Abstract
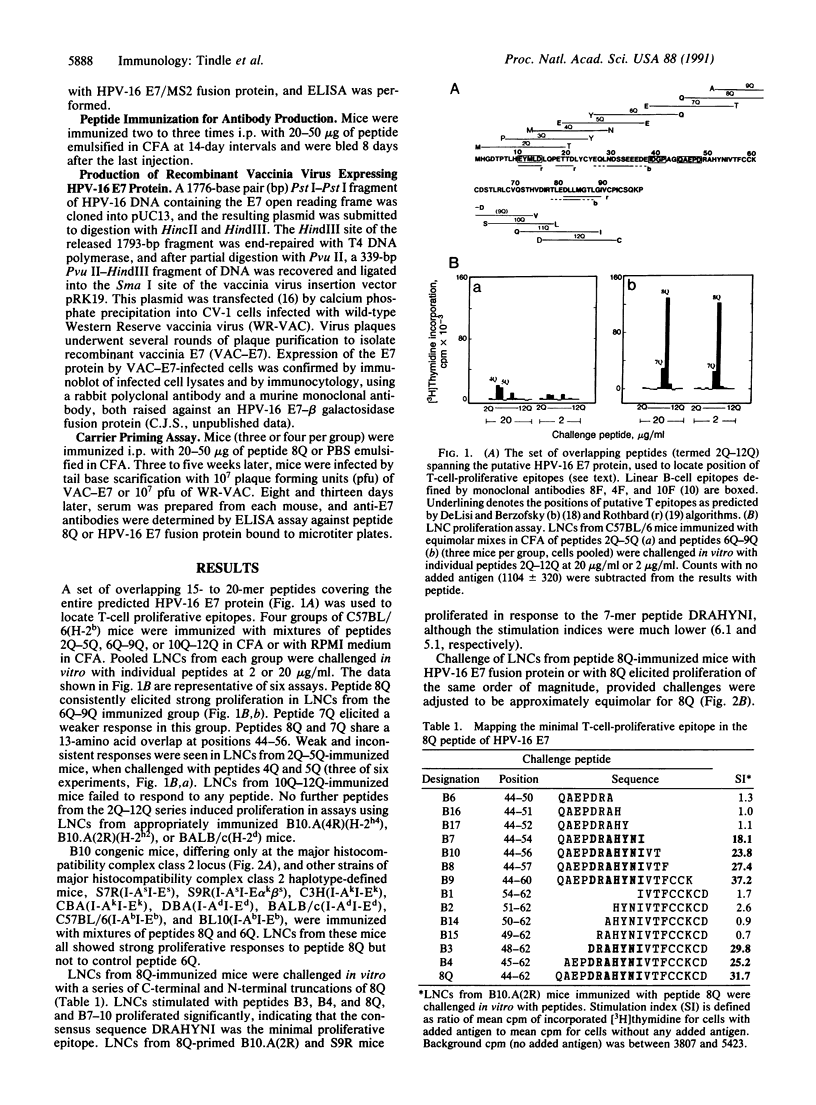
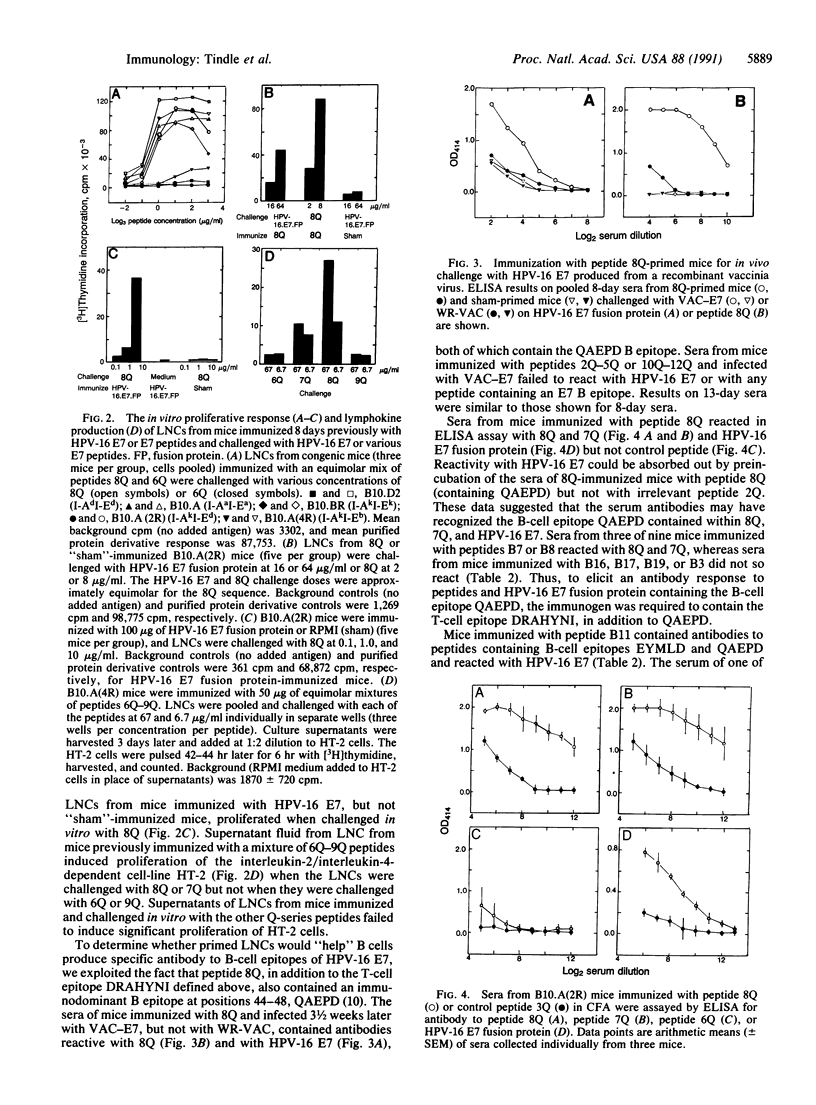
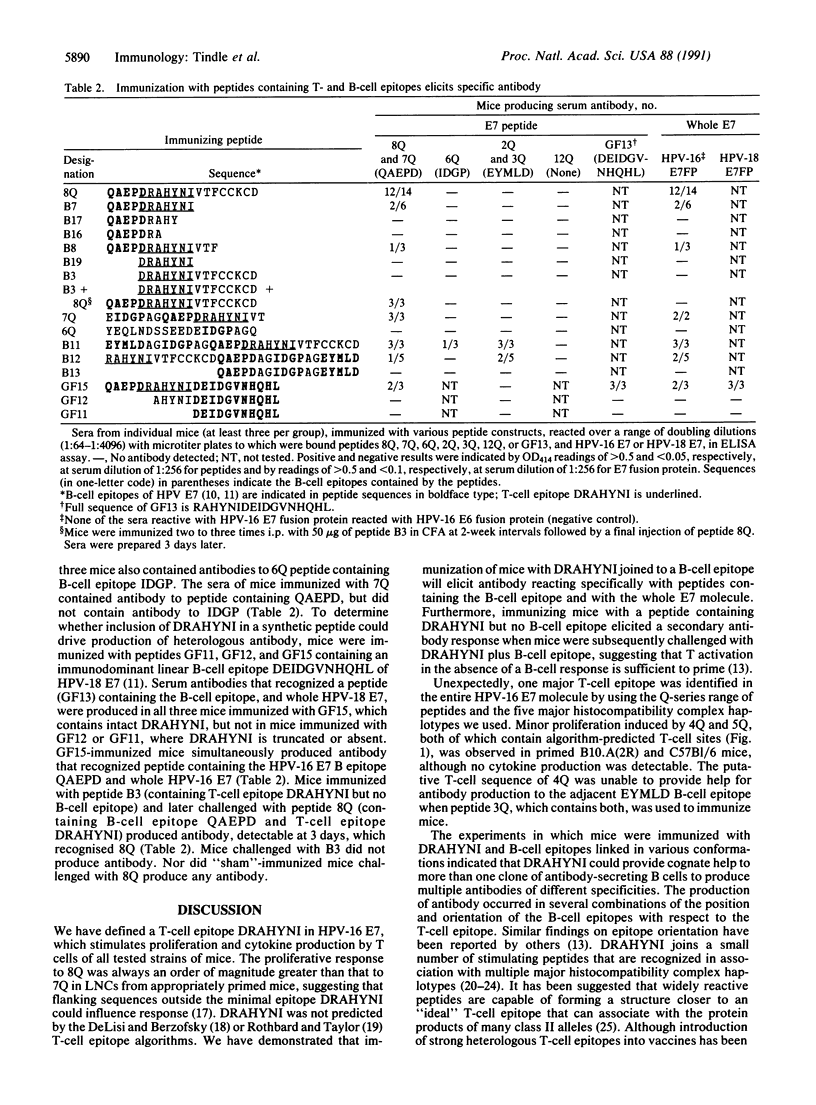
We have identified a major T-cell epitope, amino acids 48-54 (DRAHYNI, in one-letter code) in the E7 open reading frame protein of human papillomavirus (HPV) type 16. Lymph node cells from mice immunized with synthetic peptides containing DRAHYNI proliferated and produced interleukin when challenged in vitro with peptide or whole HPV-16 E7 fusion protein. The T epitope was recognized in association with all five major histocompatibility complex class II I-A and I-E alleles tested. Synthetic peptides consisting of DRAHYNI linked to major B-cell epitopes on the E7 molecule formed immunogens capable of eliciting strong antibody responses to HPV-16 E7. The T epitope could provide help for the production of antibody to several B epitopes simultaneously, including a B epitope of HPV-18 E7 protein. Mice immunized with a peptide containing DRAHYNI and B epitope and, at a later date, infected with recombinant vaccinia E7 virus, displayed secondary antibody responses to E7. Because E7 has a role in cell transformation and is the most abundant viral protein in HPV-associated neoplastic cervical epithelial cells, the data have implications for vaccine strategies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Browne H. M., Churcher M. J., Stanley M. A., Smith G. L., Minson A. C. Analysis of the L1 gene product of human papillomavirus type 16 by expression in a vaccinia virus recombinant. J Gen Virol. 1988 Jun;69(Pt 6):1263–1273. doi: 10.1099/0022-1317-69-6-1263. [DOI] [PubMed] [Google Scholar]
- Cease K. B., Margalit H., Cornette J. L., Putney S. D., Robey W. G., Ouyang C., Streicher H. Z., Fischinger P. J., Gallo R. C., DeLisi C. Helper T-cell antigenic site identification in the acquired immunodeficiency syndrome virus gp120 envelope protein and induction of immunity in mice to the native protein using a 16-residue synthetic peptide. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4249–4253. doi: 10.1073/pnas.84.12.