Abstract
Background:
Pediatric infectious endophthalmitis is a serious sight-threatening disease for children. The purpose of this study was to investigate the etiology, microbiological spectrum, and visual outcomes of infectious endophthalmitis in children at a single institution in China.
Methods:
It is a retrospective study of the medical records of all patients under 14 years of age with histories of infectious endophthalmitis, treated at a single institution from January 1, 2009 to January 1, 2015. The clinical characteristics, etiology, microbiological spectrum, and management, as well as the visual outcomes, were analyzed. The Kappa test and Chi-square test were used in the statistical evaluation.
Results:
A total of 271 children were identified, with a mean age of 5.61 ± 2.93 years (range 5 months to 14 years). Ocular trauma (94.8%) and previous ocular surgery (3.0%) were the most common etiologies. Overall, 147 (54.2%) cases had positive cultures, and 176 organisms were isolated from these patients. A single species was isolated in 120 (81.6%) cases, with multiple organisms in 27 (18.4%) cases, and the most commonly identified organisms were coagulase-negative Staphylococcus and Streptococcus species, comprising 29.5% and 26.8% of the isolates, respectively. Moreover, of 176 isolates, 142 (80.8%) were Gram-positive organisms, 23 (13.0%) were Gram-negative organisms, and 11 (6.2%) were fungi. The final visual outcomes were 20/200 or better in 66 (24.4%) eyes, counting fingers to 20/200 in 34 (12.5%), hand motions in 30 (11.1%), light perception in 33 (12.2%), no light perception in 32 (11.8%), and 9 (3.3%) eyes were enucleated or eviscerated. The visual outcomes were not available in 67 (24.7%) patients.
Conclusions:
Penetrating ocular trauma is the most frequent cause of pediatric endophthalmitis in China. Streptococcus and Staphylococcus species are the most commonly identified organisms in exogenous pediatric endophthalmitis whereas Fusarium species are commonly seen in endogenous endophthalmitis. In this research, in spite of aggressive management with antibiotics and vitrectomy, the visual prognosis was found to be generally poor.
Keywords: Endophthalmitis, Ocular Trauma, Pediatric, Retina
Introduction
Pediatric infectious endophthalmitis is a rare but serious condition that results in visual impairment and blindness in a high percentage of cases. It can cause great suffering and economic burden to the children's families. Endophthalmitis has been reported in approximately 4.0% to 30.0% of open-globe injuries and might be more prevalent in developing countries.[1,2] In the pediatric population, the reported incidence of endophthalmitis following an open-globe injury ranges from 2.0% to 54.2%.[3,4,5,6] Unlike adults, who might complain of pain or blurred vision, children might be unable to either recognize or explain their symptoms, so it is more difficult to provide prompt diagnoses and treatments in the pediatric age group.
Worldwide, infectious endophthalmitis is an important cause of blindness. However, it is not as common in the pediatric age group as in adults, and less research has been devoted to this group. Available literature on pediatric endophthalmitis is scarce, especially for large sample studies. Thordsen et al.[7] reported 16 cases of pediatric endophthalmitis in 2008, and Al-Rashaed et al.[8] reported 49 cases of pediatric endophthalmitis in 2006. We carried out a 271-case retrospective study on this condition. In this study, we described the etiology, microbiological spectrum, management, and visual outcomes of infectious endophthalmitis in children at a single institution in China during the past six years, aiming to improve the diagnosis, treatment, and final visual prognosis of this unique set of patients.
Methods
This was a retrospective, noncomparative case series. In this study, we reviewed all of the medical records of patients under 14 years of age, with diagnoses of infectious endophthalmitis, treated at a single institution from January 1, 2009 to January 1, 2015. This study was approved by the ethics committee of the same institution.
The diagnosis of infectious endophthalmitis was based on patient histories, ophthalmic examinations, and supplementary examinations. The cases were divided into posttraumatic group (n = 257), postoperative group (n = 8), endogenous group (n = 2), infectious corneal ulcer group (n = 1), and agnogenic group (n = 3) with regard to etiology.
Information was extracted from both inpatient and outpatient records, including patient's age and gender, etiology, the time of primary hospitalization and initial treatment regimen, clinical symptoms and signs, vitreous and aqueous culture results, follow-up treatment, and final visual acuity (VA).
The vitreous and aqueous samples were obtained via either needle aspiration or pars plana vitrectomy (PPV), and the specimens were sent to the Microbiology Department for direct smears and cultures. All the patients received immediate intravenous and topical antibiotics as required, and further drug treatment was guided by the culture results. Intravitreal antibiotic injections were given to most patients as soon as possible. A PPV was considered if there was significant deterioration within 24 h, or if there was no therapeutic response within 48 h after the first intravitreal antibiotic injection. Moreover, a concurrent intravitreal injection of sensitive antibiotics could be administered at the same time. Early PPV was required in cases with the existence of an intraocular foreign body (IOFB), traumatic retinal detachment, displacement of the lens, and so on. Moreover, the organisms were divided by virulence type into nonvirulent coagulase-negative Staphylococcus, Corynebacterium, and Propionibacterium, with the other cultured organisms considered to be virulent.
