Abstract
Over a decade ago, it was proposed that the regulation of tubular repair in the kidney might involve the recapitulation of developmental pathways. Although the kidney cannot generate new nephrons after birth, suggesting a low level of regenerative competence, the tubular epithelial cells of the nephrons can proliferate to repair the damage after AKI. However, the debate continues over whether this repair involves a persistent progenitor population or any mature epithelial cell remaining after injury. Recent reports have highlighted the expression of Sox9, a transcription factor critical for normal kidney development, during postnatal epithelial repair in the kidney. Indeed, the proliferative response of the epithelium involves expression of several pathways previously described as being involved in kidney development. In some instances, these pathways are also apparently involved in the maladaptive responses observed after repeated injury. Whether development and repair in the kidney are the same processes or we are misinterpreting the similar expression of genes under different circumstances remains unknown. Here, we review the evidence for this link, concluding that such parallels in expression may more correctly represent the use of the same pathways in a distinct context, likely triggered by similar stressors.
Keywords: ischemia-reperfusion, kidney development, transcriptional profiling, tubular repair, acute tubular injury, Maladaptive repair
The mammalian kidney, the metanephros, is the third pair of excretory organs to form during embryogenesis and the only one to persist in the postnatal animal. Derived from the intermediate mesoderm, the formation of this organ involves a complex interaction between the branching ureteric bud, which will form the collecting ducts, and the surrounding mesenchyme, which gives rise to all epithelial cell types within the nephrons. These cell populations engage in a process of reciprocal induction in order to drive maximal organogenesis.1 As well as giving rise to the epithelial components of the kidney, the mesenchyme also forms the surrounding interstitium, including the pericytes, and at least a portion of the endothelial population that will form the renal vasculature. The molecular pathways expressed during normal kidney morphogenesis in the mouse, including the cell types expressing each pathway and the timing of this expression, have been extensively chronicled and functionally investigated.2–6 As a result, the major transcriptional networks and growth factor signaling pathways critical for establishment of the branching ureteric epithelium and differentiation of the metanephric mesenchyme have been identified.1,7 Although also expressed in the development of many other organs, key roles have been identified for the WNT, FGF, BMP, Notch, and Hedgehog growth factor signaling pathways, and the HOX, PAX, FOX, SIX, and SOX transcription factor families, in kidney morphogenesis.
Although the final patterned and segmented nephrons contain numerous functionally distinct cell types, lineage tracing has shown that these cell types are all formed from a single progenitor population, the Six2-expressing cap mesenchyme.8 However, this population is not present in the postnatal kidney. All of the approximately 1 million nephrons9 present within the human postnatal kidney arise before birth.10 Similarly in rodents, nephrogenesis ends in the immediate perinatal period (week 1) before the terminal differentiation of the nephrogenic mesenchyme.11,12 What triggers this cessation of nephrogenesis is not understood, however this is accompanied by substantial changes in gene expression, with the loss of expression of key cap mesenchyme and ureteric tip genes.11,13
The loss of the nephron progenitor population limits the options for regeneration in the postnatal organ. Despite this, the postnatal mammalian kidney can undergo substantial repair in response to AKI. The nature of this injury response has been the focus of research for more than two decades. Although long appreciated that acute injury initiates rapid and robust proliferation of the tubular epithelium,14–16 there is an ongoing debate about whether this reparative capacity is elicited by a renal progenitor cell (RPC) population17–19 or via the proliferation of the mature epithelium in response to injury.14–16,20–25 What has been reported is the re-expression of key genes previously expressed during kidney development. This observation has been used to support both models of repair, with the suggestion that the response to AKI represents a recapitulation of development.
Although the postnatal kidney can apparently repair after acute injury, in both humans and mice repeated episodes of AKI progressively result in an aberrant signaling between tubular epithelium and the surrounding nontubular cell types, resulting in interstitial fibrosis and loss of microvasculature (rarefaction).21,26,27 Indeed, even a single severe episode of acute injury, such as delayed graft function after transplantation, can elicit this response.28 Such fibrosis is a hallmark of CKD. Evidence that an episode of AKI can contribute to future CKD has again focused attention on the repair process itself and understanding why incomplete repair occurs. Further, there has been particular interest in the prolonged expression of growth factor signaling pathways previously active during kidney development, although in this case it is proposed that the prolonged reactivation of such pathways is detrimental to tissue pathology.
In this review, we will discuss the current view on what occurs in the postnatal kidney in response to injury, whether this repair is mediated by a progenitor population or represents the response of a mature epithelium to stress, and examine the evidence that expression of key developmental pathways during the repair process, or indeed during maladaptive repair, represents a recapitulation of kidney development.
The Process of Tubular Repair in the Injured Kidney
The response of the adult kidney has been predominantly investigated in rodent models of acute injury. Perhaps the most commonly used model is ischemia-reperfusion injury (IRI).29 As in humans, this murine model shows a rapid decline in renal function accompanied by a loss of proximal tubular brush border and inflammation. In mice, this is also accompanied by the formation of tubular protein casts. Within the tubules, cellular proliferation is evident within 24–48 hours of injury, and the epithelial morphology is apparently restored after 5–7 days. Studies of the pathologic changes observed after ischemic injury describe four histologic stages to this response (Figure 1).30 During the initial stress-response phase, the epithelia have been reported to undergo both apoptosis and necrosis (Figure 1). In more recent studies, particularly in AKI associated with inflammation, a number of other forms of specialized cell death have also been reported, including necroptosis, anoikis, autophagic cell death, and pyroptosis.31–33 Many of these are more closely linked to the inflammatory component of kidney injury, which is often less marked in experimental models, such as toxic injury or IRI. The second phase of the injury response is marked by tubular flattening and dedifferentiation with upregulation of vimentin and a loss of epithelial morphology. This is followed by a period of pronounced proliferation and expression of growth factors, including IGF1, HGF, and FGFs. During the fourth phase of resolution, an apparently normal histologic appearance of the epithelium is restored.30 Whereas in most animal models, the tubular morphology is substantially normalized and evidence of inflammatory responses is resolved within a week, no study has definitively distinguished between initial epithelial cells still present in the tubules and new epithelium arising through a repair mechanism. In the setting of severe AKI, when there is an initial normalization event, a second and molecularly distinct response has been reported to occur around days 10–14 after injury, which may contribute to chronic inflammation and fibrosis.34 This is poorly understood and, as yet, not well characterized.
Figure 1.
Response of the renal epithelium to AKI. (1) In response to acute hypoxia or toxic injury, cells within the tubular epithelium undergo cell death. Initially regarded as necrosis or apoptosis, this is now known to include a number of other forms of regulated cell death, including anoikis, autophagic cell death, and pyroptosis.31–33 (2) Remaining viable epithelial cells are seen to undergo flattening and have been described as dedifferentiated. Such cells show expression of vimentin but remain E-cadherin positive. (3) Rapid and robust proliferation occurs within the remaining tubular epithelial cells. Recently, this early event has been shown to be accompanied by the expression of the transcription factor Sox9.63,64 This precedes the expression of Pax2, Wnt signaling and Notch signaling within the epithelium. (4) Epithelial cells regain normal polarity and re-establish the tubular histology before returning to a relatively quiescent state. Repeated acute injury as well as chronic injury results in the persistent expression of Wnt and Notch signaling pathways within the epithelium, which results in interstitial fibrosis.
