Abstract
Randomized controlled trials suggest that protein restriction may retard the progression of CKD toward ESRD. However, the effects of dietary protein intake level and the food sources of dietary protein on the risk of ESRD in the general population remain unclear. We investigated these effects in the Singapore Chinese Health Study, a prospective population-based cohort that recruited 63,257 Chinese adults aged 45–74 years from 1993 to 1998. We collected habitual diet information via a validated semiquantitative food frequency questionnaire and identified ESRD via record linkage with a nationwide registry. In all, 951 cases of ESRD occurred over a mean follow-up of 15.5 years. Regarding total protein intake, compared with the lowest quartile, the three higher quartiles combined had a hazard ratio for ESRD of 1.24 (95% confidence interval [95% CI], 1.05 to 1.46), but the dose-dependent association across the quartiles was not statistically significant (Ptrend=0.16). Red meat intake strongly associated with ESRD risk in a dose-dependent manner (hazard ratio for highest quartile versus lowest quartile,1.40 [95% CI, 1.15 to 1.71; Ptrend<0.001]). Intake of poultry, fish, eggs, or dairy products did not associate with risk of ESRD. In substitution analysis, replacing one serving of red meat with other food sources of protein associated with a maximum relative risk reduction of 62.4% (95% CI, 33.1 to 78.9; P<0.01). Our study shows that red meat intake may increase the risk of ESRD in the general population and substituting alternative sources of protein may reduce the incidence of ESRD.
Keywords: end-stage renal disease, diet, red meat, protein, population-based study
ESRD is the final stage of CKD and is defined by the need for renal replacement therapy (dialysis or transplantation).1,2 The incidence and prevalence of all stages of CKD, including ESRD, are rising globally with nearly 500 million people estimated to have CKD,3 posing significant stress on worldwide public health systems.4 Evidence from animal and human studies suggests that high protein intake, especially animal-sourced protein, may accelerate decline in GFR.5–7 There is also evidence from randomized controlled trials that dietary protein restriction may retard the progression of disease among patients with advanced CKD.8–11 Current guidelines recommend the restriction of dietary protein intake as part of the management of CKD.1,2,12 However, the evidence on the benefit of dietary protein restriction, especially limiting specific sources of protein intake, on the risk of ESRD or decline in renal function in the general population remains unclear.13–17
We therefore analyzed data from the Singapore Chinese Health Study, a prospective cohort of Chinese adults in Singapore, to examine the relationship between habitual dietary intakes of major food sources of protein and incident ESRD. This population-based prospective cohort was linked with the Singapore Renal Registry, which maintains nationwide records of all patients with ESRD in Singapore. We also sought to explore whether the increased risk of ESRD with any particular food sources of protein, if present, could be modified by replacing with other sources of dietary protein using the method of substitution analyses previously described.18,19 We hypothesized that risk of progression to incident ESRD would be higher with habitual intake of red meat compared with other food sources of proteins in the general Chinese population.
Results
After a mean follow-up of 15.5 (SD 4.3) years among 60,198 participants (932,646 person-years), there were 951 incident ESRD cases. The mean age at ESRD diagnosis was 69.3 (SD 8.6; range 47.6–92.6) years. The distributions of selected characteristics among the quartiles of red meat intake are shown in Table 1. Compared with the lowest quartile of red meat intake, participants with highest quartile were slightly younger at recruitment (55.7 years versus 56.5 years), more likely to be of Hokkien descent, and less likely to be physically active (31% versus 39% had at least 30 minutes of moderate activity, vigorous activity or strenuous sports per week). Participants who had the highest quartile of red meat intake also had lower prevalence of self-reported hypertension (22% versus 24%), but had higher prevalence of self-reported diabetes mellitus (9% versus 7%) at baseline compared with those in the lowest quartile of red meat intake. We also noted that participants who had the highest quartile of red meat intake also had significantly higher total protein intake (65.3 versus 53.1 g/d), but significantly lower intake of fruits (169.5 versus 232.8 g/d) and vegetables (107.9 versus 113.8 g/d) compared with those in the lowest quartile of red meat intake (Table 1). Interestingly, it was noted that subjects who were in the second and third quartiles of red meat intake were less educated (27% and 26%, respectively) and less likely to smoke (27% and 29%, respectively) compared with their counterparts who were in the lowest quartile (31% had secondary or higher education, and 31% were ever smokers) or those in the highest quartile of meat intake (30% had secondary or higher education, and 35% were ever smokers).
Table 1.