Statistical analysis
The statistical analysis was performed with SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA), and descriptive statistics were obtained to determine the proportions. Continuous variables were expressed as mean ± standard deviation (SD). The Kappa test was used to evaluate the consistency between the direct smears and culture results of the specimens. The differences in the culture positivity in different years were also investigated using the Chi-square test. Moreover, the Chi-square test was used to evaluate the visual prognoses in different groups and to determine whether there was a relationship between the virulence of organism and visual prognosis. A value of P < 0.05 was considered statistically significant when the association between two groups was investigated. There were significant statistic differences between any two of the three groups if P < 0.0167.
Results
Clinical characteristics
Overall, 271 eyes of 271 children were identified (185 males [68.3%] and 86 females [31.7%]), with a mean age of 5.61 ± 2.93 years (range, 5 months to 14 years).
The distributions of the patient ages and onset years are illustrated in Table 1. From these data, we could note that the age of peak incidence was 3–6 years, more specifically 4–5 years. Of the 271 eyes in this study, 146 were right eyes and 125 were left eyes.
Table 1.
The distribution of patient's age and onset year (n = 271)
| Patients (years) | Onset year, n (Male/Female) | ||||||
|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | Total | |
| <1 | 0/0 | 0/0 | 0/1 | 0/0 | 2/0 | 0/0 | 3 (2/1) |
| 1≤ age <2 | 1/1 | 0/0 | 2/1 | 1/1 | 3/0 | 0/3 | 13 (7/6) |
| 2≤ age <3 | 1/2 | 3/1 | 0/0 | 2/1 | 3/0 | 1/4 | 18 (10/8) |
| 3≤ age <4 | 3/1 | 5/3 | 5/2 | 2/1 | 4/2 | 5/3 | 36 (24/12) |
| 4≤ age <5 | 5/2 | 3/1 | 2/1 | 8/0 | 7/4 | 5/3 | 41 (30/11) |
| 5≤ age <6 | 4/1 | 6/2 | 3/4 | 2/3 | 3/2 | 6/2 | 38 (24/14) |
| 6≤ age <7 | 4/1 | 3/1 | 3/0 | 6/1 | 4/1 | 3/1 | 28 (23/5) |
| 7≤ age <8 | 5/3 | 6/3 | 2/3 | 3/2 | 3/0 | 2/0 | 32 (21/11) |
| 8≤ age <9 | 1/0 | 1/2 | 1/2 | 2/4 | 1/0 | 2/0 | 16 (8/8) |
| 9≤ age <10 | 3/2 | 1/0 | 2/0 | 4/1 | 1/1 | 0/0 | 15 (11/4) |
| 10≤ age <11 | 0/0 | 2/0 | 2/0 | 3/1 | 0/1 | 1/0 | 10 (8/2) |
| 11≤ age <12 | 1/0 | 1/0 | 3/1 | 0/1 | 0/0 | 2/0 | 9 (7/2) |
| 12≤ age <13 | 2/0 | 0/1 | 2/1 | 1/0 | 0/0 | 1/0 | 8 (6/2) |
| 13≤ age <14 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 1 (1/0) |
| 14 | 2/0 | 0/1 | 0/0 | 0/0 | 0/0 | 0/0 | 3 (2/1) |
| Total | 45 (32/13) | 46 (31/15) | 44 (28/16) | 50 (34/16) | 42 (31/11) | 44 (28/16) | 271 (184/87) |
The etiologies of pediatric infectious endophthalmitis in this study are illustrated in Table 2, with the most common being ocular trauma, accounting for 257 (94.8%) of the 271 cases. Of these 257 cases, 21 had retained IOFBs, of which 11 cases had vitreous foreign bodies, four cases had ocular wall foreign bodies, four cases had anterior chamber foreign bodies, and two cases had corneal foreign bodies. Previous ocular surgery, as the second leading cause, included eight (3.0%) cases. Of the six remaining cases of endophthalmitis, one was secondary to an infectious corneal ulcer, two were endogenous, and three were agnogenic, as shown in Table 2.
Table 2.
Etiology for pediatric infectious endophthalmitis (n = 271)
| Etiology | Number of patients, n | Percentage (%) |
|---|---|---|
| Ocular trauma | 257 | 94.8 |
| Scissors | 56 | 20.6 |
| Disposable syringe | 40 | 14.8 |
| Plant branch | 31 | 11.4 |
| Metal wire/flake/stick | 17 | 6.3 |
| Crabstick | 17 | 6.3 |
| Pencils | 13 | 4.8 |
| Toys | 9 | 3.3 |
| Prod | 8 | 3.0 |
| Fireworks | 8 | 3.0 |
| Rope | 6 | 2.2 |
| Glasses | 6 | 2.2 |
| Iron nail | 4 | 1.5 |
| Fingernail | 4 | 1.5 |
| Explosion | 4 | 1.5 |
| Others | 34 | 12.4 |
| Previous ocular surgery | 8 | 3.0 |
| Cataract surgery | 5 | 1.8 |
| Glaucoma-filtering surgery | 2 | 0.8 |
| PPV | 1 | 0.4 |
| Infectious corneal ulcer | 1 | 0.4 |
| Endogenous | 2 | 0.7 |
| Agnogenic | 3 | 1.1 |
PPV: Pars plana vitrectomy.
