Abstract
The organic anion-transporting polypeptides represent an important family of drug uptake transporters that mediate the cellular uptake of a broad range of substrates including numerous drugs. Doxorubicin is a highly efficacious and well-established anthracycline chemotherapeutic agent commonly used in the treatment of a wide range of cancers. Although doxorubicin is a known substrate for efflux transporters such as P-glycoprotein (P-gp; MDR1, ABCB1), significantly less is known regarding its interactions with drug uptake transporters. Here, we investigated the role of organic anion transporting polypeptide (OATP) transporters to the disposition of doxorubicin. A recombinant vaccinia-based method for expressing uptake transporters in HeLa cells revealed that OATP1A2, but not OATP1B1 or OATP1B3, and the rat ortholog Oatp1a4 were capable of significant doxorubicin uptake. Interestingly, transwell assays using Madin-Darby canine kidney II cell line cells stably expressing specific uptake and/or efflux transporters revealed that OATP1B1, OATP1B3, and OATP1A2, either alone or in combination with MDR1, significantly transported doxorubicin. An assessment of polymorphisms in SLCO1A2 revealed that four variants were associated with significantly impaired doxorubicin transport in vitro. In vivo doxorubicin disposition studies revealed that doxorubicin plasma area under the curve was significantly higher (1.7-fold) in Slco1a/1b−/− versus wild-type mice. The liver-to-plasma ratio of doxorubicin was significantly decreased (2.3-fold) in Slco1a/1b2−/− mice and clearance was reduced by 40% compared with wild-type mice, suggesting Oatp1b transporters are important for doxorubicin hepatic uptake. In conclusion, we demonstrate important roles for OATP1A/1B in transporter-mediated uptake and disposition of doxorubicin.
Introduction
Organic anion-transporting polypeptides (OATP/Oatp; SLCO/Slco) are sodium-independent uptake transporters that mediate the cellular uptake of a broad range of substrates (Hagenbuch and Meier, 2004). OATP1A/1B transporters are highly expressed in organs such as liver, kidney, and small intestine in both human and mice (König et al., 2000a,b; Cheng et al., 2005; Lee et al., 2005). OATP1A2 (SLCO1A2) is currently thought to be expressed on the apical membranes of intestinal enterocytes, distal nephrons in the kidney, cholangiocytes lining the bile ducts in the liver, and capillary endothelial cells at the blood-brain barrier (Gao et al., 2000; Lee et al., 2005; Glaeser et al., 2007) and thereby mediates the oral bioavailability, renal secretion, and central nervous system distribution of drug substrates. OATP1B1 and OATP1B3 (SLCO1B1 and SLCO1B3) are predominantly expressed at the basolateral membranes of hepatocytes in the liver and have been implicated to play key roles in the hepatic uptake and plasma clearance of a number of drug substrates and toxins (König et al., 2000a,b; Hagenbuch and Gui, 2008). Of note, OATP1A/1B transporters have been found to be expressed in a number of cancer tissues, including breast, gastrointestinal, colon, ovarian, pancreatic, lung, prostate, and bone cancers, and cancer cell lines such as breast, prostate, and ovarian cancer cell lines (Abe et al., 2001; Ballestero et al., 2006; Miki et al., 2006; Meyer zu Schwabedissen et al., 2008; Liedauer et al., 2009; Svoboda et al., 2011; Arakawa et al., 2012). Although not extensively investigated to date, the expression of OATP1A/1B transporters in cancer tissues may facilitate tumor uptake of endogenous compounds that drive proliferation and growth. Alternatively, OATPs may be considered as important therapeutic targets in anticancer drug design because of their high transport activity for many cancer drugs, and their overexpression in certain cancers may facilitate tumor accumulation of such drugs (Thakkar et al., 2015).
Historically, the typical substrates of OATPs, including OATP1A/1B transporters, were believed to be mainly polar and anionic drugs (Hagenbuch and Meier, 2003, 2004). Interestingly, however, they have been found to transport a large number of structurally divergent compounds (Kullak-Ublick et al., 1995; Kullak-Ublick et al., 2001), including many drugs in clinical use, such as methotrexate (anion), paclitaxel (bulky hydrophobic), and fexofenadine (zwitterion) (van de Steeg et al., 2010, 2011). The anthracycline doxorubicin is a chemotherapeutic agent that is used in the treatment of a wide range of cancers, including non-Hodgkin's and Hodgkin's lymphoma, multiple myeloma, lung, ovarian, gastric, thyroid, breast, sarcoma, and pediatric cancers (Weiss, 1992; Cortés-Funes and Coronado, 2007). The major mechanisms by which doxorubicin exerts its pharmacologic effects involve intercalation into DNA, leading to inhibition of the DNA synthesis or poisoning of topoisomerase II (TOP2A), and generation of free radicals, leading to DNA and cell membrane damage (Gewirtz, 1999). Because doxorubicin is a hydrophobic weak base and cation at physiologic pH, it had not been considered a potential substrate for OATP transporters. Recently, however, Durmus et al. (2014) demonstrated that OATP1A/1B transporters play a substantial role in the in vivo disposition of doxorubicin. However, although doxorubicin is widely known to be a substrate for ABC-family drug efflux transporters such as P-glycoprotein (P-gp; MDR1), MRP2, and BCRP (Allen et al., 1999; van Asperen et al., 2000; Vlaming et al., 2006), significantly less is known regarding its interactions with drug uptake transporters that could mediate its cellular uptake and clearance.
In this study, we aimed to define the relevant role of OATP transporters to the disposition of doxorubicin through in vitro model cell systems in HeLa and Madin-Darby canine kidney II (MDCKII) cell lines and in vivo using an Oatp1a/1b knockout mouse model. We identified OATP1A2, OATP1B1, and OATP1B3 to be capable of transporting doxorubicin by screening an array of drug uptake transporters transiently expressed in HeLa cells and by using custom generated stably transduced transporter-expressing MDCKII polarized cells. Additional insights into the importance of hepatic OATP1B transporters to the hepatic uptake and clearance of doxorubicin in vivo were attained using a mouse model. Our data support significant roles for OATP transporters in the hepatic uptake, clearance, and plasma exposure of doxorubicin.
Materials and Methods
Chemicals and Reagents
Radiolabeled [3H]doxorubicin (1.2 Ci/mmol; >98% purity) was obtained from Moravek Biochemicals (Brea, CA). Unlabeled doxorubicin (>97% purity) was obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals and reagents, unless stated otherwise, were obtained from Sigma-Aldrich research and were of the highest grade available.
Cell Culture and Virus Preparation
HeLa cells, purchased from ATCC (Manassas, VA) in July 2012, were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin (Invitrogen). The polarized Madin-Darby canine kidney II (MDCKII) cells were purchased from Sigma-Aldrich (January 2013). The transporter-expressing and vector-transfected MDCKII cells were grown in Dulbecco's modified Eagle's medium containing high glucose and l-glutamine (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin (Invitrogen). All cells used in this study were maintained in a 5% CO2 atmosphere at 37°C in a humidified incubator.
Preparation of viral stock of vtf-7 virus was prepared as described previously (Ho et al., 2004). Briefly, HeLa cells grown to near confluence in 25-cm tissue culture plates were infected with 1 plaque-forming unit/10 cells. After an incubation period of 48 hours at 37°C, the infected cells were pelleted, homogenized, and recovered through centrifugation, followed by tittering of viral stock as described by Blakely et al. (1991).
Generation of Wild-Type and Variant OATP1A2 Expression Plasmids
Generation of pEF6/V5-His/OATP1A2 wild type (SLCO1A2*1), a plasmid containing the full open reading frame (ORF) of human SLCO1A2 cDNA in the pEF6/V5-His-TOPO vector (Invitrogen) was described previously (Lee et al., 2005). In addition, the pEF6/V5-His/Oatp1a1 and -Oatp1a4 plasmids containing rat uptake transporters and the identified six nonsynonymous allelic variants of SLCO1A2 [T38C (rs10841795, I13T, *2), A516C (rs11568563, E172D, *3), G559A (rs11045959, A187T, *4), A382T (rs11568567, N128Y, *5), A404T (rs45502302, N135I, *6], and C2003G [rs11568557, T668S, *7)] packaged into pEF6/V5-His-TOPO vector were also created previously (Lee et al., 2005). In brief, OATP1A2 variant plasmids were generated by Site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) by using OATP1A2 cDNA sequences in pEF6/V5-His/OATP1A2 [wild type (WT)] plasmid as a template. All plasmids were constructed without the stop codon to generate epitope-tagged proteins including V5 when ligated into the pEF6/V5-His-TOPO vector.
To generate pcDNA3.1(+)/OATP1A2-V5 (WT) plasmid, the full ORF of SLCO1A2 cDNA was amplified by polymerase chain reaction (PCR) using pEF6/V5-His/OATP1A2 plasmid as the template and then ligated into the pcDNA3.1(+) vector (Invitrogen). The PCR primer pairs (5′-CTAGCTAGCACCATGGGAGAAACTGAG-3′ and 5′-CCGCTCGAGCGGTTACAATTTAGTTTTC-3′) were designed to amplify the SLCO1A2 ORF with the stop codon. Subsequently, both pcDNA3.1(+)/OATP1A2 and pEF6/V5-His/OATP1A2 constructs were double-digested with BsrG 1 and Pme I. Finally, the pcDNA3.1(+)/OATP1A2-V5 (WT) plasmid was constructed by inserting cDNA fragments [BsrG 1 site (449) of OATP1A2 cDNA sequences to Pme 1 site of pEF6/V5-His-TOPO vector containing V5] obtained from pEF6/V5-His/OATP1A2 plasmids into cDNA sequences of pcDNA3.1(+)/OATP1A2 deleted from BsrG1 site (449) of OATP1A2 cDNA to Pme 1 site of pcDNA3.1(+)vector by double-digestion. Herein, we describe OATP1A2-V5 as OATP1A2. All plasmids were verified by sequencing in the DNA Sequencing Facility (VANTAGE) at Vanderbilt University Medical Center.