Baseline characteristics of cohort participants by intake of red meat (energy-adjusted) (n=60,198)
| Characteristics | Quartiles of Red Meat Intake | |||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Red meat intake (g/d), median (interquartile range) | 12.5 (6.5–16.3) | 24.2 (21.8–26.5) | 33.4 (30.9–36.1) | 48.8 (43.3–58.3) |
| Number of participants | 15,143 | 15,199 | 14,909 | 14,947 |
| Number of ESRD cases, n (%) | 185 (1) | 255 (2) | 252 (2) | 259 (2) |
| Age at recruitment (y), mean (SD) | 56.5 (7.8) | 56.9 (8.1) | 56.5 (8.1) | 55.7 (7.9) |
| Body mass index (kg/m2), mean (SD) | 23.0 (3.3) | 23.1 (3.2) | 23.2 (3.2) | 23.2 (3.3) |
| Male, n (%) | 7534 (50) | 5968 (39) | 5895 (40) | 7384 (50) |
| Dialect, n (%) | ||||
| Cantonese | 7682 (51) | 7032 (46) | 6504 (44) | 6658 (45) |
| Hokkien | 7461 (50) | 8167 (54) | 8405 (56) | 8289 (56) |
| Secondary school or higher, n (%) | 4648 (31) | 4129 (27) | 3940 (26) | 4441 (30) |
| Ever smoker, n (%) | 4705 (31) | 4143 (27) | 4296 (29) | 5221 (35) |
| Hypertension, n (%) | 3620 (24) | 3815 (25) | 3469 (23) | 3311 (22) |
| Diabetes, n (%) | 1118 (7) | 1388 (9) | 1420 (10) | 1405 (9) |
| Coronary heart disease, n (%) | 649 (4) | 668 (4) | 607 (4) | 534 (4) |
| Stroke, n (%) | 259 (2) | 228 (2) | 216 (1) | 184 (1) |
| Physical activitya, n (%) | 5866 (39) | 4975 (33) | 4437 (30) | 4631 (31) |
| Alcohol intake, n (%) | ||||
| Nondrinkers | 13,165 (87) | 13,636 (90) | 13,413 (90) | 12,976 (87) |
| Weekly drinkers | 1299 (9) | 1094 (7) | 1110 (7) | 1423 (10) |
| Daily drinkers | 679 (5) | 469 (3) | 386 (3) | 548 (4) |
| Total protein intakeb (g/d), mean (SD) | 53.1 (10.3) | 57.6 (7.9) | 60.5 (7.6) | 65.3 (9.0) |
| Vegetables (g/d), mean (SD) | 113.8 (69.7) | 110.6 (48.6) | 109.5 (44.6) | 107.9 (48.1) |
| Fruits (g/day), mean (SD) | 232.8 (195.2) | 210.7 (138.0) | 194.8 (126.0) | 169.5 (129.50) |
P values based on the chi-squared test for categorical variables and one-way ANOVA for continuous variables, all P values <0.01.
Physical activity defined as any weekly moderate activity, vigorous activity or strenuous sports lasting at least 30 minutes (yes or no).
Total protein intake from both plant and animal sources.
Total protein intake, which included protein from both plant and animal sources, was positively associated with incidence of ESRD in a model that adjusted for basic demographic characteristics (i.e., age, gender, dialect, educational level, and year of interview), with a hazard ratio (HR) of 1.55 (95% confidence interval [95% CI], 1.28 to 1.87; Ptrend<0.001) when comparing the highest quartile with the lowest quartile intake. However, the HR was attenuated to 1.19 (95% CI, 0.98 to 1.44) after adjusting for other lifestyle and comorbidity factors. Compared with the lowest quartile, the HR for the three higher quartiles combined was 1.24 (95% CI, 1.05 to 1.46), but the dose dependent association across the quartiles was not statistically significant (Ptrend=0.16) after adjusting for lifestyle and comorbidity factors (Table 2). We also conducted sensitivity analysis to determine the relationship between absolute protein intake per day and total protein intake/body weight, and ESRD risk. For both variables, the risk of ESRD increased with increasing quartile intake but the risk estimates comparing the extreme quartiles and the dose-dependent association across the quartiles did not reach statistical significance (data not shown).
Table 2.
Hazard ratios (95% CI) for risk of ESRD according to dietary protein intakes (n=60,198)
| Protein (% kcal/d) | Quartiles of Energy-Adjusted Protein Intake | P for Trenda | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Total protein | |||||
| Cases | 185 | 255 | 252 | 259 | |
| Person-years | 233,198 | 231,836 | 233,469 | 234,143 | |
| Multivariate model 1 | 1.00 | 1.40 (1.16–1.70) | 1.43 (1.18–1.74) | 1.55 (1.28–1.87) | <0.001 |
| Multivariate model 2 | 1.00 | 1.28 (1.05–1.55) | 1.25 (1.03–1.52) | 1.19 (0.98–1.44) | 0.16 |
| Total protein | Q1 | Q2 to Q4 combined | |||
| Cases | 185 | 766 | |||
| Person-years | 233,198 | 699,448 | |||
| Multivariate model 1 | 1.00 | 1.46 (1.24–1.72) | |||
| Multivariate model 2 | 1.00 | 1.24 (1.05–1.46) | |||
Hazard ratios (95% confidence interval) presented for each quartile of protein intake (energy-adjusted) for all multivariate models. Multivariate model 1: adjusted for age, gender, dialect, educational level, and year of interview. Multivariate model 2: model 1 plus body mass index, physical activity, smoking status, alcohol use, baseline history of diabetes, baseline history of hypertension, baseline history of stroke, baseline history of heart attack, and total energy intake.
Linear trend was tested by assigning to participants the median value of the quartile and assessing this as a continuous variable.
When analyzing the individual food sources of protein intakes, we observed a strong, dose-dependent, positive association with red meat, even after adjusting for lifestyle/comorbidity risk factors of ESRD and other food sources of protein: the HR comparing the extreme quartiles was 1.40 (95% CI, 1.15 to 1.71; Ptrend<0.001) for red meat intake (Table 3). When we adjusted further for total protein intake, the positive dose response relationship between red meat intake and ESRD risk was maintained: the HR comparing the extreme quartiles was 1.38 (95% CI, 1.11 to 1.71; Ptrend<0.001), suggesting that the increased risk associated with red meat intake was not confounded by higher overall dietary protein intake.
Table 3.
