Abstract
Background:
Traditional Chinese medicine wogonin plays an important role in the treatment of leukemia. Recently, the application of drug-coated magnetic nanoparticles (MNPs) to increase water solubility of the drug and to enhance its chemotherapeutic efficiency has attracted much attention. Drugs coated with MNPs are becoming a promising way for better leukemia treatment. This study aimed to assess the possible molecular mechanisms of wogonin-coated MNP-Fe3O4 (Wog-MNPs-Fe3O4) as an antileukemia agent.
Methods:
After incubated for 48 h, the antiproliferative effects of MNPs, wogonin, or Wog-MNPs-Fe3O4 on K562/A02 cells were determined by methyl thiazolyl tetrazolium (MTT) assay. The apoptotic rates of K562/A02 cells treated with either wogonin or Wog-MNPs-Fe3O4 were determined by flow cytometer (FCM) assay. The cell cycle arrest in K562/A02 cells was determined by FCM assay. The elementary molecular mechanisms of these phenomena were explored by Western blot and reverse transcriptase polymerase chain reaction (RT-PCR).
Results:
With cell viabilities ranging from 98.76% to 101.43%, MNP-Fe3O4 was nontoxic to the cell line. Meanwhile, the wogonin and Wog-MNPs-Fe3O4 had little effects on normal human embryonic lung fibroblast cells. The cell viabilities of the Wog-MNPs-Fe3O4 group (28.64–68.36%) were significantly lower than those of the wogonin group (35.53–97.28%) in a dose-dependent manner in 48 h (P < 0.001). The apoptotic rate of K562/A02 cells was significantly improved in 50 μmol/L Wog-MNPs-Fe3O4 group (34.28%) compared with that in 50 μmol/L wogonin group (23.46%; P < 0.001). Compared with those of the 25 and 50 μmol/L wogonin groups, the ratios of G0/G1-phase K562/A02 cells were significantly higher in the 25 and 50 μmol/L Wog-MNPs-Fe3O4 groups (all P < 0.001). The mRNA and protein expression levels of the p21 and p27 in the K562/A02 cells were also significantly higher in the Wog-MNPs-Fe3O4 group compared with those of the wogonin group (all P < 0.001).
Conclusions:
This study demonstrated that MNPs were the effective drug delivery vehicles to deliver wogonin to the leukemia cells. Through increasing cells arrested at G0/G1-phase and inducing apoptosis of K562/A02 cells, MNPs could enhance the therapeutic effects of wogonin on leukemia cells. These findings indicated that MNPs loaded with wogonin could provide a promising way for better leukemia treatment.
Keywords: Apoptosis, Cell Cycle, Leukemia Cell, Magnetic Nanoparticle, Wogonin
Introduction
Leukemia is a hematologic malignancy caused by abnormal hematopoietic stem cell clones. Unfortunately, more and more leukemia cases were diagnosed from 2007 to 2011 in China. With poor outcomes, leukemia is considered as the sixth leading cause of cancer-related deaths in males and females in China.[1] The first line of treatment for leukemia is chemotherapy. Unfortunately, some patients undergoing chemotherapy suffer from one or more side effects and complications, and the survival rate is low because of increased drug resistance; furthermore, numerous patients experience relapse after remission. As a treatment for leukemia, stem cell transplantation is costly for considerable patients. As such, only a few patients can be successfully treated with stem cell transplantation, which also poses health risks. Therefore, novel therapeutic strategies should be developed to improve therapeutic effects and prolong the disease-free survival of patients with leukemia.
Wogonin [5,7-dihydroxy-8-methoxyflavone; Figure 1a and 1b], a bioflavonoid extracted from the root of Scutellaria baicalensis Georgi, a kind of traditional Chinese medicine (TCM), elicits multiple pharmacological effects, including cytotoxic effects against human cancer cell lines;[2,3,4,5,6] this bioflavonoid also provides therapeutic effects on some hematologic malignancies, such as leukemia, mostly by inducing apoptosis and cell cycle arrest in vitro.[7,8,9,10] Wogonin can also inhibit the proliferation of tumor cells in vivo.[7,11] Compared with conditional chemotherapy drugs, wogonin is an optimum natural anticancer candidate, which is barely toxic or nontoxic to normal cells.[12] However, its low solubility in water remains a problem and restricts clinical administration.
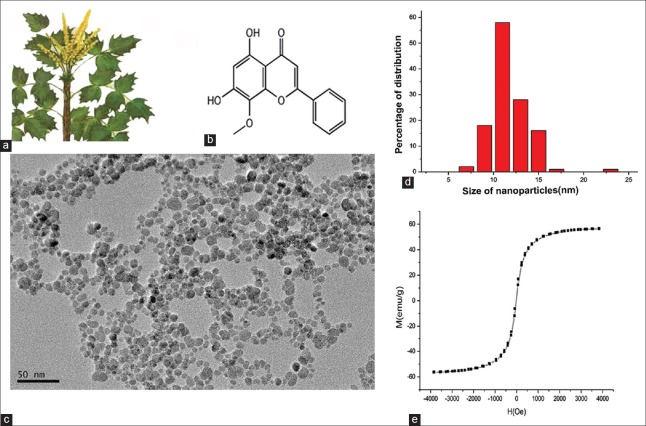
Figure 1.
Characteristics of wogonin and MNPs. (a) Scutellaria baicalensis Georgi. (b) Molecular structure of wogonin, C16H12O5. (c) Size and morphology of particles characterized by transmission electron microscope. (d) Diameter distribution of magnetic nanoparticles. (e) Magnetic properties of particles investigated by vibrating sample magnetometer. H: Magnetic field intensity; M: Magnetic susceptibility; MNP: Magnetic nanoparticles.
