Abstract
Background:
Sepsis is the leading cause of death among critically ill patients. Herein, we conducted a national survey to provide data on epidemiology and treatment of sepsis in the clinical practice in China, which has no detailed epidemiological data available on sepsis.
Methods:
This was a prospective cross-sectional survey from December 1, 2015 to January 31, 2016 in all provinces/municipalities of the mainland of China. The primary outcome of this study was the incidence of sepsis, and the secondary outcome was its etiology in China. Patients with sepsis admitted to the Intensive Care Units were included in this study. The demographic, physiological, bacteriological, and therapeutic data of these patients were recorded. The incidence of sepsis was estimated using the data from the sixth census in China, reported by the Chinese National Health and Family Planning Commission and the National Bureau of Statistics as the standard population. The independent risk factors for increased mortality from sepsis were calculated.
Conclusions:
This study indicated the incidence and outcome of sepsis in China. It also showed the most common etiology of different sites and types of infection, which could guide empiric antibiotic therapy. Moreover, it provided information on the independent risk factors for increased mortality due to sepsis. The findings provide evidence to guide clinical management and may help improve the outcome in septic patients.
Trial Registration:
ClinicalTrials.gov, NCT02448472; https://clinicaltrials.gov/show/NCT02448472.
Keywords: Epidemiology, Incidence, National Survey, Outcome, Sepsis
Introduction
Sepsis is the leading cause of death among critically ill patients.[1,2] It is the third most common cause of death in the USA following heart disease and cancer, with 230,000–370,000 people dying from the disease annually.[3] In addition, recent studies have shown that the incidence of sepsis has been increasing every year.[4,5] Seymour et al.[4] performed a retrospective cohort study from 2000 to 2009 and found that the crude rate of hospitalization due to severe sepsis increased by 11.8%/year. Similar results were obtained in Australia and New Zealand.[5]
Most data on the epidemiological characteristics of sepsis were derived from Western countries, especially the USA.[6,7,8,9,10,11,12,13,14,15] However, there is significant disparity among studies, and the true incidence of sepsis worldwide remains controversial. For example, Finfer et al.[12] reported that the incidence of severe sepsis was 11.8/100 Intensive Care Unit (ICU) admissions in Australia. In contrast, the incidence of severe sepsis was 42/100 ICU admissions in the study of Adrie et al.[13] Angus et al.[6] found 300 septic patients per 100,000 population in the USA. However, Blanco et al.[10] found 25 septic patients per 100,000 population in Spain. In the USA, different studies yielded different results.[3] Martin et al.[8] estimated an incidence of severe sepsis of 81/100,000 while Dombrovskiy et al.[14] reported an incidence of 134/100,000. Sepsis in different regions and at different times may differ.
Moreover, the etiology of sepsis also differed among different epidemiological studies. For example, in the EPIC II study,[16] the pathogens causing sepsis included Gram-positive microorganisms (69.8%), Gram-negative microorganisms (62.2%), and fungi (17.0%). However, the incidence of infections caused by multidrug-resistant organisms is much higher in China than in Western countries. In contrast to the EPIC II study, a study in China indicated different pathogens causing sepsis.[17] The Gram-negative microorganisms, Gram-positive microorganisms, and fungi accounted for 62.5%, 14.5%, and 2.2%, respectively. In addition, EPIC II study found significant regional differences in the pathogens isolated from microbiological cultures.[16] Therefore, the data could not be extrapolated directly to the Chinese Healthcare System. Hence, real epidemiological data on sepsis in China were required to know its incidence and outcome in Chinese population.
In China, two studies[17,18] were conducted to investigate the epidemiology of sepsis. Cheng et al.[18] reported the characteristics of severe sepsis in surgical patients in 10 relevant local hospitals. However, because of the limitations of location and type, the study could not reflect the actual incidence of sepsis in China. Zhou et al.[17] conducted an epidemiological study on severe sepsis and septic shock in a mixed ICU, which also could not reflect the real characteristics of sepsis in China. Moreover, the sample sizes of these two studies were small, and they both calculated the incidence of sepsis based on ICU patients rather than the entire population. Therefore, we undertook the Chinese Epidemiological Study of Sepsis to determine the epidemiology and outcomes of sepsis.
A national survey on septic patients in China, who were treated in mixed ICUs from December 1, 2015 to January 31, 2016, was conducted to provide data on the epidemiology and treatment of sepsis in clinical practice. The primary outcome of this study was the incidence of sepsis, and the secondary outcome was its etiology in China.