Measurement of Doxorubicin Transport Kinetics
Doxorubicin transport kinetics were evaluated in HeLa cells transiently expressing human OATP1A2 and murine Oatp1a4 transporters. To measure transport kinetics, radiolabeled doxorubicin uptake during the linear phase (first 3 minutes) was assessed in the presence of various concentrations (0.1–100 µM) of unlabeled compound. Transporter-dependent uptake was determined in parallel experiments as the difference in drug uptake between transporter and parental plasmid DNA-transfected HeLa cells. Michaelis-Menten-type nonlinear curve fitting was carried out to estimate the maximal uptake rate (Vmax) and concentration at which half the maximal uptake occurs (Km) for OATP1A2 and Oatp1a4 (PrismTM, GraphPad, San Diego, CA). All experiments were carried out in duplicate on at least 2–3 experimental days.
Generation of Stably Transfected MDCKII-OATP1A2 and MDCKII-OATP1A2/MDR1 Cell Lines
MDCKII cells stably expressing single (OATP1B1, OATP1B3 and MDR1) and double (OATP1B1/MDR1 and OATP1B3/MDR1) transporters, including control cells (MDCKII-Co), were generated and characterized previously (Lee et al., 2015). For this study, single (MDCKII-OATP1A2)- and double (MDCKII–OATP1A2/MDR1)-transfected cells were also generated using the same methods as conducted previously (Lee et al., 2015). Briefly, both parent MDCKII cells and MDCKII-MDR1 cells were transfected or retransfected with the plasmids pcDNA3.1(+)/OATP1A2 using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Between 32 and 48 hours after transfection, the cells were split and divided into 10-cm culture dishes with fresh media containing G-418 sulfate (800 µg/ml; Mediatech, Manassas, VA). After additional selection with G-418 sulfate, screening for single colonies of transfectants was conducted by immunoblot analysis using anti-V5 antibody (Invitrogen) to identify cell clones with the OATP1A2 protein expression. All expression values were normalized to the protein β-actin. Consequently, cell clones with the highest protein expression comparable with the expression of the control cell lines were chosen and used for the following transport studies.
Crude Cell Membrane Fractions and Immunoblot Analysis
Protein expressions from MDCKII-OATP1A2 and -OATP1A2/MDR1 cells, including control cells, were examined by immunoblot analysis of plasma membrane enriched preparations. Crude cell membrane fractions were conducted as described previously with minor modification (Kim et al., 1998; Lee et al., 2015). Briefly, cells were scraped, collected, and homogenized in 5 mM Hepes (pH 7.2) cell lysis buffer containing 1× protease inhibitor cocktails (Roche). The lysates were centrifuged at 800 g for 10 minutes to remove cell debris, and the supernatant was then centrifuged at 30,000 g at 4°C for 30 minutes to obtain membrane pellets. The membrane pellets were dissolved in the same lysis buffer and quantified by the Bicinchoninic Acid Assay Protein Assay Kit (Thermo Scientific, Grand Island, NY). Each 20 µg of extracted membrane proteins was subjected to 10% SDS polyacrylamide gels and transferred to nitrocellulose membranes (PerkinElmer, Boston, MA). After preincubation in PBS containing 0.05% Tween 20 and 5% nonfat dry milk, the blot was incubated with the monoclonal mouse anti-V5 antibody (1:2,000 dilution, Invitrogen) overnight. The blot was then washed with PBS containing 0.05% Tween 20 for 15 minutes (3 × 5 minutes) and then incubated for 1 hour with the secondary antibody, an anti-mouse IgG conjugated with horseradish peroxidase (1:10,000 dilution, Promega, Madison, WI). The same blot was stripped and in turn reprobed with different antibodies, such as the monoclonal mouse anti-MDR1 antibody (1:1,000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA) and anti-Na+/K+-ATPase α antibody (1:5,000 dilution, Santa Cruz Biotechnology), overnight at 4°C, washed, followed by the same secondary antibody as above. The anti-Na+/K+-ATPase α antibody was used as a marker of cell membrane proteins. Finally, the protein bands on the blots were detected using the Western lightning plus enhanced chemiluminescence (PerkinElmer).
Immunofluorescence Confocal Microscopy
To confirm the membrane localization of OATP1A2 and MDR1 in MDCKII-MDR1 cells transfected stably with pcDNA 3.1 (+)/OATP1A2 cDNA constructs, the MDCKII cell monolayers were fixed in 4% paraformaldehyde on transwell filter membranes for 15 minutes at room temperature. The fixed monolayers were subsequently incubated in 0.2% Triton X-100 for 2 minutes at room temperature, washed three times with PBS, followed by incubation with 5% bovine serum albumin (BSA) in PBS for 1 hour at room temperature to block nonspecific antibody binding. The monolayers were then washed three times with PBS and incubated overnight at 4°C in a 1:200 dilution (in 1% BSA in PBS) of polyclonal rabbit anti-OATP1A2 antibody (Santa Cruz Biotechnology) and/or a 1:500 dilution of monoclonal mouse anti-MDR1 antibody (Santa Cruz Biotechnology). After incubation, the monolayers were washed with PBS containing 0.05% Tween 20 for 15 minutes (3 × 5 minutes) and then incubated for 1 hour at room temperature in 1:1000 dilutions (in 1% BSA in PBS) of secondary antibodies, Alexa Fluor 488-conjugated anti-rabbit IgG and/or Alexa Fluor 546-conjugated anti-mouse IgG (Invitrogen). Subsequently, nuclei were stained with 5,000-fold diluted DAPI (4',6-diamidino-2-phenylindole) for 5 minutes, and cells were then washed three times with PBS (3 × 5 minutes). Finally, the MDCK monolayers were transferred and mounted on glass slides with ProLong Gold antifade reagent (Invitrogen) and imaged by Olympus FV-1000 inverted confocal microscope (Olympus, Center Valley, PA) with 60× oil immersed lens (Plan Apo VC N.A. 1.40, Olympus). Image analysis and processing were performed with FV10-ASW 1.7 (Olympus) and Adobe Photoshop software (Adobe, San Jose, CA). Imaging experiments and data analyses were performed in part through the use of the Vanderbilt University Medical Center Cell Imaging Shared Resource.
Transport Assays
Recombinant Vaccinia-Based Uptake Transport Assay.
Doxorubicin transport assays using a recombinant vaccinia-based method for recombinant transporter expression were conducted as described previously (Lee et al., 2005). Briefly, HeLa cells grown in 12-well plates (0.8 × 106 cells/well) were infected with vaccinia (vtf-7) at a multiplicity of infection of 10 plaque-forming units/cell in Opti-MEM I medium (reduced serum, Invitrogen) and allowed to adsorb for 30 minutes at 37°C. Cells in each well were then transfected with 1 µg transporter cDNA (wild type or variants) packaged in pEF6/V5-His-TOPO vector (Invitrogen), along with Lipofectin (Invitrogen) and incubated at 37°C for 16 hours. The parental plasmid lacking any insert was used as control. Uptake (radioactivity) of doxorubicin was measured after an incubation of 10 minutes. Total radioactivity was determined after the addition of cell lysates to vials containing 5 ml of Biodegradable Scintillation Cocktail (GE Healthcare, Pittsburgh, PA). Retained cellular radioactivity was quantified by liquid scintillation counter (PerkinElmer, Shelton, CT). Transport activities were expressed in the percentage compared with the vector control. All experiments were carried out in duplicate on at least 2 to 3 experimental days. In each set of experiments, taurocholate uptake into cells transfected to express sodium/taurocholate cotransporting polypeptide was included as a positive control for transfection and expression efficiency. In each set of experiments, doxorubicin uptake into the cells transfected with the vector only was included as a negative control to ensure that results were not confounded by an effect of the transfection process.
Transwell-Based Vectorial Transport Assay.