In the cases associated with ocular trauma, the most common source was scissors (56 cases, 21.8%), followed by disposable syringes (40 cases, 15.6%), with a descending incidence from 22.7% in 2011 to 6.8% in 2014 [Figure 1]. The remaining causes are shown in Table 2. In this group, the time from the onset of ocular trauma to the ophthalmological examination was within 12 h in 41 cases, 12–24 h in 76 cases, 24–48 h in 39 cases, 2–7 days in 57 cases, more than seven days in 41 cases (range, 1–60 days), and an unknown injury time in three cases. There were eight cases associated with previous ocular surgery. Three of the five cases undergoing cataract surgery developed endophthalmitis within one month of the surgery while the other two presented with delayed onset at two months and one year. In the two cases associated with glaucoma surgery, endophthalmitis developed six months and two years after the surgery, respectively, due to blebitis. Only one case, which was diagnosed as persistent hyperplastic primary vitreous, developed endophthalmitis two days after the PPV.
Figure 1.
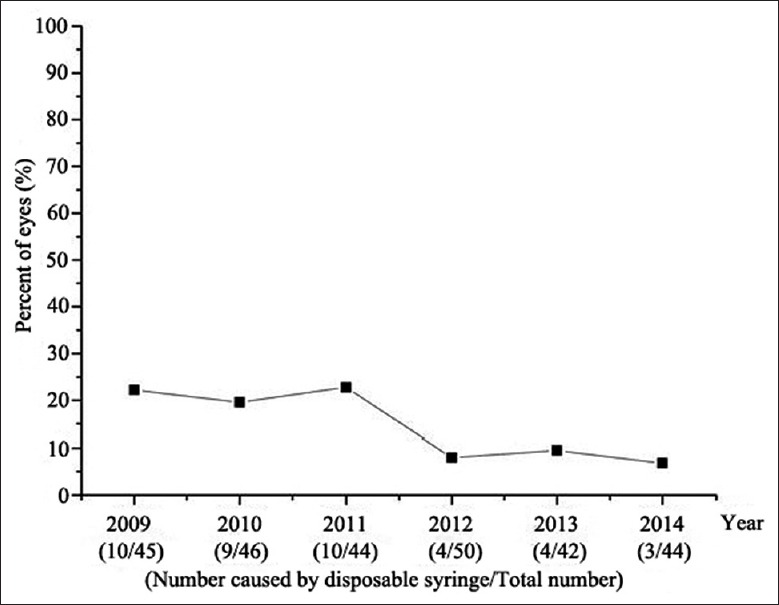
Percentage of cases caused by disposable syringe.
Endogenous causes of endophthalmitis are the least common in children. In our series, one case was found in an immunocompromised patient with leukemia, and another occurred in an infant with Candida sepsis.
The patients’ visual acuities at the time of the original presentation are illustrated in Table 3. The initial VA could not be obtained because of noncooperation in 134 (49.4%) patients.
Table 3.
Relationship between initial VA and final VA
| Final VA | Initial VA, n | ||||||
|---|---|---|---|---|---|---|---|
| NA | NLP | LP | HM | CF–20/200 | 20/200 or better | Total | |
| 20/200 or better | 24 | 0 | 5 | 10 | 12 | 15 | 66 |
| CF–20/200 | 11 | 1 | 7 | 12 | 3 | 0 | 34 |
| HM | 13 | 2 | 6 | 9 | 0 | 0 | 30 |
| LP | 13 | 0 | 13 | 5 | 2 | 0 | 33 |
| NLP | 11 | 5 | 16 | 0 | 0 | 0 | 32 |
| EE | 4 | 1 | 4 | 0 | 0 | 0 | 9 |
| NA | 58 | 0 | 0 | 5 | 4 | 0 | 67 |
| Total | 134 | 9 | 51 | 41 | 21 | 15 | 271 |
VA: Visual acuity; NA: Not available; NLP: No light perception; LP: Light perception; HM: Hand motions; CF: Counting fingers; EE: Enucleated or eviscerated.
Microbiology results
In all, there were 176 organisms isolated from 271 patients during this research, and 147 (54.2%) cases had positive cultures [Table 4]. The most common organism identified was Staphylococcus epidermidis, found in 24.5% (36/147) of the cases, followed by other coagulase-negative staphylococci in 10.9% (16/147), viridans streptococci in 11.6% (17/147), and Staphylococcus aureus in 8.8% (13/147). Moreover, the most common fungal species were Fusarium species (5/147). The isolates from this study included Gram-positive organisms in 80.8%, Gram-negative isolates in 13.0%, and fungi in 6.2%. Furthermore, one single species was isolated in 120 (81.6%) eyes, with multiple organisms found in 27 (18.4%) eyes.
Table 4.
Organisms isolated in pediatric infectious endophthalmitis
| Organism | Number of isolates, n | Percentage (%) |
|---|---|---|
| Gram-positive organisms | 142 | 80.8 |
| Staphylococcus epidermidis | 36 | 20.5 |
| Staphylococcus aureus | 13 | 7.4 |
| Other Staphylococcus | 16 | 9.1 |
| Streptococcus viridans | 17 | 9.7 |
| Group A Streptococcus | 13 | 7.4 |
| Other Streptococcus | 17 | 9.7 |
| Bacillus cereus | 9 | 5.1 |
| Aerococcus viridans | 6 | 3.4 |
| Bacillus subtilis | 4 | 2.3 |
| Corynebacterium minutissimum | 3 | 1.7 |
| Enterococcus species | 3 | 1.7 |
| Other Gram-positive bacteria | 5 | 2.8 |
| Gram-negative organisms | 23 | 13.0 |
| Enterobacter cloacae | 4 | 2.3 |
| Pseudomonas aeruginosa | 3 | 1.7 |
| Vibrio fluvialis | 3 | 1.7 |
| Klebsiella pneumoniae | 2 | 1.1 |
| Other Gram-negative bacteria | 11 | 6.2 |
| Fungi | 11 | 6.2 |
| Fusarium species | 5 | 2.8 |
| Aspergillus species | 3 | 1.7 |
| Candida species | 3 | 1.7 |
| Total, n | 176 | – |
–: No data.