With the rapid development of magnetic nanoparticles (MNPs), the above problems might be resolved. MNPs, exhibiting biocompatibility, low toxicity, biodegradability, and high volume-to-surface ratios, are potential safe materials commonly used in medical applications.[13] With the improvement of drug solubility,[14] magnetic-targeted drug delivery,[15] and magnetic-targeting hyperthermia,[16] MNPs may be considered as an efficient drug delivery vehicles, especially for cancer treatment. MNPs have been used as diagnostic tools and contrast agents in magnetic resonance imaging; MNPs also play an important role in the detection of tumor-related conditions, such as tumor micrometastasis.[17,18,19]
In this study, a wogonin-coated MNP-Fe3O4 (Wog-MNPs-Fe3O4) drug delivery system was proposed for tumor therapy. This study aimed to assess the feasibility and advantages of Wog-MNPs-Fe3O4 as an antileukemia agent. The possible molecular mechanisms were also investigated.
Methods
Main materials
Wogonin (provided by Jiangsu Key Lab Carcinogenesis and Intervention, China Pharmaceutical University, Nanjing, China) was dissolved in dimethylsulfoxide (DMSO) and stored at −20°C. The solution was diluted as needed in Roswell Park Memorial Institute (RPMI) 1640 medium. The following kits were used: Annexin V-fluorescein isothiocyanate apoptosis detection kit (KeyGen Biotech Co., Ltd., Nanjing, China); methyl thiazolyl tetrazolium (MTT; Sigma-Aldrich, USA); CycleTEST Plus DNA Reagent Kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China); and reverse transcriptase polymerase chain reaction (RT-PCR) kit (Takara Biotechnology, Japan). Monoclonal antibodies, including p21, p27, and β-actin antibodies, were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA). All the other chemicals were of analytical grade.
Preparations of wogonin-coated magnetic nanoparticle-Fe3O4
MNPs-Fe3O4 were prepared by co-precipitating FeCl2 and FeCl3 at a 1:2 molar ratio in an alkali ammonia solution.[10] Various wogonin concentrations were mixed into MNPs through mechanical absorption polymerization and maintained in a refrigerator at 4°C for more than 48 h to prepare Wog-MNPs-Fe3O4.
Cell culture
Leukemia cell line K562/A02 cells (Jiangsu Institute of Hematology, Suzhou, China) and human embryonic lung fibroblast (HELF) cells (Shanghai Institute of Cells, Chinese Academy of Sciences, Shanghai, China) were cultured in a humidified atmosphere containing 5% CO2 at 37°C in RPMI 1640 supplemented with 10% fetal bovine serum (Sijiqing, Hangzhou, China), 100 µg/ml streptomycin (Sigma-Aldrich, USA), and 100 U/ml penicillin (Sigma-Aldrich, USA). The cells in the logarithmic growth phase were used in the experiments. K562/A02 and HELF cells (1 × 106/ml) in the log phase were seeded onto 96-well plates incubated with MNPs, wogonin, or Wog-MNPs-Fe3O4 for 24, 48, and 72 h; the concentrations of MNPs, wogonin, or Wog-MNPs-Fe3O4 were regulated simultaneously. The nontreated K562/A02 cells were set as the blank group (A) and the K562/A02 cells treated with 35 µg/ml MNPs as the negative group (B). Meanwhile, other experimental groups for K562/A02 cells were treated with 25 µmol/L wogonin (C); 25 µmol/L Wog-MNPs-Fe3O4 (D); 50 µmol/L wogonin (E); and 50 µmol/L Wog-MNPs-Fe3O4 (F).
MTT assay for K562/A02 cell proliferation
In vitro cytotoxicity was determined by the MTT assay. After treatment, MTT solutions were added to each well at 37°C for 4 h. DMSO was added to the solubilized crystals, and optical density at 570 nm (A570) was recorded. Cell viability (%) was calculated as follows: Atest group/Acontrol group × 100. Cell inhibition rate (%) was defined as follows: (1 − Atest group/Acontrol group) × 100. Each assay was repeated at least thrice. In this assay, we determined the cell compatibility of MNPs at different concentrations. To detect the cytotoxicity to human normal cells, we also tested the cell viability of HELF cells when treated with wogonin and Wog-MNPs-Fe3O4. Subsequently, we chose a constant concentration, i.e., 35 µg/ml of MNPs to coat different concentrations of wogonin so that we could assess the cell inhibition effect of wogonin when combining MNPs.
Apoptosis assay of wogonin and wogonin-coated magnetic nanoparticle-Fe3O4 for K562/A02 cells
After 48 h of treatment, cells were collected, washed, and centrifuged. Subsequently, 500 µl of binding buffer and 5 µl of annexin V-fluorescein isothiocyanate solution were added. The resulting mixture was kept in dark at room temperature for 15 min. Analyses were conducted using a FACS Vantage Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Cell cycle analysis of wogonin and wogonin-coated magnetic nanoparticle-Fe3O4 for K562/A02 cells
The K562/A02 cells were treated with the same drug systems, collected, washed, and centrifuged; propidium iodide and ribonuclease were added for 30 min. Cell cycle analysis was performed using a CycleTEST plus DNA reagent kit. Flow cytometry analysis was performed as previously described.
