Abstract
Background:
Our previous studies have demonstrated that the levels of 5-hydroxytryptamine (5-HT) and 5-HT 2A receptor (5-HT2AR) in serum and platelet were associated with depression and myocardial infarction (MI), and pretreatment with ginseng fruit saponins (GFS) before MI and depression had an effect on the 5-HT system. In this study, the effects of GFS on the 5-HT system in the Sprague-Dawley (SD) rats with MI, depression, and MI + depression were evaluated.
Methods:
A total of eighty SD rats were allocated to four groups: MI, depression, MI + depression, and control groups (n = 20 in each group). Each group included two subgroups (n = 10 in each subgroup): Saline treatment subgroup and GFS treatment subgroup. The levels of 5-HT, 5-HT2AR, and serotonin transporter (SERT) were quantified in serum, platelet lysate, and brain tissue through the enzyme-linked immunosorbent assay method, respectively.
Results:
Compared with those in the saline treatment subgroups, the levels of 5-HT in serum and platelet lysate statistically significantly increased in the GFS treatment subgroups of MI, depression, and MI + depression groups (serum: all P = 0.000; platelet lysate: P = 0.002, 0.000, 0.000, respectively). However, the 5-HT levels in brain homogenate significantly decreased in the GFS treatment subgroups compared with those in the saline treatment subgroups in MI and depression groups (P = 0.025 and 0.044 respectively), and no significant difference was observed between saline and GFS treatment subgroups in MI + depression group (P = 0.663). Compared with that in GFS treatment subgroup of control group, the 5-HT2AR levels in the platelet lysate significantly decreased in GFS treatment subgroups of MI, depression, and MI + depression groups (all P = 0.000). Compared to those in the saline treatment subgroups, the serum SERT levels significantly decreased in the GFS treatment subgroups in MI, depression, and MI + depression groups (P = 0.009, 0.038, and P = 0.001, respectively), while the SERT levels of platelet lysate significantly decreased in GFS treatment subgroup of MI group (P = 0.000), significantly increased in GFS treatment subgroup of depression group (P = 0.019), and slightly changed in GFS treatment subgroup of MI + depression group (P = 0.219). No significant changes for SERT levels in brain homogenate could be found between the saline and GFS treatment subgroups in MI, depression, and MI + depression groups (P = 0.421, 0.076 and P = 0.642).
Conclusions:
This study indicated that GFS might inhibit the reuptake of 5-HT from serum to platelet according to decreased 5-HT2AR in platelet and SERT in serum and platelet. The change of 5-HT in serum after GFS treatment was inconsistent with that in the brain. It seemed that GFS could not pass through the blood-brain barrier to affect the central serotonergic system.
Keywords: 5-hydroxytryptamine 2A Receptor, Depression, Ginseng Fruit Saponins, 5-hydroxytryptamine, Myocardial Infarction, Serotonin Transporter
Introduction
Coronary heart disease (CHD) and depression seriously threaten human physical and mental health worldwide.[1] Depression is considered as an independent risk factor for mortality and morbidity in patients following myocardial infarction (MI).[2,3,4] Platelet dysfunction was demonstrated as one cardiovascular risk factor for depression led by MI while the underlying physiological mechanism that links depression and CHD remains poorly understood.[5] Heightened susceptibility to platelet activation may be a mechanism by which depression is a significant risk factor for ischemic heart and cerebrovascular disease and/or mortality after myocardial infarction.[6] Platelet activation is a primary physiological response to limit bleeding after acute vascular insults. Platelet activation occurs in MI or chronic heart disease.[7] Depression in somatically healthy patients has been proved to associate with platelet activation.[5,7]
Serotonin, also known as 5-hydroxytryptamine (5-HT), acts as both a neurotransmitter and a neurohormone that potentially impact depression and CHD. Serotonin has been shown to be involved in the platelet activation in depression and MI.[8,9,10,11] The biomarker of platelet activation is not confirmed yet. After stimulation of agonists, given that platelets release high concentrations of 5-HT and the activated platelets contain less 5-HT, the concentration of 5-HT in whole blood is recommended as a parameter to evaluate the extent of platelet activation.[5,12,13,14,15,16] The selective serotonin reuptake inhibitors are the most commonly used antidepressant drugs that target the plasma membrane serotonin transporter (SERT). The 5-HT 2A receptor (5-HT2AR) as a broadly existed receptor of 5-HT is vital for depression-related diseases.[9]
Ginseng fruit saponins (GFS) is a kind of steroid compounds that exists in the traditional Chinese medicine. As an active ingredient of ginseng fruit, GFS plays a crucial role in the treatment of CHD through multiple metabolic pathways including inhibition of platelet aggregation.[17,18,19] The study of Hao et al.[20] showed that Rb1 as an important ingredient of GFS can increase the neural 5-HT concentration and decrease immobility time in the forced swimming test (FST).