The postnatal tubular epithelium was traditionally regarded as nonproliferative unless subjected to injury. In fact, there is evidence for slow turnover of the postnatal tubular epithelium even in the absence of injury.15 However, it is accepted that the ability of the injured epithelium to return to normal relies upon the extensive proliferation of cells within the tubules. The S3 segment of the proximal tubule was first noted as the site of the most proliferative response, with proliferating cell nuclear antigen–positive cells also expressing vimentin, suggestive of a transition from an epithelial to a mesenchymal state in response to injury.14 The concept that such dedifferentiated cells might represent a progenitor population was suggested.14 Subsequent studies concluded that any differentiated tubular cell could proliferate in response to injury,15,20 and that up to 40% of the cells within the S3 proximal tubule rest in G1 rather than G0, and are therefore poised to rapidly reenter the cell cycle.16 It was assumed, therefore, that any epithelial cell could reenter the cell cycle as required, facilitating the repopulation of tubules from which some cells had been lost. The concept that there may also be a contribution of cells to the epithelium from nonepithelial cell populations was disregarded after lineage tracing studies suggested that only tubular epithelial cells (derived from the Six2+ cap mesenchyme) contributed to the repair process.35 Given the evidence of histologic change in the tubular epithelium, this was viewed as a process of dedifferentiation followed by redifferentiation that was available to any surviving mature epithelial cell. However, the molecular mechanisms governing the morphologic changes leading to repair have been more challenging to dissect. Early analyses of the transcriptional changes in response to injury noted the expression of genes also reported to be expressed during development. On the basis of the expression of the mesenchymal markers, Vimentin, and early tubular markers (such as Pax2)21,30,36,37 within such dedifferentiated epithelial cells, repair was viewed as the return of mature tubular epithelial cells to a more primitive developmental state.
Evidence for a Progenitor Population in Tubular Repair
Whereas repair was viewed as the dedifferentiation and redifferentiation of the mature epithelium, a counter view has been proposed in which the proliferative repair observed results from the specific response of a progenitor cell population within the renal epithelium. In this model, repair is the role of a specific cell population. Indeed, this view of repair has also been used as evidence that repair recapitulates development. In this model, a developmental progenitor state persists and is reactivated by injury to recapitulate tubular morphogenesis.
The presence of such RPCs within the kidney tubular epithelium was initially described in adult human kidneys on the basis of their expression of CD133+ and CD24+,38,39 markers regarded as indicative of a stem/progenitor cell state. Evidence for such an epithelial progenitor was then also reported in mice and rats.40 It was suggested that the presence of these CD133+CD24+ cells, now often referred to as RPCs and regarded as representing a population present during development, indicated that a renal stem cell population is not lost after development has been completed. Although these RPCs are unable to trigger a de novo nephrogenesis process, once isolated they were reported to give rise to tubular epithelium as well as podocytes,19,41–43 suggesting progenitor properties. Notably, these cells were also described to exhibit high Notch signaling activity, with this being a critical component for the differentiation of RPCs toward the podocyte lineage.44 In addition, the expression of the developmental marker, Pax2, has been also been reported in this population, suggesting that it could represent a retained tubular progenitor.45
More recently, the use of lineage tracing analysis has contributed to our understanding of the location and cell types contributing to tubular repair in the mouse kidney. Rinkevich et al.46 proposed the presence of segment-restricted renal progenitors, with these present within the glomerulus as well as the tubular compartment. Using lineage analysis, individually marked cells were shown to clonally expand and continuously contribute to the turnover of a specific tubular segment. It was suggested that segment-specific progenitor types existed as individual labeled cells were not seen to contribute to repair in adjacent segments. Although this study was consistent with a model in which cellular replacement was elicited by residual segment-specific progenitors, this could not disprove clonal expansion of a mature epithelial cell that had reentered the cell cycle. However, it did show evidence that the proliferative response of the epithelium was, in part, mediated by canonical Wnt signaling, a pathway also driving epithelial proliferation during kidney development.47 Both the Wnt and Notch signaling pathways are downregulated once nephrogenesis is completed.48–51 However, numerous studies have now identified activation of these pathways within the tubular epithelium in response to acute injury. The involvement of these pathways during repair would also support a model in which early developmental markers play an active role in postnatal kidney epithelial turnover.
Although there is now significant literature around the characterization of RPCs in mice and humans, there is still no consensus as to whether these cells represent a stable subpopulation within the tubular epithelium activated in response to injury or a transient property available to any differentiated epithelial cell. This is, in part, confused by the identification criteria in mice versus humans. In humans, a CD24+ scattered tubular cell (STC) population can be identified within normal human kidney tubular epithelium.52 This STC is identified based upon a particular morphologic appearance compared with the rest of the tubular cells. STCs show a flattened profile and loss of epithelial polarity, similar to the dedifferentiated state of an epithelial cell in response to damage. Hence, the STC may represent the human RPC, supporting the notion of a stable resident progenitor population in the human kidney.52 Indeed, electron microscopy evaluation of human kidneys identified a population of STCs in the absence of injury.52 The presence of the same cell type in mice has not been proven because of a lack of the CD24 epitope in this species. In an attempt to address this controversy, lineage tracing driven from a marker of the mature functional epithelium, the solute channel Slc34a1, provided evidence that the mouse kidney does not contain a stable stem cell population.53 Instead, Kusaba et al.22 argued that reparative cells arise from any mature epithelial tubular cell, re-expressing “stem cell” markers as a result of local injury. This supports the model that fully differentiated epithelial cells are responsible for proximal tubular repair rather than a preexisting stem cell population.
As similar debate continues in the literature around the capacity for podocyte turnover in the postnatal animal. Here again, there is lineage-based evidence supporting a capacity for turnover of podocytes from the parietal epithelium and renin-producing cells of the juxtaglomerular complex in the face of injury,54–56 but counter evidence to the contrary.57 Focusing specifically on the question of tubular turnover, although it is possible that humans and mice do not use the same approach to kidney tubular repair, either model can be used to argue recapitulation of development during tissue turnover on the basis of the re-expression of developmental signaling pathways such as Wnt/β-catenin and Notch.
Maladaptive Repair after Repeated Injury
Although the kidney epithelium is able to robustly repair in response to acute injury, repeated episodes of damage eventually lead to interstitial fibrosis and tubular loss, which results in an irreversible loss of nephrons. The role of proximal tubular injury itself in triggering maladaptive repair was examined by Grgic et al.,58 who reported a transgenic mouse model where the expression of the diphtheria toxin (DT) receptor was driven by the Six2 promoter. Consistent with other reports, this model showed that after a single administration of nanomolar concentrations of DT, mice promptly developed inflammatory cell infiltration and robust tubular cell proliferation followed by total repair of damaged structures. Conversely, the exposure of the same mice to repetitive doses of DT over a period of three weeks led to maladaptive repair, characterized by an increase of macrophage infiltrates, myofibroblast proliferation, loss of vasculature (rarefaction), and fibrosis. These data demonstrated that repeated epithelial injury elicits a progressive scarring response rather than one that resolves via proliferative repair between each short-term insult.21,58 As a result of the loss of renal reparative capacity, injured tubular epithelial cells may be arrested at the G2/M stage of the cell cycle, displaying a senesce–associated secretory phenotype where cytokine and growth factor production by the epithelium promotes the persistence of a chronic inflammatory response. As a consequence, several histologic changes occur. Renal pericytes detach, resulting in subsequent capillary loss. Some reports suggest that it is these detached pericytes that transform into myofibroblasts, increasing extracellular deposition.59,60 Other studies suggest that the origin of myofibroblasts in CKD includes proliferating resident fibroblasts (50%), bone marrow–derived myofibroblasts (35%), endothelial cells (10%), and some epithelial-to-mesenchymal transition.61 More recently, a specific Gli1+ fraction of the perivascular population has been reported as the source of myofibroblastic expansion.62 Irrespective of origin, both an increase in myofibroblasts and capillary rarefaction are essential contributors to chronic kidney damage.
