Abstract
Background:
The medium-to-long-term use of antimuscarinics alone or in combination with an α-blocker in men with an enlarged prostate is still controversial. This double-blind, placebo-controlled, randomized clinical trial aimed to investigate the efficacy and safety of medium-to-long-term use of tolterodine extended release (ER) with or without tamsulosin in patients with benign prostate hyperplasia (BPH) and larger prostate size.
Methods:
Totally, 152 patients (age ≥50 years) with BPH, International Prostate Symptom Score (IPSS) ≥12, quality-of-life (QoL) score ≥3, and total prostate volume ≥25 ml were enrolled in this study. The patients were randomized into four groups (n = 38 in each) to receive tolterodine ER placebo plus tamsulosin placebo, 0.2 mg tamsulosin plus tolterodine ER placebo, 4 mg tolterodine ER plus tamsulosin placebo, or tolterodine ER plus tamsulosin once daily for 24 weeks. IPSS (total, storage, and voiding subscales), QoL, maximum urinary flow rate (Qmax), and postvoid residual volume (PVR) were collected at baseline, and at weeks 4, 12, and 24.
Results:
Compared with placebo, tolterodine ER plus tamsulosin significantly improved total IPSS (−7.15, −12.20, and −14.66 vs. −3.51, −5.78, and −7.23), storage IPSS (−3.56, −5.63, and −6.66 vs. −1.52, −1.21, and −2.43), voiding IPSS (−2.88, −5.10, and −6.48 vs. −1.52, −3.03, and −2.97), QoL (−1.21, −2.40, and −3.21 vs. −0.39, −1.41, and −1.60), Qmax (2.21, 7.97, and 9.72 ml/s vs. 2.15, 2.44, and 2.73 ml/s), and PVR (−17.88, −26.97, and −27.89 ml vs. −12.03, −11.16, and −16.73 ml) at weeks 4, 12, and 24, respectively; the differences were all statistically significant (P < 0.05). Adverse events (AEs) were not increased with treatment progression. Tolterodine ER alone did not improve total IPSS (−4.61, −6.79, and −5.70), voiding IPSS (−0.64, −1.83, and −1.45), QoL (−0.69, −1.21, and −1.41), or Qmax (−0.79, 2.83, and 1.11 ml/s), compared with placebo (all P > 0.05). However, a gradual increase in PVR (10.03, 10.41, and 12.89 ml) and more urinary AEs suggestive of urinary retention (11/38 vs. 4/38) were observed.
Conclusion:
Medium-to-long-term use of tolterodine ER plus tamsulosin should be recommended in patients with BPH and an enlarged prostate volume.
Trial Registration:
www.chictr.org.cn, ChiCTR-TRC-09000596; http://www.chictr.org.cn/showproj.aspx?proj=8939.
Keywords: Organ Size, Prostatic Hyperplasia, Therapeutics, Tolterodine Tartrate
Introduction
Benign prostate hyperplasia (BPH) is one of the most prevalent diseases in older men.[1] The enlarged prostate blocks the bladder outflow orifice, leading to a series of lower urinary tract symptoms (LUTS) including voiding, storage, and postmicturition.[2,3] Medical therapy is predominantly used as the first-line treatment in BPH cases. In particular, the most commonly used drugs are α-blockers and 5-α-reductase inhibitors; the former can decrease prostate smooth muscle tone and the latter can shrink the prostate.[2] However, the two drugs, used alone or in combination, are not effective in relieving storage symptoms in most patients with BPH. Storage symptoms are commonly attributed to detrusor overactivity, which may occur either secondary to or independent from BPH.[4,5] Therefore, antimuscarinic therapy may be warranted for patients with BPH whose storage symptoms cannot be effectively treated by these two typical drugs.[6]
Traditionally, using antimuscarinics in elder men with BPH, especially in patients with larger prostate volume, has been avoided because of concerns about aggravating dysuria, increasing postvoid residual volume (PVR), and precipitating acute urinary retention (AUR).[7] However, several recent clinical studies have demonstrated the efficacy and safety of antimuscarinics alone or in combination with an α-blocker in patients with BPH.[6,8] Most of these trials, such as the TIMES and ADAM studies, however, are short-term investigations lasting approximately 12 weeks.[9,10] Because BPH is a chronic disease requiring medium-to-long-term drug therapy, it is clinically more relevant and important to investigate the efficacy and safety of medium-to-long-term use of antimuscarinics alone or in combination with an α-blocker.
The NEPTUNE II study demonstrated a good tolerance and efficacy of long-term treatment with solifenacin plus tamsulosin in men with storage and voiding symptoms, with a low incidence of AUR.[11] However, the NEPTUNE II study was open-labeled with no control arm, and the patients enrolled had a <75 ml prostate volume. Therefore, the efficacy and safety of the long-term use of antimuscarinics alone or in combination with an α-blocker in men with an enlarged prostate is still unclear.