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T., Morgenstern J. P., Crawford L., Banks L. Continued expression of HPV-16 E7 protein is required for maintenance of the transformed phenotype of cells co-transformed by HPV-16 plus EJ-ras. EMBO J. 1989 Feb;8(2):513–519. doi: 10.1002/j.1460-2075.1989.tb03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl H. C., Dietzschold B., Gore M., Otvos L., Jr, Larson J. K., Wunner W. H., Koprowski H. Induction of rabies virus-specific T-helper cells by synthetic peptides that carry dominant T-helper cell epitopes of the viral ribonucleoprotein. J Virol. 1989 Jul;63(7):2885–2892. doi: 10.1128/jvi.63.7.2885-2892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X. M., Liew F. Y., Tite J. P. Identification and characterization of T helper epitopes in the nucleoprotein of influenza A virus. J Immunol. 1989 Nov 1;143(9):3007–3014. [PubMed] [Google Scholar]
- Good M. F., Maloy W. L., Lunde M. N., Margalit H., Cornette J. L., Smith G. L., Moss B., Miller L. H., Berzofsky J. A. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science. 1987 Feb 27;235(4792):1059–1062. doi: 10.1126/science.2434994. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Valentine S., Dietzschold B., Burns-Purzycki C. Overlapping T cell antigenic sites on a synthetic peptide fragment from herpes simplex virus glycoprotein D, the degenerate MHC restriction elicited, and functional evidence for antigen-Ia interaction. J Exp Med. 1988 Feb 1;167(2):275–287. doi: 10.1084/jem.167.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochmus-Kudielka I., Schneider A., Braun R., Kimmig R., Koldovsky U., Schneweis K. E., Seedorf K., Gissmann L. Antibodies against the human papillomavirus type 16 early proteins in human sera: correlation of anti-E7 reactivity with cervical cancer. J Natl Cancer Inst. 1989 Nov 15;81(22):1698–1704. doi: 10.1093/jnci/81.22.1698. [DOI] [PubMed] [Google Scholar]