Hazard ratios (95% CI) for risk of ESRD according to food sources of protein (n=60,198)
| Food Sources of Protein (g/d) | Quartiles of Energy-Adjusted Food Intake | P for Trenda | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Red meat | |||||
| Cases | 194 | 220 | 267 | 270 | |
| Person-years | 236,141 | 236,256 | 230,243 | 230,006 | |
| Multivariate model 1 | 1.00 | 1.11 (0.91–1.35) | 1.39 (1.16–1.68) | 1.47 (1.22–1.77) | <0.001 |
| Multivariate model 2 | 1.00 | 1.01 (0.83–1.24) | 1.29 (1.06–1.56) | 1.36 (1.13–1.64) | <0.001 |
| Multivariate model 3 | 1.00 | 1.03 (0.84–1.26) | 1.32 (1.07–1.61) | 1.40 (1.15–1.71) | <0.001 |
| Poultry | |||||
| Cases | 232 | 243 | 253 | 223 | |
| Person-years | 230,852 | 232,599 | 232,016 | 237,180 | |
| Multivariate model 1 | 1.00 | 1.02 (0.85–1.22) | 1.14 (0.95–1.36) | 1.07 (0.89–1.29) | 0.37 |
| Multivariate model 2 | 1.00 | 0.92 (0.77–1.12) | 1.04 (0.86–1.26) | 0.99 (0.82–1.20) | 0.77 |
| Multivariate model 3 | 1.00 | 0.88 (0.72–1.06) | 0.95 (0.78–1.16) | 0.90 (0.74–1.09) | 0.49 |
| Fish and shellfish | |||||
| Cases | 204 | 251 | 237 | 259 | |
| Person-years | 229,112 | 231,006 | 235,816 | 236,713 | |
| Multivariate model 1 | 1.00 | 1.21 (1.01–1.46) | 1.14 (0.94–1.37) | 1.27 (1.06–1.53) | 0.03 |
| Multivariate model 2 | 1.00 | 1.16 (0.96–1.39) | 1.06 (0.87–1.27) | 1.12 (0.93–1.34) | 0.44 |
| Multivariate model 3 | 1.00 | 1.12 (0.93–1.35) | 1.01 (0.84–1.23) | 1.07 (0.89–1.30) | 0.71 |
| Eggs | |||||
| Cases | 228 | 261 | 242 | 220 | |
| Person-years | 235,386 | 235,980 | 232,203 | 229,078 | |
| Multivariate model 1 | 1.00 | 1.12 (0.94–1.34) | 1.09 (0.91–1.30) | 1.03 (0.86–1.24) | 0.95 |
| Multivariate model 2 | 1.00 | 1.08 (0.89–1.30) | 1.07 (0.88–1.30) | 1.06 (0.87–1.28) | 0.68 |
| Multivariate model 3 | 1.00 | 1.04 (0.86–1.26) | 1.02 (0.84–1.23) | 1.00 (0.83–1.22) | 0.94 |
| Dairy products | |||||
| Cases | 212 | 249 | 246 | 244 | |
| Person-years | 237,538 | 238,186 | 226,046 | 230,876 | |
| Multivariate model 1 | 1.00 | 1.12 (0.93–1.35) | 1.17 (0.97–1.41) | 1.20 (0.99–1.45) | 0.18 |
| Multivariate model 2 | 1.00 | 1.03 (0.84–1.26) | 1.14 (0.93–1.41) | 1.09 (0.90–1.32) | 0.56 |
| Multivariate model 3 | 1.00 | 1.03 (0.84–1.26) | 1.14 (0.92–1.40) | 1.11 (0.92–1.35) | 0.38 |
| Soy and legumes | |||||
| Cases | 243 | 253 | 243 | 212 | |
| Person-years | 229,680 | 232,728 | 232,980 | 237,259 | |
| Multivariate model 1 | 1.00 | 1.01 (0.84–1.20) | 0.99 (0.83–1.19) | 0.89 (0.74–1.07) | 0.18 |
| Multivariate model 2 | 1.00 | 0.95 (0.79–1.14) | 0.95 (0.79–1.15) | 0.82 (0.68–0.99) | 0.05 |
| Multivariate Model 3 | 1.00 | 0.94 (0.78–1.13) | 0.95 (0.78–1.14) | 0.83 (0.69–1.01) | 0.07 |
Hazard ratios (95% confidence interval) presented for each quartile of food intake (energy-adjusted) for all multivariate models. Multivariate model 1: adjusted for age, gender, dialect, educational level and year of interview. Multivariate model 2: model 1 plus body mass index, physical activity, smoking status, alcohol use, baseline history of diabetes, baseline history of hypertension, baseline history of stroke, baseline history of heart attack and total energy intake. Multivariate model 3: model 2 plus energy adjusted intake of vegetable, fruits, red meat, poultry, fish and shellfish, eggs, dairy products, soy foods, and nonsoy legumes.
Linear trend was tested by assigning to participants the median value of the quartile and assessing this as a continuous variable.
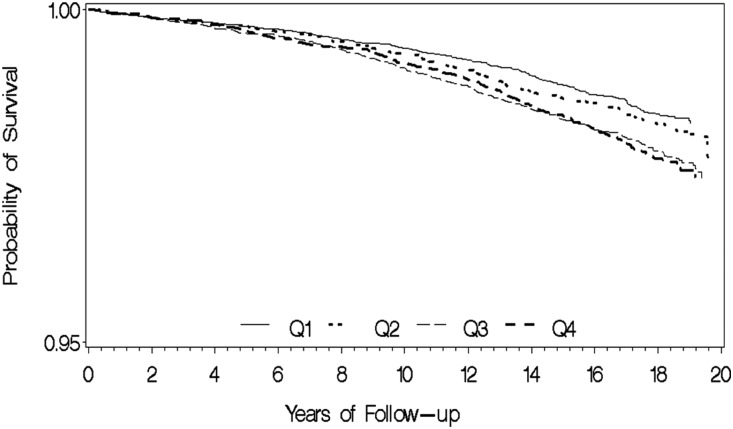
Conversely, there was an inverse relationship between consumption of soy food and legumes and risk of ESRD that was of borderline statistical significance: the HR comparing the extreme quartiles was 0.83 (95% CI, 0.69 to 1.01; Ptrend=0.07) in an overall adjusted model that included basic demographic factors, lifestyle/comorbidity risk factors, and other food sources of protein. Figure 1 illustrates ESRD-free survival by quartile intake of red meat. Although event rate was low, the probability of ESRD-free survival was higher among those with lower intake (quartiles 1 and 2) compared with those with higher intake (quartiles 3 and 4) (log-rank P<0.001). No significant association with risk of ESRD was observed with the intake of poultry, fish and shellfish, eggs, and dairy products (Table 3).