Vectorial transport assays of doxorubicin were conducted as described previously (Lee et al., 2015). MDCKII cells were seeded onto 12-Transwell (diameter 12 mm; pore size 0.4 mm; Corning Incorporated, Kennebunk, ME) at an initial density of 0.4 × 106 cells per well and grown for total 4 days. At 24 hours before the transport study is conducted the cell culture medium was replaced with culture medium supplemented with 10 mM sodium butyrate to induce transporter expression (Chen et al., 1997). After washing cells with prewarmed uptake buffer Opti-MEM 1 medium (reduced serum, Invitrogen), 0.8 ml of uptake buffer was added to the basal (for OATP1A2 cells) and apical (for OATP1B cells) compartments of the cell monolayers, respectively. Sequentially, the same amount of uptake buffer containing 1.0 and 0.2 µM of radiolabeled doxorubicin was added to the opposite sides, the apical and basal compartments, of the OATP1A2- and OATP1B-expressing cell monolayers, respectively. Consequently, as a control, the experiments were repeated in the opposite directions as well. Cells were then incubated at 37°C for given time points, 0.5, 1, 2, and 3 hours. At each time point, aliquots (50 µl) were taken from both the apical and basal compartments. The aliquots obtained from given time points were added to vials containing 5 ml of biodegradable scintillation cocktail (Amersham Biosciences), and the radioactivity of doxorubicin was then measured by liquid scintillation counter (PerkinElmer). After taking final media post-3 hour incubation, the incubation buffer was removed and uptake was terminated by adding ice-cold PBS buffer and washed twice with PBS buffer. Cells were then lysed with 0.2% SDS solution containing 1× protease inhibitor cocktail (Roche, Indianapolis, IN), followed by protein assay with bicinchoninic acid assay kit (Thermo Fisher Scientific). Meanwhile, to measure intracellular doxorubicin, cells in transwells were incubated as described above and the proper plates at designated time points were removed from the incubator. Cells were then washed and lysed, followed by determining doxorubicin radioactivity and protein concentration. All experiments were performed a minimum of three times in tetraplicates.
Doxorubicin Distribution in Slco1a/1b―/― Mice
Male Slco1a/1b−/− mice, 8–13 weeks of age and male WT littermate controls were used to determine doxorubicin distribution. Male wild-type (WT) and male Oatp1a/1b cluster knockout mice [(FVB.129P2-Del(Slco1b2-Slco1a5)1Ahs] were purchased from Taconic (Hudson, NY). All mice were housed and handled according to institutional guidelines complying with Vanderbilt Animal Care Program, DAC. All mice were kept in a temperature-controlled environment with a 12-hour reverse dark/light cycle and received a standard diet, Purina Rodent Diet #5LOD or #5LJ5 ad libitum. Radiolabeled doxorubicin (1 mg/kg, sp act 1.2 Ci/mmol) dissolved in 0.9% saline solution was injected intravenously into the tail vein of mice. Blood samples from each mouse were drawn from the saphenous vein at 5, 15, 30, 60, minutes postinjection. After 120 minutes, mice were anesthetized with isofluorane, blood was obtained by cardiac puncture, and organs were harvested, weighed, and homogenized with PBS (1% wt/vol BSA). Total radioactivity was determined after the addition of plasma (10 μl) or tissue homogenates (500 μl) to vials containing 5 ml of biodegradable scintillation cocktail (Amersham Biosciences). Doxorubicin clearance after intravenous injection was calculated as dose/AUC, where AUC is the area under the plasma concentration-time profile from t = 0 to ∞. All animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health. The protocols for the animal experiments were approved by Vanderbilt Animal Care and Use Committee, IACUC.
Data Fitting and Statistical Analysis
Parameters for saturation kinetics (Vmax and Km) were estimated by nonlinear curve fitting using Prism (GraphPad Software, Inc.). Determination of statistical differences between group parameters was determined using Student’s t test, Mann-Whitney U test, one-way analysis of variance, analysis of variance (using Tukey-Kramer multiple comparison test), or Fisher’s exact test, as appropriate. A P value of <0.05 was taken to be the minimum level of statistical significance.
Results
Doxorubicin Uptake Is Mediated by OATP1A/1a Transporters in a HeLa Model Cell System
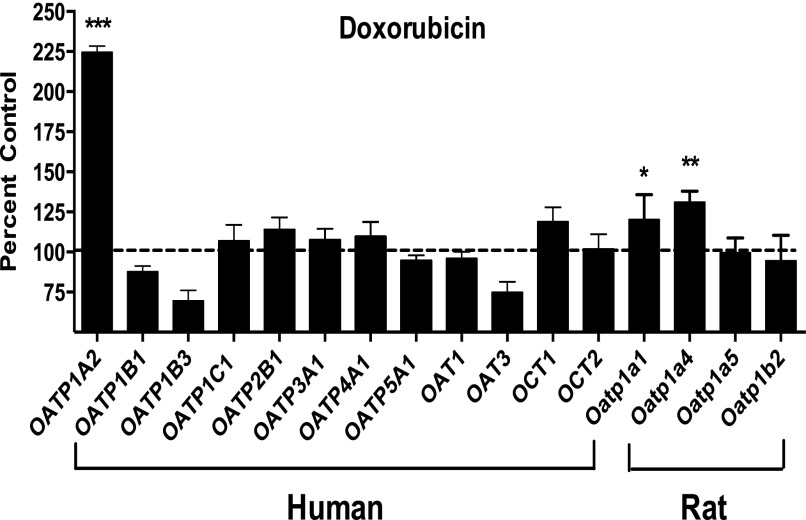
By using a recombinant vaccinia-based method in HeLa cells, a panel of uptake transporters, including multiple human OATPs, was expressed individually and evaluated for doxorubicin transport. After a 10-minute incubation period, we identified OATP1A2 as capable of doxorubicin transport with more than twofold increase in uptake compared with vector control (P < 0.001) (Fig. 1). The rodent orthologs to human OATP1A2, rat Oatp1a1 and Oatp1a4, also showed efficient doxorubicin transport (P < 0.05 for Oatp1a1; P < 0.01 for Oatp1a4). Other human OATPs including OATP1B1, OATP1B3, and OATP2B1, and rat Oatp1b2 showed no significant doxorubicin uptake. Other known drug uptake transporters, such as the organic anion transporters OAT1 and OAT3 and organic cation transporters OCT1 and OCT2, demonstrated no doxorubicin transport.
Fig. 1.
OATP-mediated doxorubicin transport in vitro in HeLa cells. A panel of uptake transporters was assessed for doxorubicin (0.2 µM) transport activity at 10 minutes. Vector control (pEF6) was used as reference. Human OATP1A2, but not OATP1B1 and OATP1B3, was capable of transporting doxorubicin. Rat Oatp1a1 and Oatp1a4 also transport doxorubicin. Data were expressed as percentage of cellular uptake compared with vector control (mean ± S.E., n = 6). *P < 0.05; **P < 0.01; ***P < 0.001.
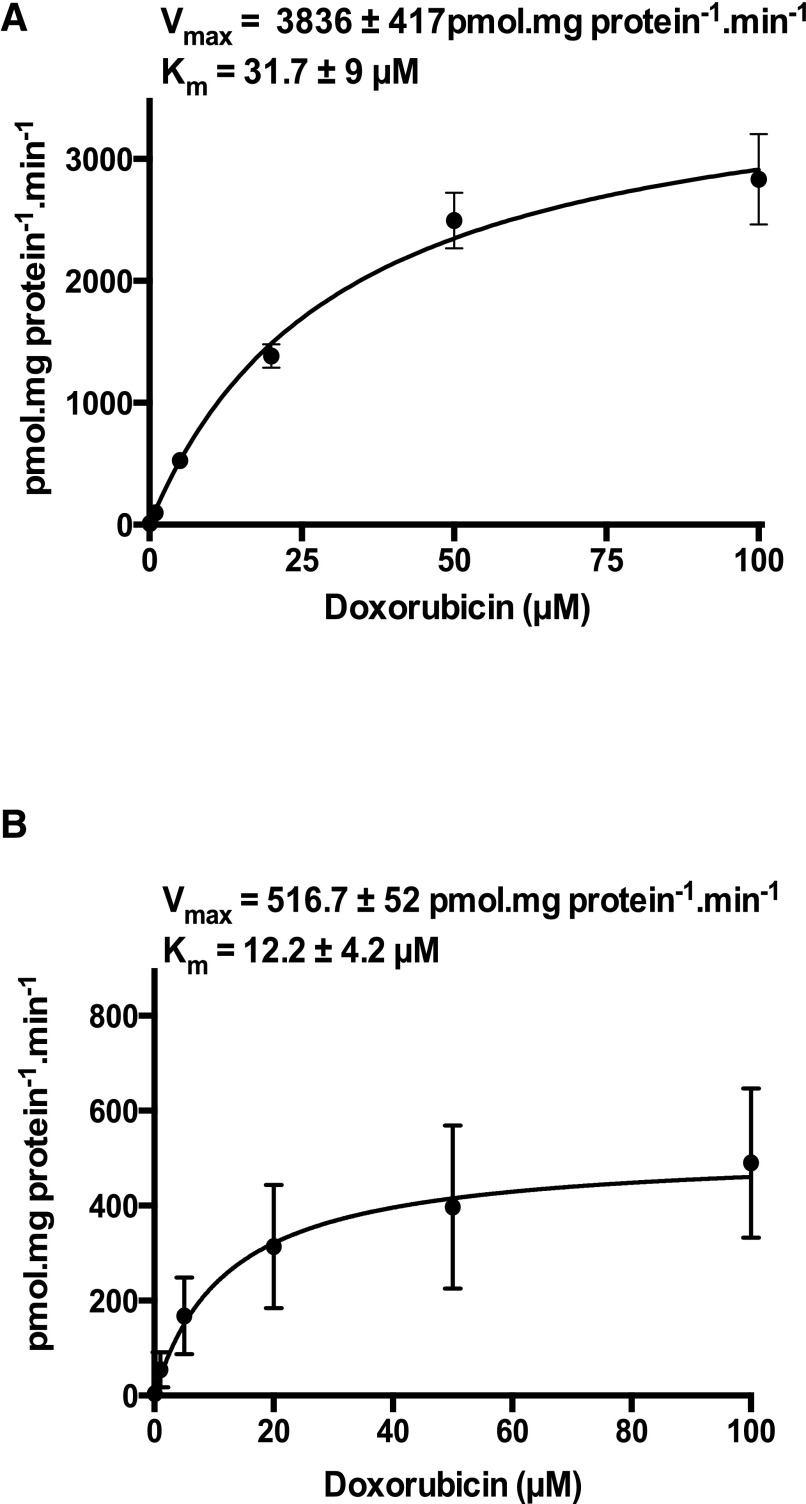
Time course experiments indicated significant accumulation of doxorubicin over control in OATP1A2- and Oatp1a4-expressing HeLa cells up to 30 minutes after incubation (data not shown). To better understand the pharmacokinetic parameters of doxorubicin transport mediated by OATP1A/1a transporters (human OATP1A2 and its closest rodent ortholog rat Oatp1a4), kinetic analysis was performed. Radiolabeled doxorubicin uptake was assessed in the presence of varying concentrations of unlabeled doxorubicin (0.1–100 µM for OATP1A2 and Oatp1a4) for estimation of Km and Vmax. Uptake transport of doxorubicin in HeLa cells transfected with OATP1A2 or Oatp1a4 cDNA constructs was saturable with Km of 31.7 ± 9 and 12.2 ± 4.2 µM, respectively, and maximum velocity Vmax of 3836 ± 417 and 516.7 ± 52 pmol⋅mg protein−1⋅min−1, respectively (Fig. 2, A and B).
