Abstract
Randomized trials of rituximab in primary membranous nephropathy (PMN) have not been conducted. We undertook a multicenter, randomized, controlled trial at 31 French hospitals (NCT01508468). Patients with biopsy-proven PMN and nephrotic syndrome after 6 months of nonimmunosuppressive antiproteinuric treatment (NIAT) were randomly assigned to 6-month therapy with NIAT and 375 mg/m2 intravenous rituximab on days 1 and 8 (n=37) or NIAT alone (n=38). Median times to last follow-up were 17.0 (interquartile range, 12.5–24.0) months and 17.0 (interquartile range, 13.0–23.0) months in NIAT-rituximab and NIAT groups, respectively. Primary outcome was a combined end point of complete or partial remission of proteinuria at 6 months. At month 6, 13 (35.1%; 95% confidence interval [95% CI], 19.7 to 50.5) patients in the NIAT-rituximab group and eight (21.1%; 95% CI, 8.1 to 34.0) patients in the NIAT group achieved remission (P=0.21). Rates of antiphospholipase A2 receptor antibody (anti–PLA2R-Ab) depletion in NIAT-rituximab and NIAT groups were 14 of 25 (56%) and one of 23 (4.3%) patients at month 3 (P<0.001) and 13 of 26 (50%) and three of 25 (12%) patients at month 6 (P=0.004), respectively. Eight serious adverse events occurred in each group. During the observational phase, remission rates before change of assigned treatment were 24 of 37 (64.9%) and 13 of 38 (34.2%) patients in NIAT-rituximab and NIAT groups, respectively (P<0.01). Positive effect of rituximab on proteinuria remission occurred after 6 months. These data suggest that PLA2R-Ab levels are early markers of rituximab effect and that addition of rituximab to NIAT does not affect safety.
Keywords: rituximab, idiopathic membranous nephropathy, randomized controlled trial, severe adverse event, anti-PLA2R antibody, anti-THSD7A antibody
Membranous nephropathy (MN) accounts for about 20% of cases of nephrotic syndrome in adults and is the leading glomerulopathy recurring after kidney transplantation.1 Thickening of glomerular capillary walls results from subepithelial formation of immune deposits containing IgG, the membrane attack complex of complement, which is the major mediator of proteinuria, and antigens. Primary forms of MN, improperly called primary membranous nephropathy (PMN), represent 70%–80% of all patients. A major breakthrough was the identification of the podocyte antigen phospholipase A2 receptor (PLA2R) as the target of circulating antibodies in about 70% of PMN, which confirmed that the disease was autoimmune in nature.2
The optimal treatment of patients with PMN is still a matter of debate3,4; 30%–40% of affected patients will undergo spontaneous, usually partial remission usually within 1 year from disease onset, whereas about one third will progress to ESRD.5,6 Treatments with corticosteroids and alkylating agents significantly increase the rates of remission and slow renal function loss in patients with persistent nephrotic syndrome.7–11 Calcineurin inhibitors induce remission in a majority of patients, but relapse rates exceed 50%; also, renal toxicity is a concern.10,12–14 The latest Kidney Disease Improving Global Outcomes (KDIGO) guidelines restricted the indication of alkylating agents to patients at high risk of progression and considered calcineurin inhibitors as an alternative therapy.15 In patients with even more restricted indications of alkylating agents, the rate of serious adverse events (SAEs), particularly malignancy, was higher in patients who received long-term immunosuppression than in those with supportive therapy.16
Both the evidence that B cells play a key role in the pathogenesis of PMN and drug toxicity led to target B cells with rituximab.17 Rituximab induced remission of nephrotic syndrome in 60%–80% of the patients with long-lasting proteinuria, despite blockade of the renin-angiotensin system,18–21 and in patients who had previously failed other treatments. Reduction of PLA2R-Ab titer preceded remission of proteinuria by several months, which suggested a causal relationship.22,23 A previous study showed that a B cell–driven approach with only one or two infusions of rituximab of 375 mg/m2 per week could allow reducing cost in comparison with the standard four weekly infusions.24
Because of the lack of randomized, controlled trials (RCTs) using rituximab and the high rate of spontaneous remission, this trial was designed to evaluate the efficacy of rituximab given to all patients at a standard dose (375 mg/m2) in two infusions added to supportive therapy compared with supportive therapy alone in patients with persistent nephrotic syndrome.
Results
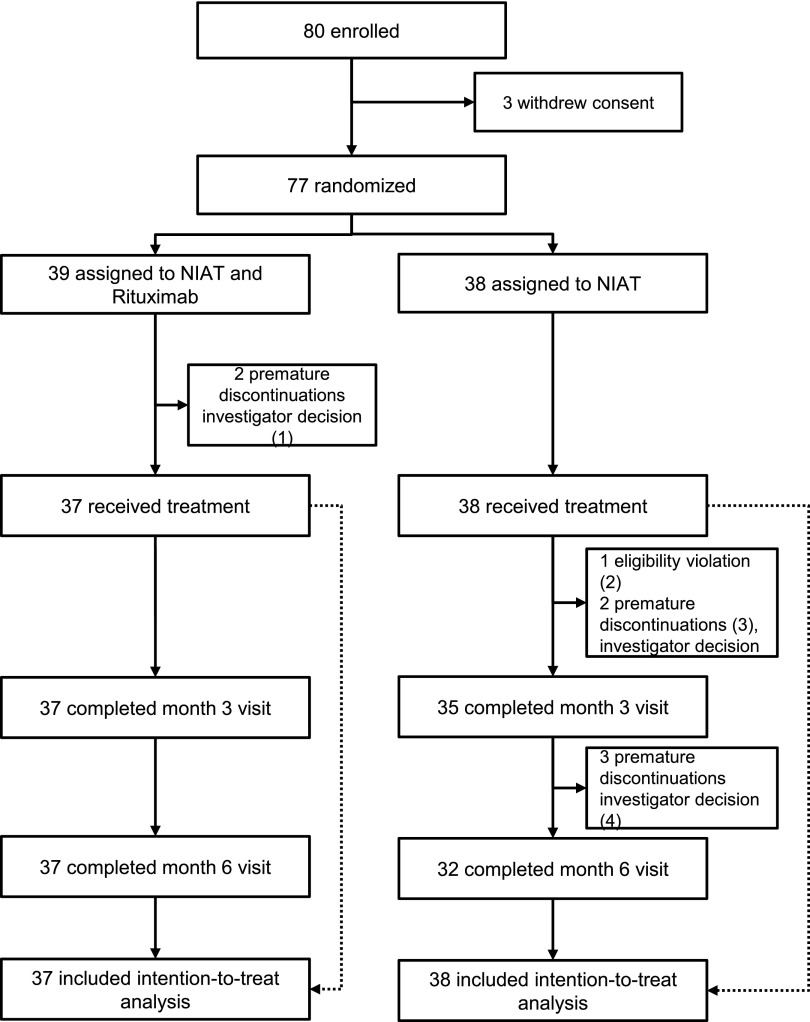
Between January 17, 2012, and July 3, 2014, 80 patients were enrolled in the RCT phase (Figure 1). Three patients withdrew their consent before randomization. Thirty-nine patients were assigned to the nonimmunosuppressive antiproteinuric treatment (NIAT)-rituximab group, and 38 patients were assigned to the NIAT only group. Thirty-seven patients in the NIAT-rituximab group and 38 in the NIAT group received the assigned treatment. Baseline characteristics in the two groups were similar (Table 1).