Figure 1.
Kaplan–Meier survival curves show probability of ESRD-free survival according to the quartile (Q) intake of red meat. Log-rank P value for difference in survival was <0.001.
We repeated our analysis by including an additional 107 CKD deaths, which were not registered as ESRD cases in the Singapore Renal Registry, with the 951 ESRD cases as a composite outcome, and continued to observe a strong, dose-dependent, positive association with red meat, but not with other sources of protein (Table 4). Compared with the lowest quartile, the HR for the three higher quartiles combined was 1.20 (95% CI, 1.00 to 1.43) for total protein intake and compared with the lowest quartile, the HR for the fourth quartile of red meat intake was 1.41 (95% CI, 1.17 to 1.71; Ptrend<0.001). Finally, we repeated the analysis with 15,082 cases of total deaths and ESRD as a composite outcome, and the dose-dependent, positive association with red meat remained unchanged. Compared with the lowest quartile, the HR for the highest quartile of red meat intake was 1.11 (95% CI, 1.06 to 1.17; Ptrend<0.001).
Table 4.
Hazard ratios (95% confidence intervals) for risk of ESRD and CKD deaths according to food sources of protein (n=60,198)
| Food Sources of Protein (g/d) | Quartiles of Energy-Adjusted Food Intake | P for Trenda | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Total protein | |||||
| Cases | 214 | 277 | 270 | 297 | |
| Person-years | 233,198 | 231,836 | 233,469 | 234,143 | |
| Multivariate model 1 | 1.00 | 1.20 (1.00–1.44) | 1.16 (0.97–1.40) | 1.20 (1.00–1.43) | 0.09 |
| Red meat | |||||
| Cases | 218 | 243 | 296 | 301 | |
| Person-years | 236,141 | 236,256 | 230,243 | 230,006 | |
| Multivariate model 2 | 1.00 | 1.02 (0.84–1.23) | 1.31 (1.08–1.59) | 1.41 (1.17–1.71) | <0.001 |
| Poultry | |||||
| Cases | 259 | 277 | 274 | 248 | |
| Person-years | 230,852 | 232,599 | 232,016 | 237,180 | |
| Multivariate model 2 | 1.00 | 0.90 (0.75–1.07) | 0.93 (0.77–1.12) | 0.91 (0.76–1.09) | 0.47 |
| Fish and shellfish | |||||
| Cases | 227 | 282 | 258 | 291 | |
| Person-years | 229,112 | 231,006 | 235,816 | 236,713 | |
| Multivariate model 2 | 1.00 | 1.13 (0.95–1.35) | 1.00 (0.83–1.20) | 1.10 (0.92–1.32) | 0.56 |
| Eggs | |||||
| Cases | 257 | 291 | 263 | 247 | |
| Person-years | 235,386 | 235,980 | 232,203 | 229,078 | |
| Multivariate model 2 | 1.00 | 1.03 (0.86–1.24) | 0.99 (0.82–1.19) | 1.01 (0.84–1.21) | 0.99 |
| Dairy products | |||||
| Cases | 242 | 274 | 271 | 271 | |
| Person-years | 237,538 | 238,186 | 226,046 | 230,876 | |
| Multivariate model 2 | 1.00 | 0.98 (0.80–1.18) | 1.07 (0.88–1.31) | 1.06 (0.88–1.28) | 0.47 |
| Soy and legumes | |||||
| Cases | 268 | 276 | 272 | 242 | |
| Person-years | 229,680 | 232,728 | 232,980 | 237,259 | |
| Multivariate model 2 | 1.00 | 0.93 (0.78–1.11) | 0.97 (0.81–1.16) | 0.88 (0.73–1.05) | 0.18 |
Hazard ratios (95% confidence interval) presented for each quartile of food intake (energy-adjusted) for all multivariate models. Multivariate model 1: adjusted for age, gender, dialect, educational level, and year of interview plus body mass index, physical activity, smoking status, alcohol use, baseline history of diabetes, baseline history of hypertension, baseline history of stroke, baseline history of heart attack, and total energy intake.
Multivariate model 2: model 1 plus energy adjusted intake of vegetable, fruits, red meat, poultry, fish and shellfish, eggs, dairy products, soy foods, and nonsoy legumes.
Linear trend was tested by assigning to participants the median value of the quartile and assessing this as a continuous variable.
A four-year lag sensitivity analysis with a total of 57,864 participants and 811 cases yielded similar results as the complete dataset (Supplemental Table 1). The positive relations of total protein and red meat with ESRD incidence remained largely unchanged. For example, the HR for the upper three quartile intakes of total protein combined, compared with the lowest quartile, was 1.49 (95% CI, 1.25 to 1.78) in the basic model and 1.29 (95% CI, 1.07 to 1.54) in the model that adjusted for lifestyle and comorbidity risk factors of ESRD. The strong dose-dependent association between red meat intake and ESRD risk remained clear in the four-year lag sensitivity analysis (Supplemental Table 2): the risk estimates for quartile 2, quartile 3 and quartile 4 intakes were 1.06 (95% CI, 0.85 to 1.32), 1.36 (95% CI, 1.09 to 1.70), and 1.49 (95% CI, 1.20 to 1.86), respectively, Ptrend<0.001, in the overall adjusted model. No significant associations could be found for the other food sources of protein intake with risk of ESRD.