Methods
Study design and participants
This study, which has already been registered (https://clinicaltrials.gov/show/NCT02448472), was a prospective cross-sectional survey in all provinces/municipalities of the mainland of China. The study was approved by the Ethics Committees of Zhongda Hospital, Southeast University (No. 2015ZDSYLL044.0), and the enrolled hospitals [Table 1]. Patients were enrolled in this study after receiving informed consent from the patient or their guardian. All case report forms (CRFs) and study reports were assigned a unique identifier number and the initial alphabets of the first and family names of the patient to maintain confidentiality.
Table 1.
Research settings and names of ethics committees
| Research setting | The name of ethics committee | Approve registration number |
|---|---|---|
| The First Affiliated Hospital of Wannan Medical College | Ethics Committee of the First Affiliated Hospital of Wannan Medical College | (2015)18 |
| The First Affiliated Hospital of Bengbu Medical College | Ethics Committee of the First Affiliated Hospital of Bengbu Medical College | (2015)003 |
| Beijing Friendship Hospital, Capital Medical University | Medical Ethic Committee of Beijing Friendship Hospital | 2015–P2–081–01 |
| Chinese People's Liberation Army General Hospital | IRB of Chinese People's Liberation Army General Hospital | S2015–079–01 |
| Fujian Provincial Hospital | Ethics Committee of Fujian Provincial Hospital | K2015–024–01 |
| The First Hospital of Lanzhou University | Ethics Committee of the First Hospital of Lanzhou University | LDYYLL2015–0081 |
| The First People's Hospital of Foshan | Medical Ethics Committee of the First People's Hospital of Foshan | (2015)26 |
| The First Affiliated Hospital of Sun Yat-sen University | Medical Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University | (2015)164 |
| The First Affiliated Hospital of Guangxi Medical University | Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University | (2015)18 |
| The Affiliated Hospital of Guizhou Medical University | Ethics Committee of the Affiliated Hospital of Guizhou Medical University | (2015)56 |
| Hainan General Hospital | Medical Ethics Committee of Hainan General Hospital | (2015)87 |
| Hebei General Hospital | Hebei General Hospital Ethics Committee Application | (2015)17 |
| Fourth Hospital of Hebei Medical University | IRB of Fourth Hospital of Hebei Medical University | 2015NEC048 |
| Henan Provincial People's Hospital | Medical Ethics Committee of Henan Provincial People's Hospital | (2015)29 |
| The First Affiliated Hospital of Zhengzhou University | Ethics Committee of the First Affiliated Hospital of Zhengzhou University | 2015ZDSYLL044.0 |
| The Second Affiliated Hospital of Harbin Medical University | Ethics Committee of the second Affiliated Hospital of Harbin Medical University | 2015–195 |
| Renmin Hospital of Wuhan University | Ethics Committee of Renmin Hospital of Wuhan University | 2015K–023 |
| Zhongnan Hospital, Wuhan University | Ethics Committee of Zhongnan Hospital of Wuhan University | 2015063 |
| The Third Xiangya Hospital of Central South University | IRB of the Third Xiangya Hospital of Central South University | 15109 |
| Xiangya Hospital, Central South University | Ethics Committee of Xiangya Hospital, Centre South University | 201511120 |
| The First Hospital of Jilin University | IRB of the First Hospital of Jilin University | (2015) 2015–203 |
| Zhongda Hospital, School of Medicine, Southeast University | Ethics Committee of Zhongda Hospital, Southeast University | 2015ZDSYLL044.0 |
| Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing University | IRB of Nanjing Drum Tower Hospital Affiliated Nanjing University Medical College | 2015–108–01 |
| The First Affiliated Hospital of Nanjing Medical University | Ethics Committee of the First Affiliated Hospital of Nanjing Medical University | 2015–SR–194 |
| The First Affiliated Hospital of Nanchang University | Ethics Committee of the First Affiliated Hospital of Nanchang University | (2015)026 |
| The First Affiliated Hospital of China Medical University | Medical Ethics Committee of the First Affiliated Hospital of China Medical University | (2015)2015–143–2 |