Developmental Gene Pathways in Kidney Repair
There is a long history of studies into changes in gene expression during the damage/repair response in the kidney.30,36,37,63,64 Indeed, the identification of the key biomarkers of the initial damage response, KIM1 and NGAL, were on the basis of studies of differential gene changes early in the damage response period. Early studies described the re-expression of mesenchymal markers, such as Vimentin and Ncam, in damaged tubular epithelium.37 This could also be regarded simply as a loss of epithelial morphology during injury. However, the expression of a number of nephrogenic genes has been reported in association with the initial proliferative response to injury. These include PAX2, FGF2, BMP7, and LHX1.37 In studies investigating the efficacy of compounds in renal injury, upregulation of HGF, WT1, PAX2, and BMP7 have been reported to associate with improved renal function and an increase in CD133/CD24 RPCs.65 Indeed, the use of BMP7 to oppose the fibrotic activity of TGFβ1 has been extensively investigated as a therapy.66
A number of transcription factors have been reported in association with renal injury. Of significant interest has been the transcription factor, Pax2. Initially expressed during kidney development in both the ureteric epithelium and the forming nephrons,67 postnatal expression of Pax2 is confined to the collecting duct, except in response to acute tubular injury, where it has been reported in the proliferating epithelium.37,68 Indeed, the presence of Pax2 expression has been associated with positivity for CD24 in rat renal epithelium after injury,69 with this interpreted as a return to a progenitor state. However, there have been no studies exploring the role of Pax2 or the requirement for Pax2 in the reparative response, and hence the significance of this expression remains unclear.
The upregulation of many developmental growth factor pathways has also been reported in response to kidney injury. These include HGF, IGF, VEGF, BMP, Wnt, and Notch pathway genes. During development, VegfA expression from the podocytes initiates the formation of the glomerular capillaries by drawing in endothelial progenitors from the surrounding stroma.70 The continued production of VEGF by the podocytes during postnatal life has now been shown to be critical for normal renal function.71 The re-expression of both VegfA and the angpoietin receptor, Tie2, has also been associated with the early proliferative phase of the AKI response.37 Villanueva et al.36 investigated the role of FGF2 (bFGF) in a rat model of ischemic injury, concluding that the delivery of FGF2 during the hypoxic insult accelerated this developmental gene expression pattern and reduced evidence for a mesenchymal change within the epithelial population. No analysis of the long-term outcome of injury was reported.
Notch and Wnt Pathway in Development, Repair, and Chronic Fibrosis
Two pathways investigated in some detail during kidney repair are the Wnt and Notch signaling pathways. During kidney development, Notch signaling plays a critical role in vascular development, mesangial maturation, collecting duct determination, and nephron segmentation, particularly the patterning of the proximal tubules and glomeruli.72 Wnt signaling initiates the mesenchyme-to-epithelial transition required to initiate nephron formation. This transient Wnt signaling activates the Notch effector genes Jag1 and Dll1 within the renal vesicles,6 possibly via induction of Lhx1.73 Expression of these and other Notch pathway effectors, Notch1 and Notch2, as well as target genes, including Hey1 and Hey5, are initiated within the renal vesicle and then persist as nephron segmentation occurs.4,74 Although Notch1 is not critical within the nephron, loss of Notch2 or Rbpj within the nephrons prevents formation of S-shaped bodies, with a particular loss of proximal tubules and glomeruli.75 Jag1 expression is seen in the progenitors of the medial S-shaped body, which expands to give rise to the loop of Henle.76 In the healthy postnatal kidney at rest, both Wnt and Notch signaling persists, although in cell types/locations distinct from that seen during development. Notch expression appears highest in the endothelial cells,77 whereas Wnt4 expression/β-catenin activity becomes restricted to the collecting duct epithelium.78
Expression of both Wnt and Notch signaling is increased in the renal epithelium in response to AKI.47 Mice lacking Wnt coreceptors Lrp5/6 showed more extensive injury when subjected to AKI, as was also the case after β-catenin deletion from the epithelial compartment.79 Expression of Wnt4 was reported as associated with increased cell proliferation, seen as increased cyclin D1, and localized to the proximal tubules in a rat model of IRI.80 Overexpression of Wnt4 or β-catenin within a tubular cell line (LLC-PK1) was also seen to increase proliferation.80 Hence, it was proposed that canonical Wnt signaling is required for renal tubular proliferation and repair, a role akin to that proposed during development. This has been subsequently reinvestigated, with a contrary conclusion suggesting no evidence for proximal tubular Wnt4 expression but robust induction of Wnt4 within medullary interstitial cells.78 Other Wnt ligands have been proposed to contribute during repair but in a fashion distinct from that played during development. For example, it has been shown that macrophages recruited as part of the inflammatory response to injury improve repair via the secretion of Wnt7b.81 In contrast, during development Wnt7b is expressed within the collecting duct and regulates medullary development by controlling the plane of cell division.82
The role of Notch in the repair process after AKI is less clear. It has been proposed that reactivation of Notch in response to injury supports a stem/progenitor cell–mediated process.44 Notch2 is upregulated in IRI, and inhibition of γ-secretase, which is responsible for activation of the pathway, does slow recovery.83
As well as a proposed role in reparative actions in response to AKI, prolonged activity of both pathways has also been associated with fibrotic change in other organs.84,85 In the kidney, increased Wnt expression/β-catenin signaling is associated with CKD in mouse models, such as unilateral ureteric obstruction.47 Notch expression within the postnatal tubular epithelium has also been associated with interstitial fibrosis, with this response blocked via pharmacologic inhibition of the Notch pathway or genetic notch pathway deletion.86 Continued activation of Wnt and Notch pathways within the podocytes leads to a pathologic response, resulting in glomerulosclerosis and albuminuria.47 In the unilateral ureteric obstruction model, many Wnt ligands are increased in expression and there is also evidence of increased β-catenin expression in the epithelial cells of the tubules.87 Overexpression of the Wnt inhibitor, Dkk1, has been reported to reduce expression of the canonical Wnt pathway and also reduce interstitial fibrosis in this model.87 Prolonged expression of Wnt4 within interstitial cells, a phenomenon seen in response to kidney injury, ultimately results in a transition of these cells to a myofibroblastic phenotype characteristic of chronic disease.78 However, deletion of Wnt4 did not reduce fibrosis in this model. Prolonged Notch1 expression has been associated with albuminuria, glomerulosclerosis, and fibrosis,88 and activation of the Notch intracellular domain results in fibrosis.86 Consistent with this, deletion of Notch3 reduced fibrosis.89 The link between these observations and specific roles of these Notch ligands during development is not clear.
Is Re-Expression Equivalent to Recapitulation of Development?
Although this concept of re-expression of developmental genes to drive postnatal repair is strongly evident in the literature, this view is not without caveats. All of the pathways discussed above, including both transcription factors and growth factor signaling pathways, are involved in the development of almost every organ in the body and are not unique to the developing or the damaged kidney. Conversely, there are other genes uniquely present during kidney development that are not involved in tubular repair postnatally. For example, expression of the Six2 transcription factor, which is expressed in the nephrogenic mesenchyme, is lost as nephrogenesis ceases.11 Although lineage tracing shows that all of the epithelial cells of the nephron arise from a Six2-expressing cell type, the dedifferentiation seen in response to postnatal injury does not involve re-expression of this gene.90 Consistent with this is an apparent complete loss of nephrogenic potential in the postnatal kidney of mammals. This is in contrast to lower vertebrates, such as bony fish and skates, in which injury evokes the formation of complete new nephrons. Interestingly, profiling of kidney tubule regeneration in adult zebrafish suggests that the nephron progenitors in the fish express wt1a, pax2a, and lhx1a,91 all orthologs of genes expressed in the pretubular aggregate/early nephron in the mammalian kidney. Such genes are also expressed during the postnatal response to injury in mammals.