We conducted sensitivity analysis with 239 incident ESRD cases arising from 42,039 participants without baseline history of diabetes, hypertension, coronary heart disease, or stroke (Supplemental Table 3). In this subpopulation, which included only 25% of ESRD cases, there was no clear association of ESRD risk with total protein intake (HR, 1.28 [95% CI, 0.87 to 1.90; Ptrend=0.26]) when comparing the two extreme quartiles, although the risk was still higher in the upper three quartiles combined compared with the lowest quartile (HR, 1.40; 95% CI, 1.01 to 1.92). However, intake of red meat continued to display a strong dose-dependent association with ESRD risk (Supplemental Table 4). The risk estimates for quartiles 2, 3, and 4 intakes were 1.16 (95% CI, 0.79 to 1.72), 1.17 (95% CI, 0.78 to 1.75), and 1.47 (95% CI, 0.99 to 2.17), respectively (Ptrend=0.06), in the overall adjusted model. All other food sources of protein examined, including soy and legumes, had null associations with ESRD risk in this subpopulation. The same positive association with red meat was also observed in participants with at least one underlying comorbidity risk factor for ESRD, namely diabetes, hypertension, coronary heart disease, or stroke. The risk estimates for quartiles 2, 3, and 4 intakes were 0.98 (95% CI, 0.77 to 1.24), 1.36 (95% CI, 1.08 to 1.72), and 1.42 (95% CI, 0.13 to 1.80), respectively (Ptrend=0.003), in the overall adjusted model.
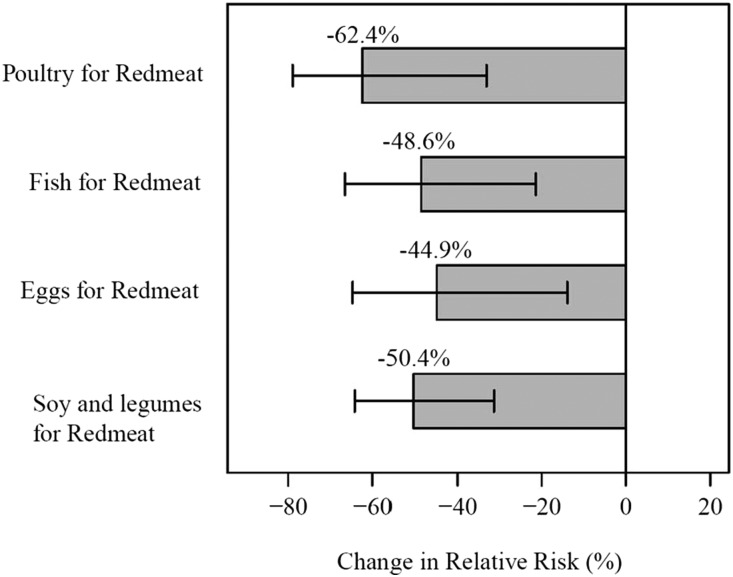
Exploring the effect of food replacement, we observed that substituting one serving of red meat with either one serving of poultry, fish, eggs, or soy and legumes resulted in a significant decline of ESRD risk. Figure 2 shows the percentage change in ESRD risk associated with the various food substitutions. Substituting one daily serving of red meat with poultry resulted in the highest reduction of ESRD risk of 62.4% (95% CI, 33.1 to 78.9; P<0.01). Substituting one daily serving of red meat with fish was associated with 48.6% lower risk of ESRD risk (95% CI, 21.4 to 66.5; P<0.01), whereas replacing one daily serving of red meat with soy and legumes or eggs resulted in 50.4% (95% CI, 31.3 to 64.2; P<0.01) and 44.9% (95% CI, 13.9 to 64.7; P<0.01) reduction in ESRD risk, respectively. Similar reductions in ESRD risk were observed when we replaced 100 g servings of red meat with 100 g of poultry, fish, eggs, or soy and legumes (results not shown).
Figure 2.
Substituting one serving of red meat with one serving of poultry, fish, eggs, or soy and legumes resulted in significant percent reduction in relative risk of ESRD; all P<0.01.
Discussion
Our study found that among the different food sources of protein examined, red meat intake was strongly associated with an increased risk of ESRD in a dose-dependent manner. No association was found with intakes of poultry, fish, eggs, or dairy products, whereas soy and legumes appeared to be slightly protective. Substituting one serving of red meat with other sources of protein significantly reduced the risk of ESRD.
Our finding of a strong positive relationship between red meat intake and ESRD incidence is largely consistent with the results of studies in other populations that have examined the effect of animal protein or nondairy meat protein. In an ecological study from Japan, there was a positive correlation between annual ESRD incidence and animal protein intake by geographical regions.17 The Nurses’ Health Study in the United States found that women with a mostly Western-style diet, which consisted of a higher intake of red and processed meats, saturated fats, and sweets, had a higher risk of decline in eGFR rate than the women who kept to a mostly Dietary Approaches to Stop Hypertension (DASH)-style diet, which consisted of a higher intake of vegetables, fruits, and whole grains.20 A dietary pattern consisting mostly of processed and fried foods, organ meats, and sweets, also known as the Southern-style diet, has also been found to be associated with an increased risk of mortality in patients with CKD.21
The decreased risk of CKD incidence or progression and death with a healthier dietary pattern was also observed by Dunkler et al. who studied participants from the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET). They found that subjects who consumed a “high intake of healthy foods, such as vegetables, and lower intake of unhealthy foods, such as fried foods” had lower risks of developing CKD. However, they also found that a higher intake of meat decreased CKD incidence or progression, which contrasts with the results of our study.22 We noted that ONTARGET involved a high risk population, which consisted entirely of subjects with diabetes, for a comparatively short period of time of 5.5 years. In contrast, only about 10% of our study subjects had diabetes at recruitment, and our study subjects were studied for a median of 15.5 years. Interestingly, whereas increased intake of animal-sourced protein was associated with increased intake of plant-sourced protein in the ONTARGET trial, participants who ate more meat in our study were found to eat less soy, fruits, and vegetables. Hence, these differences may explain the discrepancy between the findings of the ONTARGET trial and our study. In our study, the positive relationship between red meat and ESRD risk was present in those with or without existing comorbidities that strongly correlated with CKD, suggesting the propensity of meat to worsen CKD progression in those with underlying comorbidity risk factors as well as to cause CKD de novo in apparently healthy individuals without known risk factors for CKD.