| The Affiliated Hospital of Inner Mongolia Medical University | Medical Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University | (2015)003 |
| General Hospital of Ningxia Medical University | Ethics Committee of General Hospital of Ningxia Medical University | 2015–127 |
| The Affiliated Hospital of Qinghai University | Medical Ethics Committee of the Affiliated Hospital of Qinghai University | (2015)001 |
| Shandong Provincial Hospital Affiliated to Shandong University | Medical Ethics Committee of Shandong Provincial Hospital affiliated to Shandong University | (2015)15 |
| The Affiliated Hospital of Qingdao University | Medical Ethics Committee of the Affiliated Hospital of Qingdao University | QYFYEC KY2015–001–005 |
| First Hospital of Shanxi Medical University | Ethics Committee of First Hospital of Shanxi Medical University | (2015)Y09 |
| The First Affiliated Hospital of Xi’an Jiaotong University | Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University | (2015)169 |
| Shanghai Changzheng Hospital | Biomedical Ethics Committee of Shanghai Changzheng Hospital | 2015SL023 |
| Shanghai General Hospital | Shanghai General Hospital IRB | 2015K058 |
| West China Hospital, Sichuan University | West China Hospital, Sichuan University Clinical Trials and Biomedical Ethics Committee | (2015)198 |
| Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital | The Ethics Committee of the Hospital of Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital | (2015)01 |
| Tianjin Third Central Hospital | The Ethics Committee of Tianjin Third Central Hospital | TCH–YL–26 |
| People's Hospital of Tibet Autonomous Region | Medical Ethics Committee of People's Hospital of Tibet Autonomous Region | ME–TBHP–2015–08 |
| The First Affiliated Hospital of Xinjiang Medical University | Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University | 20151023–05 |
| The First Affiliated Hospital of Kunming Medical University | Ethics Committee of the First Affiliated Hospital of Kunming Medical University | (2015)L10 |
| The Second Affiliated Hospital Zhejiang University School of Medicine | Ethics Committee of the Second Affiliated Hospital Zhejiang University School of Medicine | (2015)063 |
| Zhejiang Hospital | Medical Ethics Committee of Zhejiang Hospital | (2015)24K |
| The First Affiliated Hospital of Chongqing Medical University | Medical Ethics Committee of the First Affiliated Hospital of Chongqing Medical University | (2015)20152701 |
IRB: Institutional Review Board.
Settings
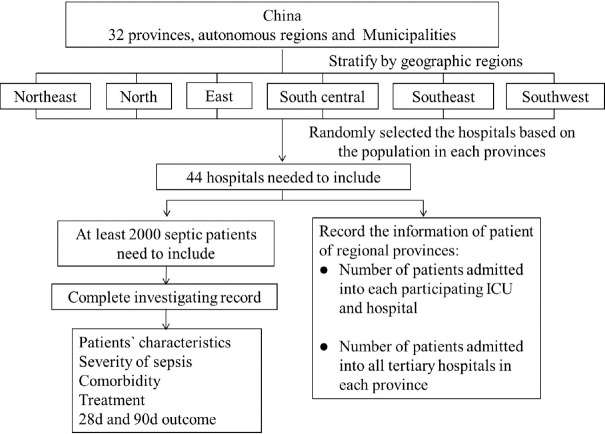
The study was conducted in the mainland of China. In each province/municipality, one to three tertiary hospitals were enrolled according to the regional population from the data of the sixth census in China in 2010 [Figure 1]. The study was conducted in the ICU of the hospital. All septic patients from December 1, 2015 to January 31, 2016, were enrolled.
Figure 1.
Detailed flowchart of the Chinese Epidemiological Study of Sepsis. ICU: Intensive Care Unit.
Participants
Stratified randomized framework
To reach every patient, the two-stage optimum stratified group sampling framework was designed under two assumptions:
All sepsis patients were admitted to the tertiary hospitals in China, so that patients admitted to these hospitals represented the whole patient group in China.
All sepsis patients were admitted to the ICU of the participating hospitals.
The sampling frame of this national cross-sectional survey was hospital. The inclusion criteria for hospital, which was the basic sample unit, were as follows:
Tertiary hospitals with mixed ICU, which is the medical center of the provincial patients;
More than 70% of admitted patients were local residents;
The mixed ICU could cover all the critical patients of the individual hospital;
The beds in the mixed ICU were ≥20;
The faculty agreed to participate in the survey.
In the first stage, all provinces/municipalities in the mainland of China were included in the survey without sampling. In the second stage, group optimum sampling was performed at the hospital level based on the population [Figure 1]. In every province/municipality, one or more hospital(s) were included based on the population of that province. The hospital in each province that met the inclusion criteria was selected by randomization.