Decreased 5-HT in the brain has been linked to depression, while excessive 5-HT in the blood caused platelet aggregation which is associated with MI. The 5-HT in the periphery is able to pass the blood-brain barrier. Moreover, platelet is considered to be the mirror of the brain for the similar characteristics of biochemistry and morphology. It was reported that the ingredients of GFS, such as Re and Rg1, could cure MI or depression. According to the above evidences, we assumed GFS may play an important role in regulating the 5-HT concentration in periphery and brain of MI + depression rats. This study was to quantify the levels of 5-HT, 5-HT2AR, and SERT in serum, platelet, and brain homogenate to evaluate the effects of GFS on serotonin and its regulator (5-HT2AR, SERT) in the Sprague-Dawley (SD) rats with MI, depression, and MI + depression.
Methods
Experimental animals
A total of eighty SD rats (body weight: 250–300 g, Pharmaceutical Base, Jiangsu Province, China) were allocated to four groups: MI, depression, MI + depression, and control groups (n = 20 in each group). Each group included saline treatment subgroup and GFS treatment subgroup (n = 10 in each subgroup). Rats were administered with 2 ml saline or GFS (Jilin Ji’an Yisheng Pharmaceutical Co. Ltd.) at 2 mg/100 g once a day, through gavages for 4 weeks, then were sacrificed. All SD rats were housed at a temperature of 22 ± 2°C and a humidity of 45 ± 5%, with free access to food and water.
Establishment of depression model
The modified FST in this study was used, which was similar to the method reported by Porsolt et al.[21] Briefly, each SD rat was placed in a cylinder (40 cm in height, 20 cm in diameter) containing water at 30 cm deep, 25°C. The depth of water used in this FST was modified from 15 to 30 cm so that the adult rats could not touch the bottom to avoid immobility. After a pretest session of 15 cm depth for 15 min, the rats were dried (under a warm air current) and were exposed to the modified FST for 5 min after 24 h and repeated.
Establishment of myocardial infarction model
The SD rats were anesthetized with ketamine (40 mg/kg) and xylazine (1 mg/kg) by intramuscular injection and then were conducted with a surgical operation.[22] After anesthesia, the rats were placed at the position of the spine. After disinfecting, the thorax of the rat was opened in the fourth intercostal space. The left anterior descending artery was cauterized at the midpoint through the starting point and the cardiac apex. After cauterization, the air in the thorax was squeezed out by the forefinger, and the thorax was closed with the suture. Sixteen (80%) rats survived after MI operation.
Establishment of myocardial infarction + depression model
The SD rats were firstly conducted with MI surgery as described above.[22] Sixteen (80%) rats survived after MI operation. Three days later, the MI rats were conducted with the modified FST mentioned above.
Sample collection and storage
The blood samples were collected from tail vein and were placed into chilled tubes containing ethylenediaminetetraacetic acid. Whole blood was aliquoted and stored at −70°C. The blood was centrifuged for 15–30 min at 200 ×g at room temperature to obtain platelet-rich plasma (PRP) as described above,[23] then PRP was aspirated and sonicated for 10 s three times on ice. To prepare isolated platelets, PRP was centrifuged for 10 min at 2100 ×g at 4°C and then was disposed.[23]
5-hydroxytryptamine, 5-hydroxytryptamine 2A receptor, and serotonin reuptake transporter detection
The levels of 5-HT, 5-HT2AR, and SERT were detected using enzyme-linked immunosorbent assay method.
Statistical analysis
Statistical analyses were performed using the SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). The values of 5-HT, 5-HT2AR, and SERT were presented as mean ± standard error (SE). Comparisons among three or more groups were assessed using one-way analysis of variance (ANOVA). Comparisons between two subgroups were assessed using independent samples t-test. A P < 0.05 was considered statistically significant.