Although a gene expressed during repair may have been expressed during development, it may also be acting within a different context during postnatal injury. It may be expressed by a cell type not present during development or signal through a downstream pathway distinct from that active during morphogenesis. By way of illustration, there has been considerable focus on the role of Wnt signaling during repair and the persistence of Wnt signaling in maladaptive repair. Many Wnt ligands are expressed during development, denoting specific cellular compartments. These include Wnt11 in the ureteric tip and Wnt9b in the initiation of nephron formation.92,93 During development, Wnt4 is required for completion of the mesenchyme-to-epithelial transition required for nephron formation.51 During this process, Wnt4 acts via the noncanonical Wnt signaling pathway.94,95 After birth, Wnt4 expression is seen in the medullary collecting duct. In response to injury, however, Wnt4 expression is activated within interstitial pericyte-like cells, initiating their proliferation and myofibroblastic transformation and thereby contributing to renal fibrotic change.78 This can be mimicked via the activation of canonical/β-catenin signaling within this pericyte population,78 suggesting a distinct role for Wnt4 in postnatal injury. Similarly, during development, Shh is expressed in the ureteric epithelium and signals to Ptch1 within the surrounding stroma.96 During kidney injury, Ihh is upregulated, activating downstream Gli effector expression by pericytes resulting in fibrotic change.97 This ligand is not regarded as playing a major role in kidney development. Hence, there are differences as well as similarities between development and repair.
Recent Transcriptional Analysis of Kidney Repair: Does This Change Our View?
Many of the early analyses of gene expression during renal repair examined genes or pathways one at a time. Recent advances in cell tracing and transcriptional profiling have improved the resolution with which such expression changes can be examined. In the most detailed analysis of gene expression changes after acute renal injury performed to date, the ribosomally-associated transcripts present in individual cell types were compared between sham and 24-hour IRI in mice.98 This approach was taken to identify those genes reaching translation, and hence actually affecting protein production. Although many of the developmental genes previously identified as upregulated in injury were identified, including Notch1/2, Jag1, and Ret, others were downregulated (including Lhx1, Fgf20, Gli2, and Ptch1 in epithelial cells and Ptch1 and Smo in vascular pericytes/fibroblasts) or apparently unaffected (no change in Pax2 in any cell type). Importantly, a number of genes that were upregulated did so in unexpected cell types. For example, upregulation of Wnt4, Wnt9b, Wnt11, Bmp7, and Vegfa were restricted to the endothelial cell population, a cell type not known to express these genes during development. This would suggest that the roles of such genes in response to injury are distinct from their roles in development.98,99 Some of these unexpected changes may reflect the restriction of this analysis to a single early time point, however, overall this paints quite a different picture of the response process. What had been assumed to be the return of epithelial cell types to a progenitor state followed by the recapitulation of normal morphogenetic programs is more likely to represent the independent activation of similar gene pathways for a distinct role.
It was this study that identified c-Myc and N-Myc, regulators of cellular proliferation, and Sox9, a transcription factor with broad developmental roles in a number of tissues, as the most highly upregulated genes within the tubular epithelium of the injured kidney.98 Kumar et al.63 investigated this further, showing that Sox9 upregulation preceded the expression of accepted biomarkers of kidney injury, Lcn2 (Ngal)100 and Kim1,101 suggesting a very early role in the response phase.63 Although Sox9 expression levels peaked at 24–48 hours, as did serum creatinine levels, elevation in expression persisted for 28 days despite a return to normal of renal functional readouts. A similar induction of Sox9 was seen in response to a second injury model; unilateral ureteric obstruction.63 Cells induced to express this transcription factor appeared as rare individual cells most frequently within the proximal tubules, but also in the distal tubule. Sox9+ cells represented a subset of the KIM1+ epithelium and 40% showed evidence of proliferation. At 28 days post injury, those tubules showed a failure to return to maximal lotus tetragonolobus lectin levels and contained cells that were Sox9+KIM1+. This would suggest a persistence of an altered gene expression profile long after an apparent return to a normal tubular histology (Figure 2). Lineage tracing showed that cells induced by injury to express Sox9 made major contributions to the proximal distal and loop of Henle epithelium. However, this analysis also suggested that any proximal tubule cell can induce Sox9 in response to injury rather than supporting the concept that these represent a privileged progenitor subtype. To investigate how critical Sox9 is to the repair process, the gene was conditionally deleted from mature proximal tubule cells using inducible Cre driven from the Slc34a1 promoter. An inability for these cells to express Sox9 in response to injury appeared to slow, but not block renal repair; however, fibrotic indices were worse in this instance.
Figure 2.
Induction of Sox9 in response to renal injury and during repair (Kumar et al.63). (A) Before injury, no evidence of expression of Sox9 or KIM1 is seen in the tubular epithelium. (B) Upon injury (unilateral ureteric obstruction or IRI), there is rapid upregulation of Sox9 (yellow nuclei) which preceded expression of Kim1 (black cell border). Cells induced to express Sox9 represented a subset of the injured epithelium as evidenced by cells inducing Kim1 without evidence of Sox9. (C) Sox9 expression levels peaked at 24–48 hours, as did serum creatinine levels and apoptosis. Approximately 40% of Sox9 cells showed evidence of proliferation (dark blue). (D) Elevation in expression of Sox9 persisted for 28 days despite a return to normal of renal functional readouts. At 28 days post injury, those tubules showing a failure to return to maximal labeling lotus tetragonolobus lectin and contained cells that were Sox9+KIM1+. Adapted with permission from Macmillan Publishers Ltd: Nature Reviews Nephrology, Ref. 122, copyright 2013.
A second study into renal repair processes64 took a slightly different approach. They commenced with the isolation of proliferative fractions of tubular epithelial cells from whole postnatal mouse kidneys, identifying an upregulation of Sox9 in such lines. Using lineage tracing, they also concluded that early nephron cells that express Sox9 during development go on to give rise to epithelial cells along the nephron, but not within the glomerulus.
Specifically focusing on the profile of key developmental genes across the response to folic acid–induced renal injury in postnatal mice, they then showed an early and significant peak in Sox9 expression that preceded the re-expression of other developmentally expressed genes, including Pax8, Lgr4, and Foxd1, all of which continued to increase in expression across 7 days post injury. Upregulation of cell cycle genes appeared to follow rather than precede Sox9. After postnatal injury, the Sox9+ population was also positive for Lgr4/5 expression and Wnt responsive. On the basis of these properties, and evidence of an in vitro multilineage capacity, Kang et al concluded that the Sox9+ cells represent a progenitor cell type.64
Although both studies identified the upregulation of Sox9 as an early event after AKI, they came to distinct conclusions, with Kumar et al.63 arguing that any tubular epithelial cell can respond in this way and Kang et al.64 arguing for the presence of a progenitor population. Whether representing a dedifferentiation of mature epithelium or the differential response of a residual progenitor population, does this represent a recapitulation of development? Sox9 is certainly expressed during kidney development, both in the ureteric epithelium and in the elongating and segmenting nephrons.102 Previous studies investigating the role of this gene in development have focused on the collecting duct. No defect is observed in Sox9 mutant mice unless accompanied by a deletion of the paralog, Sox8, which is also expressed in the developing kidney epithelium.102 Sox9 expression, however, extends into the forming nephrons. Kang et al.64 reported that during development in vivo, the majority of Sox9+ cells are proliferating (85%), suggestive of a progenitor population. Both studies demonstrated, using lineage analysis, that cells that have expressed Sox9 during development do contribute to most of the nephron segments, with the exception of the glomerulus. Although consistent with the concept that injured renal epithelium does re-express genes that have been previously expressed during development, this may be insufficient information to conclude that what is occurring is the recapitulation of a developmental process.
Parallel Gene Expression Because of Similar Triggers of Gene Regulation
So can repair be viewed as a recapitulation of development, or are we over-interpreting the use of similar pathways in a different cellular context? An alternate hypothesis is that the apparent re-expression of developmental genes reflects a congruence of the physiologic conditions able to initiate the expression of similar sets of genes. This would suggest that similar signals are active during both development and in response to acute injury. Here, we will investigate this as a hypothesis using one example: hypoxia.