Patient recruitment
In every included hospital, during the 2-month study period, all sequential septic patients admitted to the ICU were enrolled. Patients with sepsis at ICU admission or during the ICU stay were included in the study. Patients with sepsis were enrolled only once during the study. The detailed flowchart of the study design is shown in Figure 1.
Data collection
Each patient admitted to the ICU underwent the enrollment procedure. When the patient was diagnosed with sepsis, medical information was recorded in the CRF. The patient was followed up for 90 days or death after inclusion. The demographic, physiological, bacteriological, and therapeutic data were collected. The Acute Physiology and Chronic Health Evaluation II score and Sequential Organ Failure Assessment (SOFA) score of the first 24 h after inclusion was calculated to evaluate the severity of sepsis. The SOFA score was recalculated in the follow-up days. Since quick SOFA (qSOFA) is the new criteria to assess the severity of sepsis, the qSOFA score was also recorded. The data recorded are shown in Table 2. The day of inclusion was defined as day 1.
Table 2.
Data collected during the study
| Information that needed to be recorded | Evaluation time | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 14 | Day 28 | Day 90 | |
| Informed consent | ● | |||||
| Inclusion criteria | ● | |||||
| Exclusion criteria | ● | |||||
| General information | ● | |||||
| Vital sign | ● | ● | ● | |||
| Microbiological information | ● | ● | ● | ● | ||
| Antimicrobial drugs | ● | ● | ● | ● | ||
| APACHE II score | ● | |||||
| SOFA score | ● | ● | ● | ● | ||
| qSOFA score | ● | |||||
| Lactate | ● | ● | ● | ● | ||
| Coagulation | ● | ● | ● | ● | ||
| Organ support | ● | |||||
| Death/survival | ● | |||||
APACHE II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; qSOFA: Quick Sequential Organ Failure Assessment. “●” means the data needs to record in the corresponding time.
The follow-up data of patients were recorded for 90 days after inclusion. The 28- and 90-day mortality was calculated, and the survival curve was generated.
The etiology of sepsis was recorded. The most common etiology of different sites and types of infection (hospital-acquired infection, community-acquired infection, or health care-associated infection) was obtained, which could guide empiric antibiotic therapy.
Risk factors, such as age, SOFA score, biomarkers, and etiology related to increased mortality, were recorded. The number of deaths was compared with the number of survivors, and the independent risk factors associated with increased mortality in sepsis were determined after univariate and multivariate logistic regression analyses.
Data sources
The data on septic patients were obtained from the medical records. The number of patients admitted to the participating hospital was acquired from the hospital information system. The number of patients admitted to all tertiary hospitals was obtained from the report form of Health and Family Planning Commission of each province. The information on the population of each province was acquired from the report of Chinese National Health and Family Planning Commission and National Bureau of Statistics.
Definition
Sepsis was defined as a suspected or confirmed infection that met two or more criteria for a systemic inflammatory response.[19] Infection was defined according to the definitions of the International Sepsis Forum[20] and adjudicated by the attending physician. The criteria for systemic inflammatory response syndrome (SIRS) were as follows:
Core temperature <36.0°C or >38.0°C;
Heart rate >90 beats/min;
Respiratory rate >20 breaths/min or partial pressure of arterial carbon dioxide <32 mmHg (1 mmHg = 0.133 kPa) or the requirement for invasive mechanical ventilation for an acute process;
White cell count >12.0 × 109/L or <4.0 × 109/L or >10% immature band forms.
Severe sepsis was defined as sepsis with the presence of at least a severe and acute sepsis-related organ dysfunction according to the severe sepsis campaign guideline.[21] Septic shock was defined as sepsis-induced refractory hypotension or hypoperfusion. Refractory hypotension was defined as a systolic blood pressure <90 mmHg or a mean arterial pressure <65 mmHg after the infusion of ≥30 ml/kg fluids. Hypoperfusion was defined as a blood lactate level ≥4.0 mmol/L.
Primary outcome variable
Sepsis incidence was the primary outcome. Based on the aforementioned two assumptions, the variables that populated the incidence of the study sample to the national population were as follows: number of patients with sepsis admitted to the participating hospitals; number of patients admitted to the participating hospitals; number of patients admitted to the hospitals in China; population in China.
The data from the 2014 report of the Chinese National Health and Family Planning Commission and the National Bureau of Statistics were used as the standard population. All septic patients were assumed to be admitted to the ICU in each participating hospital. The data on all patients admitted to the participating hospitals and all tertiary hospitals were also recorded. Then, the number of all septic patients in mainland China during the study period was calculated. The formula used was as follows:
N = n × b/a (1)
where N represents all septic patients in mainland China during the study period; n represents all included septic patients in all participating hospitals during the study period; and b and a represent all patients admitted to the participating hospitals and all tertiary hospitals during the study period, respectively.