Methods
Ethics approval
This double-blind, placebo-controlled, randomized study (www.chictr.org.cn, ChiCTR-TRC-09000596) was approved by our Local Ethical Committee (No. 2008717). This study was conducted from December 17, 2009 to March 30, 2015 (patient recruitment from July 1, 2010 to September 30, 2014), at Peking University Wu Jieping Urology Medical Center and Beijing Shijitan Hospital in China. All participants provided informed consent to participate in the study.
Patient selection
Participants eligible for the study inclusion were Chinese men aged ≥50 years, with an International Prostate Symptom Score (IPSS) ≥12, quality-of-life (QoL) score ≥3, and total prostate volume (TPV) ≥25 ml. The cutoff point for TPV was selected because Asians have a smaller prostate relative to Europeans. Patients were excluded from the study if they had neurologic bladder dysfunction, bladder outlet obstruction from causes other than BPH, PVR ≥200 ml, maximum urinary flow rate (Qmax) ≤5 ml/s, significant hepatic or renal disease, history of postural hypotension and glaucoma, history of AUR requiring catheterization, use of an indwelling catheter or self-catheterization program, history of prostatic surgery or other invasive therapy, history of prostate cancer, or serum total prostate-specific antigen (PSA) level ≥10 ng/ml. Other exclusion criteria included treatments with an α-receptor antagonist within 2 weeks of screening, antimuscarinics, antispasmodics, or saw palmetto within 1 month, a 5α-reductase inhibitor within 3 months of screening, and any condition contraindicated for antimuscarinic and α-receptor antagonist use.
Study design
At the initial evaluation, BPH symptoms were observed through IPSS and QoL scores. In addition, Qmax was assessed using uroflowmetry, TPV using transrectal ultrasound of the prostate, and PVR using ultrasound. Serum PSA examination was also performed. After the initial evaluation, eligible participants were randomized (1:1:1:1 randomization ratio, using a random number table) to receive tolterodine extended release (ER) placebo plus tamsulosin placebo, 0.2 mg tamsulosin plus tolterodine ER placebo, 4 mg tolterodine ER plus tamsulosin placebo, or tolterodine ER plus tamsulosin once daily for 24 weeks. Placebo had the same pack, color, and odor as the corresponding drug. Patients were instructed to take the medication once daily at bedtime. At the second (week 4), third (week 12), and final (week 24) visits, all patients completed the IPSS (total, storage, and voiding subscales), QoL, Qmax, and PVR. Adverse events (AEs) were classified by investigators according to their severity and relationship to the study. The corresponding treatments were given at every visit. The patient disposition in the study is briefly summarized in Figure 1. Data were collected by one special investigator and analyzed by one appointed statistician.
Figure 1.
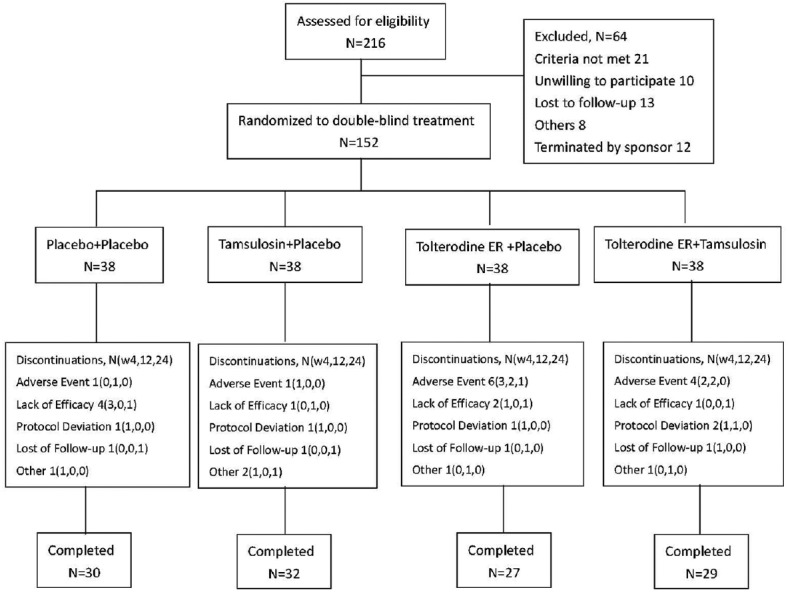
Disposition of patients with benign prostate hyperplasia and an enlarged prostate size assigned to the study treatment. ER: Extended release; N: Number; W: Weeks.