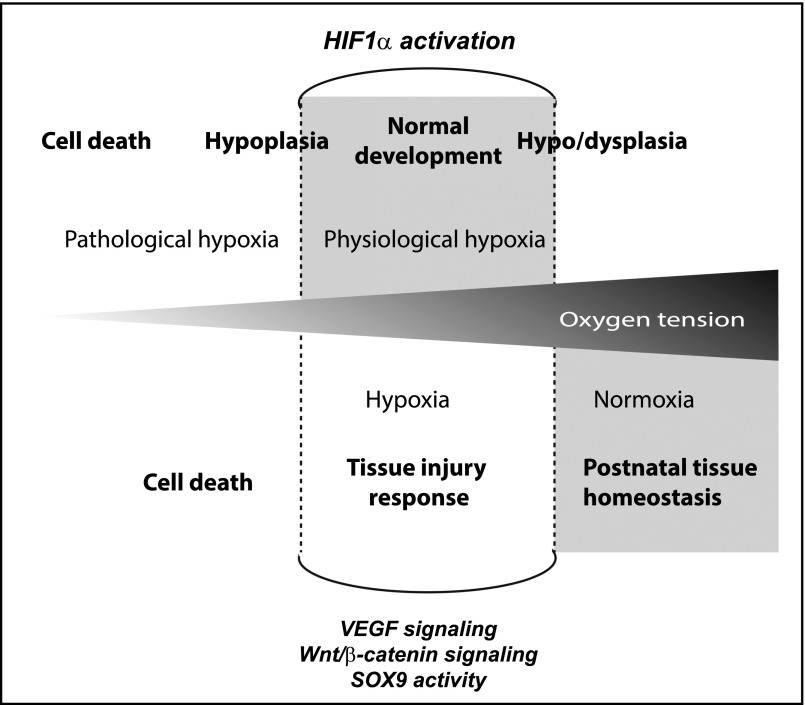
The majority of studies supporting the concept of developmental reiteration are on the basis of acute ischemic injury. This is because of the experimental reproducibility of the IRI model in rodents. The relevance of this model to humans is not well understood as biopsies are rarely taken from patients with AKI.103 Nevertheless, IRI, by definition, affects tissue oxygen tension and ischemia post transplantation, and severe hemorrhage would represent an ischemic injury in a human setting. It is well understood that a reduction in oxygen levels activates the HIF1α pathway by reducing the proteasomal degradation of HIF1α and HIF2α proteins.104 This allows heterodimerization with HIF1β, resulting in specific transcriptional regulation. Whereas the role of this hypoxia pathway is often portrayed as an injury response to a pathologic decline in oxygen tension, such as may occur in a damaged kidney, recent data suggests that this same pathway is maximally active during development.105
Normal fetal development occurs at lower oxygen tensions than that experienced by a fully vascularized mature organ.105–107 Estimates of oxygen tension within the developing embryo suggest that development is occurring at the equivalent of 8% oxygen. At this oxygen tension, the HIF1α pathway is active and likely to be regulating the expression of many of the molecular pathways integral to normal morphogenesis and organogenic patterning. Recent studies on the effect of gestational hypoxia on fetal development revealed maximal HIF1α pathway activity during normal kidney development. When this oxygen tension was reduced further into a pathologic hypoxic range, the HIF1α pathway no longer played a role. Instead, the cells showed evidence of endoplasmic reticulum stress and initiation of the unfolded protein response.105 This resulted in substantial defects in development because of a suppression of canonical Wnt signaling.105 Hence, canonical Wnt signaling activity during development appears to be influenced by oxygen tension, and is maximally active at oxygen tensions lower than the postnatal organ. Intriguingly, in vitro organ cultures of embryonic mouse kidneys show improved levels of morphogenesis at 8% oxygen compared with 21% oxygen, with improvements in both ureteric branching and subsequent nephron induction.105 Similar studies using rat have also reported increased ureteric branching in response to reduced oxygen tension (5% oxygen).108 Maximal levels of HIF1α pathway activity were evident at 8% oxygen, with HIF1α activity falling at both higher and lower oxygen tension.105 In a similar study, the deletion of HIF1α in the ureteric epithelium of the developing mouse embryo significantly reduced branching morphogenesis.109 Paradoxically, this study also showed evidence for induction of Vegfa and Bmp4 within the mesenchyme, which could elicit the opposite response: a reduction in ureteric branching.109 They suggest that the net result will depend upon the degree of hypoxia, potentially explaining some of the contradictory results reported across differing levels of oxygen in in vitro studies (Figure 3).110
Figure 3.
Potential role of hypoxia in development versus disease. Reduced oxygen tension induces the stabilization of the HIF1α protein, enabling heterodimerization with HIF1β to enable HIF-mediated transcriptional regulation. This transcriptional complex is known to intersect with key signaling pathways, including Wnt and Notch signaling.123,124 Hypoxia is a common component of AKI. During development, the oxygen tension at the tissue level is also low, with this physiologic hypoxia important for optimal morphogenesis.105 In vitro and in vivo studies suggest that further reductions or indeed elevations in oxygen tension adversely affect development.105,109,110 It is likely that other transcription factors active during development also respond to this reduced oxygen tension. The transcriptional similarities between normal development and acute injury may therefore reflect the similarities in oxygen tension.
Although the effect of hypoxia on nephrogenesis is incompletely understood,110 an association between low oxygen tension/HIF1α pathway activity and expression of key developmental genes is strongly supported in the literature. The physiologic hypoxia present during kidney development has been shown to regulate the expression of Wt1, Vegfa, Bmp4, c-met, and Ccnd1, all of which are known to be re-expressed during AKI. HIF1α activity regulates the expression of Gdnf and Gfrα1 during kidney development.108 HIF1α has been shown to upregulate Sox9 directly in model of Perthes disease, an ischemic osteonecrosis of the femur,111 also placing Sox9 in the list of hypoxia-responsive targets. Re-expression of Wt1 in postnatal injury has also been linked with HIF1α activity,112 and hypoxia directly upregulates Pax2 in a model of renal cell carconima.113
Not all studies reporting re-expression of developmental genes used the IRI model. However, in humans, acute renal failure is strongly regarded as involving hypoxia because of either IRI, sepsis or hemorrhage.114 The rarefaction that occurs as a result of acute injury is also likely to promote prolonged tissue hypoxia, which could in turn drive prolonged gene expression, resulting in maladaptive repair. Hence, the ischemic nature of AKI is often regarded as representing an obvious common denominator to both development and acute injury. Overall, this is consistent with a view that parallel patterns of gene expression seen during development and injury response represent the initiation of common regulatory networks. If we accept the premise that hypoxia is the common feature of development and ischemic injury, a better understanding of this common triggering mechanism may facilitate better treatment of AKI. By way of example, recent studies into the protective role of zinc preloading during IRI suggests that this is mediated via downregulation of HIF1α.115 Hence, a suppression of this response pathway reduces the potential ischemic response. The challenge is ensuring that we mount the appropriate degree of response to elicit efficient and complete tubular repair and avoid maladaptive responses that can result in fibrosis. More importantly, we need to understand whether this similarity is generalizable to all forms of repair after AKI.