Finally, the incidence (R) of sepsis was calculated according to the number of septic patients and population (N) as follows:
R = 6 × N/P (2)
where R represents the incidence of sepsis and P represents the population in mainland China.
Investigator training
Each participating center had one or more ICU physicians who received training for investigating the medical records. The training program involved the methods and process of the study, and the definition, diagnosis, classification, and outcome of sepsis. Actual medical records of sepsis were provided for practice to assess the outcome of the training. A manual of procedures with detailed instructions was provided for administering the records.
Data quality assurance
A data surveillance panel organized by clinical experts was responsible for monitoring every patient admitted to the participating ICU to ensure that all septic patients were sequentially included. The panel met periodically to identify patients with suspected diagnosis of sepsis. The cases were summarized by the panel secretary using the electronic data capture system. Using this strategy, the inclusion and exclusion of borderline cases was finally decided.
A monitoring team screened the patients admitted to the participating ICU during the study period to avoid missing any septic patient. They checked the medical records for any wrong data in the CRF. Then, the data were put into an electronic database. Double entry was used to test the input accuracy. Data cleaning was performed by two ICU fellows and confirmed by two senior ICU physicians. In case of missing or wrong data, the investigator was asked to refill the data. Two biostatisticians independently conducted the analysis to avoid possible statistical errors.
Statistical analysis
All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, NC, USA). Continuous variables were presented as the mean ± standard deviation (SD) and the median (interquartile range) based on the distribution of quantitative variables. The Student's t-test for independent groups was applied to data with a normal distribution. The differences in categorical variables were assessed using the Chi-square test or the Fisher's exact test, and the differences in abnormally distributed quantitative variables were analyzed using the Mann-Whitney U-test. The association between the all-cause 90-day mortality and relevant covariates was analyzed with a binomial logistic regression model. All P values were two-tailed. A value of P < 0.05 was considered statistically significant.
Discussion
The present study was the first large national survey on sepsis in the mainland of China. It provided the real epidemiological data on sepsis in China, including incidence, infection site, etiology, treatment, and outcome. It also provided the information on different regions and comparison of the data between different regions. The information provided in this study might differ from the information provided by previous studies.
The present study also showed the most common etiology of different sites and types of infection, which could guide empiric antibiotic therapy. In addition, it provided information on the independent risk factors for increased mortality due to sepsis.
In the present study, sepsis was defined as infection with SIRS criteria, and the latest definition was not used. Therefore, nearly one-eighth of all septic patients might be missing according to the studies conducted in Australia and New Zealand.[22] However, the new definition was unpublished at the time of conducting the present study. The new sepsis definition better explains the pathophysiology. It not only emphasizes the organ dysfunction and circulatory failure but also cellular metabolism abnormalities. The new definition is easier to remember and may help the clinicians to recognize sepsis earlier and initiate rapid action. However, the new definition was based on the patients from high-income countries, especially in the USA,[23] which may not be applicable in other geographical regions. In addition, SOFA score indicates the severity of sepsis but may not be good diagnostic criteria for sepsis. Vincent et al.[24] noted that qSOSA was not a replacement for SIRS and was not a part of the definition of sepsis. The new definition generated extensive discussion and controversy and needs continued reevaluation.[23,25]
In our study, we used the sepsis 1.0 criteria to include the patients. However, we also recorded the SOFA and qSOFA scores of all included patients. We could analyze these data to compare the sepsis definitions 1.0 and 3.0. We could also determine which definition is better based on our data, especially in Chinese septic patients.
The present study had some unique strengths. An obvious advantage was the national survey in each province based on the population of the mainland of China, which enabled data collection on sepsis across all geographical boundaries in China. In addition, the sample size of septic patients was much larger than that in previous studies conducted in China. Finally, the team involved included ICU specialists, investigators, monitors, and statisticians, who formulated a series of measures to ensure data quality and avoid errors. Hence, the study could provide real epidemiological data on sepsis in China.