Results
5-hydroxytryptamine levels in different tissues
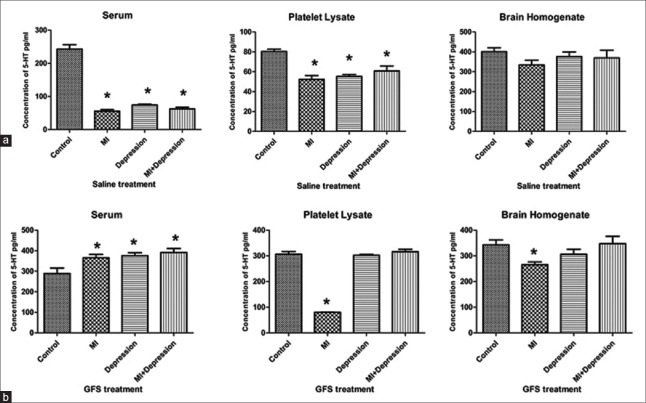
After treatment with saline, the serum 5-HT levels in subgroups of MI, depression, and MI + depression groups decreased significantly compared with that in the subgroup of control group (all P = 0.000), and the same trend could be found for 5-HT of the platelet lysate between saline treatment subgroups of MI, depression, MI + depression and control groups (P = 0.000, P = 0.000, and P = 0.001, vs. control group, respectively). However, no significant differences in the 5-HT levels of brain homogenate could be found between the saline treatment subgroups of MI, depression, MI + depression and control groups (P = 0.100, P = 0.532, and P = 0.426, vs. control group, respectively; Figure 1a).
Figure 1.
Concentrations of 5-HT in serum, platelet lysate, and brain homogenate after saline (a) and GFS (b) treatments in MI, depression, MI + depression, and control groups. *P < 0.05, versus controls. n = 10 in each subgroup of control and depression groups, and n = 8 in each subgroup of MI and MI + depression groups. 5-HT: 5-hydroxytryptamine; MI: Myocardial infarction; GFS: Ginseng fruit saponins.
After treatment with GFS, the serum 5-HT levels in the subgroups of MI, depression, and MI + depression groups significantly increased compared with that in subgroup of control group (P = 0.016, P = 0.007, and P = 0.003, respectively). Compared with that in subgroup of control group, the 5-HT levels in platelet lysate in subgroup of MI group decreased significantly (P = 0.000), but slightly changed in the subgroups of depression and MI + depression groups (P = 0.705 and P = 0.417, respectively); and the same trend could be found for 5-HT of the brain homogenate between GFS treatment subgroups of MI, depression, MI + depression and control groups (P = 0.015, P = 0.227, and P = 0.868, vs. control group; Figure 1b).
Compared to those in the saline treatment subgroups, the levels of 5-HT in serum and platelet lysate statistically significantly increased in the GFS treatment subgroups of MI, depression, MI + depression groups (all P < 0.05). However, the 5-HT levels in brain homogenate significantly decreased in the GFS treatment subgroups of MI and depression groups compared with those in the saline treatment subgroups (P = 0.025 and 0.044, respectively), and no significant difference was observed between saline and GFS treatment subgroups of MI + depression group (P = 0.663; Table 1).
Table 1.
The 5-HT levels in serum, platelet lysate, and brain homogenate after saline and GFS treatments (pg/ml)
| Items | Saline treatment subgroup | GFS treatment subgroup | F | P |
|---|---|---|---|---|
| Serum | ||||
| Controls | 242.94 ± 32.00 | 289.09 ± 74.70 | 3.318 | 0.184 |
| MI | 55.92 ± 11.58 | 365.16 ± 41.28 | 10.348 | 0.000 |
| Depression | 74.63 ± 4.97 | 375.50 ± 35.90 | 16.165 | 0.000 |
| MI + depression | 62.49 ± 10.20 | 391.66 ± 41.55 | 3.128 | 0.000 |
| Platelet lysate | ||||
| Controls | 80.38 ± 5.52 | 306.49 ± 24.91 | 9.805 | 0.000 |
| MI | 52.25 ± 8.57 | 80.30 ± 0.85 | 16.600 | 0.002 |
| Depression | 55.18 ± 4.85 | 302.10 ± 7.12 | 0.179 | 0.000 |
| MI + depression | 60.78 ± 11.16 | 315.99 ± 18.99 | 0.870 | 0.000 |
| Brain homogenate | ||||
| Controls | 400.85 ± 47.67 | 342.71 ± 47.47 | 0.130 | 0.060 |
| MI | 334.05 ± 57.49 | 265.01 ± 27.96 | 2.519 | 0.025 |
| Depression | 376.26 ± 56.84 | 306.27 ± 47.73 | 0.634 | 0.044 |
| MI + depression | 369.39 ± 95.62 | 347.65 ± 70.24 | 0.295 | 0.663 |
The data are shown as mean ± SE. n = 10 in each subgroup of control and depression groups, and n = 8 in each subgroup of MI and MI + depression groups. 5-HT: 5-hydroxytryptamine; MI: Myocardial infarction; GFS: Ginseng fruit saponins; SE: Standard error.