Figure 1.
Flow chart of the trial. The flow chart shows that premature discontinuation occurred in five patients within 3 months after inclusion: (1) two remissions at day 1 or inclusion; (2) one in NIAT for <6 months; (3) one lost to follow-up and one diagnosed with a pulmonary neoplasia; and (4) three treatment shifts between 3 and 6 months: two received rituximab or steroids because of deterioration of clinical condition and one was referred to another center.
Table 1.
Baseline characteristics
| Characteristic | NIAT-Rituximab Group, n=37 | NIAT Group, n=38 | Total, n=75 |
|---|---|---|---|
| Age, yr | 53.0 (42.0–63.0) | 58.5 (43.0–64.0) | 56 (42.0–64.0) |
| Men, no. (%) | 28 (75.7) | 24 (63.2) | 52 (69.3) |
| Weight, kg | 76.0 (70.0–85.0) | 76.5 (67.0–85.0) | 76.0 (67.0–85.0) |
| BP, mmHg | |||
| Systolic | 124 (110–140) | 125 (117–140) | 125 (115–140) |
| Diastolic | 77 (68–82) | 76 (70–81) | 76 (70–81) |
| Creatinine, μmol/L | 98.1 (73.4–122.9) | 91.1 (74.3–122.0) | 93.8 (76.9–122.9) |
| eGFR, ml/min per 1.73 m2 | 66.7 (55.4–82.5) | 72.7 (58.1–88.6) | 68.6 (55.4–88.6) |
| Protein-to-creatinine ratio, mg/g | 7680.0 (4584.3–10,399.0) | 7195.1 (5363.1–8965.1) | 7363.2 (4702.9–9735.0) |
| Albumin level, g/L | 22 (18–25) | 22 (20–26) | 22 (19–26) |
| Median time since biopsy-proven diagnosis, mo | 8 (6–13) | 8 (6–11) | 8 (6–13) |
| PLA2R-Ab–positive patients (ELISA), no. (%) | 27 (73.0) | 28 (73.7) | 55 (73.3) |
| PLA2R-Ab titer (ELISA), RU/mla | 40.5 (0.0–275.5) | 43.3 (0.0–457.5) | 40.5 (0.0–440.9) |
| Diuretics, no. (%) | 32 (86.5) | 32 (84.2) | 64 (85.3) |
| ACE inhibitor and/or ARB, no. (%) | |||
| ACE inhibitor | 16 (43.2) | 14 (38.9) | 30 (41.1) |
| ARB | 12 (32.4) | 8 (22.2) | 20 (27.4) |
| ACE inhibitor and ARB | 9 (24.3) | 14 (38.9) | 23 (31.51) |
| Statins, no. (%) | 31 (83.8) | 26 (68.4) | 57 (76.0) |
Data are shown as n (%) or median (IQR). eGFR was calculated according to the MDRD equation. ACE, angiotensin-converting enzyme; ARB, angiotensin 2 receptor blocker.
Median and IQR of PLA2R-Ab titer in all patients with and without PLA2R-Ab.
Primary End Point
The 6-month trial failed to achieve the primary end point. Thirteen patients (35.1%; 95% confidence interval [95% CI], 19.7% to 50.5%) in the NIAT-rituximab group and eight patients (21.1%; 95% CI, 8.1% to 34.0%) in the NIAT group achieved proteinuria remission at month 6 after randomization (P=0.21; odds ratio [OR], 2.0; 95% CI, 0.7 to 5.7) (Table 2). Results were not sensitive to missing data replacement.
Table 2.
Efficacy outcome variables
| Variable | NIAT-Rituximab Group, n=37 | NIAT Group, n=38 | P Value |
|---|---|---|---|
| Remission, complete and partiala | 13 (35.1; 19.7 to 50.5) | 8 (21.1; 8.1 to 34.0) | 0.21 |
| Protein-to-creatinine ratio, mg/g | |||
| Baseline | 7680.0 (4584.3–10,399.0) | 7195.1 (5363.1–8965.1) | |
| 3 mo | 4814.4 (3205.5–7398.6) | 4832.1 (2424.9–7911.9) | 0.94b |
| 6 mo | 3531.2 (1796.6–6469.4) | 5265.8 (2500.1–7690.7) | 0.18b |
| Serum albumin level, g/L | |||
| Baseline | 22 (18–25) | 22 (20–26) | |
| 3 mo | 27 (21–31) | 23 (19–27) | 0.10b |
| 6 mo | 30 (26–34) | 24 (20–29) | 0.029b |
| Serum creatinine, μmol/L | |||
| Baseline | 98.1 (82.2–122.9) | 91.1 (74.3–122.0) | |
| 3 mo | 94.6 (78.7–114.0) | 100.8 (81.3–115.8) | 0.88b |
| 6 mo | 94.6 (75.1–130.8) | 97.2 (76.0–126.4) | 0.67b |
| eGFR, ml/min per 1.73 m2 | |||
| Baseline | 66.7 (55.4–82.5) | 72.7 (58.1–88.6) | |
| 3 mo | 66.7 (57.2–87.1) | 68.9 (45.7–89.7) | 0.95b |
| 6 mo | 65.6 (51.0–89.0) | 72.5 (52.4–89.7) | 0.75b |
| PLA2R-Ab–positive patients, ELISA | |||
| Baseline | 27 (73.0) | 28 (73.7) | |
| Day 8 | 18 (52.9) | 17 (68.0) | 0.25c |
| 3 mo | 11 (31.4) | 25 (83.3) | <0.001c |
| 6 mo | 13 (36.1) | 24 (75.0) | 0.001c |
| PLA2R-Ab–depleted patients | |||
| 3 mo | 14/25 (56.0) | 1/23 (4.3) | <0.001b |
| 6 mo | 13/26 (50.0) | 3/25 (12.0) | 0.004b |
| PLA2R-Ab titer (all patients), RU/ml | |||
| Baseline | 40.5 (0.0–275.5) | 43.3 (0.0–457.5) | |
| Day 8 | 27.1 (0.0–126.1) | 65.5 (0.0–345.5) | 0.24c |
| 3 mo | 0.0 (0.0–49.1) | 54.6 (16.5–278.4) | <0.001c |
| 6 mo | 0.0 (0.0–34.0) | 45.7 (7.6–262.2) | 0.002c |
| PLA2R-Ab titer (positive patients),d RU/ml | |||
| Baseline | 102.5 (36.1–672.5) | 199.5 (24.2–491.4) | |
| Day 8 | 63.2 (12.9–382.0) | 163.5 (34.7–438.5) | 0.41c |
| 3 mo | 0.0 (0.0–60.5) | 77.5 (30.3–325.9) | 0.003c |
| 6 mo | 8.3 (0.0–73.5) | 62.9 (16.6–449.3) | 0.01c |
| Post hoc composite end point at 6 mo | 15 (40.5; 24.7 to 56.4) | 5 (13.2; 2.4 to 23.9) | <0.01 |
| CD19, per mm3e | |||
| 3 mo | 11 (2.0–22.0) | NA | |
| 6 mo | 61 (34.0–100) | NA |
Data are shown as n (%), n (%; 95% CI), or median (IQR). eGFR was calculated according to the MDRD equation. NA, not available.