We also found that the substitution of one serving of red meat per day with a serving of other sources of protein may reduce the risk of ESRD. Poultry, fish, eggs, soy, and legumes were all protective relative to red meat. High dietary acid load has been shown to be associated with higher incidence of ESRD in the general population in the United States.23 Conversely, plant-based proteins (e.g., soy and legumes) and fruits and vegetables have a high basal load.24 Therapy with alkali has been shown to slow the progression to ESRD in randomized controlled trials.25 Published data have shown that red meat generally yields greater acid production than other animal-sourced protein, although the difference is not that dramatic.26 Hence, a plausible explanation could be that red meat may produce endogenously more acid that other protein-rich food, and the differential ability to induce endogenous acid and base offers one explanation for the observed association between red mean and ESRD risk in our study population. Other explanations include the roles of nitrites, nitrates, heme iron, advanced glycation endproducts (AGEs), and advanced lipoxidation endproducts (ALEs) found in red meat.27 Specifically, dietary AGEs and ALEs have been postulated to play a role in diabetes mellitus due to their oxidative properties, and it has been shown that patients with CKD and ESRD have higher levels of circulatory AGEs and ALEs.28 Finally, previously published results have shown that the methods of cooking meats in this population were similar, with boiling or steaming being the most common method.29 Hence, we do not think that the method of cooking of red meat plays a significant role in the risk of ESRD, nor does it explain the differences in ESRD risks with different types of meat found in this study. Future studies are needed to investigate the underlying mechanisms as to how other compounds present in red meat may deteriorate renal function.
The strengths of our study include its prospective design, large sample size, and long-term and complete follow-up of outcomes. The dietary intake was assessed using a validated food frequency questionnaire that was developed specifically for this population and demonstrated to be internally consistent and reproducible.30 We also had nearly complete morbidity assessment with objectively obtained records on the incidence of ESRD using the nationwide Singapore Renal Registry, which has a high rate of follow up and strict definitions of ESRD that follow international guidelines.31 The age-specific rates for the Singapore population for treated ESRD in those above 70 years old was close to 1000 per million population.31 In this cohort, the rates were 1090 per million for men, and 967 per million for women. Hence, the rate in this cohort was comparable with the national rate. Lag sensitivity analysis and the exclusion analysis of patients with existing chronic diseases known to contribute to CKD yielded similar results to those derived from the entire cohort, lending support for an underlying role of red meat in the pathogenesis of and progression to ESRD. The substitution analysis demonstrates the amount of avoidance in the proportion of ESRD in a population with the replacement of red meat with other food sources of protein.
The limitations of our study are those related to the nature of an observation study, the use of self-report, and one-time assessment of diet, which could lead to misclassification bias due to measurement error. However, given the prospective design, the potential misclassification error is unlikely to be different between ESRD cases and noncase participants. Hence, such nondifferential misclassification with respect to disease status could likely result in an underestimation of risk. Although we performed multivariate Cox regression analysis with a comprehensive adjustment for known lifestyle and comorbidity risk factors for ESRD and all other food sources of protein, we cannot completely rule out residual confounding due to unmeasured potential confounders. As reported previously, red meat intake in this population consisted mainly of pork (97%).29 Thus, generalizability to other common sources of red meat including beef and lamb needs further evaluation. We also did not collect any biomarkers as part of this study and were not able to measure the progression of kidney disease, if any, over time. Information on CKD deaths that were not due to ESRD may not be comprehensive as many laboratories in Singapore still do not routinely report eGFR, and therefore earlier stages of CKD as an underlying diagnosis of death may be under-reported. However, the data on our primary outcome of incident ESRD, including deaths from ESRD, were comprehensive. Finally, given the observational nature of the study, any inference on causality should be made with caution.
In summary, protein intake from red meat is positively associated with increased ESRD risk. Substituting red meat with other food sources of protein may reduce the risk of developing ESRD, especially in high-risk populations. Future studies are warranted to confirm our findings and to investigate the underlying mechanisms as to how the acid load or other compounds present in red meat may aggravate the progression of CKD.
Concise Methods
Study Population
As described previously, the Singapore Chinese Health Study is a population-based cohort of 63,257 Chinese adults, aged 45–74 years during recruitment from April 1993 to December 1998.30 The study recruited participants who resided in government housing estates, facilities in which 86% of Singaporeans resided during the recruitment period. We also restricted the participants to those belonging to the two major dialect groups, Hokkien and Cantonese, who originated from the contiguous provinces of Fujian and Guangdong in the southern part of China. At recruitment, after obtaining informed consent, trained interviewers conducted face-to-face interviews in participants’ homes using a structured, scanner-readable questionnaire that ascertained information on demographics, height, weight, tobacco use, physical activity, and medical history. Habitual diet in the preceding year was captured using a validated 165-item food frequency questionnaire.30
The present study was approved by the Institutional Review Board of the National University of Singapore. All participants gave informed consent.