5-hydroxytryptamine 2A receptor levels in different tissues
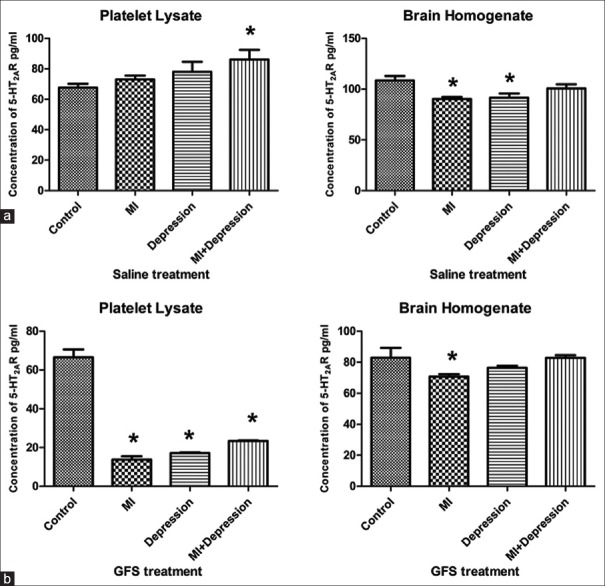
Compared with that in the saline treatment subgroup of control group, the 5-HT2AR levels of platelet lysate significantly increased in saline treatment subgroup of MI + depression group (P = 0.016) and also increased in saline treatment subgroups of MI and depression groups but without significant differences (P = 0.466 and P = 0.154, respectively). On the contrary, compared with that in the saline treatment subgroup of control group, the 5-HT2AR levels in brain homogenate significantly decreased in the saline treatment subgroups of MI and depression groups (P = 0.003 and P = 0.006, respectively), but no significant change could be found between the saline treatment subgroups of MI + depression and control groups (P = 0.164; Figure 2a).
Figure 2.
Concentrations of 5-HT2AR in platelet lysate and brain homogenate after saline (a) and GFS (b) treatments in MI, depression, MI + depression, and control groups. *P < 0.05, versus control group. n = 10 in each subgroup of control and depression groups, and n = 8 in each subgroup of MI and MI + depression groups. 5-HT2AR: 5-hydroxytryptamine 2A receptor; MI: Myocardial infarction; GFS: Ginseng fruit saponins.
Compared with that in the GFS treatment subgroup of control group, the 5-HT2AR levels of platelet lysate significantly decreased in the GFS treatment subgroups of MI, depression, and MI + depression groups (all P = 0.000). However, compared with that in the GFS treatment subgroup of control group, the 5-HT2AR levels of brain homogenate significantly decreased in the GFS treatment subgroup of MI group (P = 0.036), but no significant differences could be found between GFS treatment subgroups of depression, MI + depression and control groups (P = 0.210 and P = 0.984, respectively; Figure 2b).
Compared to those in the saline treatment subgroups, the 5-HT2AR levels of platelet lysate statistically significantly decreased in the GFS treatment subgroups of MI, depression, and MI + depression groups (all P = 0.000). The same trend could also be found for 5-HT2AR levels in brain homogenate between saline and GFS treatment subgroups of MI, depression, and MI + depression groups (P = 0.000, 0.014, and 0.006, respectively; Table 2).
Table 2.
The 5-HT2AR levels in platelet lysate and brain homogenate after saline and GFS treatments (pg/ml)
| Items | Saline treatment subgroup | GFS treatment subgroup | F | P |
|---|---|---|---|---|
| Platelet lysate | ||||
| Control | 67.60 ± 6.33 | 66.59 ± 9.92 | 0.874 | 0.838 |
| MI | 73.08 ± 5.54 | 13.79 ± 3.47 | 0.522 | 0.000 |
| Depression | 78.03 ± 16.06 | 17.09 ± 0.82 | 10.697 | 0.000 |
| MI + depression | 86.15 ± 15.45 | 23.34 ± 0.79 | 10.854 | 0.000 |
| Brain homogenate | ||||
| Control | 108.52 ± 10.87 | 82.95 ± 14.27 | 0.435 | 0.008 |
| MI | 90.27 ± 5.31 | 70.71 ± 3.14 | 1.542 | 0.000 |
| Depression | 91.56 ± 10.23 | 76.40 ± 2.86 | 7.162 | 0.014 |
| MI + depression | 100.64 ± 10.33 | 82.85 ± 3.88 | 5.071 | 0.006 |
The data are shown as mean ± SE. n = 10 in each subgroup of control and depression groups, and n = 8 in each subgroup of MI and MI + depression groups. 5-HT2AR: 5-hydroxytryptamine 2A receptor; MI: Myocardial infarction; GFS: Ginseng fruit saponins; SE: Standard error.