Efficacy assessment
The primary efficacy outcomes were the dynamic improvement of the eligible BPH patients’ IPSS (total index, storage subscale, and voiding subscale) and QoL scores during the 24-week combined treatment of tolterodine ER and tamsulosin. The other efficacy outcomes were the improvement of the total IPSS, storage IPSS, voiding IPSS, and QoL with tolterodine ER alone and tamsulosin alone.
Safety assessment
The dynamic changes of Qmax and PVR during the study period were measured and evaluated at every visit, and other AEs associated with drugs were also evaluated by investigators.
Statistical analysis
Normal distribution data were shown as mean ± standard deviation (SD), and skewed distribution data were shown as median (Q1, Q3). One-way analysis of variance (ANOVA) was used to analyze the normal distribution data of the four treatment groups. Kruskal-Wallis test was used for the skewed distribution data, and multiple comparison rank-sum tests were used to analyze the improvement of each clinical characteristic at different visits in the four treatment groups. P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS software, version 19.0 (SPSS Inc., USA) and Excel software (Microsoft Excel 2007).
Similar to the study by Lim et al.,[12] the sample size was determined based on the assumption that a mean difference of 3.5 in the total IPSS (mean of total IPSS of three treatment groups − mean of placebo group) and SD of 4.5 among the groups; 26 patients per group with a statistical power of 80%.
Results
Patient population
A total of 152 patients with BPH and an enlarged prostate volume were randomly assigned to the four treatment groups, of which 118 completed the study [Figure 1]. Baseline demographic and clinical characteristics were similar among the four treatment groups [Table 1].
Table 1.
Baseline demographic and clinical characteristics of patients with BPH and an enlarged prostate volume in the four treatment groups
| Characteristics | Placebo | Tamsulosin | Tolterodine ER | Tolterodine ER + tamsulosin | Statistics | P |
|---|---|---|---|---|---|---|
| Age (years) | 69.5 ± 6.5 | 69.3 ± 7.5 | 68.4 ± 7.4 | 70.8 ± 7.8 | 0.721* | 0.541 |
| Duration of BPH (years) | 8.0 (4.8, 11.0) | 9.0 (6.5, 10.3) | 7.5 (3.0, 11.0) | 6.0 (4.8, 13.5) | 4.326† | 0.228 |
| IPSS-t | 19.1 ± 4.6 | 17.8 ± 5.2 | 17.6 ± 5.1 | 18.8 ± 4.9 | 0.842* | 0.473 |
| IPSS-s | 7.5 ± 2.6 | 7.8 ± 3.5 | 7.3 ± 2.8 | 8.3 ± 2.4 | 0.974* | 0.407 |
| IPSS-v | 8.0 ± 2.7 | 7.7 ± 3.0 | 7.9 ± 2.9 | 7.8 ± 2.3 | 0.051* | 0.985 |
| QoL | 4.3 ± 0.9 | 4.2 ± 0.8 | 4.1 ± 0.9 | 4.3 ± 1.0 | 0.282* | 0.839 |
| Qmax (ml/s) | 12.0 ± 3.3 | 12.1 ± 3.7 | 11.8 ± 3.2 | 11.9 ± 2.9 | 0.091* | 0.965 |
| TPV (ml) | 43.6 ± 18.1 | 42.3 ± 16.6 | 38.8 ± 15.7 | 42.4 ± 13.8 | 1.145* | 0.333 |
| PSA (ng/µl) | 2.4 ± 1.3 | 2.3 ± 1.2 | 2.1 ± 1.2 | 2.5 ± 1.1 | 0.901* | 0.443 |
| PVR (ml) | 14.5 (10.8, 40.0) | 35.0 (20.0, 52.5) | 20.0 (10.0, 35.0) | 20.0 (12.8, 35.0) | 6.616† | 0.085 |
Data were shown as mean ± SD or median (Q1, Q3). *One-way analysis of variance; †Kruskal-Wallis test. ER: Extended release; IPSS-t: Total International Prostate Symptom Score; IPSS-s: Storage International Prostate Symptom Score; IPSS-v: Voiding International Prostate Symptom Score; QoL: IPSS quality of life; TPV: Total prostate volume; PVR: Postvoid residual volume; BPH: Benign prostate hyperplasia; SD: Standard deviation; PSA: Prostate-specific antigen.