Complete and partial remissions were defined according to 2012 KDIGO criteria on the basis of proteinuria; composite end point was defined as reduction of proteinuria >50% and increase of serum albumin >30%.
P value <0.03 indicates statistical significance (Bonferroni correction).
P value <0.02 indicates statistical significance (Bonferroni correction).
Patients with at least one positive detection of PLA2R-Ab at any time.
Normal range (100–500/mm3).
Secondary End Points
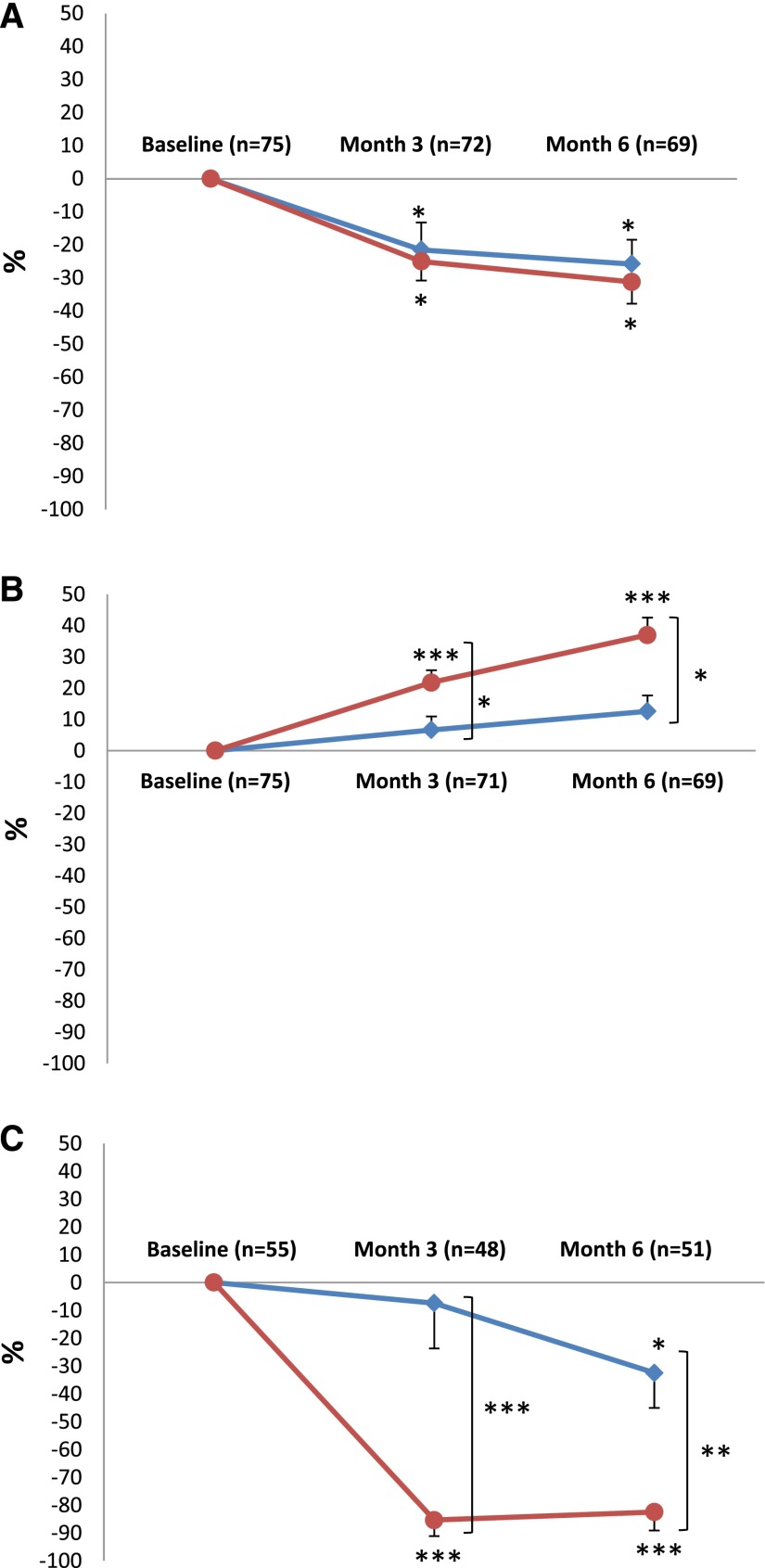
Protein-to-creatinine ratio decreased similarly in both groups at months 3 and 6 (Figure 2A, Table 2). Percentage increase of serum albumin was significantly higher at months 3 and 6 in the NIAT-rituximab group (Figure 2B). Serum creatinine and eGFR by Modification of Diet in Renal Disease (MDRD) formula (Table 2), serum triglycerides, total and LDL cholesterol, body weight, and need for diuretic therapy did not differ at months 3 and 6 between the two groups (Supplemental Table 1).
Figure 2.
The secondary end points are expressed as percentage changes in proteinuria, serum albumin, and PLA2R-Ab with time. Mean±SEM percentage changes from baseline in (A) proteinuria, (B) serum albumin, and (C) anti–PLA2R-Ab levels. Please note that C shows percentage changes of PLA2R antibodies in the subset of patients who had PLA2R-Ab at baseline. Bonferroni correction was applied; P value <0.02 indicates statistical significance. NIAT is shown by blue lines, and NIAT-rituximab is shown by red lines. *P<0.02; **P<0.001; ***P<0.001.
PLA2R-Ab was detected in 27 (73%) and 28 (74%) patients at baseline in the NIAT-rituximab and the NIAT groups, respectively (Table 1). As early as month 3, rates of PLA2R-Ab–positive patients (31% versus 83%; P<0.001) and PLA2R-Ab titers (0.0; interquartile range [IQR], 0.0–49.1 RU/ml versus 54.6; IQR, 16.5–278.4 RU/ml; P<0.001) were lower in the NIAT-rituximab group than in the NIAT alone, respectively (Table 2). No further decrease in the rate of PLA2R-Ab–positive patients was observed between months 3 and 6, and the difference between PLA2R-Ab titer at months 3 and 6 was 0.0 (IQR, 0.0–19.8) in the NIAT-rituximab group. No change in the rates of PLA2R-Ab–positive patients and PLA2R-Ab titers occurred between baseline, and months 3 and 6 in the NIAT group (Table 2).
In the subgroup of patients who were initially positive for PLA2R-Ab, significant decreases of the titers of PLA2R-Ab were observed at month 3 (0.0; IQR, 0.0–60.5 RU/ml; P<0.001) and month 6 (8.3; IQR, 0.0–73.5 RU/ml; P<0.001) compared with baseline (102.5; IQR, 36.1–672.5 RU/ml) in the NIAT-rituximab group and only at month 6 (62.9; IQR, 16.6–49.3 RU/ml versus 199.5; IQR, 24.2–491.4 RU/ml at baseline; P=0.02) in the NIAT alone group. Percentage decrease of PLA2R-Ab titer was significantly higher in the NIAT-rituximab group at months 3 and 6 (Figure 2C).