Serotonin transporter levels in different tissues
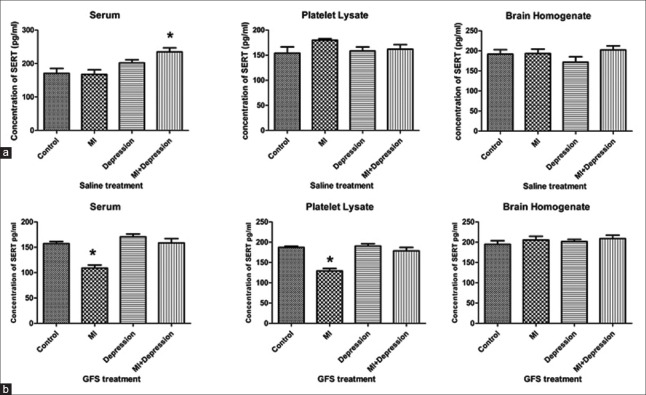
After treatment with saline, the serum SERT levels in a subgroup of MI + depression group significantly increased compared with that in the subgroup of control group (P = 0.002), but no significant differences could be found between the saline treatment subgroups of MI, depression and control groups (P = 0.871 and 0.092, respectively). There were no significant differences in SERT levels of platelet lysate and brain homogenate between the saline treatment subgroups of MI, depression, MI + depression and control groups (platelet lysate: P = 0.095, 0.758, and 0.558; brain homogenate: P = 0.912, 0.234, and 0.515; Figure 3a).
Figure 3.
Concentrations of SERT in serum, platelet lysate, and brain homogenate after saline (a) and GFS (b) treatments in MI, depression, MI + depression, and control groups. *P < 0.05, versus control group. n = 10 in each subgroup of control and depression groups, and n = 8 in each subgroup of MI and MI + depression groups. SERT: Serotonin transporter; MI: Myocardial infarction; GFS: Ginseng fruit saponins.
After treatment with GFS, the serum concentration of SERT significantly decreased in subgroup of MI group (P = 0.000), compared with that in the subgroup of controls, but it slightly increased in the subgroup of depression and MI + depression groups without significant difference (P = 0.144 and P = 0.857). In platelet lysate, the SERT levels significantly decreased in the subgroup of MI group (P = 0.000) and slightly changed in the subgroups of depression and MI + depression groups (P = 0.698 and 0.299), compared with that in the subgroup of control group. There were no significant differences in SERT levels of brain homogenate between the subgroups of MI, depression, MI + depression and control groups (P = 0.359, 0.561, and 0.235; Figure 3b).
Compared to those in the saline treatment subgroups, the serum SERT levels significantly decreased in the GFS treatment subgroups of MI, depression, and MI + depression groups (P = 0.009, 0.038, and P = 0.001), while the SERT levels of platelet lysate significantly decreased in GFS treatment subgroup of MI group (P = 0.000), significantly increased in GFS treatment subgroup of depression group (P = 0.019), and slightly changed in GFS treatment subgroup of MI + depression group (P = 0.219). No significant changes for SERT levels in brain homogenate could be found between the saline and GFS treatment subgroups in MI, depression, and MI + depression groups (MI: F = 0.232, P = 0.421; depression: F = 3.835, P = 0.076; MI + depression: F = 0.047, P = 0.642; Table 3).
Table 3.
The SERT levels in serum, platelet lysate, and brain homogenate after saline and GFS treatments (pg/ml)
| Items | Saline treatment subgroup | GFS treatment subgroup | F | P |
|---|---|---|---|---|
| Serum SERT | ||||
| Control | 170.56 ± 33.40 | 157.09 ± 10.35 | 6.351 | 0.428 |
| MI | 167.51 ± 30.76 | 108.96 ± 12.25 | 5.455 | 0.009 |
| Depression | 202.02 ± 23.32 | 170.62 ± 11.06 | 1.850 | 0.038 |
| MI + depression | 234.76 ± 29.59 | 158.60 ± 18.85 | 1.133 | 0.001 |
| Platelet lysate SERT | ||||
| Control | 154.15 ± 30.67 | 187.12 ± 7.91 | 96.909 | 0.046 |
| MI | 179.95 ± 5.73 | 129.09 ± 12.25 | 1.517 | 0.000 |
| Depression | 158.24 ± 19.85 | 190.46 ± 10.88 | 1.775 | 0.019 |
| MI + depression | 161.94 ± 22.19 | 178.62 ± 18.98 | 0.172 | 0.219 |
| Brain homogenate SERT | ||||
| Control | 191.69 ± 25.01 | 194.61 ± 19.81 | 0.381 | 0.843 |
| MI | 193.50 ± 23.98 | 205.53 ± 20.70 | 0.232 | 0.421 |
| Depression | 171.60 ± 30.52 | 201.47 ± 11.95 | 3.835 | 0.076 |
| MI + depression | 202.48 ± 22.35 | 208.86 ± 19.2 | 0.047 | 0.642 |
The data are shown as mean ± SE. n = 10 in each subgroup of control and depression groups, and n = 8 in each subgroup of MI and MI + depression groups. SERT: Serotonin transporter; GFS: Ginseng fruit saponins; MI: Myocardial infarction; SE: Standard error.