In the posttraumatic group, coagulase-negative staphylococci were the most common isolates, including Staphylococcus epidermidis, Staphylococcus auricularis, and Staphylococcus warneri, comprising 36 (21.3%), 4 (2.4%), and 4 (2.4%) of the 169 isolates, respectively, in this group. In the postoperative group, four (50.0%) of eight cases had positive culture results, and the isolated organisms were Staphylococcus epidermidis, Streptococcus viridans, Bacillus cereus, and Klebsiella rhinoscleromatis. In both the groups, the Staphylococcus species were the most common isolates, comprising 61 (36.1%) of the 169 isolates, whereas they comprised 2 (40.0%) of the 5 isolates in the postoperative group.
In the 271 specimens, 29.9% (81 cases) of the direct smears were positive, and 54.2% (147 cases) of the cultures were positive. In all, 68 cases of 271 specimens were positive in both direct smears and cultures, showing low consistency between the direct smears and culture results (Kappa was 0.343, P < 0.05). The differences in culture positivity in different years were not statistically significant (P = 0.721).
Treatment and outcomes
All of the patients received immediate intravenous and topical antibiotics as soon as they were treated in our hospital. Vancomycin and ceftazidime or amphotericin B were used as intravitreal antibiotics, depending on the intraoperative direct smear results. PPV with intravitreal injection of antibiotics was directly performed in 121 (44.6%) patients due to an existing IOFB, severe vitreous hemorrhage/inflammation, retinal detachment, and serious lens injury. Fifty (18.5%) of the patients were treated with only vitreous sampling and antibiotic injections, one (0.4%) patient was treated with intravenous and topical antibiotics alone, 94 (34.7%) patients were initially treated with intravitreal injections of antibiotics, eventually undergoing PPV with repeated injections of intravitreal antibiotics because of persistent progressive endophthalmitis. Five (1.8%) eyes had to be enucleated or eviscerated due to devastating endophthalmitis and panophthalmitis that did not respond to the treatments, or that presented the risk of further spread of inflammation.
Visual prognosis
The mean follow-up time was 18.7 months (range 5 months to 4 years), and the distributions of the initial and final visual acuities are presented in Table 3. The final VA could not be obtained in 67 (24.7%) patients because the children were too young to distinguish letters or were lost to follow-up. Of the 271 eyes, only 66 (24.4%) patients achieved final visual acuities of 20/200 or better, but 74 (27.3%) patients had a final VA of no more than light perception. In this study, we did not find any statistically significant association between delay in primary repair and posttraumatic endophthalmitis [Table 5].
Table 5.
Relationship between primary hospitalization time and final VA
| Primary hospitalization time | Final VA, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| EE | NLP | LP | HM | CF–20/200 | 20/200 or better | Total | |
| <24 h | 1 (1.1) | 11 (12.2) | 11 (12.2) | 12 (13.3) | 20 (22.2) | 35 (38.9) | 90 (47.1) |
| 1–7 days | 6 (8.5) | 10 (14.1) | 13 (18.3) | 11 (15.5) | 10 (14.1) | 21 (29.6) | 71 (37.2) |
| >7 days | 2 (6.7) | 8 (26.7) | 7 (23.3) | 5 (16.7) | 3 (10.0) | 5 (16.7) | 30 (15.7) |
| Total | 9 (4.7) | 29 (15.2) | 31 (16.2) | 28 (14.7) | 33 (17.3) | 61 (31.9) | 191 (100.0) |
VA: Visual acuity; EE: Enucleated or eviscerated; NLP: No light perception; LP: Light perception; HM: Hand motions; CF: Counting fingers.
In this series, despite aggressive management with antibiotics and PPVs, the final visual prognosis was poor. To determine if differences in the operative method can affect the final VA, a comparison of the use of PPV and intravitreal antibiotic injection was performed between three groups as shown in Table 6 (Groups a, b, and c). The percentage of children with a final VA of no light perception (NLP) was higher in Group a (25.0%) than that in Group b (2.6%), which is statistically significant. However, a higher percentage of children in Group b (68.4%) than that in Group a (25.0%) had a final VA of 20/200 or better, which was statistically significant.
Table 6.