International Prostate Symptom Score
Total International Prostate Symptom Score
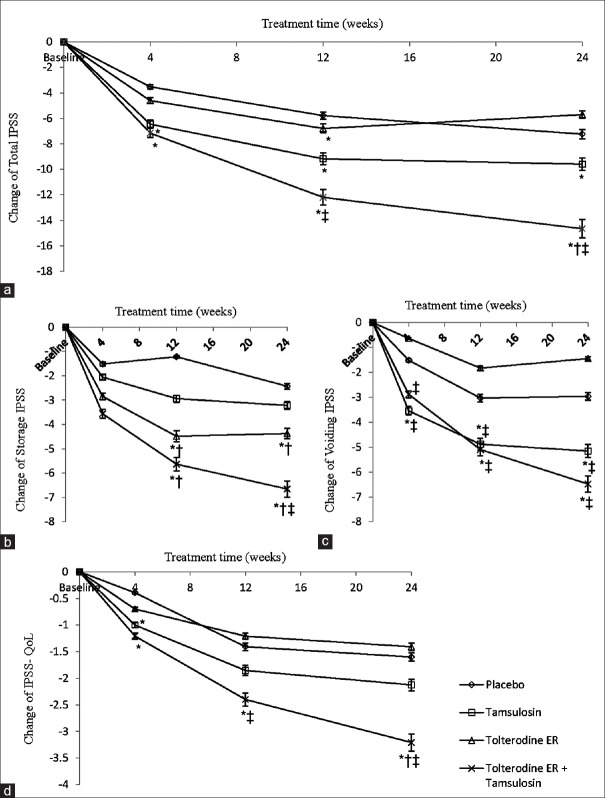
The mean total IPSS improved from baseline in all the four treatment groups with treatment progression [Figure 2a]. In placebo group, the reduction of mean total IPSS from baseline at weeks 4, 12, and 24 was −3.51, −5.78, and −7.23, respectively; in tamsulosin group, the reduction was −6.45, −9.17, and −9.59, respectively; tolterodine ER group was −4.61, −6.79, and −5.70, respectively; and tolterodine ER plus tamsulosin group was −7.15, −12.20, and −14.66, respectively. Compared with the placebo group, the reduction in total IPSS was significantly greater in the tamsulosin group at weeks 4 (P = 0.002), 12 (P < 0.001), and 24 (P = 0.014); tolterodine ER group at week 12 (P = 0.028, difference score, 1.1); tolterodine ER plus tamsulosin group at weeks 4 (P = 0.001), 12 (P < 0.001), and 24 (P < 0.001). Greater improvements in the total IPSS were observed in the tolterodine ER plus tamsulosin group versus the tolterodine group at weeks 12 (P = 0.025) and 24 (P < 0.001) versus tamsulosin group at week 24 (P < 0.001).
Figure 2.
Mean change from baseline in total International Prostate Symptom Score (IPSS) (a), IPSS storage subscale (b), IPSS voiding subscale (c), and IPSS-quality of life (QoL) (d) of the four treatment groups. *P < 0.05 versus placebo; †P < 0.05 versus tamsulosin; ‡P < 0.05 versus tolterodine extended release (ER).
Compared with results at week 12, only the tolterodine ER plus tamsulosin group of the four treatment groups presented persistent and statistically significant improvement in the total IPSS at week 24 (P = 0.010).
Storage International Prostate Symptom Score
The mean IPSS for storage symptoms also decreased progressively from baseline in all treatment groups as the study progressed [Figure 2b]. From baseline, the mean descent of storage IPSS in placebo group at weeks 4, 12, and 24 was −1.52, −1.21, and −2.43, respectively; tamsulosin group was −2.06, −2.94, and −3.22, respectively; tolterodine ER group was −2.85, −4.48, and −4.37, respectively; and tolterodine ER plus tamsulosin group was −3.56, −5.63, and −6.66, respectively. Compared with the placebo group, statistically significant improvements in storage IPSS were observed in the tolterodine ER group at weeks 12 (P < 0.001) and 24 (P < 0.001). Improvements in tolterodine ER plus tamsulosin group appeared at weeks 12 (P < 0.001) and 24 (P < 0.001), but not in tamsulosin group. The improvements became statistically significant in the tolterodine ER group and tolterodine ER plus tamsulosin group at weeks 12 (P < 0.001, P < 0.001) and 24 (P = 0.001, P < 0.001), compared with tamsulosin group. The significant improvements were only observed in the tolterodine ER plus tamsulosin group at week 24 (P = 0.034), compared with tolterodine ER group.
In addition, the improvement of storage IPSS in tolterodine ER plus tamsulosin group at week 24 was statistically significant (P = 0.024), compared with results at week 12; these data were not significant in the other three treatment groups.
Voiding International Prostate Symptom Score
From baseline, the mean voiding IPSS in the four treatment groups decreased as treatment went on [Figure 2c]. The mean improvement of voiding IPSS in placebo group at weeks 4, 12, and 24 was −1.52, −3.03, and −2.97, respectively; tamsulosin group was −3.54, −4.88, and −5.16, respectively; tolterodine ER group was −0.64, −1.83, and −1.45, respectively; and tolterodine ER plus tamsulosin group was −2.88, −5.10, and −6.48, respectively. Compared with placebo group, the improvements were statistically significant in the tamsulosin group at weeks 4 (P = 0.022), 12 (P < 0.001), and 24 (P = 0.001) as well as in tolterodine ER plus tamsulosin group at weeks 12 (P = 0.003) and 24 (P < 0.001). Compared with the tolterodine ER group, the tamsulosin group and tolterodine ER plus tamsulosin group achieved statistically significant improvements at weeks 4 (P = 0.001, P = 0.008), 12 (P < 0.001, P < 0.001), and 24 (P < 0.001, P < 0.001).