Fig. 2.
Doxorubicin transport kinetics. Transport kinetics of OATP1A2 (A) and Oatp1a4 (B) after transient heterologous expression in HeLa cells at varying concentrations of doxorubicin (0.1–100 µM). Parameters for saturation kinetics (Vmax and Km) were estimated by Michaelis-Menten-type nonlinear curve fitting. Data are expressed as mean ± S.E. (n = 8).
OATP1A2 Variants Differentially Transport Doxorubicin in Vitro
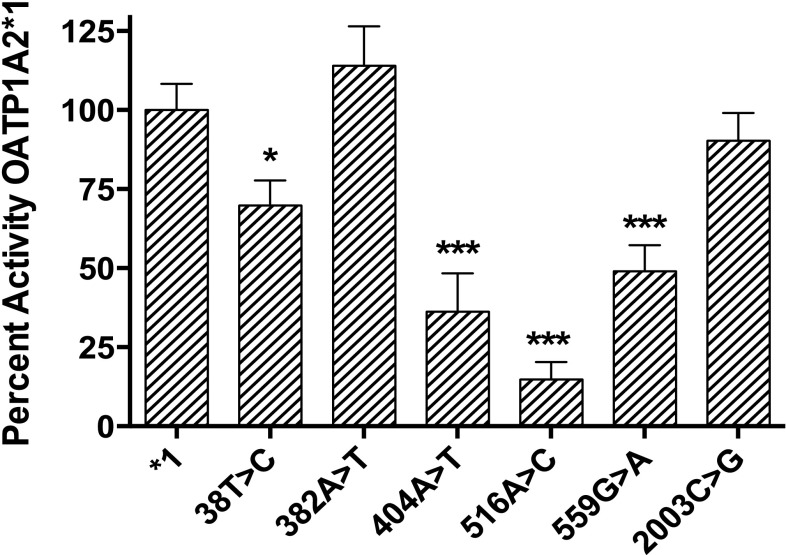
To examine the effect on doxorubicin transport mediated by OATP1A2 polymorphisms, a panel of six known OATP1A2 nonsynonymous variants in addition to wild-type OATP1A2 (*1) was expressed in HeLa cells and evaluated for differential transport of doxorubicin in vitro. Several variants, including 38T>C, 404A>T, 516A>C, and 559G>A, were associated with significantly impaired doxorubicin uptake compared with the WT reference allele, SLCO1A2*1. In contrast, the other two variants, 382A>T and 2003C>G, exhibited no significant difference compared with the WT reference control (1*) (Fig. 3).
Fig. 3.
OATP1A2 variants differentially transport doxorubicin in vitro. Uptake of radiolabeled doxorubicin (0.2 µM) at 10 minutes in HeLa cells transfected with OATP1A2 variants was assessed relative to wild-type OATP1A2*1. Several variants, including 38T>C, 404A>T, 516A>C, and 559G>A, were associated with significantly impaired doxorubicin transport. Data are expressed as percentage of cellular uptake by OATP1A2*1 (mean ± S.E., n = 6). *P < 0.05; ***P < 0.001.
Membrane Localization of OATP1A2 in OATP1A2- and OATP1A2/MDR1-Transfected MDCKII Cells
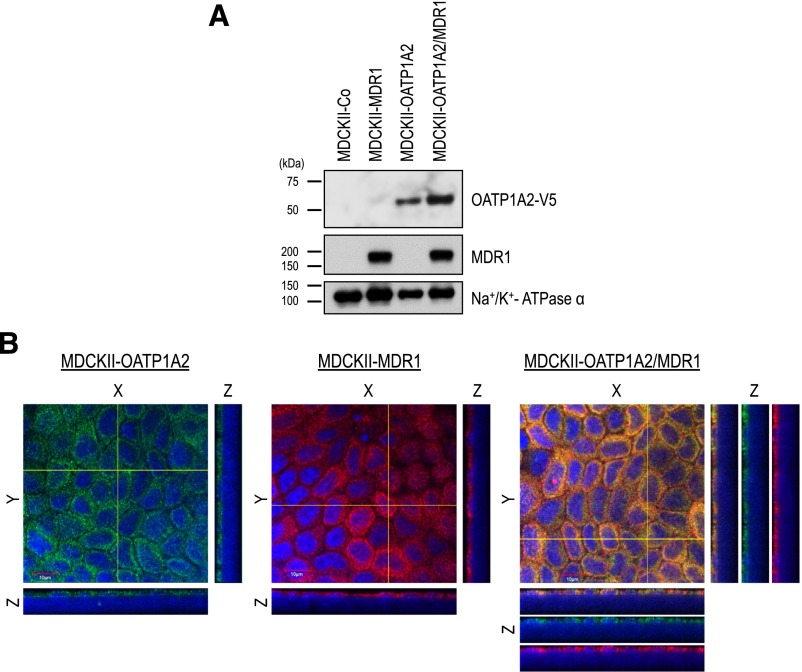
The transcriptional or protein expression of constitutive OATP1A2 as well as hepatic OATP1Bs is absent in MDCKII cells (König et al., 2000a,b; Goh et al., 2002). Hence, we used a MDCKII model cell system to examine the directional drug transport activity mediated by OATP transporters. We generated MDCKII cell lines stably expressing an uptake transporter OATP1A2. The protein expression of OATP1A2 and MDR1 was characterized in OATP1A2-expressing MDCKII-WT and MDCKII-MDR1 cells. The expression of OATP1A2 and MDR1 in single or double transfectants was confirmed by immunoblot analysis (Fig. 4A). OATP1A2 was detected as a single protein band with an unglycosylated form MW ∼58 kDa (Lee et al., 2005) in all lanes of OATP1A2-transfected cells. We were also able to detect human MDR1 with the predicted molecular mass of ∼170 kDa as described previously (Lee et al., 2015), whereas any proteins of interest were not detectable in control cells (MDCKII-Co). The plasma membrane protein marker Na+/K+-ATPase α was detected in all cell lines.
Fig. 4.
Protein expression of OATP1A2 and MDR1 in single (OATP1A2 and MDR1)- and double (OATP1A2/MDR1)-transfected MDCKII cells. Crude membrane proteins were prepared and applied to SDS-PAGE, followed by immunoblot analysis (A). An anti-V5 antibody detected OATP1A2 as a single band with MW ∼58 kDa. MDR1 showed the predicted MW ∼170 kDa. To confirm cell surface expression of OATP1A2 and MDR1, immunofluorescence confocal microscopy (B) was conducted. Membrane localization of human OATP1A2 and MDR1 in single-transfected MDCKII-OATP1A2 and MDCKII-MDR1 cells and double-transfected MDCKII-OATP1A2/MDR1 cells is shown. Cells stably transfected with OATP1A2 cDNA constructs were single or double stained with a rabbit polyclonal anti-human OATP1A2 antibody (green fluorescence) and/or a mouse monoclonal anti-human MDR1 antibody (red fluorescence). Nuclei were stained with DAPI (blue fluorescence). Pictures are single optical sections (X/Y) (center) with X/Z (bottom) and Y/Z (right) projections, respectively. Dotted line indicates the position where Y-Z and X-Z images locate. Scale bar = 10 μm (in X-Y image). 60× objective.
In addition to immunoblot analysis, to confirm cellular membrane localization of OATP1A2 and MDR1 in stably transfected MDCKII cells, we performed immunofluorescence confocal microscopy. As shown in Fig. 4B (left and middle), OATP1A2 and MDR1 were localized on the apical membranes of MDCKII-OATP1A2 and MDCKII-MDR1 cells, respectively. Transporter expression at the apical membranes of cells was reconfirmed by colocalization of human OATP1A2 and MDR1 in double transporter-expressing MDCKII-OATP1A2/MDR1 cells (Fig. 4B, right).