Relationship between operative method and final VA
| Operative method | Final VA, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| EE | NLP | LP | HM | CF–20/200 | 20/200 or better | Total | |
| Primary PPV + intravitreal antibiotic injection (a) | 2 (2.1) | 24 (25.0) | 18 (18.8) | 14 (14.6) | 14 (14.6) | 24 (25.0) | 96 (48.0) |
| Intravitreal antibiotic injection only (b) | 0 (0) | 1 (2.6) | 4 (10.5) | 2 (5.3) | 5 (13.2) | 26 (68.4) | 38 (19.0) |
| Initial intravitreal antibiotic injection + subsequent PPV with repeated injections (c) | 3 (4.5) | 8 (12.1) | 10 (15.2) | 14 (21.2) | 15 (22.7) | 16 (24.2) | 66 (33.0) |
| χ2, P | |||||||
| a versus b | 0.804, 0.370 | 8.976, 0.003* | 1.342, 0.247 | 2.249, 0.134 | 0.045, 0.831 | 21.944, <0.0001* | – |
| a versus c | 0.793, 0.373 | 4.092, 0.043 | 0.354, 0.552 | 1.202, 0.273 | 1.765, 0.184 | 0.012, 0.913 | – |
| b versus c | 1.779, 0.182 | 2.747, 0.097 | 0.443, 0.506 | 4.712, 0.030 | 1.422, 0.306 | 19.550, <0.0001* | – |
In all, 265 cases were given the above operative method, of which 200 cases had final VA (not available: 65). The difference between any two of the three groups was significant at the level *P<0.0167 at baseline tested by Chi-square test. –: No data; PPV: Pars plana vitrectomy; VA: Visual acuity; EE: Enucleated or eviscerated; NLP: No light perception; LP: Light perception; HM: Hand motions; CF: Counting fingers.
In the postoperative group, 5 (62.5%) of 8 eyes attained final visual outcomes of 20/200 or better, compared with 60 (23.3%) of 257 in the posttraumatic group; this difference was statistically significant (P < 0.05).
Overall, 56 (31.8%) of 176 isolates were nonvirulent organisms whereas 120 (68.2%) were virulent. Of 35 children who had nonvirulent organisms, three (8.6%) had a final VA of NLP, compared with 24 (28.2%) of 85 children who had virulent organisms (P = 0.019), while 13 (37.1%) of 35 children who had nonvirulent organisms had a final VA of 20/200 or better, compared with 16 (18.8%) of 85 children who had virulent organisms (P = 0.033). However, in other final VA groups, the signification was not detected [Table 7].
Table 7.
Relationship between organism virulence type and final VA
| Virulence type | Final VA, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| EE | NLP | LP | HM | CF–20/200 | 20/200 or better | Total | |
| Nonvirulent | 0 | 3 (8.6) | 7 (20.0) | 5 (14.3) | 7 (20.0) | 13 (37.1) | 35 (29.2) |
| Virulent | 7 (8.2) | 24 (28.2) | 13 (15.3) | 16 (18.8) | 9 (10.6) | 16 (18.8) | 85 (70.8) |
| χ2 | 3.061 | 5.497 | 0.395 | 0.354 | 1.900 | 4.540 | – |
| P | 0.080 | 0.019* | 0.530 | 0.552 | 0.168 | 0.033* | – |
The difference between groups was significant at the level *P<0.05 at baseline tested by Chi-square test. –: No data; VA: Visual acuity; EE: Enucleated or eviscerated; NLP: No light perception; LP: Light perception; HM: Hand motions; CF: Counting fingers.
Discussion
Pediatric infectious endophthalmitis is a rare but serious complication that might result from trauma, intraocular surgery, or systemic infection. In this series of cases, ocular trauma was the most common leading cause. In contrast to sharp tools and objects thrown at the child which were the most common etiologies in developed countries, scissors and disposable syringes attained the first two places in China.[9,10] Recently, the percentage of cases caused by disposable syringes had decreased slightly, which might be related to the increased emphasis on medical waste management, as well as parents being more involved in the care of their children.
In this study, 21 (8.2%) eyes had retained IOFBs, which are thought to be a significant high-risk factor of endophthalmitis. A previous study by Essex et al.[11] reported that the frequency of endophthalmitis after an open-globe injury was 13.0% in cases with IOFBs, compared with 4.4% in IOFB-negative cases in the whole population. However, IOFB is not as common in adults as in children.
Previous ocular surgery was the second leading cause of endophthalmitis, especially in cataract and glaucoma-filtering surgeries because congenital cataracts and congenital glaucoma were found to be the most common eye diseases in children.[12,13,14] With regard to cataract surgery-related endophthalmitis, the operation process itself and early postoperative nursing were the key factors.[15] The predisposing factors might include inadequate disinfection, leakage of the incision, vitreous loss, and loss of sutures.[16] The use of a 5.0% povidone-iodine solution has been proved to be effective in decreasing the incidence of infectious endophthalmitis.[17] It is also recommended that selected prophylactic antibiotics are injected into the anterior chamber at the end of a cataract operation.
With regard to glaucoma-filtering-surgery-related endophthalmitis, the occurrence of delayed onset symptoms was closely related to thin-wall-filtering blebs. Mitomycin C, which has been defined as a dangerous risk factor, was used in both of the cases in our series that progressed to endophthalmitis.[18,19,20] However, the use of mitomycin C was essential in some patients. The results reported by Giampani et al.[21] showed that trabeculectomy with mitomycin C was safe and effective for the treatment of congenital glaucoma, and that the frequency of bleb-related endophthalmitis was no higher than that described in adults. In some studies in developed countries, postoperative endophthalmitis was the most common form, and Benz et al.[22] showed that postoperative endophthalmitis accounts for 72.0% of all the endophthalmitis cases. This result was quite different from ours.
The low consistency between direct smears and culture results means that some of the cases had positive smear results but negative cultural results, while other cases had the opposite results. This suggests that the combined application of smears and cultures could improve the diagnosis rate.