Assessment of Diet and Covariates
The semiquantitative food frequency questionnaire included 165 commonly consumed food items in this population which were categorized into rice and noodle, meats (pork/beef/mutton, poultry, fish, and shellfish), vegetables, fruits, soy foods, legumes, dairy products, beverages, condiments, and preserved foods. The participants were instructed to select from eight frequency categories (ranging from “never or hardly ever” to “two or more times a day”) and three portion sizes (small, medium, large) with the aid of photographs. As previously described, the dietary nutrients of the food items were derived from the Singapore Food Composition Database, which was developed together with this cohort study and is a food-nutrient database that lists the levels of 96 nutritive/non-nutritive compounds per 100 g of cooked food and beverages in the Singaporean Chinese diet.30
The food frequency questionnaire was subsequently validated using two 24-hour recalls and readministration of the food frequency questionnaire among 810 cohort participants. The correlation coefficient by these two methods for each dietary component ranged between 0.24 and 0.79, and the differences between mean values of most pairs for energy and nutrients were within 10% of each other’s values.30
Incident ESRD Cases and CKD Mortality Cases
ESRD cases were identified by linking the cohort database with the population-based Singapore Renal Registry. The Singapore Renal Registry has been shown to be comprehensive in its recording of ESRD cases since 1999.31 Multiple sources are used to identify ESRD cases, and these include laboratory records, hospital records, and listings of patients on dialysis. Since 2011 notifications of ESRD have been made compulsory under the National Registry of Diseases Act. Consistent with other international guidelines, the registry defines ESRD as meeting at least one of the following criteria: (1) serum creatinine level ≥880 µmol/L (10 mg/dl), (2) eGFR<15 ml/min per 1.73 m2 (based on either the Modification of Diet in Renal Disease Study equation, Cockcroft Gault equation, or 24-hour creatinine clearance), (3) undergoing hemodialysis or peritoneal dialysis, or (4) has undergone kidney transplant. Criteria 1–3 had to be persistent for >3 months to qualify as ESRD.2,31 CKD death was defined according to the International Classification of Diseases, Ninth Revision, codes 585.0–586.9 that were recorded as the primary cause of death in death certificates. The team of nurses performed audits of ESRD cases annually to ensure data accuracy of at least 95% and inter-rater reliability (κ coefficient) of ≥75%. As of 31 Dec 2012, only 47 subjects (0.07%) from our cohort were known to be lost to follow-up due to migration out of Singapore or for other reasons. This suggests that emigration among these subjects was negligible. Thus the follow-up via linkage with nationwide registries can be considered to be virtually complete.
The current analysis included data from 60,198 participants after excluding 110 participants who were diagnosed with ESRD, and another 1927 participants who had a diagnosis of cancer, at baseline. A further 1022 participants who reported extreme calorie intakes (<600 or >3000 kcal/d for women, and <700 or >3700 kcal/d for men) were also excluded from the analysis.
Statistical Analyses
Primary outcome was defined as incident ESRD and secondary outcome was defined as incident ESRD or CKD deaths in this study. Person-years for each participant were calculated from date of baseline interview to date of reported ESRD, lost-to-follow-up, death, or December 31, 2012, whichever occurred first. The differences between baseline characteristics by ESRD status were examined using the chi-squared test for categorical variables and t test for continuous variables. The dietary intakes of protein and food sources were analyzed in quartiles of density intake. Cox proportional hazards regression was used to calculate the HR and its 95% CI for developing ESRD by using the lowest quartile of dietary intake as the reference group. The proportional hazards assumption was met and no significant interactions with time were observed for the variables of interest in our study.
The energy-adjusted intakes of food, protein, and soy protein were computed using the residual method to control for potential confounding by total energy intake and to remove extraneous variation due to total energy intake.32 This method involved using linear regression to regress the food variable with total energy intake to obtain the residuals. Then, the value of the food variable at the mean population’s energy intake was added to the residuals to make it more interpretable. The quartile levels of each energy-adjusted intake for food/nutrient were derived from their respective distributions among all the study participants in the cohort. Total energy intake (kcal/d) was also included as a covariate in this residual model method.32 Tests for trend were performed by using median values of intake in the quartile categories as continuous variables in the Cox regression models.
The selection of potential confounders was based primarily on prior consideration of their associations with either dietary intake or risk of ESRD in this population. Three different analytical models were used: (1) model 1 (a basic model) included age (years), sex, year of baseline interview (1993–1995, 1996–1998), dialect (Hokkien/Cantonese), and educational level (none, primary school, secondary school, or higher); (2) model 2 included all variables in model 1 and the following variables: smoking status (never, ever), alcohol consumption (none, occasionally, weekly, daily), body mass index (<20.0, 20.0–23.9, 24.0–27.9, ≥28.0 kg/m2), physical activity (defined as any weekly moderate activity, vigorous activity or strenuous sports lasting at least 30 minutes, yes or no), self-reported histories of physician-diagnosed hypertension, diabetes, coronary heart disease, and stroke (yes or no for each disease), and total energy intake; and (3) model 3 included all variables in model 1 and energy-adjusted intakes of red meat, poultry, fish and shellfish, eggs, dairy products, soy foods, legumes, vegetables, and fruits (all in quartiles).
We modeled the effects of substituting a serving of red meat for an alternative food such as poultry, fish, eggs, soy, and legumes using the method described by Halston et al.19 and Bernstein et al.18 Briefly, the substitution analysis was performed by simultaneously including all dietary variables in continuous values in the same multivariable model. The relative risk was then estimated by considering the exponential of the difference in their coefficients. The corresponding 95% CI was then derived based on the covariance between the two food items. To facilitate the substitution analysis, we further defined one serving as 90 g red meat, fish, or poultry, 53 g eggs, and 80 g soy and legumes based on the dietary definitions from the Singapore Health Promotion Board serving size guide.33
Sensitivity analysis was conducted by repeating our analysis on subjects with at least 4 years of follow-up (n=57,864) and those without self-reported history of diabetes, hypertension, coronary heart disease, and stroke (n=42,039), which are common comorbidities associated with ESRD. We also repeated the substitution analysis by “substituting” 100 g of red meat for 100 g of an alternative food source (i.e., poultry, fish, eggs, or soy and legumes).
All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC), and statistical significance was based on two-sided probability of 0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Siew-Hong Low of the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study and Kazuko Arakawa and Renwei Wang for the development and maintenance of the cohort study database. Finally, we acknowledge the founding Principal Investigator of the Singapore Chinese Health Study, Mimi C. Yu.