Reverse transcriptase polymerase chain reaction assay
After the cells were incubated, the cells were lysed and 4 µg of RNA was extracted with TRIzol. The total RNA was added to reverse transcriptase buffer containing 25 mmol/L MgCl2, 10 mmol/L deoxyribonucleotide triphosphates, 50 pmol/µl random 9 mers, 40 U/µl RNase inhibitor, and 5 U/µl avian myeloblastosis virus reverse transcriptase to prepare a final total volume of 25 µl. After 24 h of treatment, the total RNA was isolated and used to synthesize cDNA. RT-PCR was then performed to determine the expression levels of p21, p27, and GAPDH (internal control). The designed PCR primers are shown in Table 1. The PCR products were arranged in terms of size by agarose gel electrophoresis. Densitometry was conducted to quantify the different bands by using Quantity One (BioRad). The ratio was calculated and compared with that of the internal control gene, and the results were plotted graphically.
Table 1.
The designed PCR primers of genes
| Primers | Sequences (5’–3’) | Amplification fragment (bp) |
|---|---|---|
| GAPDH | Forward: AAGGTCGGAGTCAACGGATTT Reverse: AGATGATGACCCTTTTGGCTC |
352 |
| p21 | Forward: TTAGCAGCGGAACAAGGAGT Reverse: AGAAACGGGAACCAGGACAC |
252 |
| p27 | Forward: TTGCCCGAGTTCTACTACAGA Reverse: CATTCCATGAAGTCAGCGATA |
461 |
PCR: Polymerase chain reaction; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Western blot analysis
Western blot analysis was conducted according to the standard protocols.[20] In brief, proteins extracted from each group were size fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to a polyvinylidene fluoride membrane. Primary monoclonal antibodies, including p21, p27, or β-actin antibodies, were detected using Western blot analysis, then horseradish peroxidase-conjugated anti-rabbit secondary antibody was detected. The protein band was observed by an enhanced chemiluminescence detection system (Amersham BioSciences UK Ltd., UK).
Statistical analysis
All experimental data were described as mean ± standard deviation (SD). Student's t-test or one-way analysis of variance (ANOVA) was used for evaluating differences. All analyses were performed using SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Results
Characterization of magnetic nanoparticles
The size and morphological characteristics of the nanoparticles were characterized by transmission electron microscopy. Nanoparticles exhibited a nearly spherical shape [Figure 1c]. In Figure 1d, the diameter of the nanoparticles ranged from 5.3 nm to 25.4 nm, and the mean size was 12.2 ± 4.1 nm. The mean hydrodynamic diameter of the nanoparticles was 30.2 ± 7.5 nm, and the mean zeta potential of the nanoparticles was −42.7 ± 9.8 mV. These results suggested that MNPs were stable in a colloidal solution under the influence of a magnet. The magnetic properties of the synthesized MNPs were investigated using a vibrating sample magnetometer (VSM) at room temperature [Figure 1e]. The magnetic saturation (Ms) value of the MNPs was 56.4 emu/g. The MNPs also showed a fast response to the applied magnetic field of 1000 Oe; these results also indicated that these particles exhibited excellent magnetic properties. Therefore, MNPs were an effective tool to improve the solubility of wogonin because wogonin did not precipitate in the colloidal suspension of the Wog-MNPs-Fe3O4 drug delivery system as previously reviewed.
Cell viability and inhibition rate evaluated by MTT assay
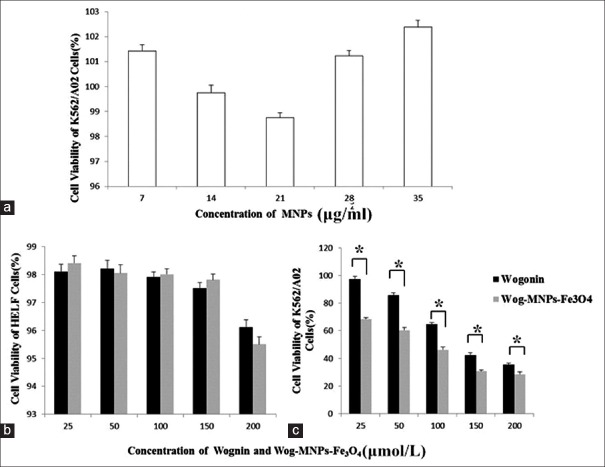
The MTT assay revealed that the MNPs alone for 48 h did not significantly influence the cell viability of K562/A02 cells. The cell viability ranged from 98.76% to 101.43%, which confirmed the low cytotoxicity and good biocompatibility of MNPs [Figure 2a]. According to the inhibition rate of K562/A02 cells treated with different concentrations of wogonin or Wog-MNPs-Fe3O4 for 48 h [Table 2], the cell viability of K562/A02 cells treated with wogonin and Wog-MNPs-Fe3O4 both changed in dose- and time-dependent manner [35.53–97.28% for wogonin and 28.64–68.36% for Wog-MNPs-Fe3O4; Figure 2c]. As shown in Table 2, Wog-MNPs-Fe3O4 could more effectively inhibit the growth of K562/A02 cells than wogonin alone (P < 0.01). The cytotoxicities of the wogonin and Wog-MNPs-Fe3O4 for HELF cells were found to be dose dependent. Low cytotoxicity was observed with survival rate >95.16%. Compared with the cytotoxicity on K562/A02 cells, the wogonin and Wog-MNPs-Fe3O4 had little effect on HELF cells [Figure 2b].
Figure 2.