Vectorial Doxorubicin Transport Is Mediated by OATP1A2 and OATP1B Uptake and MDR1 Efflux in a MDCKII Cell Model System
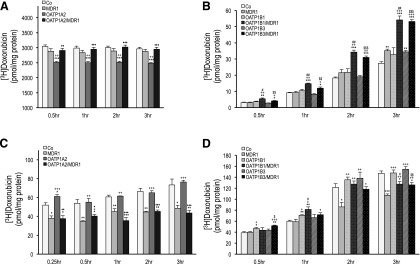
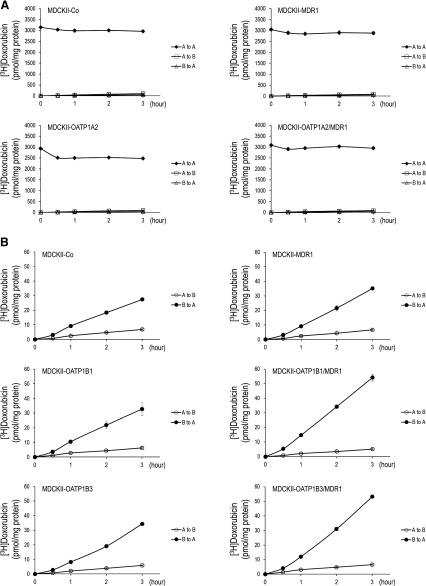
Doxorubicin is a known substrate of the efflux transporter MDR1 (van Asperen et al., 2000). To evaluate directional transport of doxorubicin in vitro, radiolabeled doxorubicin was administered to the apical (for OATP1A2 cells) and basal (for OATP1B cells) compartments of monolayers of control (MDCKII-Co), single (MDCKII-OATP1A2, -OATP1B1, -OATP1B3, and -MDR1)- and double (MDCKII-OATP1A2/MDR1, -OATP1B1/MDR1, and -OATP1B3/MDR1)-transfected cells, respectively. The vectorial transport of doxorubicin in OATP1A2-expressing MDCKII cells is shown in Fig. 5A. Compared with control cells (MDCKII-Co), there was significantly reduced doxorubicin retention in the apical compartment in MDCKII-OATP1A2 cells at all time points assessed (P < 0.001), reflecting OATP1A2-mediated uptake. Moreover, the apical doxorubicin concentration was markedly greater in double (MDCKII-OATP1A2/MDR1)-transfected cells than in single (MDCKII-OATP1A2)-transfected cells (P < 0.01 at 0.5 hour; P < 0.001 at 1–3 hours), reflecting MDR1-mediated efflux. Likewise, for OATP1B-expressing MDCKII cells, the amount of doxorubicin translocated into the apical side, in this case from the basal compartment, was significantly higher in double (MDCKII-OATP1B1/MDR1 and -OATP1B3/MDR1)-transfected cells than in single (MDCKII-OATP1B1 and -OATP1B3)-transfected cells at all time points assessed (P < 0.05 at 0.5 hour, P < 0.01 at 1–3 hours versus MDCKII-OATP1B1 cells; P < 0.01 at 0.5 and 1 hour, P < 0.001 at 2 and 3 hours versus MDCKII-OATP1B3 cells, Fig. 5B) as well as compared with MDCKII-MDR1 and MDCKII-Co cells, reflecting OATP1B-mediated uptake and MDR1-mediated efflux. Collectively, these results reflect active doxorubicin uptake by OATP1A2, OATP1B1, and OATP1B3 and active doxorubicin efflux by MDR1. As expected, corresponding experiments evaluating doxorubicin transepithelial transport from apical to basal and basal to apical for OATP1A2 (Fig. 6A) and apical to basal for OATP1B1 and OATP1B3 (Fig. 6B) demonstrated no significant differences in transport among control, single or double-transfected cell lines.
Fig. 5.
Vectorial transcellular transport and intracellular accumulation of doxorubicin. Radiolabeled doxorubicin (1.0 µM for OATP1A2; 0.2 µM for OATP1Bs) was administered to the apical (for OATP1A2) and basal (for OATP1Bs) compartment of monolayers of MDCKII-control (Co), single (MDR1, OATP1A2, OATP1B1, and OATP1B3)- and double (OATP1A2/MDR1, OATP1B1/MDR1, and OATP1B3/MDR1)-transfected cell lines. After incubation at given time points, translocation of doxorubicin into the apical compartment (A and B) and intracellular doxorubicin accumulation (C and D) are shown. Apical doxorubicin was significantly lower in MDCKII-OATP1A2 cells compared with control at all time points (A). Apical doxorubicin was significantly higher in double-transfected MDCKII-OATP1B1/MDR1 and MDCKII-OATP1B3/MDR1 cells compared with control at all time points (B). Intracellular doxorubicin was significantly higher in MDCKII-OATP1A2 (C) and MDCKII-OATP1B1 (D) cells at early time points. Data are expressed as mean ± S.E. (n = 4 for both studies). *P < 0.05, **P < 0.01, and ***P < 0.001 versus MDCKII-Co; +P < 0.05, ++P < 0.01, and +++P < 0.001 versus MDCKII-MDR1; ♦P < 0.05, ♦♦P < 0.01, and ♦♦♦P < 0.001 versus MDCKII-OATP1A2; #P < 0.05 and ##P < 0.01 versus MDCKII-OATP1B1; $P < 0.05, $$P < 0.01, and $$$P < 0.001 versus MDCKII-OATP1B3 cells.
Fig. 6.
Unidirectional transport of doxorubicin. Radioactive doxorubicin (A, 1.0 µM for OATP1A2; B, 0.2 µM for OATP1Bs) was administered to the apical or basal compartments of monolayers of transporter-expressing MDCKII cells in transwells. The opposite compartments were added with media without radioactive doxorubicin. Cells were then incubated and equal amount (50 µl) of media were taken out from both compartments at given time points, followed by measurement for doxorubicin radioactivity. There were no significant differences in doxorubicin transport among control, single-, or double-transfected cell lines for OATP1A2 (A to B, B to A) or OATP1B1/1B3 (A to B). Data are expressed as mean ± S.E. (n = 4). A to A, apical to apical; A to B, apical to basal; B to A, basal to apical.
To examine intracellular doxorubicin, radiolabeled doxorubicin was administrated to the apical (for OATP1A2 cells) and basal (for OATP1B cells) compartments of cell monolayers in transwells as described above. Cells were analyzed for intracellular accumulation of radiolabeled doxorubicin at given time points. As shown in Fig. 5C, there was significantly more intracellular doxorubicin accumulation in MDCKII-OATP1A2 cells compared with MDCKII-Co cells at 0.25 hour only with no significant difference at all subsequent time points. Intracellular doxorubicin concentration was markedly reduced in MDCKII-OATP1A2/MDR1 cells compared with MDCKII-OATP1A2 cells at all time points assessed, revealing active doxorubicin efflux by MDR1 at later time points. Meanwhile, in OATP1B-expressing MDCKII cells, the intracellular concentration of doxorubicin revealed active doxorubicin uptake in MDCKII-OATP1B1 compared with MDCKII-Co cells up to 1 hour but not in MDCKII-OATP1B3 cells (Fig. 5D). The intracellular accumulation of doxorubicin was significantly lower in double (MDCKII-OATP1B1/MDR1 and -OATP1B3/MDR1)-transfected cells than in single (MDCKII-OATP1B1 and -OATP1B3)- or MDCKII-Co-transfected cells at 3 hours postadministration of radiolabeled doxorubicin (P < 0.05 for OATP1B1; P < 0.01 for OATP1B3; P < 0.05 for Co).