The study also had several limitations. First, it only included septic patients admitted to the ICU, which could exclude any patient with sepsis admitted in the other departments or not hospitalized. However, epidemiological studies measured the “treated incidence” rather than the actual incidence.[1] Previous studies showed that part of septic patients were treated in emergency department. However, most of them are treated in the ICU.[6,26] The incidence of sepsis in China may be calculated. Second, to obtain high-quality data, the study only included tertiary hospitals, and most of them were university hospitals, which could bias the results. However, most critically ill patients went to tertiary hospitals for treatment in China, which might reduce the bias.
Financial support and sponsorship
This study was supported by the grants form Hongri Medical Research and Education Fund (No. WH2015-01-01).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51. doi: 10.1056/NEJMra1208623. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–6. doi: 10.1016/S2213-2600(14)70061-X. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 3.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–74. doi: 10.1097/CCM.0b013e31827c09f8. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 4.Seymour CW, Rea TD, Kahn JM, Walkey AJ, Yealy DM, Angus DC. Severe sepsis in pre-hospital emergency care: Analysis of incidence, care, and outcome. Am J Respir Crit Care Med. 2012;186:1264–71. doi: 10.1164/rccm.201204-0713OC. doi: 10.1164/rccm.201204-0713OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand 2000-2012. JAMA. 2014;311:1308–16. doi: 10.1001/jama.2014.2637. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 6.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. CUB-Réa Network. Current epidemiology of septic shock: The CUB-Réa Network. Am J Respir Crit Care Med. 2003;168:165–72. doi: 10.1164/rccm.2201087. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- 8.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 9.Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland 1996 to 2004: Secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care. 2006;10:R42. doi: 10.1186/cc4854. doi: 10.1186/cc4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco J, Muriel-Bombín A, Sagredo V, Taboada F, Gandía F, Tamayo L, et al. Incidence, organ dysfunction and mortality in severe sepsis: A Spanish multicentre study. Crit Care. 2008;12:R158. doi: 10.1186/cc7157. doi: 10.1186/cc7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez F, Barrera L, De La Rosa G, Dennis R, Dueñas C, Granados M, et al. The epidemiology of sepsis in Colombia: A prospective multicenter cohort study in ten university hospitals. Crit Care Med. 2011;39:1675–82. doi: 10.1097/CCM.0b013e318218a35e. doi: 10.1097/CCM.0b013e318218a35e. [DOI] [PubMed] [Google Scholar]
- 12.Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med. 2004;30:589–96. doi: 10.1007/s00134-004-2157-0. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- 13.Adrie C, Alberti C, Chaix-Couturier C, Azoulay E, De Lassence A, Cohen Y, et al. Epidemiology and economic evaluation of severe sepsis in France: Age, severity, infection site, and place of acquisition (community, hospital, or intensive care unit) as determinants of workload and cost. J Crit Care. 2005;20:46–58. doi: 10.1016/j.jcrc.2004.10.005. doi: 10.1016/j.jcrc.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 15.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–72. doi: 10.1164/rccm.201504-0781OC. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–9. doi: 10.1001/jama.2009.1754. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Qian C, Zhao M, Yu X, Kang Y, Ma X, et al. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS One. 2014;9:e107181. doi: 10.1371/journal.pone.0107181. doi: 10.1371/journal.pone.0142287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med. 2007;35:2538–46. doi: 10.1097/01.CCM.0000284492.30800.00. doi: 10.1097/01.CCM.0000284492.30800.00. [DOI] [PubMed] [Google Scholar]
- 19.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. doi: 10.1097/00003246-199206000-00025. [PubMed] [Google Scholar]
- 20.Calandra T, Cohen J. International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538–48. doi: 10.1097/01.ccm.0000168253.91200.83. doi: 10.1097/01.CCM.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- 21.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629–38. doi: 10.1056/NEJMoa1415236. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 23.Abraham E. New definitions for sepsis and septic shock: Continuing evolution but with much still to be done. JAMA. 2016;315:757–9. doi: 10.1001/jama.2016.0290. doi: 10.1001/jama.2016.0290. [DOI] [PubMed] [Google Scholar]
- 24.Vincent JL, Martin GS, Levy MM. qSOFA does not replace SIRS in the definition of sepsis. Crit Care. 2016;20:210. doi: 10.1186/s13054-016-1389-z. doi: 10.1186/s13054-016-1389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson SQ. New sepsis criteria: A change we should not make. Chest. 2016;149:1117–8. doi: 10.1016/j.chest.2016.02.653. doi: 10.1016/j.chest.2016.02.653. [DOI] [PubMed] [Google Scholar]
- 26.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117–23. doi: 10.1001/jama.273.2.117. [PubMed] [Google Scholar]