Viability of cells treated with different concentrations of wogonin or Wog-MNPs-Fe3O4. (a) Cell viability of leukemia K562/A02 cells treated with different concentrations of MNPs for 48 h. (b) Cell viability of HELF cells treated with different concentrations of wogonin or Wog-MNPs-Fe3O4 for 48 h. (c) Cell viability of leukemia K562/A02 cells treated with different concentrations of wogonin or Wog-MNPs-Fe3O4 for 48 h. *P < 0.05. MNP: Magnetic nanoparticle; Wog-MNPs-Fe3O4: Wogonin-coated magnetic nanoparticle-Fe3O4; HELF: Human embryonic lung fibroblast.
Table 2.
Inhibition rates of leukemia K562/A02 cells treated with different concentrations of wogonin or Wog-MNPs-Fe3O4 for 48 h (%)
| Concentrations | Wogonin group | Wog-MNPs-Fe3O4 group | t | P |
|---|---|---|---|---|
| 25 μmol/L | 2.72 ± 1.93 | 31.64 ± 1.02 | 22.94 | <0.001 |
| 50 μmol/L | 14.26 ± 1.84 | 39.57 ± 1.79 | 17.07 | <0.001 |
| 100 μmol/L | 35.14 ± 0.99 | 53.71 ± 1.84 | 15.39 | <0.001 |
| 150 μmol/L | 57.83 ± 1.79 | 69.41 ± 1.09 | 9.57 | <0.001 |
| 200 μmol/L | 64.47 ± 0.99 | 71.36 ± 1.63 | 6.25 | 0.003 |
The data are shown as mean ± SD. Wog-MNPs-Fe3O4: Wogonin-coated magnetic nanoparticle-Fe3O4; SD: Standard deviation.
Apoptosis assay by flow cytometry analysis
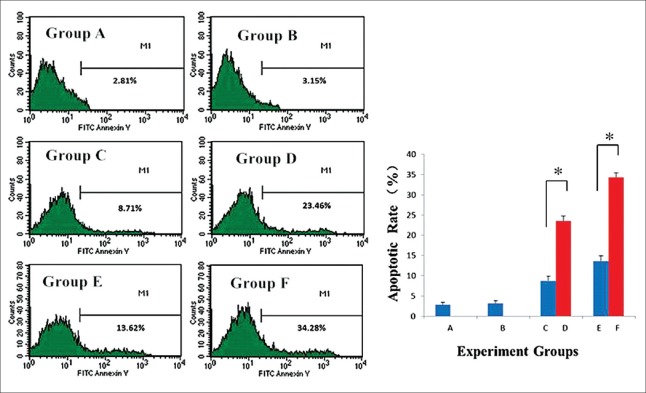
We conducted flow cytometry analysis to determine the apoptotic rate of K562/A02 cells [Figure 3]. After 48 h of culture, the total apoptotic rate was 2.81% in the blank group. The apoptotic rate of the K562/A02 cells treated with wogonin and Wog-MNPs-Fe3O4 increased in a concentration-dependent manner. The apoptotic rates of the K562/A02 cells increased to 8.71% and 13.62% after cultured with 25 and 50 µmol/L of wogonin for 48 h, respectively, and the apoptotic rates increased to 23.46% and 34.28% after cultured with 25 and 50 µmol/L of Wog-MNPs-Fe3O4 for 48 h, respectively; the differences between wogonin and Wog-MNPs-Fe3O4 groups at the same concentration were statistically significant (t = 401.13, P < 0.001 for 25 µmol/L and t = 590.37, P < 0.001 for 50 µmol/L). These findings suggested that MNPs could strengthen the effect of wogonin on cell apoptosis.
Figure 3.
Apoptotic rates of K562/A02 cells with different treatments for 48 h. *P < 0.001. Group A: Nontreated K562/A02 cells as the blank group; Group B: The K562/A02 cells treated with 35 μg/ml MNPs as the negative group; Group C: K562/A02 cells treated with 25 μmol/L wogonin; Group D: K562/A02 cells treated with 25 μmol/L Wog-MNPs-Fe3O4; Group E: K562/A02 cells treated with 50 μmol/L wogonin; Group F: K562/A02 cells treated with 50 μmol/L Wog-MNPs-Fe3O4; Wog-MNPs-Fe3O4: Wogonin-coated magnetic nanoparticle-Fe3O4; MNPs: Magnetic nanoparticles.
Cell cycle arrest evaluated by flow cytometry analysis
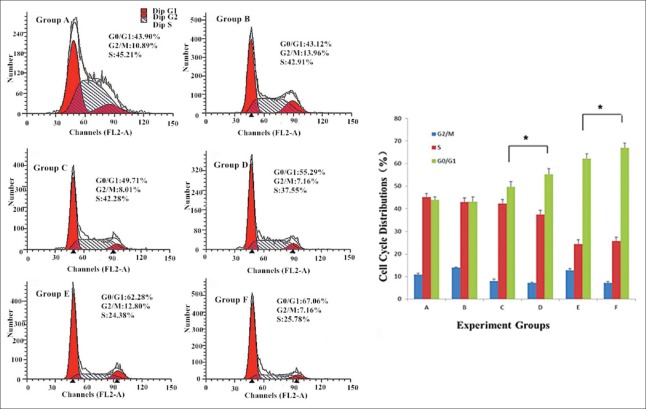
The effects of wogonin and wogonin-MNPs on the cell cycle progression of the K562/A02 cells were evaluated by flow cytometry analysis since cell proliferation is regulated by the cell cycle. After the K562/A02 cells were cultured for 48 h, the ratios of G0/G1- and S-phase cells were approximately 43.90% and 45.21% in the blank group, respectively. In the negative group, MNPs slightly affected the K562/A02 cell cycle, with 43.12% of G0/G1-phase cells and 42.91% of S-phase cells. Compared with the blank and negative groups, the ratios of G0/G1-phase cells in the wogonin and Wog-MNPs-Fe3O4 groups increased dose dependently. Compared with those of the 25 and 50 µmol/L wogonin groups (49.71% and 62.28%), the K562/A02 cells arrested in G0/G1-phase were improved to 55.29% and 67.06% when combining MNPs. These results suggested that the ratios of G0/G1-phase cells in the Wog-MNPs-Fe3O4 groups were significantly higher than those in the wogonin groups at the same concentration [t = 31.34, P < 0.001 for 25 µmol/L; t = 82.58, P < 0.001 for 50 µmol/L; Figure 4].