Slco1a/1b−/− Mice Significantly Alter the Hepatic Disposition of Doxorubicin
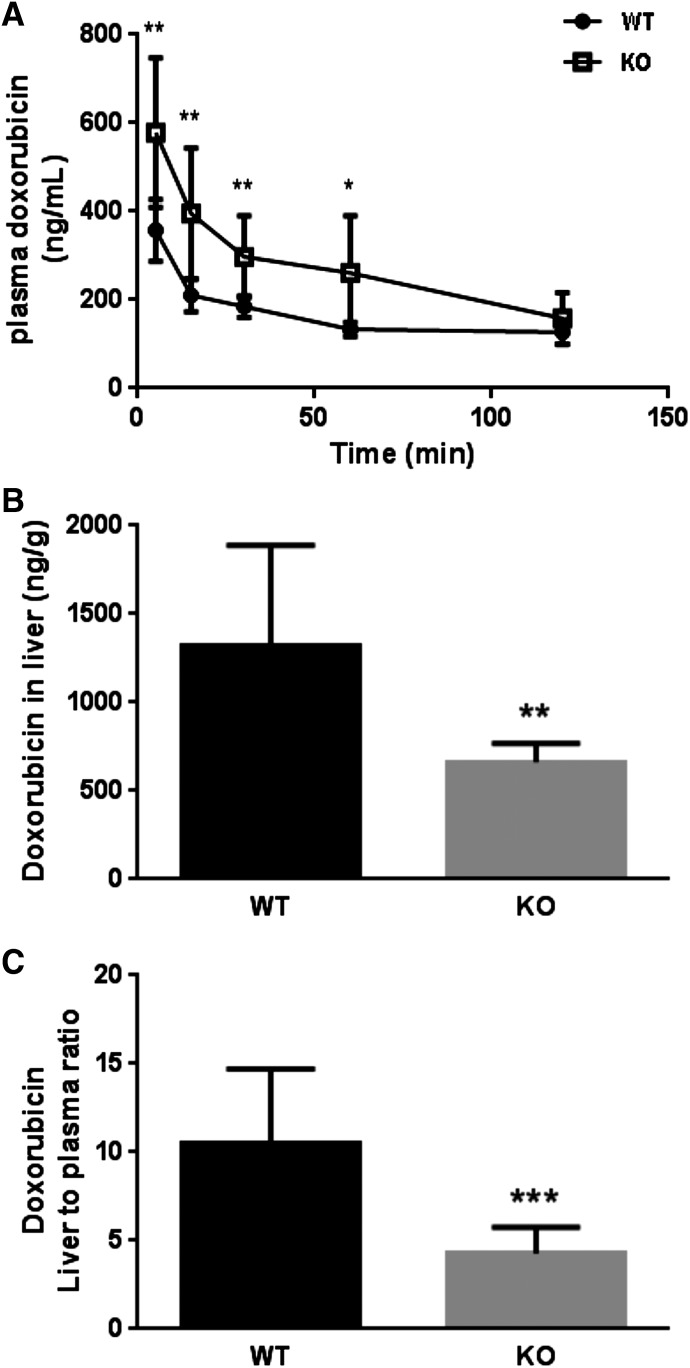
To further evaluate the relevance of OATP1A/1B transporters to doxorubicin disposition, we sequentially examined the in vivo effect of Oatp1a/1b deficiency in mice on doxorubicin disposition up to 120 minutes after intravenous administration of radiolabeled doxorubicin. Male Slco1a/1b−/− mice and male WT littermate controls were used for doxorubicin distribution experiments. Plasma doxorubicin was significantly increased in the Oatp1a/1b−/−mice compared with the WT mice up to 60 minutes (Fig. 7A), leading to a 2.0-fold higher plasma concentration (133.52 versus 260.72 ng/ml, P < 0.05). At early time points 5, 15, and 30 minutes after administration, plasma doxorubicin levels were significantly increased by 1.6 (P < 0.01)-, 1.9 (P < 0.01)-, and 1.6-fold (P < 0.01) in Oatp1a/1b−/− mice compared with WT. The total plasma AUC in Slco1a/1b−/− mice was approximately 1.7-fold greater than in WT mice (mean doxorubicin plasma AUC ± S.D.: WT, 18,399 ± 587.9 ng·min/ml; Oatp1a/1b−/−, 31,779 ± 5006 ng·min/ml, P < 0.01). Whereas plasma doxorubicin AUC was significantly higher in Slco1a/1b−/− versus wild-type mice, liver doxorubicin concentrations in Slco1a/1b−/− mice were significantly decreased compared with WT mice (P < 0.01) (Fig. 7B). Accordingly, the liver-to-plasma ratio of doxorubicin was 2.3-fold lower in Slco1a/1b−/− mice compared with WT mice (P < 0.001) (Fig. 7C), suggesting hepatic OATP1B transporters are involved in hepatic doxorubicin uptake. The plasma clearance of doxorubicin in Slco1a/1b−/− mice was 40% lower than WT mice (mean plasma clearance ± S.D.: WT mice, 96.47 ± 24.52 ml/h; Slco1a/1b−/− mice, 58.28 ± 20.97 ml/h; P < 0.05), indicating impaired hepatic uptake of doxorubicin in the absence of Oatp1a/1b transporters. Collectively, in vivo drug disposition studies in Slco1a/1b−/− mice suggest important roles for OATP1B transporters in the hepatic clearance of doxorubicin. In addition to liver, we examined doxorubicin disposition in several major organs such as brain and kidney, sites of Oatp1a expression. However, we did not observe any significant differences in doxorubicin concentrations in brain and kidney between Slco1a/1b−/− and WT mice (data not shown).
Fig. 7.
Doxorubicin disposition studies in wild-type and Slco1a/1b−/− mice. Mice were administered a single intravenous dose of doxorubicin (1 mg/kg, sp act 1.2 Ci/mmol). Slco1a/1b−/− mice have significantly higher doxorubicin plasma AUC than wild-type mice (A). Doxorubicin concentration in liver (B) and the liver-to-plasma ratio (C) were significantly lower in Slco1a/1b−/− mice compared with wild-type mice. Data were expressed as mean ± SD (n = 8 for WT mice; n = 6 for KO mice; 8–13 weeks of age). *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
In this study, we conducted a systematic evaluation to define relevant OATP transporters involved in doxorubicin uptake and clearance. We identified multiple OATPs, including human OATP1A2, OATP1B1, and OATP1B3, capable of transporting doxorubicin in vitro. Rat Oatp1a1 and Oatp1a4 were also associated with doxorubicin transport in vitro. Because OATP1B1 and OATP1B3 are expressed primarily at the basolateral membrane of hepatocytes (König et al., 2000a,b; Abe et al., 2001), these results suggest potential important roles for these transporters in the hepatic uptake and clearance of doxorubicin. In mice, absence of Oatp1a/1b transporters led to a substantial increase in the plasma exposure and a corresponding decrease in the hepatic uptake and clearance of doxorubicin. Accordingly, we demonstrate that OATP1A/1B transporters play significant roles in the disposition of doxorubicin.
Our initial studies revealed doxorubicin to be an efficient substrate for human OATP1A2, but not OATP1B1 and OATP1B3, when assessed in detail using a recombinant vaccinia-based method in a HeLa cell system. However, for reasons that are not well-defined, OATP-mediated uptake of certain substrates, including drugs such as doxorubicin and docetaxel, has been noted by other groups to be variably discrepant in results among in vitro systems or when comparing in vitro to in vivo transport (Smith et al., 2005; de Graan et al., 2012; Durmus et al., 2014; Lee et al., 2015). Hence, absence of doxorubicin transport in HeLa cells does not preclude it to be transported in vitro by OATP1B transporters in another system. Thus, another goal of this study was to establish an in vitro cell model system to explore OATP-mediated transcellular transport of substrate drugs and the interplay with MDR1, an efflux transporter that is known to transport doxorubicin. In this manner, establishing directional doxorubicin transport evaluating the interplay between uptake and efflux may better recapitulate drug transport that occurs in vivo. Recently, we generated and characterized MDCKII cells stably expressing OATP1B and/or MDR1 transporters (Lee et al., 2015). Similarly, we created a model cell system stably expressing OATP1A2 in MDCKII monolayers and used this to examine the effect of OATP1A2 expression and the interplay with MDR1 on transcellular transport of doxorubicin.
The membrane localization of OATP1A2 and MDR1 expressed transiently in MDCKII cells transduced by BacMam virus was previously confirmed by confocal imaging (Liu et al., 2015). Likewise, in our study, we assessed membrane expression of OATP1A2 and MDR1 in MDCKII-OATP1A2, MDCKII-MDR1, and MDCKII-OATP1A2/MDR1 cell lines by immunofluorescence confocal microscopy as well as immunoblot analysis. OATP1A2 and MDR1 were detected at the surface of MDCKII-OATP1A2 and MDCKII–MDR1 cells, respectively. Also, the surface expression pattern of OATP1A2 in double (MDCKII-OATP1A2/MDR1)-transfected cells was colocalized with MDR1, confirming the apical membrane localization of these proteins, akin to the physiologic expression of these transporters in human organs such as intestine, kidney, and brain. By using transwells, doxorubicin accumulation in the apical compartment was significantly lower in MDCKII-OATP1A2 cells compared with MDCKII-OATP1A2/MDR1 cells, revealing that MDR1-mediated efflux of doxorubicin counteracts OATP1A2-mediated uptake. To the best of our knowledge, this is the first report using polarized MDCKII cells transfected stably by OATP1A2 and MDR1 cDNA constructs for drug transport. Collectively, our data indicate doxorubicin is a good substrate for OATP1A2, which is in agreement with a previous report demonstrating OATP1A2-mediated doxorubicin transport in HEK293 cells (Durmus et al., 2014).
Mice deficient in Oatp1a/1b transporters have been generated and functionally characterized for in vivo transport of endogenous or xenobiotic OATP substrates (van de Steeg et al., 2010; Zaher et al., 2008). Durmus et al. (2014) demonstrated that doxorubicin disposition was affected by mouse and human OATP1A/1B transporters using Slco1a/1b2−/− mice and humanized transgenic mice. Slco1a/1b2−/− mice demonstrated significantly higher plasma concentrations and AUC than wild-type mice. Interestingly, liver-specific expression of OATP1A2 in Slco1a/1b2−/− mice could restore plasma levels of doxorubicin to those of wild-type mice. However, OATP1A2 is not natively expressed in human liver other than cholangiocytes (Lee et al., 2005). We performed similar doxorubicin distribution experiments in Slco1a/1b2−/− and wild-type mice. We did not witness significant differences in doxorubicin concentrations in kidneys or the brains of Slco1a/1b2−/− mice compared with their wild-type counterparts, where OATP1A2 is physiologically expressed. This could be related, in part, because of effects of native Mdr1a/1b-mediated doxorubicin efflux in these tissues counteracting Oatp1a-mediated uptake. We also cannot rule out that doxorubicin may be a substrate for other transporters expressed in these tissues that may modulate tissue-specific uptake and transport.