This study was supported by the National Institutes of Health (R01 CA144034 and UM1 CA182876).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Is Dietary Red Meat Kidney Toxic?,” on pages 5–7.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016030248/-/DCSupplemental.
References
- 1.Nesrallah GE, Mustafa RA, Clark WF, Bass A, Barnieh L, Hemmelgarn BR, Klarenbach S, Quinn RR, Hiremath S, Ravani P, Sood MM, Moist LM; Canadian Society of Nephrology : Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ 186: 112–117, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 3.Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN, Chen J, He J: A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int 88: 950–957, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Nahas M: The global challenge of chronic kidney disease. Kidney Int 68: 2918–2929, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Brenner BM, Lawler EV, Mackenzie HS: The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 49: 1774–1777, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Goraya N, Wesson DE: Dietary interventions to improve outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens 24: 505–510, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Kusano K, Segawa H, Ohnishi R, Fukushima N, Miyamoto K: Role of low protein and low phosphorus diet in the progression of chronic kidney disease in uremic rats. J Nutr Sci Vitaminol (Tokyo) 54: 237–243, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Cianciaruso B, Pota A, Bellizzi V, Di Giuseppe D, Di Micco L, Minutolo R, Pisani A, Sabbatini M, Ravani P: Effect of a low- versus moderate-protein diet on progression of CKD: follow-up of a randomized controlled trial. Am J Kidney Dis 54: 1052–1061, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Greene T, Beck GJ, Caggiula AW, Kusek JW, Hunsicker LG, Klahr S: Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. J Am Soc Nephrol 10: 2426–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Menon V, Kopple JD, Wang X, Beck GJ, Collins AJ, Kusek JW, Greene T, Levey AS, Sarnak MJ: Effect of a very low-protein diet on outcomes: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis 53: 208–217, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Academy of Nutrition and Dietetics Evidence Analysis Library. Chronic kidney disease evidence-based nutrition practice guideline. Available at: http://www.andeal.org/category.cfm?cid=14. Accessed December 12, 2015 [Google Scholar]
- 13.Friedman AN, Ogden LG, Foster GD, Klein S, Stein R, Miller B, Hill JO, Brill C, Bailer B, Rosenbaum DR, Wyatt HR: Comparative effects of low-carbohydrate high-protein versus low-fat diets on the kidney. Clin J Am Soc Nephrol 7: 1103–1111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwingshackl L, Hoffmann G: Comparison of high vs. normal/low protein diets on renal function in subjects without chronic kidney disease: a systematic review and meta-analysis. PLoS One 9: e97656, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dwyer JT, Madans JH, Turnbull B, Cornoni-Huntley J, Dresser C, Everett DF, Perrone RD: Diet, indicators of kidney disease, and later mortality among older persons in the NHANES I Epidemiologic Follow-Up Study. Am J Public Health 84: 1299–1303, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC: The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med 138: 460–467, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Motokawa M, Fukuda M, Muramatsu W, Sengo K, Kato N, Usami T, Yoshida A, Kimura G: Regional differences in end-stage renal disease and amount of protein intake in Japan. J Ren Nutr 17: 118–125, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC: Major dietary protein sources and risk of coronary heart disease in women. Circulation 122: 876–883, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB: Potato and french fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr 83: 284–290, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Fung TT, Hu FB, Curhan GC: Association of dietary patterns with albuminuria and kidney function decline in older white women: a subgroup analysis from the Nurses’ Health Study. Am J Kidney Dis 57: 245–254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutiérrez OM, Muntner P, Rizk DV, McClellan WM, Warnock DG, Newby PK, Judd SE: Dietary patterns and risk of death and progression to ESRD in individuals with CKD: a cohort study. Am J Kidney Dis 64: 204–213, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunkler D, Dehghan M, Teo KK, Heinze G, Gao P, Kohl M, Clase CM, Mann JF, Yusuf S, Oberbauer R; ONTARGET Investigators : Diet and kidney disease in high-risk individuals with type 2 diabetes mellitus. JAMA Intern Med 173: 1682–1692, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Banerjee T, Crews DC, Wesson DE, Tilea AM, Saran R, Ríos-Burrows N, Williams DE, Powe NR; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : High Dietary Acid Load Predicts ESRD among Adults with CKD. J Am Soc Nephrol 26: 1693–1700, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scialla JJ, Anderson CA: Dietary acid load: a novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis 20: 141–149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remer T, Manz F: Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 95: 791–797, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Kim Y, Keogh J, Clifton P: A review of potential metabolic etiologies of the observed association between red meat consumption and development of type 2 diabetes mellitus. Metabolism 64: 768–779, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Semba RD, Nicklett EJ, Ferrucci L: Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci 65: 963–975, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koh WP, Yang HN, Yang HQ, Low SH, Seow A: Potential sources of carcinogenic heterocyclic amines in the Chinese diet: results from a 24-h dietary recall study in Singapore. Eur J Clin Nutr 59: 16–23, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC: Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer 39: 187–195, 2001 [DOI] [PubMed] [Google Scholar]
- 31.National Registry of Diseases Office: Singapore Renal Registry Annual Registry Report 1999-2013 (Preliminary). Health Promotion Board, Singapore (Ed.), 2015 Available at: https://www.nrdo.gov.sg/docs/librariesprovider3/Publications—Kidney-Failure/singapore-renal-registry-annual-registry-report-1999-2013-preliminary.pdf?sfvrsn=0. Accessed December 15, 2015 [Google Scholar]
- 32.Willett, WC, Howe, GR, Kushi, LH: Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65: 1220S-1228S; discussion 1229S-1231S, 1997 [DOI] [PubMed]
- 33.Health Promotion Board: Build a Healthy Food Foundation. Ministry of Health, Singapore (Ed.), 2016 Available at: http://www.hpb.gov.sg/HOPPortal/health-article/2638. Accessed December 15, 2015 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.