Complete immunologic remission (full PLA2R-Ab depletion) was observed in the NIAT-rituximab group in 14 of 25 (56%) and 13 of 26 (50%) patients at months 3 and 6, respectively, compared with one of 23 (4%; P<0.001) and three of 25 (12%; P=0.004) patients, respectively, in the NIAT group (Table 2). Of the 14 rituximab-treated patients that were antibody depleted at month 3, six (43%) patients subsequently achieved the primary end point compared with only two of 11 patients (18%) without antibody depletion. PLA2R-Ab level <275 RU/ml at baseline was associated with the primary end point (OR, 4.3; 95% CI, 1.1 to 17.3; P=0.04), and this was independent from treatment group, age, sex, baseline proteinuria, serum albumin, and creatinine (Supplemental Table 2).
PLA2R-Ab was also measured at a very early time point (day 8). Rates of PLA2R-Ab positivity and PLA2R-Ab titer in the whole population and PLA2R-Ab titer in the subset of patients who were positive at baseline were similar in both treatment groups (Table 2). Among the eight patients of the NIAT-rituximab group who were PLA2R positive at baseline and achieved remission at 6 months, two were antibody depleted at day 8, and two had marked reductions (78% and 42%) of antibody titer.
Twenty patients had undetectable PLA2R-Ab at baseline (10 in the NIAT-rituximab group and 10 in the NIAT group). However, three patients in the NIAT group later developed PLA2R-Ab and were considered as having PLA2R-related PMN. At baseline, no statistical difference in age, protein-to-creatinine ratio, serum albumin, and eGFR was seen according to presence (n=55 patients) or absence (n=20) of PLA2R-Ab. The effect of rituximab on proteinuria remission did not differ according to the serologic status at baseline. Two PLA2R-Ab–negative patients were positive for antithrombospondin domain 7A antibody (anti–THSD7A-Ab). The first patient (1/100 dilution at baseline) received NIAT-rituximab and achieved remission at month 6, with sustained antibody depletion from month 3. The second patient was treated with NIAT alone and achieved partial remission at month 6 but relapsed at 1 year. In this patient, THSD7A-Ab remained detectable at low level (1/10) at any time.
CD19+ B cells remained below normal range (100–500/mm3) throughout the observation period in the NIAT-rituximab group. Median CD19+ B cell count was 11/mm3 (IQR, 2.0–22.0) at month 3 and 61/mm3 (IQR, 34.0–100) at month 6 (Table 2). Among PLA2R-Ab–positive patients at baseline, there was no difference in CD19 count between patients who were PLA2R-Ab depleted and those who were not at month 3 (P=0.76; n=23 patients) and month 6 (P=0.89; n=24 patients).
Post Hoc Composite End Point
In a post hoc analysis that includes reduction of proteinuria >50% and increase of serum albumin level >30% at month 6, 15 (41%) patients in the NIAT-rituximab group and five (13%) patients in the NIAT group achieved the composite end point at month 6 after randomization (OR, 0.22; 95% CI, 0.07 to 0.70; P<0.01) (Table 2).
Post–RCT Observational Period
Median duration from inclusion to last follow-up was 17.0 (IQR, 12.5–24.0) months and 17.0 (IQR, 13.0–23.0) months in the NIAT-rituximab and the NIAT groups, respectively. The rates of KDIGO remission occurring without modification of initial immunosuppressive treatment (NIAT-rituximab) or introduction of an immunosuppressive treatment (NIAT) were 24 of 37 (64.9%) and 13 of 38 (34.2%) in the NIAT-rituximab and the NIAT groups, respectively (OR, 3.5; 95% CI, 1.7 to 9.2; P<0.01) (Table 3). Numbers of complete remission were seven of 37 and one of 38 in the NIAT-rituximab and the NIAT groups, respectively (P=0.03). Median times to remission were 7.0 (IQR, 5.5–10.5) months (n=24) and 7.0 (4.0–13.0) months (n=13) in the NIAT-rituximab and the NIAT groups, respectively. Protein-to-creatinine ratio was lower in the NIAT-rituximab group (2194.8; IQR, 1309.8–5310.0 mg/g) than in the NIAT group (4701.1; IQR, 2027.8–8265.3; P=0.02), whereas serum albumin level was higher (32; IQR, 26–35 g/L versus 27; 20–30 g/L, respectively; P=0.03). Serum creatinine and eGFR by MDRD formula did not differ between the two groups (Table 3). In multivariate analyses, KDIGO remission was associated with PLA2R-Ab level <275 RU/ml at baseline (OR, 3.5; 95% CI, 1.1 to 10.7; P=0.03), and this was independent from treatment group, age, sex, baseline proteinuria, serum albumin, and creatinine (Supplemental Table 3). KDIGO remission was also associated with composite end point at month 6 (OR, 30.1; 95% CI, 3.9 to 262.8; P=0.001), regardless of treatment group. In the NIAT-rituximab group, CD19 counts at months 3 and 6 were not associated with remission.
Table 3.
Results of efficacy analysis at last follow-up
| End Point | NIAT-Rituximab Group, n=37 | NIAT Group, n=38 | P Value |
|---|---|---|---|
| Remission, complete and partiala | 24 (64.9; 49.5 to 80.2) | 13 (34.2; 19.1 to 49.3) | <0.01 |
| Protein-to-creatinine ratio, mg/g | 2194.8 (1309.8–5310.0) | 4701.1 (2027.8–8265.3) | 0.02 |
| Serum albumin, g/L | 32 (26–35) | 27 (20–30) | 0.03 |
| Serum creatinine, μmol/L | 101 (87–135) | 97.2 (78.5–133.5) | 0.50 |
| eGFR, ml/min per 1.73 m2 | 61.1 (48.7–83.4) | 73.1 (50.4–90.5) | 0.48 |
Data are shown as n (%; 95% CI) or median (IQR). Data were recorded before any potential modification of treatment assigned at randomization (modification of initial immunosuppressive treatment in the NIAT-rituximab group or addition of any immunosuppressive treatment in the NIAT group).
Complete and partial remissions were defined according to 2012 KDIGO criteria on the basis of proteinuria.
SAEs
Number of SAEs was comparable in both groups (Table 4). Only one SAE was related to NIAT-rituximab treatment in a patient who developed prostatitis with favorable outcome. In the rituximab group, no leukopenia was observed. Patients received a premedication protocol of 100 mg solumedrol, 1 g paracetamol, and 5 mg dexchlorpheniramine; no allergic reactions were observed.
Table 4.
SAEs according to treatment group
| SAEs | NIAT-Rituximab Group, n=37 | NIAT Group, n=38 | P Value |
|---|---|---|---|
| No. of events | 0.87 | ||
| 0 | 31 | 33 | |
| 1 | 5 | 4 | |
| ≥2 | 1 | 1 | |
| Event details | |||
| Acute renal failurea | 0 | 2 | |
| Infection | |||
| Prostatitis | 1 | 0 | |
| Pleural effusiona | 0 | 1 | |
| Cardiac and vascular disorders | |||
| Myocardial infarction | 1 | 1 | |
| Critical limb ischemia | 0 | 1 | |
| Mesenteric ischemiab | 1 | 0 | |
| Carotid endarteriectomyb | 1 | 0 | |
| Aortoiliac femoral bypassb | 1 | ||
| Cancera | 0 | 1 | |
| Others | |||
| Edema | 1 | 1 | |
| Pain and fever | 1 | 0 | |
| Diarrhea | 1 | 0 | |
| Asthma | 0 | 1 |
Data are shown as n.