The differences in voiding IPSS at week 12 versus week 24 in all the four treatment groups were not statistically significant (all P > 0.05).
Quality-of-life assessment
The mean QoL score improved consistently from baseline in the four treatment groups at weeks 4, 12, and 24; the most significant improvement was in the tolterodine ER plus tamsulosin group [Figure 2d]. The mean improvement of QoL score from baseline in placebo group at weeks 4, 12, and 24 was −0.39, −1.41, and −1.60, respectively; tamsulosin group was −1.00, −1.85, and −2.13, respectively; tolterodine ER group was −0.69, −1.21, and −1.41, respectively; tolterodine ER plus tamsulosin group was −1.21, −2.40, and −3.21, respectively. Statistically significant differences were observed in the combined group versus placebo group at weeks 4 (P = 0.008), 12 (P = 0.004), and 24 (P < 0.001); versus the tolterodine ER group at week 12 (P = 0.025); and versus the tolterodine ER group and tamsulosin group at week 24 (P < 0.001, P < 0.001). Other statistically significant differences were observed only between the tamsulosin group and the placebo group at week 4 (P = 0.030).
QoL improvement in the tolterodine ER plus tamsulosin group at week 24 was ongoing, compared with week 12; the difference was statistically significant (P = 0.013). The QoL improvement in the other three treatment groups was not significant.
Maximum urinary flow rate
The mean changes of Qmax from baseline in placebo group, tamsulosin group, tolterodine ER group, and tolterodine ER plus tamsulosin group were 2.15, 2.00, −0.79, and 2.21 ml/s, respectively, at week 4 of follow-up; 2.44, 7.12, 2.83, and 7.97 ml/s, respectively, at week 12 of follow-up; 2.73, 7.84, 1.11, and 9.72 ml/s, respectively, at week 24 of follow-up [Figure 3a]. Compared with the placebo group, a statistically significant improvement was observed in the tamsulosin group and tolterodine ER plus tamsulosin group at weeks 12 (P < 0.001, P < 0.001) and 24 (P < 0.001, P < 0.001). Compared with the tolterodine ER group, a significant improvement appeared in the two active treatment groups at weeks 4 (P = 0.013, P = 0.001), 12 (P < 0.001, P < 0.001), and 24 (P < 0.001, P < 0.001). Compared with results at week 12, the improvement at week 24 in all the four treatment groups was not statistically significant (all P > 0.05).
Figure 3.

Mean change from baseline in Qmax (a) and postvoid residual volume (b) of the four treatment groups. *P < 0.05 versus placebo; †P < 0.05 versus tamsulosin; ‡P < 0.05 versus tolterodine extended release (ER); §P < 0.05 versus tolterodine ER + tamsulosin.
Postvoid residual volume
The mean PVR was gradually reduced as treatment went on in the treatment groups, except in tolterodine ER group [Figure 3b]. The mean reduction of PVR from baseline in placebo group at weeks 4, 12, and 24 was −12.03, −11.16, and −16.73 ml, respectively; tamsulosin group was −21.17, −31.06, and −32.41 ml, respectively; tolterodine ER plus tamsulosin group was −17.88, −26.97, and −27.89 ml, respectively. The statistically significant difference of PVR reduction could be seen in the tamsulosin group and tolterodine ER plus tamsulosin group at weeks 12 (P = 0.006, P = 0.034) and 24 (P = 0.002, P = 0.050), compared with placebo group. PVR increased gradually as treatment continued in the tolterodine ER group, the mean increase volume from baseline was 10.03 ml at week 4, 10.41 ml at week 12, and 12.89 ml at week 24. Compared with the other treatment groups, the differences at weeks 4, 12, and 24 were all statistically significant (P = 0.004, P = 0.032, and P = 0.033 in the placebo group; P = 0.009, P < 0.001, and P < 0.001 in the tamsulosin group; P < 0.001, P < 0.001, and P < 0.001 in the combination group, respectively). Compared with week 12, PVR changes at week 24 were not statistically significant in all the four treatment groups (all P > 0.05).
Tolerability and safety
The most common treatment-emergent AEs were dry mouth in patients in the tolterodine ER group (7/38) and combined group (10/38), but most of them were mild to moderate and tolerated. The AEs were treated effectively by increased water consumption; only one patient in each of the two groups reported intolerable dry mouth and withdrew from the trial. Most AEs suggestive of urinary retention were reported in patients in the tolterodine ER group (11/38, one patient needed catheterization), while only 4/38 in placebo group. Other treatment-emergent AEs and AEs suggestive of urinary retention were comparable in the four treatment groups [Table 2].