In this series of pediatric endophthalmitis cases, the Streptococcus group and the coagulase-negative Staphylococci group were the most common isolates. Further, Bacillus cereus, which is well known for its many virulence factors, attained third place. Bacillus species spores have been reported to be abundant in the soil; therefore, Bacillus-related endophthalmitis usually occurs in cases of rural posttraumatic endophthalmitis.[23] It has been reported that the severity and rapidity of the progression of endophthalmitis are associated with the virulence of the infecting organism, and that more virulent organisms usually lead to worse visual outcomes, which is consistent with our results.[8,24,25,26] Another study has reported that the most common fungal isolates were Candida species.[27] This was different from our study, which might be due to the limited number of cases in our study and might not be representative of those found in larger data studies.
Staphylococcus epidermidis was the most common isolate in the postoperative group, which was also the most commonly seen normal flora in the conjunctiva. This result is consistent with those reported in previous series of endophthalmitis.[8,28,29,30] In the research carried out by the Endophthalmitis Vitrectomy Study (EVS), 94% of the isolates were found to be Gram-positive organisms. EVS also found that the coagulase-negative staphylococci from the patient's periocular skin flora contribute significantly to cataract surgery-related endophthalmitis.[29]
There were some differences in the positivity distributions of the Gram-negative organisms in different countries and districts. It has been suggested that the proportion of Gram-negative organisms in the pathogenic species of postoperative endophthalmitis is not only related to aseptic manipulation during surgery and nursing after the operation but also associated with the level of local economic development.[31,32]
We did not find any association between delay in primary repair and posttraumatic endophthalmitis. However, Thompson et al.[1] found a slight trend toward a higher incidence of infection if primary repair was delayed longer than 24 h after the initial injury compared with those eyes that received primary repair within 24 h; however, this trend did not reach significance.
The role of the PPV in the management of endophthalmitis in the pediatric population has been getting more and more attention. In this research, Group b (intravitreal antibiotic injection only) seemed to have a better visual prognosis. The reason for this might be that the patients’ conditions in Group b were relatively mild compared with the other two groups. If there was significant deterioration or no therapeutic response after intravitreal antibiotic injection alone, the PPV was performed. Therefore, it is not only our clinical impression but also that of many others, that eyes with severe forms of endophthalmitis in the pediatric population benifit from vitrectomy.[33,34,35] As reported by Al-Rashaed et al.,[8] the cases treated with primary PPV and intravitreal injection of antibiotics had better visual outcomes than those treated with antibiotics alone on presentation. Among the patients who underwent primary PPVs, 18.5% had a final visual outcome of NLP, compared with 47.1% of those patients treated with antibiotics alone. Moreover, 26.0% of the patients who underwent primary PPV attained a final visual outcome of counting fingers, compared with 5.9% of those patients who were treated with antibiotics alone.[8] All of these results supported the use of the PPV in cases of pediatric endophthalmitis.
There are several factors that account for delays in diagnosis and treatment of pediatric endophthalmitis. For one thing, children are usually poor historians, so they might not complain of symptoms on time promptly. For another, treatment might be delayed by parents, or by primary care doctors, if the presenting signs are not obvious. To prevent traumatic endophthalmitis, corresponding literature and education must be provided to adolescents and children. In this study, disposable syringes were the second most common cause of traumatic endophthalmitis. Syringe needles have strong perforating ability, and the wounds are usually tiny and self-sealing. Combined with the behavioral characteristics of children, these injuries are very easily overlooked. More attention should be paid to the risks of disposable syringe-induced traumatic endophthalmitis in our country while policies and regulations related to disposable syringe management should be further developed and perfected.
Compared with adult endophthalmitis, pediatric endophthalmitis has its own characteristics. First, the visual outcomes are generally poor. For some severe cases, even if the VA cannot be preserved, the eyeball should be retained as much as possible because the eyeball contour is important for the development of the orbit and the children's psychological health. Second, diagnosis and treatment are likely to be delayed. Third, the vitreous space of children is filled with gel, which hinders the dispersion of drugs in the vitreous cavity, and children are more likely to get retinal detachment complicated by proliferative vitreoretinopathy.
This study still has several limitations. The data are from a single institution and might not be representative of those found in other organizations. Initial diagnoses and treatments were performed by different doctors in different medical institutions. In this series, endophthalmitis following an operation had a better prognosis than that of other causes. However, this finding is limited by the small sample size and is subject to bias because it was performed in the pediatric population, so the results cannot be extrapolated to the general population of a different age group.
In summary, penetrating ocular trauma was found to be the most frequent cause of pediatric endophthalmitis in China, especially with scissors and disposable syringes. Streptococcus and Staphylococcus species were the most commonly identified organisms. In spite of aggressive management with antibiotics and PPV, endophthalmitis in the pediatric population is usually associated with a poor visual prognosis.
In this retrospective study, we provided detailed epidemiological data relating to the etiology, microbiology, management, and visual outcomes of infectious endophthalmitis in children at a single institution in China over the past 6 years. We hope that this could help promptly diagnose, guide treatment, and improve the prognoses in this special community.