The Challenges of a Reductionist Approach to Describing AKI
What we have proposed above is that similar transcriptional responses could arise from similar physiologic triggers in both development and postnatal injury. Although hypoxia is one convenient and logical example of this, clearly hypoxia-inducible responses are not necessarily pertinent to all types of postnatal kidney injury. Despite this, the concept of “transcriptional recapitulation of development during kidney repair” has been applied broadly to all AKI, irrespective of the cause. This broad generalization represents a reductionist view of the nature of the repair response that may well be incorrect. Indeed, viewing the many varied causes of AKI as one pathologic entity has been criticized as inaccurate and even counterproductive.116
There have been little transcriptional comparisons made between toxic (e.g., cisplatin or gentamycin) versus hypoxic (prolonged hemorrhage, IRI post transplantation) versus primarily inflammatory causes of AKI (interstitial nephritis, hemolytic uremic syndrome), with each resulting from different challenges and resulting in distinct pathologic changes. Two recent analyses of transcriptional responses to cisplatin versus gentamycin propose distinct transcriptional shifts in specific microRNA expression.117,118 Neither of these studies can be directly compared with the data now available for unilateral ureteric obstruction or IRI, and neither claim congruence with developmental expression changes. Even within the studies available for the transcriptional analysis of IRI in mice, reports show significant differences in transcriptional changes across a 48-hour time course despite using an identical injury.34
There are other forms of renal injury in which even less is known about the pathology, let alone the transcriptional responses. For example, renal histology post septic shock has been examined postmortem in humans and in sheep models of induced sepsis.119,120 Here, there is little evidence of tubular injury in the sheep model,120 and only focal tubular cell death in the human tissue.119 Indeed, a systematic review of histopathology after septic shock in primates and rodents suggested only mild, nonspecific histologic changes across the kidney.121 This would suggest that we are a long way from dissecting both the damage and repair processes in kidney injury, and that there may well be quite distinct responses depending upon the type of injury.
In conclusion, there is indeed some evidence for an overlap in gene expression during both normal kidney development and the acute response to postnatal renal injury. Similar pathways are also present in chronic injury and maladaptive repair. However, these pathways are active in many contexts. Although it is tempting to suggest that the response of the kidney to injury is to reinitiate developmental processes, the cell types present in the postnatal kidney are completely different to those evident during development. Hence, another way of viewing this overlap in gene expression is simply as a set of pathways triggered by specific environmental conditions. Reinterpreting this hypothesis in no way reduces the importance of studying the endogenous response to AKI, nor does it shed any further light onto whether the cells involved in repair have a particular progenitor phenotype or represent mature epithelial cells responding stochastically to a damage signal. However, more careful analysis of repair is needed to both improve the outcomes from acute injury and understand the origin of the maladaptive responses leading to chronic kidney injury.
Disclosures
None.
Acknowledgments
We thank Dr. Sanjeev Kumar for his advice on this article. M.H.L. is a Senior Principal Research Fellow of the National Health and Medical Research Council (NHMRC). P.K. is a Comisión Nacional de Investigación Científica y Tecnológica Becas-Chile postgraduate scholar.
The laboratory is supported by funding from the NHMRC (grant nos. GNT1054985 and 1063989; grant no. GNT1042093 to M.H.L.), National Institutes of Health (grant no. DK107344-01), and the Australian Research Council (grant no. SR1101002). The Murdoch Children’s Research Institute is supported by the Victorian Government’s Operational Infrastructure Support Program.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Little MH, McMahon AP: Mammalian kidney development: Principles, progress, and projections. Cold Spring Harb Perspect Biol 2012. Available at http://cshperspectives.cshlp.org/content/4/5/a008300.full. Accessed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challen G, Gardiner B, Caruana G, Kostoulias X, Martinez G, Crowe M, Taylor DF, Bertram J, Little M, Grimmond SM: Temporal and spatial transcriptional programs in murine kidney development. Physiol Genomics 23: 159–171, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Yu J, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS: Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell 15: 781–791, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, Aronow BJ, Brunskill EW, Combes AN, Tang D, Taylor D, Grimmond SM, Potter SS, McMahon AP, Little MH: Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol 332: 273–286, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Valerius MT, Duah M, Staser K, Hansard JK, Guo JJ, McMahon J, Vaughan J, Faria D, Georgas K, Rumballe B, Ren Q, Krautzberger AM, Junker JP, Thiagarajan RD, Machanick P, Gray PA, van Oudenaarden A, Rowitch DH, Stiles CD, Ma Q, Grimmond SM, Bailey TL, Little MH, McMahon AP: Identification of molecular compartments and genetic circuitry in the developing mammalian kidney. Development 139: 1863–1873, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JS, Ma W, O’Brien LL, Chung E, Guo JJ, Cheng JG, Valerius MT, McMahon JA, Wong WH, McMahon AP: Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell 23: 637–651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopan R, Chen S, Little M: Nephron progenitor cells: Shifting the balance of self-renewal and differentiation. Curr Top Dev Biol 107: 293–331, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE: Human nephron number: Implications for health and disease. Pediatr Nephrol 26: 1529–1533, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D: Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64: 777–784, 1991 [PubMed] [Google Scholar]
- 11.Rumballe BA, Georgas KM, Combes AN, Ju AL, Gilbert T, Little MH: Nephron formation adopts a novel spatial topology at cessation of nephrogenesis. Dev Biol 360: 110–122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartman HA, Lai HL, Patterson LT: Cessation of renal morphogenesis in mice. Dev Biol 310: 379–387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Brunskill EW, Potter SS, Dexheimer PJ, Salomonis N, Aronow BJ, Hong CI, Zhang T, Kopan R: Intrinsic age-dependent changes and cell-cell contacts regulate nephron progenitor lifespan. Dev Cell 35: 49–62, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witzgall R, Brown D, Schwarz C, Bonventre JV: Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogetseder A, Karadeniz A, Kaissling B, Le Hir M: Tubular cell proliferation in the healthy rat kidney. Histochem Cell Biol 124: 97–104, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M: Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol 294: C22–C28, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G: Isolation of renal progenitor cells from adult human kidney. Am J Pathol 166: 545–555, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P: Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol 18: 3128–3138, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P: Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 30: 1714–1725, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Vogetseder A, Palan T, Bacic D, Kaissling B, Le Hir M: Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol 292: C807–C813, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Bonventre JV: Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14[Suppl 1]: S55–S61, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD: Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A 111: 1527–1532, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG: Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramann R, Kusaba T, Humphreys BD: Who regenerates the kidney tubule? Nephrol Dial Transplant 30: 903–910, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lombardi D, Becherucci F, Romagnani P: How much can the tubule regenerate and who does it? An open question. Nephrol Dial Transplant 31: 1243–1250, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Humphreys BD, Bonventre JV: Pathophysiology of acute kidney injury to chronic kidney disease: Maladaptive repair. Contrib Nephrol 174: 149–155, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Ferenbach DA, Bonventre JV: Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11: 264–276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burne-Taney MJ, Yokota N, Rabb H: Persistent renal and extrarenal immune changes after severe ischemic injury. Kidney Int 67: 1002–1009, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Wei