These SAEs occurred in the same patient.
These SAEs occurred in the same patient.
Discussion
In this randomized study, we analyzed the effect of rituximab combined with NIAT in patients with PMN and severe nephrotic syndrome who had resisted maximally tolerated antiproteinuric therapy. The RCT showed that, compared with NIAT alone, addition of two infusions of rituximab to NIAT decreased PLA2R-Ab as early as month 3 and was associated with a higher percentage increase of serum albumin at months 3 and 6. However, the effect of this combined treatment on the rate of proteinuria remission (primary end point) was not observed during the RCT but was delayed to the post–RCT observational period (median time to remission of 7 months). The trial, thus, provides new biomarkers of early treatment response.
We compared NIAT-rituximab with NIAT alone, because there was no evidence-based proof of the efficacy of rituximab in PMN, although several nonrandomized studies suggested that rituximab was efficient18–21; the possibility of bias linked to a high rate of late spontaneous remissions as confirmed in this study called for a randomized trial. With this aim in mind, an ideal trial would have been a prolonged trial for >1 year. However, we considered it unethical to maintain the patients on NIAT for >6 months. Because no major complication occurred in the NIAT group, the risk taken was acceptable; however, after 6 months, PLA2R-Ab–positive patients had a markedly higher antibody titer in the NIAT group than in the NIAT-rituximab group, and it is uncertain whether a delay by 1 year in the NIAT group would affect any future response to immunosuppressive agents or rituximab therapy. Alternatively, we could have set the end point at 12 months with prespecified measures in the most aggressive forms for the patients in the NIAT group. However, this protocol would have assessed a global treatment strategy (NIAT with or without retreatment and NIAT with rituximab with or without retreatment) and not only the efficacy of rituximab added to supportive therapy. We, therefore, opted for a pragmatic approach, with a 6-month period of RCT followed by an observational phase.
This RCT failed to reach the primary end point. The lack of effect of NIAT-rituximab on the rate of proteinuria remission at 6 months has several explanations: (1) the high rate of remission (21%) in the NIAT group; (2) the lower rate of remission (35%) in the NIAT-rituximab group than we expected, because sample size was calculated from initial studies on rituximab,17,18,25 which probably overestimated the rate of remission in the NIAT-rituximab group and led to a lack of power; (3) the short duration of the RCT phase; and (4) the fact that proteinuria is a delayed marker of treatment effect.20,21,23
However, we did observe an effect of rituximab on serum albumin variation from baseline (increase) and PLA2R-Ab levels as early as month 3, which was confirmed at month 6. The increase from baseline of serum albumin contrasting with persisting high–level proteinuria at month 6 in the NIAT-rituximab group might be related to decreased tubular reabsorption of albumin when serum albumin increases26 and/or increased albumin anabolic rate in the liver resulting in increasing protein load to the glomerulus, which would offset the improving glomerular sieving function.27 We, thus, considered a post hoc composite end point with the aim to define early clinical criteria of response to rituximab, and it was a reduction of proteinuria >50% and an increase of serum albumin level >30% at month 6. A significantly higher number of patients treated with NIAT-rituximab compared with NIAT reached this composite end point at month 6. Moreover, remission defined on composite end point at month 6 was associated with proteinuria remission occurring at any time before any change of initially assigned treatment. This composite end point might, therefore, better reflect early renal outcome, although it should be validated in additional studies.
We continued to follow the patients during a post–RCT observational phase. The suspected beneficial clinical effect of rituximab at month 6 was confirmed by the data of the observational phase, which were recorded before any potential modification of treatment assigned at randomization. Proteinuria remission rate was substantially higher in the NIAT-rituximab group than in the NIAT alone group (64.9% versus 37.5%), with proteinuria dropping to a much lower level in the NIAT-rituximab group. In the patients treated with NIAT-rituximab, proteinuria remission rate and median time to remission (7 months) during the follow-up study were similar to those reported in previous nonrandomized series.20,21
Remission rate was comparable with the one achieved with the Ponticelli protocol in the same time frame (50% at 6 months8 and 32% within 1 year9) considering that, in those studies, patients were enrolled without a run-in period. It was lower than in patients treated with cyclosporin13 and tacrolimus14 who had remission rates of 75% after 26 weeks and 58% after 6 months, respectively; however, baseline serum albumin was higher by >0.5 g/dl than in our study, and these drugs are known to have an effect on glomerular hemodynamics, with a high risk of relapse at discontinuation, and be associated with a clinically relevant nephrotoxicity. The ongoing Membranous Nephropathy Trial of Rituximab (ClinicalTrials.gov no. NCT01180036) will hopefully show whether rituximab is superior to cyclosporin in terms of proteinuria remission over a 24-month period. The high rate of spontaneous remission that may occur >1 year after disease onset in our study and the relatively low rate of remission with NIAT-rituximab, as with other immunosuppressive treatments, indicate that we have not yet reached an optimal treatment in patients with persisting nephrotic syndrome. It is difficult to extrapolate what would be the remission rate with rituximab only, because all patients with persisting nephrotic syndrome are treated with NIAT according to the KDIGO recommendations.
This trial has several strengths. First, it is the first RCT in patients with PMN with monitoring of PLA2R-Ab, detected in 71% of the patients as in previous studies,28 and THSD7A-Ab.29 Rituximab associated with NIAT reduced median PLA2R-Ab titer as early as month 3 and induced complete immunologic remission in 56% and 50% of the patients at months 3 and 6, respectively. Multivariate analyses showed that PLA2R-Ab<275 RU/ml at baseline was the only factor associated with remission occurring at month 6 (primary end point) and during the post–RCT observational phase without modification of treatment assigned at randomization, regardless of treatment group and other adjustment variables. Our results also suggest that THSD7A-Ab may be useful for the monitoring of patients with MN. However, our univariate and multivariate analyses failed to identify classic predictors of long-term outcome and proteinuria remission, such as proteinuria, serum creatinine, eGFR, serum albumin, age, and sex. A possible reason is that we studied a relatively homogeneous population after a 6-month run–in period of maximally tolerated conservative therapy. This discrepant observation gives even more importance to PLA2R-Ab as a predictor of proteinuria remission in agreement with the autoimmune nature of the disease. The finding that PLA2R-Ab positivity and titer tended to decrease as early as 8 days in the NIAT-rituximab group was somewhat unexpected given that the half-life of Igs is about 3 weeks, but it confirmed previous observations by Hoxha et al.30 This suggests that PLA2R-Ab might be a very early biomarker of rituximab efficacy, although this has to be confirmed in additional prospective studies.
Second, this trial shows that B cell counts in rituximab-treated subjects do not predict proteinuria remission and confirms that PLA2R-Ab depletion rather than CD20+ depletion achieved in all patients matters for prediction of rituximab response.23 It does not tell us, however, whether the absence of immunologic remission at 3 months is because of lack of efficacy of rituximab or insufficient dosage or whether patients without antibody depletion at 6 months should be reinfused or shifted to a new generation anti–CD20 antibody or another immunosuppressant.