Discussion
The system of serotonin played a critical role in the development of CHD and depression. Major depression is proved to be associated with serotonergic neurotransmission dysfunction. A decrease of the blood 5-HT levels was considered as a marker for depression.[24,25,26] For instance, the Cleare's study[27] showed a decreased level of serotonin in the patients who were diagnosed with major depression. The symptoms of major depression have been identified as independent risk factors of cardiac morbidity and mortality for those patients who were diagnosed with ischemic heart disease.[28] The data of the heart and soul study indicated that depression was independently associated with high concentration of serotonin in whole blood that was obtained from the patients who were diagnosed with CHD.[29] The variation of 5-HT was closely connected with the upregulation or downregulation of peripheral 5-HT receptors and SERT.[30] A study by Nemeroff et al.[9] showed the decreased level of 5-HT in the hippocampus of the depression rats, while the expression of 5-HT2AR increased. The increased effects on activation of 5-HT2AR could be explained by increased amount of receptors on the platelet membrane, increased affinity of receptors, or active sensitivity in signal transmission. SERT as the transporter of 5-HT is responsible for balancing the concentration intracellular and extracellular 5-HT. In platelet, the SERT levels of plasma membrane and the uptake of 5-HT levels initially rose as the plasma 5-HT levels increased but fell below the normal limit as the plasma 5-HT level continued to rise.[31] In addition, 5-HT plays a role in vasoconstriction and vasodilatation, presenting as an inhibitor of the sympathetic nervous system at different levels. First, the release of norepinephrine can be inhibited by the level of 5-HT at the sympathetic neuroeffector junction in blood vessels through the interactions with the presynaptic inhibitory 5-HT receptor.[32] Second, the sympathetic outflow was centrally modified by the level of 5-HT,[33] and the changes in the activity of preganglionic sympathetic nerves can be observed. Third, the sympathetic ganglionic activity, such as postganglionic sympathetic nerve activity, can be modified by the level of 5-HT.[33]
Our results showed that the 5-HT level was increased after treatment with GFS due to a decreased level of 5-HT2AR and up- or down-regulated levels of SERT in serum. In the previous studies, the plasma membrane SERT levels and uptake of 5-HT initially rose when platelets were exposed to an increased level of 5-HT, followed by a reduce in the level of SERT (below baseline) led by a higher concentration of 5-HT.[34]
Some studies suggested that the level of serotonin in brain can be evaluated by the blood 5-HT level and be used as a peripheral biomarker for depression or other mental disorders. However, the results from the clinical observations were inconsistent.[25,34,35] Our study showed different variation of 5-HT levels in serum, platelet lysate, and brain homogenate after saline or GFS treatments, which suggested that this peripheral index was not a direct reflector of central 5-HT levels, which was consistent with the results of Li et al.'s study.[36]
In conclusion, this study indicated that GFS might inhibit the reuptake of 5-HT from serum to platelet according to decreased 5-HT2AR in platelet and SERT in serum and platelet. The change of 5-HT in serum after GFS treatment was inconsistent with that in the brain. It seemed that GFS could not pass through the blood-brain barrier to affect the central serotonergic system. Further studies are needed to confirm it in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Ying-Bin Ge for the great effort to the animal experiments.
Footnotes
Edited by: Xin Chen
References
- 1.World Health Organization. The Global Burden of Disease 2014 Update. [Last accessed in May 2016]. Available from: http: //www.who.int/features/factfiles/global_burden/facts/en/index3.html .