Q, Dong Z: Mouse model of ischemic acute kidney injury: Technical notes and tricks. Am J Physiol Renal Physiol 303: F1487–F1494, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S: Gene expression in early ischemic renal injury: Clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab 80: 365–376, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S: Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81: 751–761, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Sancho-Martínez SM, López-Novoa JM, López-Hernández FJ: Pathophysiological role of different tubular epithelial cell death modes in acute kidney injury. Clin Kidney J 8: 548–559, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Zhang C, Hu L, Yang C: Necroptosis in acute kidney injury: A shedding light. Cell Death Dis 7: e2125, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S, Liu J, McMahon AP: Defining the acute kidney injury and repair transcriptome. Semin Nephrol 34: 404–417, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV: Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A 108: 9226–9231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villanueva S, Cespedes C, Gonzalez A, Vio CP: bFGF induces an earlier expression of nephrogenic proteins after ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 291: R1677–R1687, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Villanueva S, Cespedes C, Vio CP: Ischemic acute renal failure induces the expression of a widerange of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol 290: R861–R870, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P: Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Romagnani P: Toward the identification of a “renopoietic system”? Stem Cells 27: 2247–2253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romagnani P, Remuzzi G: Renal progenitors in non-diabetic and diabetic nephropathies. Trends Endocrinol Metab 24: 13–20, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P: Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindgren D, Boström AK, Nilsson K, Hansson J, Sjölund J, Möller C, Jirström K, Nilsson E, Landberg G, Axelson H, Johansson ME: Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol 178: 828–837, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sallustio F, De Benedictis L, Castellano G, Zaza G, Loverre A, Costantino V, Grandaliano G, Schena FP: TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB J 24: 514–525, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Lasagni L, Romagnani P: Glomerular epithelial stem cells: The good, the bad, and the ugly. J Am Soc Nephrol 21: 1612–1619, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Romagnani P, Anders HJ: What can tubular progenitor cultures teach us about kidney regeneration? Kidney Int 83: 351–353, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Rinkevich Y, Montoro DT, Contreras-Trujillo H, Harari-Steinberg O, Newman AM, Tsai JM, Lim X, Van-Amerongen R, Bowman A, Januszyk M, Pleniceanu O, Nusse R, Longaker MT, Weissman IL, Dekel B: In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep 7: 1270–1283, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato H, Susztak K: Repair problems in podocytes: Wnt, Notch, and glomerulosclerosis. Semin Nephrol 32: 350–356, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costantini F: Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley Interdiscip Rev Dev Biol 1: 693–713, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hendry C, Rumballe B, Moritz K, Little MH: Defining and redefining the nephron progenitor population. Pediatr Nephrol 26: 1395–1406, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R: Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14: 53–67, 2014 [DOI] [PubMed] [Google Scholar]
- 51.Stark K, Vainio S, Vassileva G, McMahon AP: Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372: 679–683, 1994 [DOI] [PubMed] [Google Scholar]
- 52.Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, Berger K, Bornemann J, Gelman IH, Floege J, van der Vlag J, Wetzels JF, Moeller MJ: Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol 229: 645–659, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berger K, Bangen JM, Hammerich L, Liedtke C, Floege J, Smeets B, Moeller MJ: Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci U S A 111: 1533–1538, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pippin JW, Kaverina NV, Eng DG, Krofft RD, Glenn ST, Duffield JS, Gross KW, Shankland SJ: Cells of renin lineage are adult pluripotent progenitors in experimental glomerular disease. Am J Physiol Renal Physiol 309: F341–F358, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaverina NV, Eng DG, Schneider RR, Pippin JW, Shankland SJ: Partial podocyte replenishment in experimental FSGS derives from nonpodocyte sources. Am J Physiol Renal Physiol 310: F1397–F1413, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lasagni L, Angelotti ML, Ronconi E, Lombardi D, Nardi S, Peired A, Becherucci F, Mazzinghi B, Sisti A, Romoli S, Burger A, Schaefer B, Buccoliero A, Lazzeri E, Romagnani P: Podocyte regeneration driven by renal progenitors determines glomerular disease remission and can be pharmacologically enhanced. Stem Cell Rep 5: 248–263, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, Walz G, Moeller MJ, Grahammer F, Huber TB: Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol 25: 707–716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV: Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82: 172–183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, Duffield JS, Lin SL: Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 80: 1170–1181, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R: Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD: Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16: 51–66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar S, Liu J, Pang P, Krautzberger AM, Reginensi A, Akiyama H, Schedl A, Humphreys BD, McMahon AP: Sox9 activation highlights a cellular pathway of renal repair in the acutely injured mammalian kidney. Cell Reports 12: 1325–1338, 2015 [DOI] [PubMed] [Google Scholar]
- 64.Kang HM, Huang S, Reidy K, Han SH, Chinga F, Susztak K: Sox9-positive progenitor cells play a key role in renal tubule epithelial regeneration in mice. Cell Rep 14: 861–871, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Z, He L, Huang D, Lei S, Gao J: Icariin protects rats against 5/6 nephrectomy-induced chronic kidney failure by increasing the number of renal stem cells. BMC Complement Altern Med 15: 378, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yanagita M: Inhibitors/antagonists of TGF-β system in kidney fibrosis. Nephrol Dial Transplant 27: 3686–3691, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Rothenpieler UW, Dressler GR: Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development 119: 711–720, 1993 [DOI] [PubMed] [Google Scholar]
- 68.Imgrund M, Gröne E, Gröne HJ, Kretzler M, Holzman L, Schlöndorff D, Rothenpieler UW: Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice 1. Kidney Int 56: 1423–1431, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Jiang Y, Jiang T, Ouyang J, Zhou Q, Liang Y, Cui Y, Chen P, Huang B: Cell atavistic transition: Paired box 2 re-expression occurs in mature tubular epithelial cells during acute kidney injury and is regulated by angiotensin II. PLoS One 9: e93563, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gnudi L, Benedetti S, Woolf AS, Long DA: Vascular growth factors play critical roles in kidney glomeruli. Clin Sci (Lond) 129: 1225–1236, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, Nagy A, Ema M, Rossant J, Jothy S, Miner JH, Quaggin SE: Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol 17: 724–735, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Barak H, Surendran K, Boyle SC: The role of Notch signaling in kidney development and disease. Adv Exp Med Biol 727: 99–113, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR: Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132: 2809–2823, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Chen L, Al-Awqati Q: Segmental expression of Notch and hairy genes in nephrogenesis. Am J Physiol Renal Physiol 288: F939–F952, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R: Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 134: 801–811, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barker N, Rookmaaker MB, Kujala P, Ng A, Leushacke M, Snippert H, van de Wetering M, Tan S, Van Es JH, Huch M, Poulsom R, Verhaar MC, Peters PJ, Clevers H: Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Reports 2: 540–552, 2012 [DOI] [PubMed] [Google Scholar]
- 77.Vooijs M, Ong CT, Hadland B, Huppert S, Liu Z, Korving J, van den Born M, Stappenbeck T, Wu Y, Clevers H, Kopan R: Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development 134: 535–544, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD: Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol 24: 1399–1412, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou T, Benda C, Dunzinger S, Huang Y, Ho JC, Yang J, Wang Y, Zhang Y, Zhuang Q, Li Y, Bao X, Tse HF, Grillari J, Grillari-Voglauer R, Pei D, Esteban MA: Generation of human induced pluripotent stem cells from urine samples. Nat Protoc 7: 2080–2089, 2012 [DOI] [PubMed] [Google Scholar]