Third, we made the important observation that two infusions of rituximab were not associated with an increased risk of SAEs, which differs from all of the other current immunosuppressive therapies for PMN.
This trial has certain limitations. First, 11 patients among the 24 patients who entered remission in the NIAT-rituximab group reached the primary end point during the post–RCT observational study compared with only four in the NIAT group. However, the observational nature of the data does not provide similar strength of evidence as those provided by the randomized period. Second, one cannot exclude that some patients without circulating PLA2R-Ab at treatment onset had PLA2R-related PMN. This question could be addressed by analysis of kidney biopsies,31 which was not possible in this multicentric trial. Third, the trial was not blinded. However, data assessors were blinded to treatment allocation, and SAEs were monitored by an independent organization. Fourth, the trial was too short to determine whether the relapse rate was influenced by immunosuppressive treatment. Most of the remissions were partial. Because relapses of nephrotic syndrome and disease progression are more frequent in patients with partial remission, long-term studies with rituximab are advocated.
In conclusion, this trial shows that serum albumin and PLA2R-Ab levels are early markers of NIAT-rituximab efficacy, whereas the effect on proteinuria remission appears after 6 months. Addition of rituximab to NIAT has no effect on safety. This first RCT is another step toward the use of rituximab as first-line therapy in severe forms of PMN. It also suggests that criteria for definition of remission should include serum albumin and PLA2R-Ab levels, particularly in trials where rapid responses on drug efficacy and surrogate criteria are needed.
Concise Methods
Study Design
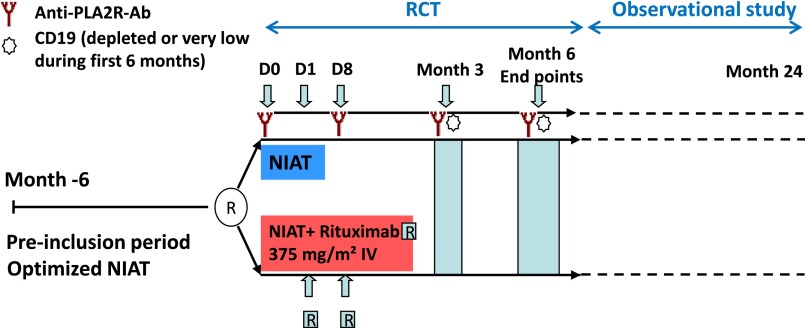
Patients were enrolled at 31 hospital nephrology units throughout France in this multicenter, open–labeled RCT. After a run-in period of 6 months, during which time patients were treated with the maximal tolerated dose of NIAT (angiotensin–converting enzyme inhibitors and/or angiotensin 2 receptor blockers, diuretics, and statin), patients were randomly assigned to 6-month therapy with NIAT plus rituximab or NIAT alone (Figure 3). The goal of the NIAT group was to determine the percentage of nonimmunosuppressant-induced remissions, which was known to be high during the first 12 months.6 We deliberately opted for a short trial of 6 months to avoid any loss of a chance of patients receiving an immunosuppressive treatment in patients who only received a supportive treatment. After the end of the randomized phase, patients were followed ≤24 months during a post–RCT observational phase. The study was approved by an institutional review board in Paris, France (Comité de Protection des Personnes Ile-de-France XI).
Figure 3.
Study design. After a pre-inclusion period of 6 months during which NIAT was optimized, patients were assigned either to NIAT plus Rituximab (375 mg/m2, two infusions at days 1 and 8) or to NIAT alone. Serum for anti-PLA2R-Ab determination was sampled at days 0 and 8, months 3 and 6, CD19 counts were determined at months 3 and 6; end points were assessed at month 6. The RCT was followed by an observational study during which follow-up was extended up to 24 months. IV, intravenous; R, rituximab.
Patients
Eligible patients were 18 years of age or older, had a biopsy-proven diagnosis established <2 years before inclusion, had a urinary protein excretion ≥3.5 g/d or a urinary protein-to-creatinine ratio ≥3500 mg/g, and had a serum albumin <30 g/L for at least 6 months, despite maximal tolerated dose of NIAT (angiotensin–converting enzyme inhibitors and/or angiotensin 2 receptor blockers, diuretics, and statin). Proteinuria was measured repeatedly before inclusion and treatment assignment to confirm persistence of full–blown nephrotic syndrome. The eGFR by MDRD formula had to be >45 ml/min per 1.73 m2. Exclusion criteria were secondary MN, pregnancy, breastfeeding, immunosuppressive treatment in the 3 preceding months, and active infectious disease. Hepatitis B serology included Hbs antigen and Hbs and Hbc antibodies. Patients with active hepatitis B and those with past hepatitis B infection without anti-Hbs antibodies were excluded. Four patients had previously received chemotherapy according to the Ponticelli protocol: one in the NIAT-rituximab group had chemotherapy completed 13 months before inclusion, and three in the NIAT group had chemotherapy completed 8 months, 2.5 years, and 6 years before inclusion. After 12 months, we deleted the time limit for the kidney biopsy, and we decreased the eGFR threshold down to 30 ml/min per 1.73 m2 to improve recruitment. Sixty-nine patients had a renal biopsy taken <2 years before inclusion. In the five remaining patients, the renal biopsies were taken 25, 26, 28, 41, and 78 months before inclusion. Seven patients had eGFR≤45 and >30 ml/min per 1.73 m2. All patients gave written informed consent.
Procedures and Follow-Up
Patients received NIAT in association with 375 mg/m2 intravenous rituximab on days 1 and 8 after randomization or NIAT alone (Figure 3). We selected this dosing schedule on the basis of previous reports of rituximab’s ability to induce proteinuria remission and CD19 depletion.18,24 At the end of the 6-month randomized phase, referring physicians were free to reinfuse rituximab or shift immunosuppressant in patients of the NIAT-rituximab group or introduce an immunosuppressant in patients of NIAT group, and patients were followed ≤24 months during an observational period. The same antiproteinuric drugs were allowed before and after randomization.
Study visits occurred at baseline, weeks 1 and 2, and months 3 and 6 during the RCT. At each study visit, clinical data and medications were recorded. Blood and urine samples were collected at baseline and months 3 and 6 for serum creatinine, serum albumin, and proteinuria-to-creatinine ratio or proteinuria excretion per day. PLA2R-Ab was measured at baseline, day 8, and months 3 and 6. CD19+ B lymphocyte counts were measured at months 3 and 6 in the NIAT-rituximab group.
During the post–RCT observational phase, visits occurred according to the habits of the clinician in charge. Proteinuria, serum albumin, serum creatinine, and immunosuppressive treatment modifications were collected.
Data were collected in each of the 31 hospital nephrology units in a paper case report form and entered in a database located at Unité de Recherche Clinique-Centre de Recherche Clinique de l'Est Parisien, an external and independent organization.