Financial support and sponsorship
This research was supported by a grant from National Natural Science Foundation for Young Scholar of China (No.81400410).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
References
- 1.Thompson WS, Rubsamen PE, Flynn HW, Jr, Schiffman J, Cousins SW. Endophthalmitis after penetrating trauma. Risk factors and visual acuity outcomes. Ophthalmology. 1995;102:1696–701. doi: 10.1016/s0161-6420(95)30807-x. doi: 10.1016/S0161-6420(95)30807-X. [DOI] [PubMed] [Google Scholar]
- 2.Boldt HC, Pulido JS, Blodi CF, Folk JC, Weingeist TA. Rural endophthalmitis. Ophthalmology. 1989;96:1722–6. doi: 10.1016/s0161-6420(89)32658-3. doi: 10.1016/S0161-6420(89)32658-3. [DOI] [PubMed] [Google Scholar]
- 3.Narang S, Gupta V, Simalandhi P, Gupta A, Raj S, Dogra MR. Paediatric open globe injuries. Visual outcome and risk factors for endophthalmitis. Indian J Ophthalmol. 2004;52:29–34. [PubMed] [Google Scholar]
- 4.Scharf J, Zonis S. Perforating injuries of the eye in childhood. J Pediatr Ophthalmol. 1976;13:326–8. [PubMed] [Google Scholar]
- 5.Junejo SA, Ahmed M, Alam M. Endophthalmitis in paediatric penetrating ocular injuries in Hyderabad. J Pak Med Assoc. 2010;60:532–5. [PubMed] [Google Scholar]
- 6.Lee CH, Lee L, Kao LY, Lin KK, Yang ML. Prognostic indicators of open globe injuries in children. Am J Emerg Med. 2009;27:530–5. doi: 10.1016/j.ajem.2008.04.004. doi: 10.1016/j.ajem.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Thordsen JE, Harris L, Hubbard GB., 3rd Pediatric endophthalmitis. A 10-year consecutive series. Retina. 2008;28(3 Suppl):S3–7. doi: 10.1097/IAE.0b013e318159ec7f. doi: 10.1097/IAE.0b013e318159ec7f. [DOI] [PubMed] [Google Scholar]
- 8.Al-Rashaed SA, Abu El-Asrar AM. Exogenous endophthalmitis in pediatric age group. Ocul Immunol Inflamm. 2006;14:285–92. doi: 10.1080/09273940600954323. doi: 10.1080/09273940600954323. [DOI] [PubMed] [Google Scholar]
- 9.Thompson CG, Kumar N, Billson FA, Martin F. The aetiology of perforating ocular injuries in children. Br J Ophthalmol. 2002;86:920–2. doi: 10.1136/bjo.86.8.920. doi: 10.1136/bjo.86.8.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beby F, Kodjikian L, Roche O, Donate D, Kouassi N, Burillon C, et al. Perforating ocular injuries in children: A retrospective study of 57 cases. J Fr Ophtalmol. 2006;29:20–3. doi: 10.1016/s0181-5512(06)73742-1. doi: 10.1016/S0181-5512(06)73742-1. [DOI] [PubMed] [Google Scholar]
- 11.Essex RW, Yi Q, Charles PG, Allen PJ. Post-traumatic endophthalmitis. Ophthalmology. 2004;111:2015–22. doi: 10.1016/j.ophtha.2003.09.041. doi: 10.1016/j.ophtha.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Good WV, Hing S, Irvine AR, Hoyt CS, Taylor DS. Postoperative endophthalmitis in children following cataract surgery. J Pediatr Ophthalmol Strabismus. 1990;27:283–5. doi: 10.3928/0191-3913-19901101-03. [DOI] [PubMed] [Google Scholar]
- 13.Waheed S, Ritterband DC, Greenfield DS, Liebmann JM, Sidoti PA, Ritch R. Bleb-related ocular infection in children after trabeculectomy with mitomycin C. Ophthalmology. 1997;104:2117–20. doi: 10.1016/s0161-6420(97)30051-7. doi: 10.1016/S0161-6420(97)30051-7. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler DT, Stager DR, Weakley DR., Jr Endophthalmitis following pediatric intraocular surgery for congenital cataracts and congenital glaucoma. J Pediatr Ophthalmol Strabismus. 1992;29:139–41. doi: 10.3928/0191-3913-19920501-04. [DOI] [PubMed] [Google Scholar]
- 15.Taban M, Behrens A, Newcomb RL, Nobe MY, Saedi G, Sweet PM, et al. Acute endophthalmitis following cataract surgery: A systematic review of the literature. Arch Ophthalmol. 2005;123:613–20. doi: 10.1001/archopht.123.5.613. doi: 10.1001/archopht.123.5.613. [DOI] [PubMed] [Google Scholar]
- 16.Herretes S, Stark WJ, Pirouzmanesh A, Reyes JM, McDonnell PJ, Behrens A. Inflow of ocular surface fluid into the anterior chamber after phacoemulsification through sutureless corneal cataract wounds. Am J Ophthalmol. 2005;140:737–40. doi: 10.1016/j.ajo.2005.03.069. doi: 10.1016/j.ajo.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 17.Packer M, Chang DF, Dewey SH, Little BC, Mamalis N, Oetting TA, et al. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37:1699–714. doi: 10.1016/j.jcrs.2011.06.018. doi: 10.1016/j.jcrs.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Ciulla TA, Beck AD, Topping TM, Baker AS. Blebitis, early endophthalmitis, and late endophthalmitis after glaucoma-filtering surgery. Ophthalmology. 1997;104:986–95. doi: 10.1016/s0161-6420(97)30196-1. doi: 10.1016/S0161-6420(97)30196-1. [DOI] [PubMed] [Google Scholar]