Figure 4.
Cell cycle distributions of K562/A02 cells with different treatments for 48 h. *P < 0.001. Group A: Nontreated K562/A02 cells as the blank group; Group B: The K562/A02 cells treated with 35 μg/ml MNPs as the negative group; Group C: K562/A02 cells treated with 25 μmol/L wogonin; Group D: K562/A02 cells treated with 25 μmol/L Wog-MNPs-Fe3O4; Group E: K562/A02 cells treated with 50 μmol/L wogonin; Group F: K562/A02 cells treated with 50 μmol/L Wog-MNPs-Fe3O4; Wog-MNPs-Fe3O4: Wogonin-coated magnetic nanoparticle-Fe3O4; MNPs: Magnetic nanoparticles.
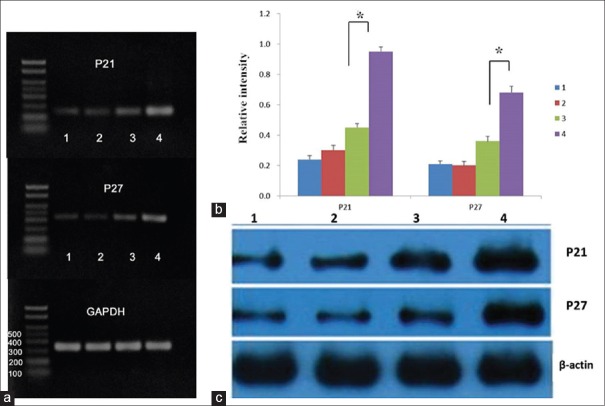
Expression of p21 and p27 evaluated by RT-PCR analysis
The results of RT-PCR for mRNA expression levels of p21 and p27 genes are shown in Figure 5a and 5b. No significant differences in mRNA expression levels of p21 and p27 genes were found between the blank and negative groups (t = 0.45, P = 0.340 for p21 and t = 0.32, P = 0.740 for p27); compared with 50 µmol/L wogonin group, 50 µmol/L Wog-MNP-Fe3O4 group significantly upregulated the mRNA expression levels of p21 and p27 genes in the K562/A02 cells (t = 1352.26, P < 0.001 for p21 and t = 819.17, P < 0.001 for p27).
Figure 5.
The mRNA and protein expression levels of p21 and p27 in K562/A02 cells using RT-PCR and Western blot analysis. (a and b) The mRNA expression of p21, p27 genes, and GAPDH after different treatments for 48 h. (c) Protein expression of p21, p27, and β-actin after different treatments for 48 h. Lane 1: Nontreated K562/A02 cells as the blank group; Lane 2: K562/A02 cells treated with 35 μg/ml MNPs as the negative group; Lane 3: K562/A02 cells treated with 50 μmol/L wogonin; Lane 4: K562/A02 cells treated with 50 μmol/L Wog-MNPs-Fe3O4. *P < 0.001. MNP: Magnetic nanoparticle; Wog-MNPs-Fe3O4: Wogonin-coated magnetic nanoparticle-Fe3O4.
Expression levels of cell cycle regulatory proteins in K562/A02 cells
After treated with MNPs, wogonin, or Wog-MNPs-Fe3O4 for 48 h, the expression levels of the cell cycle regulatory proteins in the K562/A02 cells were detected by Western blot analysis to confirm the results of RT-PCR. As shown in the Western blot analysis, the expression levels of p21 and p27 proteins in the blank group were not significantly different from those of the negative group. An obvious upregulation of both p21 and p27 proteins was observed in the 50 µmol/L Wog-MNPs-Fe3O4 group when compared with the 50 µmol/L wogonin group [Figure 5c].
Discussion
Chemotherapy plays an important role in the treatment of leukemia. Unfortunately, more and more intractable problems including chemotherapy resistance and high toxicity to normal cells associated with large chemotherapy-induced adverse effects have emerged.[21,22,23] Studies have also demonstrated that reduced drug uptake, increased proportions of multidrug resistance cells, and decreased intracellular drug proportion in leukemia cells may account for chemotherapy resistance.[21] As such, new sources of antileukemia drugs and new chemotherapeutic adjuvants should be developed to enhance the therapeutic efficacy and attenuate the adverse reactions of chemotherapy.[24] TCMs have been rarely investigated, and a few TCMs have satisfied chemotherapeutic requirements with effective antitumor efficacy and negligible toxicity.[24,25,26] Wogonin, a representative TCM with negligible toxicity to normal cells,[12] inhibits the proliferation of human cancer cells; however, the underlying molecular mechanisms remain unclear. Studies have mostly identified the association of wogonin with cell cycle arrest and apoptosis,[10] inhibition of tumor angiogenesis,[27] inhibition of tumor cell metastasis by targeting inflammatory microenvironments,[3] and antitumor immunity effect.[11,28] In this study, a novel antileukemia agent containing a nanoparticle and an extract from wogonin was designed and synthesized. Our study indicated that wogonin could dose- and time-dependently inhibit the growth of K562/A02 cells. The low solubility of wogonin in water was consistent with that described in a previous study,[10] but this parameter limited its application in further treatments of tumors.