- 80.Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, Sasaki S: Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol 14: 1223–1233, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Lin SA, Kolle G, Grimmond SM, Zhou Q, Doust E, Little MH, Aronow B, Ricardo SD, Pera MF, Bertram JF, Laslett AL: Subfractionation of differentiating human embryonic stem cell populations allows the isolation of a mesodermal population enriched for intermediate mesoderm and putative renal progenitors. Stem Cells Dev 19: 1637–1648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP: A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136: 161–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sörensen-Zender I, Rong S, Susnik N, Zender S, Pennekamp P, Melk A, Haller H, Schmitt R: Renal tubular Notch signaling triggers a prosenescent state after acute kidney injury. Am J Physiol Renal Physiol 306: F907–F915, 2014 [DOI] [PubMed] [Google Scholar]
- 84.Reuter S, Martin H, Beckert H, Bros M, Montermann E, Belz C, Heinz A, Ohngemach S, Sahin U, Stassen M, Buhl R, Eshkind L, Taube C: The Wnt/β-catenin pathway attenuates experimental allergic airway disease. J Immunol 193: 485–495, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Lam AQ, Freedman BS, Morizane R, Lerou PH, Valerius MT, Bonventre JV: Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol 25: 1211–1225, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K: Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 120: 4040–4054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murea M, Park JK, Sharma S, Kato H, Gruenwald A, Niranjan T, Si H, Thomas DB, Pullman JM, Melamed ML, Susztak K: Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int 78: 514–522, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Djudjaj S, Chatziantoniou C, Raffetseder U, Guerrot D, Dussaule JC, Boor P, Kerroch M, Hanssen L, Brandt S, Dittrich A, Ostendorf T, Floege J, Zhu C, Lindenmeyer M, Cohen CD, Mertens PR: Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. J Pathol 228: 286–299, 2012 [DOI] [PubMed] [Google Scholar]
- 90.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 91.Diep CQ, Peng Z, Ukah TK, Kelly PM, Daigle RV, Davidson AJ: Development of the zebrafish mesonephros. Genesis 53: 257–269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP: Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: 283–292, 2005 [DOI] [PubMed] [Google Scholar]
- 93.Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP: Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130: 3175–3185, 2003 [DOI] [PubMed] [Google Scholar]
- 94.Burn SF, Webb A, Berry RL, Davies JA, Ferrer-Vaquer A, Hadjantonakis AK, Hastie ND, Hohenstein P: Calcium/NFAT signalling promotes early nephrogenesis. Dev Biol 352: 288–298, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tanigawa S, Wang H, Yang Y, Sharma N, Tarasova N, Ajima R, Yamaguchi TP, Rodriguez LG, Perantoni AO: Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev Biol 352: 58–69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu J, Carroll TJ, McMahon AP: Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129: 5301–5312, 2002 [DOI] [PubMed] [Google Scholar]
- 97.Fabian SL, Penchev RR, St-Jacques B, Rao AN, Sipilä P, West KA, McMahon AP, Humphreys BD: Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol 180: 1441–1453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J, Krautzberger AM, Sui SH, Hofmann OM, Chen Y, Baetscher M, Grgic I, Kumar S, Humphreys BD, Hide WA, McMahon AP: Cell-specific translational profiling in acute kidney injury. J Clin Invest 124: 1242–1254, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chiba T, Hukriede N, de Caestecker MP: Kidney regeneration: Lessons from development. Curr Pathobiol Rep 3: 67–79, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 101.Ichimura T, Mou S: Kidney injury molecule-1 in acute kidney injury and renal repair: A review. Zhong Xi Yi Jie He Xue Bao 6: 533–538, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Reginensi A, Clarkson M, Neirijnck Y, Lu B, Ohyama T, Groves AK, Sock E, Wegner M, Costantini F, Chaboissier MC, Schedl A: SOX9 controls epithelial branching by activating RET effector genes during kidney development. Hum Mol Genet 20: 1143–1153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosen S, Stillman IE: Acute tubular necrosis is a syndrome of physiologic and pathologic dissociation. J Am Soc Nephrol 19: 871–875, 2008 [DOI] [PubMed] [Google Scholar]
- 104.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU: Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002 [DOI] [PubMed] [Google Scholar]
- 105.Wilkinson LJ, Neal CS, Singh RR, Sparrow DB, Kurniawan ND, Ju A, Grieve SM, Dunwoodie SL, Moritz KM, Little MH: Renal developmental defects resulting from in utero hypoxia are associated with suppression of ureteric beta-catenin signaling. Kidney Int 87: 975–983, 2015 [DOI] [PubMed] [Google Scholar]
- 106.Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, Raleigh JA, Chung HY, Yoo MA, Kim KW: Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: A possible signal for vessel development. Dev Dyn 220: 175–186, 2001 [DOI] [PubMed] [Google Scholar]
- 107.Dunwoodie SL: The role of hypoxia in development of the mammalian embryo. Dev Cell 17: 755–773, 2009 [DOI] [PubMed] [Google Scholar]
- 108.Tsuji K, Kitamura S, Makino H: Hypoxia-inducible factor 1α regulates branching morphogenesis during kidney development. Biochem Biophys Res Commun 447: 108–114, 2014 [DOI] [PubMed] [Google Scholar]
- 109.Schley G, Scholz H, Kraus A, Hackenbeck T, Klanke B, Willam C, Wiesener MS, Heinze E, Burzlaff N, Eckardt KU, Buchholz B: Hypoxia inhibits nephrogenesis through paracrine Vegfa despite the ability to enhance tubulogenesis. Kidney Int 88: 1283–1292, 2015 [DOI] [PubMed] [Google Scholar]
- 110.Buchholz B, Schley G, Eckardt KU: The impact of hypoxia on nephrogenesis. Curr Opin Nephrol Hypertens 25: 180–186, 2016 [DOI] [PubMed] [Google Scholar]
- 111.Zhang C, Yang F, Cornelia R, Tang W, Swisher S, Kim H: Hypoxia-inducible factor-1 is a positive regulator of Sox9 activity in femoral head osteonecrosis. Bone 48: 507–513, 2011 [DOI] [PubMed] [Google Scholar]
- 112.Wagner KD, Wagner N, Wellmann S, Schley G, Bondke A, Theres H, Scholz H: Oxygen-regulated expression of the Wilms’ tumor suppressor Wt1 involves hypoxia-inducible factor-1 (HIF-1). FASEB J 17: 1364–1366, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Luu VD, Boysen G, Struckmann K, Casagrande S, von Teichman A, Wild PJ, Sulser T, Schraml P, Moch H: Loss of VHL and hypoxia provokes PAX2 up-regulation in clear cell renal cell carcinoma. Clin Cancer Res 15: 3297–3304, 2009 [DOI] [PubMed] [Google Scholar]
- 114.Esson ML, Schrier RW: Diagnosis and treatment of acute tubular necrosis. Ann Intern Med 137: 744–752, 2002 [DOI] [PubMed] [Google Scholar]
- 115.Zhang X, Liang D, Fan J, Lian X, Zhao Y, Wang X, Chi ZH, Zhang P: Zinc attenuates tubulointerstitial fibrosis in diabetic nephropathy via inhibition of HIF through PI-3K signaling. Biol Trace Elem Res 173: 372–383, 2016 [DOI] [PubMed] [Google Scholar]
- 116.Mulay SR, Holderied A, Kumar SV, Anders HJ: Targeting inflammation in so-called acute kidney injury. Semin Nephrol 36: 17–30, 2016 [DOI] [PubMed] [Google Scholar]
- 117.Saikumar J, Hoffmann D, Kim TM, Gonzalez VR, Zhang Q, Goering PL, Brown RP, Bijol V, Park PJ, Waikar SS, Vaidya VS: Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicol Sci 129: 256–267, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee CG, Kim JG, Kim HJ, Kwon HK, Cho IJ, Choi DW, Lee WH, Kim WD, Hwang SJ, Choi S, Kim SG: Discovery of an integrative network of microRNAs and transcriptomics changes for acute kidney injury. Kidney Int 86: 943–953, 2014 [DOI] [PubMed] [Google Scholar]
- 119.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS: Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 187: 509–517, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maiden MJ, Otto S, Brealey JK, Finnis ME, Chapman MJ, Kuchel TR, Nash CH, Edwards J, Bellomo R: Structure and function of the kidney in septic shock. A prospective controlled experimental study. Am J Respir Crit Care Med 194: 692–700, 2016 [DOI] [PubMed] [Google Scholar]
- 121.Langenberg C, Gobe G, Hood S, May CN, Bellomo R: Renal histopathology during experimental septic acute kidney injury and recovery. Crit Care Med 42: e58–e67, 2014 [DOI] [PubMed] [Google Scholar]
- 122.Rabelink TJ, Little MH: Stromal cells in tissue homeostasis: Balancing regeneration and fibrosis. Nat Rev Nephrol 9: 747–753, 2013 [DOI] [PubMed] [Google Scholar]
- 123.Andersson ER, Sandberg R, Lendahl U: Notch signaling: Simplicity in design, versatility in function. Development 138: 3593–3612, 2011 [DOI] [PubMed] [Google Scholar]
- 124.Braunschweig L, Meyer AK, Wagenführ L, Storch A: Oxygen regulates proliferation of neural stem cells through Wnt/β-catenin signalling. Mol Cell Neurosci 67: 84–92, 2015 [DOI] [PubMed] [Google Scholar]