Outcomes
The primary end point was the percentage of patients with complete or partial remission of nephrotic syndrome at 6 months of follow-up. Remission was defined according to 2012 KDIGO guidelines15 as (1) complete in the case of urinary protein excretion <500 mg/d or <500 mg/g creatinine and (2) partial in the case of urinary protein excretion <3.5 g/d or <3500 mg/g creatinine and ≥500 mg/g creatinine with ≥50% reduction compared with baseline. Secondary end points were rate of proteinuria, serum albumin, serum creatinine, PLA2R-Ab levels, and SAEs. PLA2R-Ab was measured by using a quantitative ELISA (EuroImmune AG, Lubeck, Germany); THSD7A-Abs were assessed by an immunofluorescence test (EuroImmune AG). Antibody depletion was defined as complete disappearance of antibodies in PLA2R-Ab–positive patients. Because albumin level may be an earlier marker of response than end points defined by proteinuria only,23,27 we also considered a post hoc composite end point defined as reduction of proteinuria >50% and increase of serum albumin level >30% at month 6 of follow-up.
Adverse events and unexpected changes in clinical or laboratory parameters were reported in patient case report forms and monitored up to complete resolution. All SAEs were monitored by Unité de Recherche Clinique-Centre de Recherche Clinique de l'Est Parisien and reported to the sponsor.
During the observational phase, remission defined according to the KDIGO and other variables were recorded before potential modification of treatment assigned at randomization (i.e., before any amendment of initial immunosuppressive treatment in the NIAT-rituximab group or addition of an immunosuppressive treatment in the NIAT group). Follow-up was too short to record relapses.
Statistical Analyses
On the basis of previous studies,17,18,25 rituximab was effective in decreasing proteinuria as early as 3 months21,30 and achieving remission at 20 weeks17 to 1 year18,25 in 60%–80% of patients with PMN and nephrotic syndrome persisting after 6 months of supportive therapy. The trial was designed to establish whether rituximab was superior in terms of efficacy as assessed by the number of remissions. Assuming a remission rate of 20% in the NIAT group, the inclusion of 80 patients would provide 80% power at two-sided α of 0.05 to detect a 30% absolute increase in the remission rate (50%) under the assumption of 10% exclusion or dropout rates (Fisher exact test).
Baseline characteristics of the study population were expressed as frequencies and percentages for qualitative variables and medians and IQRs for continuous variables. Remission rates were expressed as frequencies or percentages and their 95% CIs. All PLA2R-Ab titers not achieving the 14-RU/ml detection threshold of the method were spiked at zero. PLA2R-Ab titer was considered as a continuous variable, a binary variable (absence/presence), or at baseline only, a categorical variable according to tertiles (<22.5 RU/ml, lowest; 22.5–275.5 RU/ml, middle; ≥275.5 RU/ml, highest). Because at univariate analysis, tertiles 1 and 2 did not show any statistically significant difference, a binary variable was created (highest versus middle/lowest). Quantitative variables were compared by a t test or a Wilcoxon rank sum test, and categorical variables were compared by a Pearson chi-squared test or a Fisher exact test.
Sensitivity analyses were performed to check the effect of replacement methods of missing values with missing data considered as (1) success (remission) in the NIAT group and failure (no remission) in the NIAT-rituximab group and (2) failure in the NIAT group and success in the NIAT-rituximab group. Additional analyses were performed with missing data being replaced by last available data (proteinuria at 3 months) and available data under the hypothesis of data missing completely at random.
Additional logistic regression analyses were performed to identify potential prognostic factors of remission. The following variables of interest were analyzed in univariate and multivariate analyses: treatment, age, sex, proteinuria, serum creatinine, serum albumin at baseline, and presence of PLA2R-Ab at baseline. In other exploratory analyses, mean percentage changes from baseline of proteinuria, serum albumin, and PLA2R-Ab levels at months 3 and 6 were plotted and compared using a Wilcoxon matched pair signed rank test.
Statistical analyses were performed blinded to treatment allocation on the basis of intention to treat and included all patients who received at least one dose of treatment and did not withdrawal consent. Safety population was defined as patients who received at least one dose of treatment.
All tests were two sided, and P values <0.05 were considered to indicate statistical significance, except when Bonferroni correction was applied and mentioned. SAS V.9.3 software (SAS Institute Inc., Cary, NC) was used for statistical analyses.
Findings from the trial are described in accordance with Consolidated Standards of Reporting Trials guidelines (www.consort-statement.org). The trial was registered as the Evaluate Rituximab Treatment for Idiopathic Membranous Nephropathy Study (Clinical Trials.gov no. NCT01508468).
Disclosures
K.D. has received grants from the French Ministry of Health and Roche (Basel, Switzerland). E.P. is a paid adviser for and has received lecture fees from Gilead Sciences (Foster City, CA); she has received research grants from Amgen, Inc. (Thousand Oaks, CA). T.S. is a paid adviser for and has received lecture fees from AstraZeneca Pharmaceuticals (Wilmington, DE), Astellas, Bayer HealthCare (Whippany, NJ), BMS, Lilly, MSD, and Sanofi US (Bridgewater, NJ); she has received research grants from AstraZeneca Pharmaceuticals, Daiichi-Sankyo, Eli Lilly (Indianapolis, IN), GlaxoSmithKline (Brentford, United Kingdom), MSD, Novartis (Basel, Switzerland), and Sanofi (Bridgewater, NJ). P.R. is a paid adviser for Otsuka Pharmaceuticals France SAS (Rueil-Malmaison, France), Bristol-Myers Squibb Company (Princetown, NJ), Amicus Therapeutics France (Paris, France), and Air Liquide (Paris, France); has received lecture fees from Shire (Belgium), Servier, Chugai Pharmaceutical Co. (Tokyo, Japan), and the Mayo Clinic; and has received research grants from Amgen SAS (Boulogne-Billancourt, France) and Otsuka Pharmaceuticals France SAS. Hoffman-La Roche (Basel, Switzerland) provided rituximab for the study.
Supplementary Material
Acknowledgments
We thank Nora Semai, Jennifer Piro, Julie Claudepierre, Benjamin Laverdant, Matthieu Le lay, Anne Seguin, Dominique Damas, and Elodie Drouet from the Clinical Research Department (Unité de Recherche Clinique - Centre de Recherche Clinique de l'Est Parisien) at Saint Antoine University Hospital (Assistance Publique-Hôpitaux de Paris, Université Pierre et Marie Curie) for trial monitoring and handling, preparation, and submission of all required research ethics and regulatory documents. We thank Emmanuel Roux for monitoring and handling the biobank at Tenon Hospital (Prof. Isabelle Brocheriou) and Christine Vial (UMR_S1155) for help in the preparation of the manuscript.
This study was funded by Programme Hospitalier de Recherche Clinique, French Ministry of Health grant AOM10089; European Research Council ERC-2012-ADG_20120314 grant agreement 322947; Agence Nationale pour la Recherche Programme Blanc SVSE1 (2012) Decision grant ANR-12-BSE1-0002-01; Fondation pour la Recherche Médicale Equipe FRM 2012 grant; and 7th Framework Programme of the European Community contract 2012-305608 (European Consortium for High-Throughput Research in Rare Kidney Diseases). The sponsor of this study was Assistance Publique-Hôpitaux de Paris (Département de la Recherche Clinique et du Développement, Clinical Research and Development Department). Rituximab was given by Hoffmann-La Roche.
This work was presented, in part, at the meeting of the American Society of Nephrology in San Diego, California (November 3–8, 2015).