Table 2.
Summary of treatment-emergent AEs among patients with benign prostate hyperplasia and an enlarged prostate size in four treatment groups, n
| Items | Placebo (N = 38) | Tamsulosin (N = 38) | Tolterodine ER (N = 38) | Tolterodine ER + tamsulosin (N = 38) |
|---|---|---|---|---|
| AEs | ||||
| Dry mouth | 1 | 2 | 7 | 10 |
| Constipation | 2 | 0 | 3 | 2 |
| Headache | 1 | 2 | 1 | 2 |
| Dizziness | 1 | 2 | 1 | 2 |
| Fatigue | 1 | 1 | 1 | 1 |
| Diarrhea | 0 | 2 | 1 | 2 |
| AEs suggestive of AUR | ||||
| Urinary retention | 1 | 0 | 3 | 1 |
| Urinary flow decreased | 1 | 0 | 3 | 1 |
| Dysuria | 1 | 0 | 2 | 1 |
| Discontinuations | 1 | 0 | 2 | 1 |
| Catheterization required | 0 | 0 | 1 | 0 |
ER: Extended release; AEs: Adverse events; AUR: Acute urinary retention.
Discussion
Starting from 1994, the approach of combination therapy with α-blockers and antimuscarinics in men with bladder outlet obstruction and overactive bladder (OAB) symptoms has become increasingly accepted.[13] The earliest report by Athanasopoulos et al.[14] set off the growing interest of antimuscarinics use in men with LUTS. Tolterodine ER is the common antimuscarinics used in clinical practice, alone or in combination with other drugs including α-blockers and 5α-reductase inhibitors. Many studies, including the TIMES study, reported that tolterodine ER and α-blockers combination therapy – not α-blockers, tolterodine ER, or placebo alone – had a significant treatment benefit, significantly improved total IPSS and QoL, and storage IPSS at the end of the study period (12 weeks) with a good tolerance.[9,15,16]
There has been reluctance to prescribe antimuscarinics in men with BPH because of the risk of precipitating urinary retention through decreased bladder contractility in the setting of bladder outlet obstruction, and the concern is greater when male patients have a larger prostate volume. Therefore, clinical trials evaluating antimuscarinic use in patients with BPH, OAB, and larger prostate volume are rare. Chung et al.[17] enrolled 51 men with BPH and a prostate size >30 ml. The patients were treated with dutasteride for >6 months to effectively reduce the prostate volume; patients with persistent OAB symptoms were administered 4 mg of tolterodine ER daily for 12 weeks. The results showed that the addition of tolterodine ER effectively reduced the frequency and urgency, IPSS and storage symptoms, and was tolerated well. Post hoc analysis data from the TIMES study stratified by median baseline prostate size (<29 vs. ≥29 ml) indicated that tolterodine ER was beneficial in patients with BPH, smaller prostate, and moderate-to-severe LUTS, including OAB symptoms. Tolterodine ER plus tamsulosin was effective regardless of prostate size, and tolterodine ER, with or without tamsulosin, was well tolerated and did not increase AUR incidence.[18]
The current study was a double-blind, placebo-controlled, randomized clinical trial investigating the effect of medium-to-long-term use of tolterodine ER with or without tamsulosin in patients with BPH and large prostate size. Similar to the TIMES study, combination therapy with tolterodine ER and tamsulosin significantly improved IPSS, storage IPSS, voiding IPSS, and QoL scores in men with larger prostate size, compared with placebo. However, unlike the TIMES study, our results indicated that tolterodine ER alone also can effectively improve storage IPSS (weeks, 12 and 24) in men with larger prostate volume. We speculate that this difference may be because of the race of the study population; older Asian men seem to more easily suffer from moderate-to-severe LUTS than Australians,[19] and therefore our patients may have better response to tolterodine ER than those in TIMES study.
The current study also indicated that the therapeutic effect of combination of tolterodine ER and tamsulosin for patients with BPH and larger prostate was maintained continuously and enhanced after 12 weeks of therapy, especially in total IPSS, storage IPSS, and QoL; the AEs or AEs suggestive of urinary retention did not increase accordingly. The significantly greater enhanced improvement was associated with treatment with tolterodine ER plus tamsulosin, compared with tolterodine alone, tamsulosin alone, or placebo. Therefore, it would be reasonable to believe that medium-to-long-term combination of tolterodine ER and tamsulosin can relieve storage and voiding symptoms simultaneously, and it is a recommendable treatment for patients with BPH and larger prostate size.