To solve these problems, some drug carriers are taken into consideration. It has been demonstrated that cadmium-telluride quantum dots (CdTe QDs) loaded with wogonin could induce the apoptosis of leukemia K562/A02 cells.[29] However, CdTe QDs displays inherent risk, suggesting the need for short- and long-term toxicity assessment of CdTe QDs.[30] Meanwhile, MNPs-Fe3O4 have been extensively investigated by virtue of their magnetic property, nontoxicity, and biocompatibility. MNPs-Fe3O4 have been successfully loaded with doxorubicin,[15,31] wogonin,[10,32] and gambogic acid.[14,25] All demonstrated that MNPs-Fe3O4 could increase the intracellular drug accumulation for cancer cells, thus improving the anticancer effects of the drug. In addition, MNPs-Fe3O4 can be applicable for cancer thermotherapy.[33] Therefore, MNPs-Fe3O4 loaded with drugs could comprehensively improve the therapeutic efficacy for cancer. Accordingly, wogonin combined with MNP-Fe3O4 were used in this work.
In our study, the mean hydrodynamic diameter of the MNPs was 30.2 ± 7.5 nm; nanoparticles larger than 100 nm were quickly eliminated through the reticuloendothelial system, whereas those smaller than 10 nm were readily cleared by glomerular filtration;[34] therefore, the size of our MNPs was suitable for biomedical applications. MNPs were not observed to settle down under the influence of magnet and thus confirmed the stability of colloidal solutions, which could improve the solubility of wogonin in water. The Ms values of the MNPs were 56.4 emu/g. The magnetic properties of the MNPs were evaluated by VSM; the results revealed the excellent magnetic properties of the MNPs. MNPs did not affect the cell viability of K562/A02 leukemia cells but could dose dependently amplify the anticancer efficacy of wogonin, including induced cell cycle arrest and apoptosis.
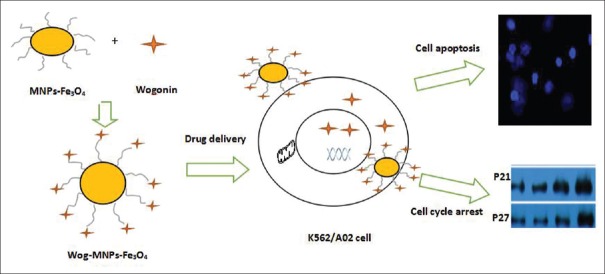
We proposed a model to analyze the possible mechanisms of wogonin-MNPs in leukemia K562/A02 cells [Figure 6]. Cell cycle is an important process in cell life activities, including cell growth and division into two daughter cells; this process is promoted and regulated by a series of activated specific protein complexes. Cell cycle is divided into four sequential phases: G1, S, G2, and M. Tumor cells are mainly characterized by the disruption of cell cycle regulation; as a result, cell proliferation is dysregulated. Thus, cell cycle analysis is necessary to investigate tumor cell proliferation and inhibition. Cell cycle progression is monitored and regulated by the G1/S and G2/M checkpoints. After the cells were treated with wogonin and Wog-MNP-Fe3O4, the K562/A02 leukemia cells were arrested in G0/G1-phase, and this finding was similar to that described in a previous study on lymphoma cells.[10] The shift of cell distribution into G0/G1-phase was significantly enlarged by Wog-MNP-Fe3O4 compared with single wogonin. Several protein complexes have been described to regulate the specific phases of the cell cycle, such as cyclins, cyclin-dependent kinases (CDK), and CDK inhibitors (CKIs). Furthermore, p21 and p27 are important cell cycle regulators belonging to CKI and related to the negative regulation of the cell cycle. p21 and p27 could block G1- to S-phase progression in the cell cycle by preventing or limiting cyclin-CDKs from phosphorylating their normal substrates. When complexed with their respective cyclin-binding partner, p21 and p27 could also block the kinase activity of CDKs. The loss of the expression or function of p21 and p27 has been identified in the progression of many human malignancies.[35] Wogonin can enhance the expression of p21 and p27 in lymphoma cells; thus, the cell cycle is blocked and the effect was significantly enlarged by MNP-wogonin.[10] Our study obtained the same conclusion, as indicated by RT-PCR analyses and confirmed by Western blot analyses.
Figure 6.
Proposed model of Wog-MNPs-Fe3O4 on K562/A02 cells. Wog-MNPs-Fe3O4: Wogonin-coated magnetic nanoparticle-Fe3O4.
Apoptosis is the programed cell death regulated by correlated genes; this process is an important component in the development of multicellular organisms. The dysregulation of apoptosis is related to cancer development when the balance between cell growth and cell death is disrupted. The inhibition of cell death can cause tumors; therefore, apoptosis activation can inhibit tumor growth. In this study, apoptosis activation can be an effective strategy to treat cancer. Our study revealed that the apoptotic rates of the K562/A02 cells treated with wogonin and Wog-MNP-Fe3O4 were higher than those in the blank and negative groups. The apoptotic rate of the K562/A02 cells treated with Wog-MNP-Fe3O4 increased significantly compared with that of K562/A02 cells treated with wogonin alone. This result indicated that MNPs can enhance the effect of wogonin on the apoptotic rate of K562/A02 cells. Apoptosis is a two-way regulatory process when apoptosis-stimulating genes and apoptosis-inhibitory genes are activated orderly. The activation of apoptosis-stimulating genes and the suppression of apoptosis-inhibitory genes activate apoptosis; as a result, cancer growth is inhibited. The p53-dependent transcriptional induction of PUMA and the oligomerization of Bax are implicated in the wogonin-induced apoptosis of prostate cancer cells.[12] Further studies should be investigated to elucidate the mechanism of the wogonin-induced apoptosis of leukemia cells.