- 2.Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–7. doi: 10.1016/s0006-3223(03)00111-2. doi: 10.1016/S0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 3.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93:1976–80. doi: 10.1161/01.cir.93.11.1976. doi: 10.1161/01.CIR.93.11.1976. [DOI] [PubMed] [Google Scholar]
- 4.Bush DE, Ziegelstein RC, Tayback M, Richter D, Stevens S, Zahalsky H, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88:337–41. doi: 10.1016/s0002-9149(01)01675-7. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 5.Schins A, Hamulyák K, Scharpé S, Lousberg R, Van Melle J, Crijns H, et al. Whole blood serotonin and platelet activation in depressed post-myocardial infarction patients. Life Sci. 2004;76:637–50. doi: 10.1016/j.lfs.2004.04.060. doi: 10.1016/j.lfs.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 6.Musselman DL, Tomer A, Manatunga AK, Knight BT, Porter MR, Kasey S, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry. 1996;153:1313–7. doi: 10.1176/ajp.153.10.1313. doi: 10.1176/ajp.153.10.1313. [DOI] [PubMed] [Google Scholar]
- 7.Yabanoglu S, Akkiki M, Seguelas MH, Mialet-Perez J, Parini A, Pizzinat N. Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. J Mol Cell Cardiol. 2009;46:518–25. doi: 10.1016/j.yjmcc.2008.12.019. doi: 10.1016/j.yjmcc.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Sanner JE, Frazier L. The role of serotonin in depression and clotting in the coronary artery disease population. J Cardiovasc Nurs. 2011;26:423–9. doi: 10.1097/JCN.0b013e3182076a81. doi: 10.1097/JCN.0b013e3182076a81. [DOI] [PubMed] [Google Scholar]
- 9.Nemeroff CB, Musselman DL. Are platelets the link between depression and ischemic heart disease? Am Heart J. 2000;140(4 Suppl):57–62. doi: 10.1067/mhj.2000.109978. doi: 10.1067/mhj.2000.109978. [DOI] [PubMed] [Google Scholar]
- 10.Markovitz JH, Matthews KA. Platelets and coronary heart disease: Potential psychophysiologic mechanisms. Psychosom Med. 1991;53:643–68. doi: 10.1097/00006842-199111000-00006. doi: 10.1097/00006842-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Skop BP, Brown TM. Potential vascular and bleeding complications of treatment with selective serotonin reuptake inhibitors. Psychosomatics. 1996;37:12–6. doi: 10.1016/S0033-3182(96)71592-X. doi: 10.1016/S0033-3182(96)71592-X. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Wallén NH, Ladjevardi M, Hjemdahl P. Effects of serotonin on platelet activation in whole blood. Blood Coagul Fibrinolysis. 1997;8:517–23. doi: 10.1097/00001721-199711000-00006. doi: 10.1097/00001721-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Vikenes K, Farstad M, Nordrehaug JE. Serotonin is associated with coronary artery disease and cardiac events. Circulation. 1999;100:483–9. doi: 10.1161/01.cir.100.5.483. doi: 10.1161/01.CIR.100.5.483. [DOI] [PubMed] [Google Scholar]
- 14.Franke L, Schewe HJ, Müller B, Campman V, Kitzrow W, Uebelhack R, et al. Serotonergic platelet variables in unmedicated patients suffering from major depression and healthy subjects: Relationship between 5HT content and 5HT uptake. Life Sci. 2000;67:301–15. doi: 10.1016/s0024-3205(00)00620-2. doi: 10.1016/S0024-3205(00)00620-2. [DOI] [PubMed] [Google Scholar]
- 15.Maurer-Spurej E, Pittendreigh C, Solomons K. The influence of selective serotonin reuptake inhibitors on human platelet serotonin. Thromb Haemost. 2004;91:119–28. doi: 10.1160/TH03-05-0330. doi: 10.1160/TH03-05-0330. [DOI] [PubMed] [Google Scholar]
- 16.Pérez V, Bel N, Celada P, Ortiz J, Alvarez E, Artigas F. Relationship between blood serotonergic variables, melancholic traits, and response to antidepressant treatments. J Clin Psychopharmacol. 1998;18:222–30. doi: 10.1097/00004714-199806000-00007. doi: 10.1097/00004714-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Mashour NH, Lin GI, Frishman WH. Herbal medicine for the treatment of cardiovascular disease: Clinical considerations. Arch Intern Med. 1998;158:2225–34. doi: 10.1001/archinte.158.20.2225. doi: 10.1001/archinte.158.20.2225. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Xu H. Integrative Western and Chinese medicine on coronary heart disease: Where is the orientation? Evid Based Complement Alternat Med 2013. 2013:459264. doi: 10.1155/2013/459264. doi: 10.1155/2013/459264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, Liu Y, Chen K. Roles and mechanisms of ginsenoside in cardiovascular diseases: Progress and perspectives. Sci China Life Sci. 2016;59:292–8. doi: 10.1007/s11427-016-5007-8. doi: 10.1007/s11427-016-5007-8. [DOI] [PubMed] [Google Scholar]