- 19.Brown RH, Yang LH, Walker SD, Lynch MG, Martinez LA, Wilson LA. Treatment of bleb infection after glaucoma surgery. Arch Ophthalmol. 1994;112:57–61. doi: 10.1001/archopht.1994.01090130067019. doi: 10.1001/archopht.1994.01090130067019. [DOI] [PubMed] [Google Scholar]
- 20.Zheng PF, Pang XQ. Bleb-associated endophthalmitis treated by sclera patch graft, vitrectomy and endoscopic cyclophotocoagulation. Chin Med J. 2012;125:3344–5. doi: 10.3760/cma.j.issn.0366-6999.2012.18.027. [PubMed] [Google Scholar]
- 21.Giampani J, Jr, Borges-Giampani AS, Carani JC, Oltrogge EW, Susanna R., Jr Efficacy and safety of trabeculectomy with mitomycin C for childhood glaucoma: A study of results with long-term follow-up. Clinics (Sao Paulo) 2008;63:421–6. doi: 10.1590/S1807-59322008000400002. doi: 10.1590/S1807-59322008000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benz MS, Scott IU, Flynn HW, Jr, Unonius N, Miller D. Endophthalmitis isolates and antibiotic sensitivities: A 6-year review of culture-proven cases. Am J Ophthalmol. 2004;137:38–42. doi: 10.1016/s0002-9394(03)00896-1. doi: 10.1016/S0002-9394(03)00896-1. [DOI] [PubMed] [Google Scholar]
- 23.Fekete T. Bacillus species and related genera other than Bacillus anthracis. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingstone; 2005. pp. 2493–6. [Google Scholar]
- 24.Verbraeken H, Rysselaere M. Post-traumatic endophthalmitis. Eur J Ophthalmol. 1994;4:1–5. doi: 10.1177/112067219400400101. [DOI] [PubMed] [Google Scholar]
- 25.Affeldt JC, Flynn HW, Jr, Forster RK, Mandelbaum S, Clarkson JG, Jarus GD. Microbial endophthalmitis resulting from ocular trauma. Ophthalmology. 1987;94:407–13. doi: 10.1016/s0161-6420(87)33447-5. doi: 10.1016/S0161-6420(87)33447-5. [DOI] [PubMed] [Google Scholar]
- 26.Al-Omran AM, Abboud EB, Abu El-Asrar AM. Microbiologic spectrum and visual outcome of posttraumatic endophthalmitis. Retina. 2007;27:236–42. doi: 10.1097/01.iae.0000225072.68265.ee. doi: 10.1097/01.iae.0000225072.68265.ee. [DOI] [PubMed] [Google Scholar]
- 27.Peyman GA, Carroll CP, Raichand M. Prevention and management of traumatic endophthalmitis. Ophthalmology. 1980;87:320–4. doi: 10.1016/s0161-6420(80)35240-8. doi: 10.1016/S0161-6420(80)35240-8. [DOI] [PubMed] [Google Scholar]
- 28.Khan S, Athwal L, Zarbin M, Bhagat N. Pediatric infectious endophthalmitis: A review. J Pediatr Ophthalmol Strabismus. 2014;51:140–53. doi: 10.3928/01913913-20140507-01. doi: 10.3928/01913913-20140507-01. [DOI] [PubMed] [Google Scholar]
- 29.Bannerman TL, Rhoden DL, McAllister SK, Miller JM, Wilson LA. The source of coagulase-negative staphylococci in the Endophthalmitis Vitrectomy Study. A comparison of eyelid and intraocular isolates using pulsed-field gel electrophoresis. Arch Ophthalmol. 1997;115:357–61. doi: 10.1001/archopht.1997.01100150359008. doi: 10.1001/archopht.1997.01100150359008. [DOI] [PubMed] [Google Scholar]
- 30.Shi XY, Zhao HS, Wei WB. Analysis of post-operative endophthalmitis after pars plana vitrectomy: A 10-year experience at a single center. Chin Med J. 2013;126:2890–3. doi: 10.3760/cma.j.issn.0366-6999.2013.07.98. [PubMed] [Google Scholar]
- 31.Eser I, Kapran Z, Altan T, Ozel Karatas M, Aydin D, Okaygun E, et al. Isolates and antibiotic sensitivity of eighty culture-proven endophthalmitis cases from Istanbul. Ophthalmologica. 2008;222:157–60. doi: 10.1159/000126077. doi: 10.1159/000126077. [DOI] [PubMed] [Google Scholar]
- 32.Anand AR, Therese KL, Madhavan HN. Spectrum of aetiological agents of postoperative endophthalmitis and antibiotic susceptibility of bacterial isolates. Indian J Ophthalmol. 2000;48:123–8. [PubMed] [Google Scholar]
- 33.Cakir M, Cekiç O, Pekel G, Yilmaz OF. Pars plana vitrectomy results of exogenous endophthalmitis in children. Eur J Ophthalmol. 2010;20:424–8. doi: 10.1177/112067211002000225. [DOI] [PubMed] [Google Scholar]
- 34.Feng X, Feng K, Hu Y, Ma Z. Clinical features and outcomes of vitrectomy in pediatric ocular injuries-eye injury vitrectomy study. Indian J Ophthalmol. 2014;62:450–3. doi: 10.4103/0301-4738.120222. doi: 10.4103/0301-4738.120222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfaro DV, Roth DB, Laughlin RM, Goyal M, Liggett PE. Paediatric post-traumatic endophthalmitis. Br J Ophthalmol. 1995;79:888–91. doi: 10.1136/bjo.79.10.888. doi: 10.1007/s00417-016-3330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