Prostate volume is an important factor that must be considered when prescribing antimuscarinics in men with BPH and OAB.[20] Some studies recommended that antimuscarinics, especially antimuscarinic monotherapy, is not suitable for men with an enlarged prostate.[6,21] The post hoc analysis in the TIMES study also indicated that tolterodine ER monotherapy was effective only in men with BPH and TPV <29 ml.[18] In the current study, which included Asian patients with ≥25 ml prostate, medium-to-long-term therapy with tolterodine ER alone did not achieved satisfactory therapeutic effectiveness in total IPSS, voiding IPSS, or QoL IPSS, compared with placebo. However, patients receiving tolterodine ER monotherapy demonstrated less improvement in Qmax, a gradual increase of PVR, and more urinary AEs, suggestive of urinary retention. Therefore, although it may be able to improve the storage LUTS to a certain degree, medium-to-long-term use of antimuscarinic monotherapy in patients with BPH and a larger prostate should be avoided.
Similar to previous clinical trials,[11,22] medium-to-long-term use of tolterodine ER, especially combined with tamsulosin, was well tolerated in the current study although the patients we selected had a relatively bigger prostate.
There were some limitations in this study. First, the number of patients selected for inclusion in the clinical trial was not as great as in previously reported multicenter studies. Although the results obtained can successfully undergo statistical analyses, a multi-center study with far more patients enrolled would be required. Second, the indices we used to assess the clinical efficacy of drugs were total IPSS, storage IPSS, voiding IPSS, and QoL IPSS, and did not include a bladder diary, urgency rating scale, or other indices such as patients’ perception of bladder condition. The reason is that we found many older men were not willing to or able to finish such observation items correctly in our clinical practice, whereas IPSS and its subscales can be easily and correctly finished under a physician's direction. Therefore, what was reported in this study reflects the real clinical practice in our medical center.
Financial support and sponsorship
This work was supported by a grant of Chinese Capital Medical Development Science Fund (No. 2007-3114).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are grateful to Dr. Yong-Pei Yu for statistical analysis and the pharmacist Jing Wu for drug management and distribution. We also acknowledge Nanjing Meirui Pharmaceutical Co. Ltd., for providing tolterodine extended release and placebo, and Kunming Jida Pharmaceutical Co. Ltd., for providing tamsulosin and placebo.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.McVary KT. BPH: Epidemiology and comorbidities. Am J Manag Care. 2006;12(5 Suppl):S122–8. [PubMed] [Google Scholar]
- 2.Burnett AL, Wein AJ. Benign prostatic hyperplasia in primary care: What you need to know. J Urol. 2006;175(3 Pt 2):S19–24. doi: 10.1016/S0022-5347(05)00310-1. doi: 10.1016/S0022-5347(05)00310-1. [DOI] [PubMed] [Google Scholar]
- 3.Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur Urol. 2006;50:1306–14. doi: 10.1016/j.eururo.2006.09.019. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Aziz KF, Lemack GE. Overactive bladder in the male patient: Bladder, outlet, or both? Curr Urol Rep. 2002;3:445–51. doi: 10.1007/s11934-002-0095-3. doi: 10.1007/s11934-002-0095-3. [DOI] [PubMed] [Google Scholar]
- 5.Liu N, Man LB, He F, Huang GL, Wang H, Li GZ, et al. Multiple factors related to detrusor overactivity in Chinese patients with benign prostate hyperplasia. Chin Med J. 2012;125:3778–81. [PubMed] [Google Scholar]
- 6.Kaplan SA, Roehrborn CG, Abrams P, Chapple CR, Bavendam T, Guan Z. Antimuscarinics for treatment of storage lower urinary tract symptoms in men: A systematic review. Int J Clin Pract. 2011;65:487–507. doi: 10.1111/j.1742-1241.2010.02611.x. doi: 10.1111/j.1742-1241.2010.02611. [DOI] [PubMed] [Google Scholar]
- 7.Abrams P, Andersson KE. Muscarinic receptor antagonists for overactive bladder. BJU Int. 2007;100:987–1006. doi: 10.1111/j.1464-410X.2007.07205.x. doi: 10.1111/j.1464-410X.2007.07205. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Zhao XF, Li HZ, Wang W, Zhang Y, Xiao H, et al. Efficacy and safety of combined therapy with terazosin and tolterodine for patients with lower urinary tract symptoms associated with benign prostatic hyperplasia: A prospective study. Chin Med J. 2007;120:370–4. [PubMed] [Google Scholar]