We and others previously reported that OATP1A2 was overexpressed in breast cancer tissues and cell lines (Miki et al., 2006; Meyer zu Schwabedissen et al., 2008). OATP1A2 mRNA expression was nearly 10-fold greater in breast cancer tissues compared with adjacent normal breast tissue. OATP1A2 is known to transport endogenous substrates such as the estrogen metabolites estrone sulfate and estradiol glucuronide, which in turn may promote cellular proliferation (Nozawa et al., 2004, 2005). Notably, one of the major active chemotherapeutic agents used in breast cancer treatment is doxorubicin (Nagar, 2010). Thus, it is plausible that its efficacy in breast cancer treatment could be related to the fact that doxorubicin is an excellent OATP1A2 substrate and hence would preferentially accumulate in breast cancer cells due to OATP1A2 overexpression. Indeed, a recent study in breast cancer patients indicated those tumors with high expression of OATP1A2 was an independent predictor of good pathologic response to anthracycline- and taxane-based chemotherapy (Hashimoto et al., 2014). This merits further investigation because these data would suggest that OATPs may be an appropriate drug target when overexpressed in tumor tissues as chemotherapeutic agents that are good OATP substrates would preferentially accumulate in tumor cells, which may enhance therapeutic efficacy.
We did not initially identify doxorubicin as a substrate for OATP1B1 or OATP1B3 when transiently overexpressed in HeLa cells. However, it was previously documented that ∼50% of doxorubicin’s disposition is mediated by biliary elimination, suggesting hepatic uptake and clearance play important roles in its disposition (Danesi et al., 2002). We further evaluated the vectorial transport of doxorubicin in transwells using polarized MDCKII cell lines stably transfected with MDR1, OATP1B1, or OATP1B3 alone and double-transfected OATP1B1/MDR1 or OATP1B3/MDR1 transporters. Notably, there was significant higher doxorubicin accumulation in the apical compartments of double-transfected MDCKII-MDR1/OATP1B1 or MDCKII-MDR1/OATP1B3 cells compared with control cells or cells transfected with MDR1, OATP1B1 or OATP1B3 alone, strongly suggesting that OATP1B1 and OATP1B3 mediate hepatic uptake of doxorubicin and MDR1 mediates hepatic efflux. In addition to OATP1A2 overexpression in breast cancer tissues and cell lines as previously noted, OATP1B1 has also been noted to be expressed in cancer tissues, including colon cancer, ovarian cancer, hepatocellular carcinoma, whereas OATP1B3 has been shown to be expressed in gastric cancer, colon cancer, and pancreatic cancers (Thakkar et al., 2015). Of course, MDR1 has long been known to be overexpressed in a number of cancers, leading to the multidrug resistance phenomenon by which many tumors develop chemotherapy resistance (Fojo et al., 1987). It would be of further interest to evaluate the roles and interplay of tumor expressed OATPs and MDR1 to doxorubicin response and/or resistance.
Durmus et al. (2014) were unable to demonstrate OATP1B-mediated doxorubicin transport when either OATP1B1 or OATP1B3 was transiently expressed in HEK293 cells. However, they demonstrated that Slco1a/1b2−/− mice demonstrated significantly higher doxorubicin plasma concentrations with 1.3-fold higher plasma AUC than wild-type mice and 4.1-fold lower liver-to-plasma ratios in Slco1a/1b2−/− mice compared with wild-type mice, suggesting OATP1B transporters play an important role in hepatic doxorubicin uptake and clearance. In further support, transgenic expression of either OATP1B1 or OATP1B3 in Slco1a/1b2−/− mice could partially restore doxorubicin plasma and liver AUC toward that of wild-type of mice. Similarly, we performed doxorubicin distribution experiments in Slco1a/1b2−/− and wild-type mice. Slco1a/1b2−/− mice exhibited up to twofold higher plasma doxorubicin concentration up to 2 hours after administration and 1.7-fold higher AUC than wild-type mice. In addition, liver concentrations were significantly lower in Slco1a/1b2−/− mice with 2.3-fold lower liver-to-plasma ratio and 40% decreased hepatic doxorubicin clearance compared with wild-type mice. OCT6 (SLC22A16), a cation uptake transporter, has also been shown to transport doxorubicin (Okabe et al., 2005) and is expressed in testis, fetal liver, bone marrow, peripheral blood leukocytes, leukemias, and some cancer cell lines, but not in liver or kidney (Gong et al., 2002). Durmus et al. (2014) also confirmed that OCT6 expression, by RT-PCR, was not detected in liver of their Slco1a/1b2−/− mice. Collectively, our results confirm that OATP1B/1b transporters are important to the hepatic uptake and clearance of doxorubicin. Of note, the differential expression of OATPs in tissues of importance to drug disposition may also contribute to doxorubicin-mediated adverse effects. For instance, as OATP1B1 and OATP1B3 are highly expressed in the liver, the recommendation that doxorubicin dose be reduced in patients with hepatic impairment would suggest the important roles these transporters play in the clearance of doxorubicin.
Significant genetic variability in OATP transporters exists and contributes to variation in drug disposition (Gong and Kim, 2013). Population pharmacokinetic studies demonstrate significant interindividual differences in doxorubicin disposition with up to 30% differences in doxorubicin clearance in oncology patients (Kontny et al., 2013). We evaluated the effect of SLCO1A2 polymorphisms on doxorubicin transport in vitro in HeLa cells. Lee et al. (2005) previously identified six nonsynonymous single nucleotide polymorphisms in exonic regions of SLCO1A2 gene from an ethnically diverse population and functionally characterized the associated variant proteins in vitro. When we examined these six polymorphisms for doxorubicin transport, four variants, including 38T>C, 404A>T, 516A>C, and 559G>A, led to significantly decreased uptake of doxorubicin in vitro. The consequences of OATP1A2 variation to drug disposition in vivo have not been determined. For example, imatinib, a Bcr-Abl tyrosine kinase inhibitor, was transported by OATP1A2 in vitro, and this process was sensitive to pH, rosuvastatin, and genetic variants, but in patients with cancer, imatinib absorption was not associated with OATP1A2 variants and was even unaffected by rosuvastatin (Eechoute et al., 2011). Another study indicated that lopinavir was transported by OATP1A2 in Xenopus laevis oocytes, but the association between SLCO1A2 polymorphisms, 38T>C and 516T>G, and plasma concentrations of lopinavir in cancer patients was not observed (Hartkoorn et al., 2010). Therefore, the relevance of OATP1A2 variants to doxorubicin disposition in vivo warrants further investigation.
In conclusion, we describe important roles for OATPs, including OATP1A2, OATP1B1, and OATP1B3, to the disposition of doxorubicin. Through a series of in vitro and in vivo experiments, we demonstrate that hepatic OATP1B transporters play significant roles in the hepatic uptake, clearance, and plasma exposure of doxorubicin. OATP1A2-mediated doxorubicin uptake may have important therapeutic implications for evaluating OATP transporters as drug targets through their overexpression in various cancer tissues. Significantly impaired doxorubicin transport by OATP1A2 polymorphic variants may contribute to the oft-witnessed wide interindividual variability in doxorubicin disposition and response, leading to important toxicological and therapeutic consequences. Accordingly, our findings reveal important new insights into the relevance of OATP1A/1B transporters to the clinical pharmacology of doxorubicin. In addition, we suggest that doxorubicin is transported by multiple OATPs, which may also play important roles on its disposition, pharmacokinetics, and toxicities.
Acknowledgments
The authors kindly acknowledge Wendy Teft for assistance with analysis of pharmacokinetic parameters in the animal studies.
Abbreviations
- AUC
area under the curve
- BSA
bovine serum albumin
- Co
control
- DAPI
4',6-diamidino-2-phenylindole
- KO
knock out
- MDCKII
Madin-Darby canine kidney II cell line
- MDR1
multidrug resistance protein 1
- OATP
organic anion transporting polypeptide
- OATP1A2
organic anion transporting polypeptide 1A2
- OATP1B1
organic anion transporting polypeptide 1B1
- OATP1B3
organic anion transporting polypeptide 1B3
- ORF
open reading frame
- PCR
polymerase chain reaction
- WT
wild type
Authorship Contributions
Participated in research design: Lee, Kim, and Ho.
Conducted experiments: Lee and Leake.
Contributed new reagents or analytic tools: Lee.
Performed data analysis: Lee and Ho.
Wrote or contributed to the writing of the manuscript: Lee, Kim, and Ho.
Footnotes
This work was supported by Hyundai Hope on Wheels, the National Institutes of Health National Institute of General Medical Sciences [Grant R01 GM099924], the National Cancer Institute [Grant CA68485], National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK20593, DK58404, DK59637], and National Eye Institute [Grant EY08126]; Cell Imaging Shared Resource (CISR) at Vanderbilt University Medical Center; and the Canadian Institutes of Health Research [Grants MOP-8975 and DSEN-PREVENT FRN-117588].