- 20.Hao K, Gong P, Sun SQ, Hao HP, Wang GJ, Dai Y, et al. Beneficial estrogen-like effects of ginsenoside Rb1, an active component of Panax ginseng, on neural 5-HT disposition and behavioral tasks in ovariectomized mice. Eur J Pharmacol. 2011;659:15–25. doi: 10.1016/j.ejphar.2011.03.005. doi: 10.1016/j.ejphar.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 22.Akbay E, Onur MA. A new modified myocardial infarction animal model. Cardiovasc Surg. 2013;1:69–71. doi: 10.5455/jcvs.2013137. [Google Scholar]
- 23.Rao ML, Hawellek B, Papassotiropoulos A, Deister A, Frahnert C. Upregulation of the platelet Serotonin2A receptor and low blood serotonin in suicidal psychiatric patients. Neuropsychobiology. 1998;38:84–9. doi: 10.1159/000026522. doi: 10.1159/000026522. [DOI] [PubMed] [Google Scholar]
- 24.Flores-Ramos M, Moreno J, Heinze G, Aguilera-Pérez R, Pellicer Graha F. Gonadal hormone levels and platelet tryptophan and serotonin concentrations in perimenopausal women with or without depressive symptoms. Gynecol Endocrinol. 2014;30:232–5. doi: 10.3109/09513590.2013.875994. doi: 10.3109/09513590.2013.875994. [DOI] [PubMed] [Google Scholar]
- 25.Sekiyama T, Nakatani Y, Yu X, Seki Y, Sato-Suzuki I, Arita H. Increased blood serotonin concentrations are correlated with reduced tension/anxiety in healthy postpartum lactating women. Psychiatry Res. 2013;209:560–5. doi: 10.1016/j.psychres.2013.03.009. doi: 10.1016/j.psychres.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Zahn D, Petrak F, Franke L, Hägele AK, Juckel G, Lederbogen F, et al. Cortisol, platelet serotonin content, and platelet activity in patients with major depression and type 2 diabetes: An exploratory investigation. Psychosom Med. 2015;77:145–55. doi: 10.1097/PSY.0000000000000145. doi: 10.1097/PSY.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 27.Cleare AJ. Reduced whole blood serotonin in major depression. Depress Anxiety. 1997;5:108–11. doi: 10.1002/(sici)1520-6394(1997)5:2<108::aid-da8>3.0.co;2-b. doi: 10.1159/000026522. [DOI] [PubMed] [Google Scholar]
- 28.Schins A, Honig A, Crijns H, Baur L, Hamulyák K. Increased coronary events in depressed cardiovascular patients: 5-HT2A receptor as missing link? Psychosom Med. 2003;65:729–37. doi: 10.1097/01.psy.0000088596.42029.10. doi: 10.1097/01.PSY.0000088596.42029.10. [DOI] [PubMed] [Google Scholar]
- 29.Wulsin LR, Musselman D, Otte C, Bruce E, Ali S, Whooley MA. Depression and whole blood serotonin in patients with coronary heart disease from the Heart and Soul Study. Psychosom Med. 2009;71:260–5. doi: 10.1097/PSY.0b013e31819cc761. doi: 10.1097/PSY.0b013e31819cc761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu MY, Ren YP, Wei WL, Tian GX, Li G. Changes of serotonin (5-HT), 5-HT2A receptor, and 5-HT transporter in the Sprague-Dawley rats of depression, myocardial infarction and myocardial infarction co-exist with depression. Chin Med J. 2015;128:1905–9. doi: 10.4103/0366-6999.160526. doi: 10.4103/0366-6999.160526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: Regulation of plasma serotonin levels. Mol Interv. 2010;10:231–41. doi: 10.1124/mi.10.4.6. doi: 10.1124/mi.10.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molderings GJ, Brüss M, Göthert M. Functional and molecular identification of 5-hydroxytryptamine receptors in rabbit pulmonary artery: Involvement in complex regulation of noradrenaline release. Pharmacol Rep. 2006;58:188–99. [PubMed] [Google Scholar]
- 33.Ramage AG, Villalón CM. 5-hydroxytryptamine and cardiovascular regulation. Trends Pharmacol Sci. 2008;29:472–81. doi: 10.1016/j.tips.2008.06.009. doi: 10.1016/j.tips.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Fisar Z, Kalisová L, Paclt I, Anders M, Vevera J. Platelet serotonin uptake in drug-naïve depressive patients before and after treatment with citalopram. Psychiatry Res. 2008;161:185–94. doi: 10.1016/j.psychres.2007.06.022. doi: 10.1016/j.psychres.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Ziegelstein RC, Parakh K, Sakhuja A, Bhat U. Platelet function in patients with major depression. Intern Med J. 2009;39:38–43. doi: 10.1111/j.1445-5994.2008.01794.x. doi: 10.1111/j.1445-5994.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Fan Y, Xiao S, Peng S, Dong X, Zheng X, et al. Decreased platelet 5-hydroxytryptamin (5-HT) levels: A response to antidepressants. J Affect Disord. 2015;187:84–90. doi: 10.1016/j.jad.2015.08.025. doi: 10.1016/j.jad.2015.08.025. [DOI] [PubMed] [Google Scholar]