- Lai M. Z., Ross D. T., Guillet J. G., Briner T. J., Gefter M. L., Smith J. A. T lymphocyte response to bacteriophage lambda repressor cI protein. Recognition of the same peptide presented by Ia molecules of different haplotypes. J Immunol. 1987 Dec 15;139(12):3973–3980. [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Woody J. N., Green N. Human T-cell clones recognize chemically synthesized peptides of influenza haemagglutinin. Nature. 1982 Nov 4;300(5887):66–69. doi: 10.1038/300066a0. [DOI] [PubMed] [Google Scholar]
- Manca F., Kunkl A., Fenoglio D., Fowler A., Sercarz E., Celada F. Constraints in T-B cooperation related to epitope topology on E. coli beta-galactosidase. I. The fine specificity of T cells dictates the fine specificity of antibodies directed to conformation-dependent determinants. Eur J Immunol. 1985 Apr;15(4):345–350. doi: 10.1002/eji.1830150408. [DOI] [PubMed] [Google Scholar]
- Matlashewski G., Schneider J., Banks L., Jones N., Murray A., Crawford L. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. EMBO J. 1987 Jun;6(6):1741–1746. doi: 10.1002/j.1460-2075.1987.tb02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., Hughes J. L., McLachlan A., Thornton G. B., Moriarty A. Hepatitis B synthetic immunogen comprised of nucleocapsid T-cell sites and an envelope B-cell epitope. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1610–1614. doi: 10.1073/pnas.85.5.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. A., Levely M. E., Mitchell M. A., Smith C. W. A 16-amino acid peptide of respiratory syncytial virus 1A protein contains two overlapping T cell-stimulating sites distinguishable by class II MHC restriction elements. J Immunol. 1989 Nov 1;143(9):2790–2796. [PubMed] [Google Scholar]
- Nicholas J. A., Mitchell M. A., Levely M. E., Rubino K. L., Kinner J. H., Harn N. K., Smith C. W. Mapping an antibody-binding site and a T-cell-stimulating site on the 1A protein of respiratory syncytial virus. J Virol. 1988 Dec;62(12):4465–4473. doi: 10.1128/jvi.62.12.4465-4473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Phelps W. C., Zhang Y. L., Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 1988 Oct;7(10):3181–3187. doi: 10.1002/j.1460-2075.1988.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Gnann J. W., Jr, Landes R., Lockshin C., Richman D., McCutchan A., Kennedy C., Oldstone M. B., Nelson J. A. T cell recognition of HIV synthetic peptides in a natural infection. J Immunol. 1989 Feb 15;142(4):1166–1176. [PubMed] [Google Scholar]