In conclusion, this study demonstrated that MNPs were the effective drug delivery vehicles to deliver wogonin to the leukemia cells. Through increasing cells arrested at G0/G1-phase and inducing apoptosis of K562/A02 cells, MNPs could enhance the therapeutic effects of wogonin on leukemia cells. These findings indicated that MNPs loaded with wogonin provided a promising way for better leukemia treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Zhao K, Wei L, Hui H, Dai Q, You QD, Guo QL, et al. Wogonin suppresses melanoma cell B16-F10 invasion and migration by inhibiting Ras-medicated pathways. PLoS One. 2014;9:e106458. doi: 10.1371/journal.pone.0106458. doi: 10.1371/journal.pone.0106458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Yao J, Wu XP, Zhao L, Zhou YX, Zhang Y, et al. Wogonin suppresses human alveolar adenocarcinoma cell A549 migration in inflammatory microenvironment by modulating the IL-6/STAT3 signaling pathway. Mol Carcinog. 2015;54(Suppl 1):E81–93. doi: 10.1002/mc.22182. doi: 10.1002/mc.22182. [DOI] [PubMed] [Google Scholar]
- 4.Tian J, Wang L, Wang L, Ke X. A wogonin-loaded glycyrrhetinic acid-modified liposome for hepatic targeting with anti-tumor effects. Drug Deliv. 2014;21:553–9. doi: 10.3109/10717544.2013.853850. doi: 10.3109/10717544.2013.853850. [DOI] [PubMed] [Google Scholar]
- 5.Huang KF, Zhuang Y, Huang YQ, Diao Y. Experimental study on inhibitory effect of wogonin on proliferation and invasion of breast cancer cells (in Chinese) China J Chin Mater Med. 2014;39:1485–9. doi: 10.4268/cjcmm20140824. [PubMed] [Google Scholar]
- 6.Ge W, Yin Q, Xian H. Wogonin induced mitochondrial dysfunction and endoplasmic reticulum stress in human malignant neuroblastoma cells via IRE1alpha-dependent pathway. J Mol Neurosci. 2015;56:652–62. doi: 10.1007/s12031-015-0530-9. doi: 10.1007/s12031-015-0530-9. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Hui H, Wang Q, Li H, Zhao K, Zhou Y, et al. Wogonin induces cell cycle arrest and erythroid differentiation in imatinib-resistant K562 cells and primary CML cells. Oncotarget. 2014;5:8188–201. doi: 10.18632/oncotarget.2340. doi: 10.18632/oncotarget.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu H, Gao F, Shu G, Xia G, Shao Z, Lu H, et al. Wogonin inhibits the proliferation of myelodysplastic syndrome cells through the induction of cell cycle arrest and apoptosis. Mol Med Rep. 2015;12:7285–92. doi: 10.3892/mmr.2015.4353. doi: 10.3892/mmr.2015.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin MG, Liu LP, Li CY, Zhang M, Chen Y, Qin J, et al. Scutellaria extract decreases the proportion of side population cells in a myeloma cell line by down-regulating the expression of ABCG2 protein. Asian Pac J Cancer Prev. 2013;14:7179–86. doi: 10.7314/apjcp.2013.14.12.7179. doi: 10.7314/APJCP.2013.14.12.7179. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Zhang H, Chen B, Xia G, Wang S, Cheng J, et al. Effect of magnetic nanoparticles on apoptosis and cell cycle induced by wogonin in Raji cells. Int J Nanomedicine. 2012;7:789–98. doi: 10.2147/IJN.S28089. doi: 10.2147/IJN.S28089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CC, Lin JJ, Wu PP, Lu CC, Chiang JH, Kuo CL, et al. Wogonin, a natural and biologically-active flavonoid, influences a murine WEHI-3 leukemia model in vivo through enhancing populations of T- and B-cells. In Vivo. 2013;27:733–8. [PubMed] [Google Scholar]
- 12.Lee DH, Kim C, Zhang L, Lee YJ. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem Pharmacol. 2008;75:2020–33. doi: 10.1016/j.bcp.2008.02.023. doi: 10.1016/j.bcp.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmoudi M, Simchi A, Milani AS, Stroeve P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J Colloid Interface Sci. 2009;336:510–8. doi: 10.1016/j.jcis.2009.04.046. doi: 10.1016/j.jcis.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Zhang H, Chen B, Yin H, Wang W. Study of the enhanced anticancer efficacy of gambogic acid on Capan-1 pancreatic cancer cells when mediated via magnetic Fe3O4 nanoparticles. Int J Nanomedicine. 2011;6:1929–35. doi: 10.2147/IJN.S24707. doi: 10.2147/IJN.S24707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akbarzadeh A, Mikaeili H, Zarghami N, Mohammad R, Barkhordari A, Davaran S. Preparation and in vitro evaluation of doxorubicin-loaded Fe3O4 magnetic nanoparticles modified with biocompatible copolymers. Int J Nanomedicine. 2012;7:511–26. doi: 10.2147/IJN.S24326. doi: 10.2147/IJN.S24326. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Döblinger M, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142:108–21. doi: 10.1016/j.jconrel.2009.10.002. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Wu W, He Q, Jiang C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res Lett. 2008;3:397–415. doi: 10.1007/s11671-008-9174-9. doi: 10.1007/s11671-008-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalkhali M, Sadighian S, Rostamizadeh K, Khoeini F, Naghibi M, Bayat N, et al. Synthesis and characterization of dextran coated magnetite nanoparticles for diagnostics and therapy. Bioimpacts. 2015;5:141–50. doi: 10.15171/bi.2015.19. doi: 10.15171/bi.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kou G, Wang S, Cheng C, Gao J, Li B, Wang H, et al. Development of SM5-1-conjugated ultrasmall superparamagnetic iron oxide nanoparticles for hepatoma detection. Biochem Biophys Res Commun. 2008;374:192–7. doi: 10.1016/j.bbrc.2008.06.126. doi: 10.1016/j.bbrc.2008.06.126. [DOI] [PubMed] [Google Scholar]