References
- Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, Nakagomi R, Adachi H, Fujiwara K, Okabe M, Suzuki T, et al. (2001) LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology 120:1689–1699. [DOI] [PubMed] [Google Scholar]
- Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. (1999) The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res 59:4237–4241. [PubMed] [Google Scholar]
- Arakawa H, Nakanishi T, Yanagihara C, Nishimoto T, Wakayama T, Mizokami A, Namiki M, Kawai K, Tamai I. (2012) Enhanced expression of organic anion transporting polypeptides (OATPs) in androgen receptor-positive prostate cancer cells: possible role of OATP1A2 in adaptive cell growth under androgen-depleted conditions. Biochem Pharmacol 84:1070–1077. [DOI] [PubMed] [Google Scholar]
- Ballestero MR, Monte MJ, Briz O, Jimenez F, Gonzalez-San Martin F, Marin JJ. (2006) Expression of transporters potentially involved in the targeting of cytostatic bile acid derivatives to colon cancer and polyps. Biochem Pharmacol 72:729–738. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Clark JA, Rudnick G, Amara SG. (1991) Vaccinia-T7 RNA polymerase expression system: evaluation for the expression cloning of plasma membrane transporters. Anal Biochem 194:302–308. [DOI] [PubMed] [Google Scholar]
- Chen WY, Bailey EC, McCune SL, Dong JY, Townes TM. (1997) Reactivation of silenced, virally transduced genes by inhibitors of histone deacetylase. Proc Natl Acad Sci USA 94:5798–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Maher J, Chen C, Klaassen CD. (2005) Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps). Drug Metab Dispos 33:1062–1073. [DOI] [PubMed] [Google Scholar]
- Cortés-Funes H, Coronado C. (2007) Role of anthracyclines in the era of targeted therapy. Cardiovasc Toxicol 7:56–60. [DOI] [PubMed] [Google Scholar]
- Danesi R, Fogli S, Gennari A, Conte P, Del Tacca M. (2002) Pharmacokinetic-pharmacodynamic relationships of the anthracycline anticancer drugs. Clin Pharmacokinet 41:431–444. [DOI] [PubMed] [Google Scholar]
- de Graan AJ, Lancaster CS, Obaidat A, Hagenbuch B, Elens L, Friberg LE, de Bruijn P, Hu S, Gibson AA, Bruun GH, et al. (2012) Influence of polymorphic OATP1B-type carriers on the disposition of docetaxel. Clin Cancer Res 18:4433–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmus S, Naik J, Buil L, Wagenaar E, van Tellingen O, Schinkel AH. (2014) In vivo disposition of doxorubicin is affected by mouse Oatp1a/1b and human OATP1A/1B transporters. Int J Cancer 135:1700–1710. [DOI] [PubMed] [Google Scholar]
- Eechoute K, Franke RM, Loos WJ, Scherkenbach LA, Boere I, Verweij J, Gurney H, Kim RB, Tirona RG, Mathijssen RH, et al. (2011) Environmental and genetic factors affecting transport of imatinib by OATP1A2. Clin Pharmacol Ther 89:816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. (1987) Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci USA 84:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. (2000) Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther 294:73–79. [PubMed] [Google Scholar]
- Gewirtz DA. (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57:727–741. [DOI] [PubMed] [Google Scholar]
- Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, et al. (2007) Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther 81:362–370. [DOI] [PubMed] [Google Scholar]
- Goh LB, Spears KJ, Yao D, Ayrton A, Morgan P, Roland Wolf C, Friedberg T. (2002) Endogenous drug transporters in in vitro and in vivo models for the prediction of drug disposition in man. Biochem Pharmacol 64:1569–1578. [DOI] [PubMed] [Google Scholar]
- Gong IY, Kim RB. (2013) Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab Pharmacokinet 28:4–18. [DOI] [PubMed] [Google Scholar]
- Gong S, Lu X, Xu Y, Swiderski CF, Jordan CT, Moscow JA. (2002) Identification of OCT6 as a novel organic cation transporter preferentially expressed in hematopoietic cells and leukemias. Exp Hematol 30:1162–1169. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Gui C. (2008) Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 38:778–801. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2003) The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta 1609:1–18. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2004) Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665. [DOI] [PubMed] [Google Scholar]
- Hartkoorn RC, Kwan WS, Shallcross V, Chaikan A, Liptrott N, Egan D, Sora ES, James CE, Gibbons S, Bray PG, et al. (2010) HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics 20:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Tatsumi S, Takeda R, Naka A, Ogane N, Kameda Y, Kawachi K, Shimizu S, Sakai M, Kamoshida S. (2014) Expression of organic anion-transporting polypeptide 1A2 and organic cation transporter 6 as a predictor of pathologic response to neoadjuvant chemotherapy in triple negative breast cancer. Breast Cancer Res Treat 145:101–111. [DOI] [PubMed] [Google Scholar]
- Ho RH, Leake BF, Roberts RL, Lee W, Kim RB. (2004) Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J Biol Chem 279:7213–7222. [DOI] [PubMed] [Google Scholar]
- Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. (1998) The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest 101:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. (2000a) Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem 275:23161–23168. [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. (2000b) A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol 278:G156–G164. [DOI] [PubMed] [Google Scholar]
- Kontny NE, Würthwein G, Joachim B, Boddy AV, Krischke M, Fuhr U, Thompson PA, Jörger M, Schellens JH, Hempel G. (2013) Population pharmacokinetics of doxorubicin: establishment of a NONMEM model for adults and children older than 3 years. Cancer Chemother Pharmacol 71:749–763. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, Meier PJ. (1995) Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology 109:1274–1282. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. (2001) Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 120:525–533. [DOI] [PubMed] [Google Scholar]
- Lee HH, Leake BF, Teft W, Tirona RG, Kim RB, Ho RH. (2015) Contribution of hepatic organic anion-transporting polypeptides to docetaxel uptake and clearance. Mol Cancer Ther 14:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, Leake BF, Kim RB. (2005) Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem 280:9610–9617. [DOI] [PubMed] [Google Scholar]
- Liedauer R, Svoboda M, Wlcek K, Arrich F, Jä W, Toma C, Thalhammer T. (2009) Different expression patterns of organic anion transporting polypeptides in osteosarcomas, bone metastases and aneurysmal bone cysts. Oncol Rep 22:1485–1492. [DOI] [PubMed] [Google Scholar]
- Liu H, Yu N, Lu S, Ito S, Zhang X, Prasad B, He E, Lu X, Li Y, Wang F, et al. (2015) Solute Carrier Family of the Organic Anion-Transporting Polypeptides 1A2- Madin-Darby Canine Kidney II: A Promising In Vitro System to Understand the Role of Organic Anion-Transporting Polypeptide 1A2 in Blood-Brain Barrier Drug Penetration. Drug Metab Dispos 43:1008–1018. [DOI] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Tirona RG, Yip CS, Ho RH, Kim RB. (2008) Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer Res 68:9338–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y, Suzuki T, Kitada K, Yabuki N, Shibuya R, Moriya T, Ishida T, Ohuchi N, Blumberg B, Sasano H. (2006) Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Res 66:535–542. [DOI] [PubMed] [Google Scholar]
- Nagar S. (2010) Pharmacokinetics of anti-cancer drugs used in breast cancer chemotherapy. Adv Exp Med Biol 678:124–132. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Suzuki M, Takahashi K, Yabuuchi H, Maeda T, Tsuji A, Tamai I. (2004) Involvement of estrone-3-sulfate transporters in proliferation of hormone-dependent breast cancer cells. J Pharmacol Exp Ther 311:1032–1037. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Suzuki M, Yabuuchi H, Irokawa M, Tsuji A, Tamai I. (2005) Suppression of cell proliferation by inhibition of estrone-3-sulfate transporter in estrogen-dependent breast cancer cells. Pharm Res 22:1634–1641. [DOI] [PubMed] [Google Scholar]
- Okabe M, Unno M, Harigae H, Kaku M, Okitsu Y, Sasaki T, Mizoi T, Shiiba K, Takanaga H, Terasaki T, et al. (2005) Characterization of the organic cation transporter SLC22A16: a doxorubicin importer. Biochem Biophys Res Commun 333:754–762. [DOI] [PubMed] [Google Scholar]
- Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. (2005) Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther 4:815–818. [DOI] [PubMed] [Google Scholar]
- Svoboda M, Wlcek K, Taferner B, Hering S, Stieger B, Tong D, Zeillinger R, Thalhammer T, Jäger W. (2011) Expression of organic anion-transporting polypeptides 1B1 and 1B3 in ovarian cancer cells: relevance for paclitaxel transport. Biomed Pharmacother 65:417–426. [DOI] [PubMed] [Google Scholar]
- Thakkar N, Lockhart AC, Lee W. (2015) Role of Organic Anion-Transporting Polypeptides (OATPs) in cancer therapy. AAPS J 17:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asperen J, van Tellingen O, Beijnen JH. (2000) The role of mdr1a P-glycoprotein in the biliary and intestinal secretion of doxorubicin and vinblastine in mice. Drug Metab Dispos 28:264–267. [PubMed] [Google Scholar]
- van de Steeg E, van Esch A, Wagenaar E, van der Kruijssen CM, van Tellingen O, Kenworthy KE, Schinkel AH. (2011) High impact of Oatp1a/1b transporters on in vivo disposition of the hydrophobic anticancer drug paclitaxel. Clin Cancer Res 17:294–301. [DOI] [PubMed] [Google Scholar]
- van de Steeg E, Wagenaar E, van der Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP, Kenworthy KE, Schinkel AH. (2010) Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest 120:2942–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaming ML, Mohrmann K, Wagenaar E, de Waart DR, Elferink RP, Lagas JS, van Tellingen O, Vainchtein LD, Rosing H, Beijnen JH, et al. (2006) Carcinogen and anticancer drug transport by Mrp2 in vivo: studies using Mrp2 (Abcc2) knockout mice. J Pharmacol Exp Ther 318:319–327. [DOI] [PubMed] [Google Scholar]
- Weiss RB. (1992) The anthracyclines: will we ever find a better doxorubicin? Semin Oncol 19:670–686. [PubMed] [Google Scholar]
- Zaher H, Meyer zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, Palandra J, Stock JL, Kim RB, Ware JA. (2008) Targeted disruption of murine organic anion-transporting polypeptide 1b2 (Oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol 74:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]