None of the funding sources played a role in the writing of the manuscript or the decision to submit it for publication.
Members of the Evaluate Rituximab Treatment for Idiopathic Membranous Nephropathy Research Group are listed in Supplemental Material.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016040449/-/DCSupplemental.
Contributor Information
Collaborators: the GEMRITUX Study Group, Vincent Audard, Pierre Bataille, Yvon Berland, Jean-Jacques Boffa, Nicolas Bouvier, Laura Braun, Frank Bridoux, Stéphane Burtey, Déborah Chaintreuil, Cindy Castrale, Gabriel Choukroun, Christian Combe, Eric Daugas, Michel Delahousse, Ariane Duval-Sabatier, Marie Essig, Isabelle Etienne, Hélène François, Denis Fouque, Denis Glotz, Michel Godin, Bertrand Gondouin, Morgane Gosselin, Maryvonne Hourmant, Aurélie Hummel, Corinne Isnard-Bagnis, Charlotte Jouzel, Bruno Hurault de Ligny, Alexandre Karras, Thomas Kofman, Philippe Lang, Sandrine Lemoine, Anne-Sophie Librez Verhoeven, Rafik Mesbah, Laurent Mesnard, Bruno Moulin, Jean-Noël Ottavioli, Marie-Noelle Péraldi, Evangeline Pillebout, Claire Pouteil-Noble, Philippe Rieu, Claire Rigothier, Jean-Philippe Ryckelynck, Djillali Sahali, Zaara Soltani, Marc Souid, Thomas Stehlé, Maxime Touzot, Pierre Trolliet, Philippe Vanhille, Céline Lebas, David Verhelst, Cecile Vigneau, Laurence Vrigneaud, and François Vrtosvnik
References
- 1.Ponticelli C, Glassock RJ: Glomerular diseases: Membranous nephropathy--a modern view. Clin J Am Soc Nephrol 9: 609–616, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck LH Jr., Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofstra JM, Fervenza FC, Wetzels JF: Treatment of idiopathic membranous nephropathy. Nat Rev Nephrol 9: 443–458, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Cravedi P, Remuzzi G, Ruggenenti P: Rituximab in primary membranous nephropathy: First-line therapy, why not? Nephron Clin Pract 128: 261–269, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Schieppati A, Mosconi L, Perna A, Mecca G, Bertani T, Garattini S, Remuzzi G: Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med 329: 85–89, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreño A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C, Pons S, Lorenzo D, Bernis C, Arrizabalaga P, Fernández-Juárez G, Barrio V, Sierra M, Castellanos I, Espinosa M, Rivera F, Oliet A, Fernández-Vega F, Praga M; Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología : Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol 21: 697–704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponticelli C, Zucchelli P, Passerini P, Cagnoli L, Cesana B, Pozzi C, Pasquali S, Imbasciati E, Grassi C, Redaelli B, Sasdelli M, Locatelli F: A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 320: 8–13, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, Melis P, Valzorio B, Sasdelli M, Pasquali S, Pozzi C, Piccoli G, Lupo A, Segagni S, Antonucci F, Dugo M, Minari M, Scalia A, Pedrini L, Pisano G, Grassi C, Farina M, Bellazzi R: A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 9: 444–450, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, Joshi K, Sakhuja V: A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol 18: 1899–1904, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Howman A, Chapman TL, Langdon MM, Ferguson C, Adu D, Feehally J, Gaskin GJ, Jayne DR, O’Donoghue D, Boulton-Jones M, Mathieson PW: Immunosuppression for progressive membranous nephropathy: A UK randomised controlled trial. Lancet 381: 744–751, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Schieppati A, Cai G, Chen X, Zamora J, Giuliano GA, Braun N, Perna A: Immunosuppression for membranous nephropathy: A systematic review and meta-analysis of 36 clinical trials. Clin J Am Soc Nephrol 8: 787–796, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattran DC, Greenwood C, Ritchie S, Bernstein K, Churchill DN, Clark WF, Morrin PA, Lavoie S; Canadian Glomerulonephritis Study Group : A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Kidney Int 47: 1130–1135, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL; North America Nephrotic Syndrome Study Group : Cyclosporine in patients with steroid-resistant membranous nephropathy: A randomized trial. Kidney Int 59: 1484–1490, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Praga M, Barrio V, Juárez GF, Luño J; Grupo Español de Estudio de la Nefropatía Membranosa : Tacrolimus monotherapy in membranous nephropathy: A randomized controlled trial. Kidney Int 71: 924–930, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group : KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 186–197, 2012 [Google Scholar]
- 16.van den Brand JA, van Dijk PR, Hofstra JM, Wetzels JF: Long-term outcomes in idiopathic membranous nephropathy using a restrictive treatment strategy. J Am Soc Nephrol 25: 150–158, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P: Rituximab for idiopathic membranous nephropathy. Lancet 360: 923–924, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E, Hladunewich M, Cattran DC: Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 73: 117–125, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Bomback AS, Derebail VK, McGregor JG, Kshirsagar AV, Falk RJ, Nachman PH: Rituximab therapy for membranous nephropathy: A systematic review. Clin J Am Soc Nephrol 4: 734–744, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC; Mayo Nephrology Collaborative Group : Rituximab therapy in idiopathic membranous nephropathy: A 2-year study. Clin J Am Soc Nephrol 5: 2188–2198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, Rambaldi A, Marasà M, Remuzzi G: Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1416–1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck LH Jr., Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, Cosio FG, Cattran DC, Salant DJ: Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruggenenti P, Debiec H, Ruggiero B, Chianca A, Pellé T, Gaspari F, Suardi F, Gagliardini E, Orisio S, Benigni A, Ronco P, Remuzzi G: Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol 26: 2545–2558, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cravedi P, Ruggenenti P, Sghirlanzoni MC, Remuzzi G: Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2: 932–937, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Ruggenenti P, Chiurchiu C, Brusegan V, Abbate M, Perna A, Filippi C, Remuzzi G: Rituximab in idiopathic membranous nephropathy: A one-year prospective study. J Am Soc Nephrol 14: 1851–1857, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Wagner MC, Campos-Bilderback SB, Chowdhury M, Flores B, Lai X, Myslinski J, Pandit S, Sandoval RM, Wean SE, Wei Y, Satlin LM, Wiggins RC, Witzmann FA, Molitoris BA: Proximal tubules have the capacity to regulate uptake of albumin. J Am Soc Nephrol 27: 482–494, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruggenenti P, Mise N, Pisoni R, Arnoldi F, Pezzotta A, Perna A, Cattaneo D, Remuzzi G: Diverse effects of increasing lisinopril doses on lipid abnormalities in chronic nephropathies. Circulation 107: 586–592, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Du Y, Li J, He F, Lv Y, Liu W, Wu P, Huang J, Wei S, Gao H: The diagnosis accuracy of PLA2R-AB in the diagnosis of idiopathic membranous nephropathy: A meta-analysis. PLoS One 9: e104936, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomas NM, Beck LH Jr., Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RA, Lambeau G: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, Helmchen U, Stahl RA: An immunofluorescence test for phospholipase-A₂-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 26: 2526–2532, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Debiec H, Ronco P: PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med 364: 689–690, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.