- 20.Babar IA, Cheng CJ, Booth CJ, Liang X, Weidhaas JB, Saltzman WM, et al. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc Natl Acad Sci U S A. 2012;109:E1695–704. doi: 10.1073/pnas.1201516109. doi: 10.1073/pnas.1201516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu P, Wang R, Ouyang J, Chen B. A new strategy for TiO2 whiskers mediated multi-mode cancer treatment. Nanoscale Res Lett. 2015;10:94. doi: 10.1186/s11671-015-0796-4. doi: 10.1186/s11671-015-0796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islambulchilar M, Asvadi I, Sanaat Z, Esfahani A, Sattari M1. Effect of taurine on attenuating chemotherapy-induced adverse effects in acute lymphoblastic leukemia. J Cancer Res Ther. 2015;11:426–32. doi: 10.4103/0973-1482.151933. doi: 10.4103/0973-1482.151933. [DOI] [PubMed] [Google Scholar]
- 23.Breen S, Ritchie D, Schofield P, Hsueh YS, Gough K, Santamaria N, et al. The patient remote intervention and symptom management system (PRISMS) – A Telehealth- mediated intervention enabling real-time monitoring of chemotherapy side-effects in patients with haematological malignancies: Study protocol for a randomised controlled trial. Trials. 2015;16:472. doi: 10.1186/s13063-015-0970-0. doi: 10.1186/s13063-015-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li-Weber M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Xu P, Li J, Shi L, Selke M, Chen B, Wang X. Synergetic effect of functional cadmium-tellurium quantum dots conjugated with gambogic acid for HepG2 cell-labeling and proliferation inhibition. Int J Nanomedicine. 2013;8:3729–36. doi: 10.2147/IJN.S51622. doi: 10.2147/IJN.S51622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong JW, Jin CY, Park C, Hong SH, Kim GY, Jeong YK, et al. Induction of apoptosis by cordycepin via reactive oxygen species generation in human leukemia cells. Toxicol In Vitro. 2011;25:817–24. doi: 10.1016/j.tiv.2011.02.001. doi: 10.1016/j.tiv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Zhao K, Song X, Huang Y, Yao J, Zhou M, Li Z, et al. Wogonin inhibits LPS-induced tumor angiogenesis via suppressing PI3K/Akt/NF-kappaB signaling. Eur J Pharmacol. 2014;737:57–69. doi: 10.1016/j.ejphar.2014.05.011. doi: 10.1016/j.ejphar.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Li XJ, Chen Z, Zhu XX, Wang J, Zhang LB, et al. Wogonin induced calreticulin/annexin A1 exposure dictates the immunogenicity of cancer cells in a PERK/AKT dependent manner. PLoS One. 2012;7:e50811. doi: 10.1371/journal.pone.0050811. doi: 10.1371/journal.pone.0050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang B, Liu H, Huang D, Mao X, Hu X, Jiang C, et al. Apoptosis induction and imaging of cadmium-telluride quantum dots with wogonin in multidrug-resistant leukemia K562/A02 Cell. J Nanosci Nanotechnol. 2016;16:2499–503. doi: 10.1166/jnn.2016.10792. doi: 10.1166/jnn.2016.10792. [DOI] [PubMed] [Google Scholar]
- 30.Ghaderi S, Ramesh B, Seifalian AM. Fluorescence nanoparticles “quantum dots” as drug delivery system and their toxicity: A review. J Drug Target. 2011;19:475–86. doi: 10.3109/1061186X.2010.526227. doi: 10.3109/1061186X.2010.526227. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Wang L, Tan X, Zhang H, Sun G. Construction of doxorubicin-loading magnetic nanocarriers for assaying apoptosis of glioblastoma cells. J Colloid Interface Sci. 2014;436:267–75. doi: 10.1016/j.jcis.2014.09.002. doi: 10.1016/j.jcis.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Cheng J, Cheng L, Chen B, Xia G, Gao C, Song H, et al. Effect of magnetic nanoparticles of Fe3O4 and wogonin on the reversal of multidrug resistance in K562/A02 cell line. Int J Nanomedicine. 2012;7:2843–52. doi: 10.2147/IJN.S32065. doi: 10.2147/IJN.S32065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai CC, Chen CC. The design of a half-bridge series-resonant type heating system for magnetic nanoparticle thermotherapy. PIERS Online. 2007;4:276–80. doi: 10.2529/PIERS070907021847. [Google Scholar]
- 34.Cole AJ, Yang VC, David AE. Cancer theranostics: The rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011;29:323–32. doi: 10.1016/j.tibtech.2011.03.001. doi: 10.1016/j.tibtech.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abukhdeir AM, Park BH. p21 and p27: Roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008;10:e19. doi: 10.1017/S1462399408000744. doi: 10.1017/ [DOI] [PMC free article] [PubMed] [Google Scholar]