- 9.Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: A randomized controlled trial. JAMA. 2006;296:2319–28. doi: 10.1001/jama.296.19.2319. doi: 10.1001/jama.296.19.2319. [DOI] [PubMed] [Google Scholar]
- 10.Chapple C, Herschorn S, Abrams P, Sun F, Brodsky M, Guan Z. Tolterodine treatment improves storage symptoms suggestive of overactive bladder in men treated with alpha-blockers. Eur Urol. 2009;56:534–41. doi: 10.1016/j.eururo.2008.11.026. doi: 10.1016/j.eururo.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Drake MJ, Chapple CH, Sokol R, Oelke M, Traudtner K, Klaver M, et al. Long-term safety and efficacy of single-tablet combinations of solifenacin and tamsulosin oral controlled absorption system in men with storage and voiding lower urinary tract symptoms: Results from the NEPTUNE study and NEPTUNE II open-label extension. Eur Urol. 2015;67:262–70. doi: 10.1016/j.eururo.2014.07.013. doi: 10.1016/j.eururo.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Lim KT, Kim YT, Lee TY, Park SY. Effects of tamsulosin, solifenacin, and combination therapy for the treatment of ureteral stent related discomforts. Korean J Urol. 2011;52:485–8. doi: 10.4111/kju.2011.52.7.485. doi: 10.4111/kju.2011.52.7.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapple CR, Smith D. The pathophysiological changes in the bladder obstructed by benign prostatic hyperplasia. Br J Urol. 1994;73:117–23. doi: 10.1111/j.1464-410x.1994.tb07477.x. doi: 10.1111/j.1464-410X.1994.tb07477. [DOI] [PubMed] [Google Scholar]
- 14.Athanasopoulos A, Gyftopoulos K, Giannitsas K, Fisfis J, Perimenis P, Barbalias G. Combination treatment with an alpha-blocker plus an anticholinergic for bladder outlet obstruction: A prospective, randomized, controlled study. J Urol. 2003;169:2253–6. doi: 10.1097/01.ju.0000067541.73285.eb. doi: 10.1097/01.ju.0000067541.73285. [DOI] [PubMed] [Google Scholar]
- 15.Gacci M, Novara G, De Nunzio C, Tubaro A, Schiavina R, Brunocilla E, et al. Tolterodine extended release in the treatment of male OAB/storage LUTS: A systematic review. BMC Urol. 2014;14:84. doi: 10.1186/1471-2490-14-84. doi: 10.1186/1471-2490-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan SA, Roehrborn CG, Chancellor M, Carlsson M, Bavendam T, Guan Z. Extended-release tolterodine with or without tamsulosin in men with lower urinary tract symptoms and overactive bladder: Effects on urinary symptoms assessed by the International Prostate Symptom Score. BJU Int. 2008;102:1133–9. doi: 10.1111/j.1464-410X.2008.07761.x. doi: 10.1111/j.1464-410x.2008.07761. [DOI] [PubMed] [Google Scholar]
- 17.Chung DE, Te AE, Staskin DR, Kaplan SA. Efficacy and safety of tolterodine extended release and dutasteride in male overactive bladder patients with prostates >30 grams. Urology. 2010;75:1144–8. doi: 10.1016/j.urology.2009.12.010. doi: 10.1016/j.urology.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Roehrborn CG, Kaplan SA, Jones S, Wangd JT, Bavendam T, Guan Z. Tolterodine extended release with or without tamsulosin in wen with lower urinary tract symptoms including overactive bladder symptoms: Effects of prostate size. Eur Urol. 2009;55:472–9. doi: 10.1016/j.eururo.2008.06.032. doi: 10.1016/j.eururo.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Homma Y, Kawabe K, Tsukamoto T, Yamanaka H, Okada K, Okajima E, et al. Epidemiologic survey of lower urinary tract symptoms in Asia and Australia using the international prostate symptom score. Int J Urol. 1997;4:40–6. doi: 10.1111/j.1442-2042.1997.tb00138.x. doi: 10.1111/j.1442-2042.1997.tb00138. [DOI] [PubMed] [Google Scholar]
- 20.Liao CH, Kuo YC, Kuo HC. Predictors of successful first-line antimuscarinic monotherapy in men with enlarged prostate and predominant storage symptoms. Urology. 2013;81:1030–3. doi: 10.1016/j.urology.2013.01.018. doi: 10.1016/j.urology.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Djavan B, Margreiter M, Dianat SS. An algorithm for medical management in male lower urinary tract symptoms. Curr Opin Urol. 2011;21:5–12. doi: 10.1097/MOU.0b013e32834100ef. doi: 10.1097/MOU.0b013e32834100ef. [DOI] [PubMed] [Google Scholar]
- 22.Chung SD, Chang HC, Chiu B, Liao CH, Kuo HC. The efficacy of additive tolterodine extended release for 1-year in older men with storage symptoms and clinical benign prostatic hyperplasia. Neurourol Urodyn. 2011;30:568–71. doi: 10.1002/nau.20923. doi: 10.1002/nau.20923. [DOI] [PubMed] [Google Scholar]