B, Lameire NH, Lombardi R, Lewington A, Feehally J, Finkelstein F, Levin N, Pannu N, Thomas B, Aronoff-Spencer E, Remuzzi G: International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 385: 2616–2643, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H, Yamamoto Y, Yokoyama S, Asai K, Mizuno K: Long-term prognostic impact after acute kidney injury in patients with acute heart failure. Int Heart J 53: 313–319, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Mielniczuk LM, Pfeffer MA, Lewis EF, Blazing MA, de Lemos JA, Mohanavelu S, Rouleau J, Fox K, Pedersen TR, Califf RM: Acute decline in renal function, inflammation, and cardiovascular risk after an acute coronary syndrome. Clin J Am Soc Nephrol 4: 1811–1817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jose P, Skali H, Anavekar N, Tomson C, Krumholz HM, Rouleau JL, Moye L, Pfeffer MA, Solomon SD: Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J Am Soc Nephrol 17: 2886–2891, 2006 [DOI] [PubMed] [Google Scholar]
- 15.James MT, Samuel SM, Manning MA, Tonelli M, Ghali WA, Faris P, Knudtson ML, Pannu N, Hemmelgarn BR: Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv 6: 37–43, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Ozrazgat-Baslanti T, Thottakkara P, Huber M, Berg K, Gravenstein N, Tighe P, Lipori G, Segal MS, Hobson C, Bihorac A: Acute and Chronic Kidney Disease and Cardiovascular Mortality After Major Surgery [published online ahead of print January 7, 2016]. Ann Surg doi: 10.1097/SLA.0000000000001582 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uyarel H, Cam N, Ergelen M, Akkaya E, Ayhan E, Isik T, Cicek G, Gunaydin ZY, Osmonov D, Gul M, Demirci D, Guney MR, Ozturk R, Yekeler I: Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction: incidence, a simple risk score, and prognosis. Arch Med Sci 4: 550–558, 2009 [Google Scholar]
- 18.Kim J-H, Yang JH, Choi S-H, Song YB, Hahn J-Y, Choi J-H, Lee SH, Gwon H-C: Predictors of outcomes of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with chronic kidney disease. Am J Cardiol 114: 1830–1835, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Narula A, Mehran R, Weisz G, Dangas GD, Yu J, Généreux P, Nikolsky E, Brener SJ, Witzenbichler B, Guagliumi G, Clark AE, Fahy M, Xu K, Brodie BR, Stone GW: Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J 35: 1533–1540, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Blair JEA, Pang PS, Schrier RW, Metra M, Traver B, Cook T, Campia U, Ambrosy A, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Konstam MA, Gheorghiade M; EVEREST Investigators : Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the EVEREST trial. Eur Heart J 32: 2563–2572, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL: Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol 9: 448–456, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen MK, Gammelager H, Jacobsen C-J, Hjortdal VE, Layton JB, Rasmussen BS, Andreasen JJ, Johnsen SP, Christiansen CF: Acute Kidney Injury and Long-term Risk of Cardiovascular Events After Cardiac Surgery: A Population-Based Cohort Study. J Cardiothorac Vasc Anesth 29: 617–625, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei Cas L: Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail 10: 188–195, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Watabe H, Sato A, Hoshi T, Takeyasu N, Abe D, Akiyama D, Kakefuda Y, Nishina H, Noguchi Y, Aonuma K: Association of contrast-induced acute kidney injury with long-term cardiovascular events in acute coronary syndrome patients with chronic kidney disease undergoing emergent percutaneous coronary intervention. Int J Cardiol 174: 57–63, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Gammelager H, Christiansen CF, Johansen MB, Tønnesen E, Jespersen B, Sørensen HT: Three-year risk of cardiovascular disease among intensive care patients with acute kidney injury: a population-based cohort study. Crit Care 18: 492, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ; COACH investigators : Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH). Eur J Heart Fail 11: 847–854, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Damman K, Perez AC, Anand IS, Komajda M, McKelvie RS, Zile MR, Massie B, Carson PE, McMurray JJV: Worsening renal function and outcome in heart failure patients with preserved ejection fraction and the impact of angiotensin receptor blocker treatment. J Am Coll Cardiol 64: 1106–1113, 2014 [DOI] [PubMed] [Google Scholar]
- 28.James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, Pannu N, Manns BJ, Klarenbach SW, Hemmelgarn BR; Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators : Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation 123: 409–416, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Choi AI, Li Y, Parikh C, Volberding PA, Shlipak MG: Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney Int 78: 478–485, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg A, Kogan E, Hammerman H, Markiewicz W, Aronson D: The impact of transient and persistent acute kidney injury on long-term outcomes after acute myocardial infarction. Kidney Int 76: 900–906, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Olsson D, Sartipy U, Braunschweig F, Holzmann MJ: Acute kidney injury following coronary artery bypass surgery and long-term risk of heart failure. Circ Heart Fail 6: 83–90, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Tsai M-L, Mao C-T, Chen D-Y, Hsieh I-C, Wen M-S, Chen T-H: Short- and long-term major cardiovascular adverse events in carotid artery interventions: a nationwide population-based cohort study in Taiwan. PLoS One 10: e0121016, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giacoppo D, Madhavan MV, Baber U, Warren J, Bansilal S, Witzenbichler B, Dangas GD, Kirtane AJ, Xu K, Kornowski R, Brener SJ, Généreux P, Stone GW, Mehran R: Impact of Contrast-Induced Acute Kidney Injury After Percutaneous Coronary Intervention on Short- and Long-Term Outcomes: Pooled Analysis From the HORIZONS-AMI and ACUITY Trials. Circ Cardiovasc Interv 8: e002475, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Rydén L, Ahnve S, Bell M, Hammar N, Ivert T, Sartipy U, Holzmann MJ: Acute kidney injury after coronary artery bypass grafting and long-term risk of myocardial infarction and death. Int J Cardiol 172: 190–195, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Lindsay J, Apple S, Pinnow EE, Gevorkian N, Gruberg L, Satler LF, Pichard AD, Kent KM, Suddath W, Waksman R: Percutaneous coronary intervention-associated nephropathy foreshadows increased risk of late adverse events in patients with normal baseline serum creatinine. Catheter Cardiovasc Interv 59: 338–343, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Holzmann MJ, Rydén L, Sartipy U: Acute kidney injury and long-term risk of stroke after coronary artery bypass surgery. Int J Cardiol 168: 5405–5410, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Wu V-C, Wu C-H, Huang T-M, Wang C-Y, Lai C-F, Shiao C-C, Chang C-H, Lin S-L, Chen Y-Y, Chen Y-M, Chu T-S, Chiang W-C, Wu K-D, Tsai P-R, Chen L, Ko W-J; NSARF Group : Long-term risk of coronary events after AKI. J Am Soc Nephrol 25: 595–605, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harel Z, Wald R, Bargman JM, Mamdani M, Etchells E, Garg AX, Ray JG, Luo J, Li P, Quinn RR, Forster A, Perl J, Bell CM: Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 83: 901–908, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151: 264–269, 2009 [DOI] [PubMed]
- 41.Mehta R, Kellum J, Shah S, Molitoris B, Ronco C, Warnock D, Levin A, the Acute Kidney Injury Network: Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed]
- 42.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup : Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kidney Disease: Improving Global Outcomes (KDIGO): KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 109: S1–S99, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwarzer G, Carpenter J, Rücker G: Empirical evaluation suggests Copas selection model preferable to trim-and-fill method for selection bias in meta-analysis. J Clin Epidemiol 63: 282–288, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.