- Seedorf K., Oltersdorf T., Krämmer G., Röwekamp W. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987 Jan;6(1):139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvey L. A., Tindle R. W., Geysen H. M., Haller C. J., Smith J. A., Frazer I. H. Identification of B-epitopes in the human papillomavirus 18 E7 open reading frame protein. J Immunol. 1990 Nov 1;145(9):3105–3110. [PubMed] [Google Scholar]
- Singer A., Walker P. G., McCance D. J. Genital wart virus infections: nuisance or potentially lethal? Br Med J (Clin Res Ed) 1984 Mar 10;288(6419):735–737. doi: 10.1136/bmj.288.6419.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S. J., Murray K. Immunogenicity of peptide fusions to hepatitis B virus core antigen. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6283–6287. doi: 10.1073/pnas.86.16.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindle R. W., Smith J. A., Geysen H. M., Selvey L. A., Frazer I. H. Identification of B epitopes in human papillomavirus type 16 E7 open reading frame protein. J Gen Virol. 1990 Jun;71(Pt 6):1347–1354. doi: 10.1099/0022-1317-71-6-1347. [DOI] [PubMed] [Google Scholar]
- Tindle R. W., Zhou W. D., Saul A., Frazer I. H. The molecular specificity of linear B-epitopes in the E7 open reading frame protein of human papillomavirus 16 defined by monoclonal antibodies. Pept Res. 1990 Jul-Aug;3(4):162–166. [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Vacchio M. S., Berzofsky J. A., Krzych U., Smith J. A., Hodes R. J., Finnegan A. Sequences outside a minimal immunodominant site exert negative effects on recognition by staphylococcal nuclease-specific T cell clones. J Immunol. 1989 Nov 1;143(9):2814–2819. [PubMed] [Google Scholar]
- Yasumoto S., Burkhardt A. L., Doniger J., DiPaolo J. A. Human papillomavirus type 16 DNA-induced malignant transformation of NIH 3T3 cells. J Virol. 1986 Feb;57(2):572–577. doi: 10.1128/jvi.57.2.572-577.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



