Abstract
Bio-guided screening is an important method to identify bioactive compounds from fungi. In this study we applied a fast digital time-lapse microscopic method for assessment of the antibacterial properties of secondary metabolites from the fungal genus Fusarium. Here antibacterial effects could be detected for antibiotic Y, aurofusarin, beauvericin, enniatins and fusaric acid after six hours of cultivation. The system was then used in a bio-guided screen of extracts from 14 different Fusarium species, which had been fractionated by HPLC. In this screen, fractions containing the red pigments aurofusarin and bikaverin showed effects against strains of Lactobacillus and Bifidobacterium. The IC50 for aurofusarin against Lactobacillus acidophilus was 8 µM, and against Bifidobacterium breve it was 64 µM. Aurofusarin only showed an effect on probiotic bacteria, leading to the speculation that only health-promoting bacteria with a positive effect in the gut system are affected.
Keywords: Fusarium, mycotoxins, secondary metabolites, bioactivity, polyketides, pigments, antibiotics, bio-guided assays, antibacterial, aurofusarin
1. Introduction
Filamentous fungi represent a rich source of secondary metabolites used in the battle for survival in natural habitats and as infection facilitators during host pathogenesis. Each fungus has a specific arsenal intended to inhibit growth of competing organisms such as bacteria. Several antibacterial compounds have been isolated from fungi, with penicillin, produced by Penicillium and Aspergillus species, being the most famous [1]. In the search for novel antibacterial secondary metabolites, several bio-guided approaches can be applied such as fractionating the secondary metabolome by reverse-phase liquid chromatography [2,3] or by explorative solid-phase extraction [4,5]. The fractions are then often assessed for bioactivity through traditional techniques (e.g., spectrophotometry). The recent development in real-time monitoring systems has enabled accurate bacterial susceptibility testing within a few minutes or hours [6].
In the present study we used the oCelloScope real-time microscopy system to detect antibacterial effects of compounds from Fusarium species. The Fusarium genus comprises a group of pathogenic fungi that are found throughout the world. Some Fusarium species are able to colonize a wide range of plant species, whereas others are pathogens of insects and mammals. Members of the genus contain typically more than 30 different secondary metabolite gene clusters, of which several are species-specific [7]. Some secondary metabolites from Fusarium have been shown to possess an antibacterial effect, including antibiotic Y, beauvericin, enniatins and fusaric acid [8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Others such as T-2 toxin, diacetoxyscirpenol and deoxynivalenol have not been shown to have an antibacterial effect [22]. There is a huge potential to discover novel secondary metabolites in Fusarium as the genetic information shows that only one-fourth of the potentially produced compounds have been identified.
2. Results and Discussion
Seventeen available secondary metabolites known to be produced by Fusarium were used in an initial experiment to set up the oCelloScope system for screening for antibacterial activity of fungal compounds against Lactobacillus acidophilus, Escherichia coli, Staphylococcus aureus and Salmonella typhimurium (Supplementary Figures S1–S4). In this screen, fusaric acid, beauvericin, enniatins, antibiotic Y and aurofusarin exhibited antibacterial activity against one or more of the four bacterial species. To evaluate IC50 values for the five secondary metabolites with antibacterial potential, we used the oCelloScope system armed with the SESA algorithm, which is optimized to detect bacterial growth in liquid suspensions when the total bacteria number is low (Table 1).
Table 1.
IC50 values for the five secondary metabolites with antibacterial effect. Bacteria were incubated with of secondary metabolites (2–256 µM) for 6 h (-: no effect).
| L. acidophilus | E. coli | Staph. aureus | Salm. typhimurium | |
|---|---|---|---|---|
| Antibiotic Y | - | - | 32 μM | - |
| Beauvericin | 32 μM | - | 32 μM | - |
| Enniatin mix | 32 μM | - | 64 μM | 128 μM |
| Fusaric acid | 64 μM | 64 μM | - | - |
| Aurofusarin | 8 μM | - | - | - |
Strong inhibitions of bacterial growth could be determined within the first hour of the experiment, whereas milder inhibitions were observed after 4–6 h of incubation (Video S1). Visual inspection of the generated movies could also be used to detect precipitation of antibiotic Y at high concentrations (Video S1).
The most potent compound was aurofusarin with an IC50 value of 8 µM against L. acidophilus. Antibiotic Y, beauvericin, enniatins, and fusaric acid had weak antibiotic effects, in accordance with other studies [8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Comparison of IC50 or MIC values between different studies is never straightforward due to differences in methods, solubility and purity of the tested compounds. We did not observe any antibiotic effect of the 12 remaining compounds: cyclosporine, diacetoxyscirpenol, deoxynivalenol, fumonisin B1, fusarin C, fusarielin A, fusarielin H, moniliformin, nivalenol, neosolaniol, T-2 toxin, and zearalenone (Figures S1–S4); this is also in agreement with earlier reports. Cyclosporine has been reported to enhance the ability of E. coli to stick to human epithelial cells [23]. Fusarielin H has not been tested before and showed no effect.
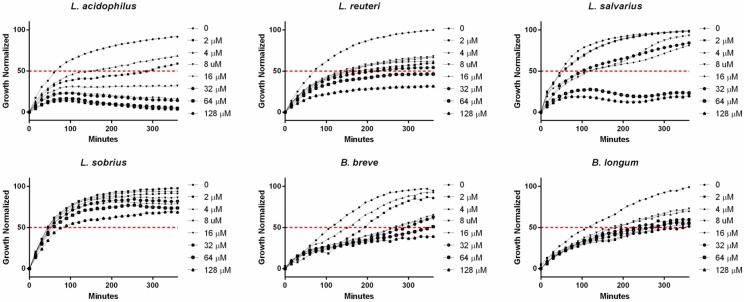
The antibacterial activity of aurofusarin against L. acidophilus was further investigated using strains of four additional Lactobacillus species and two of the related genus Bifidobacterium. Aurofusarin inhibited growth of all strains with IC50 values of: Lactobacillus acidophilus 8 µM, Lactobacillus reuteri 64 µM, Lactobacillus salivarius 32 µM, Lactobacillus sobrius >128 µM, Bifidobacterium breve 64 µM, and Bifidobacterium longum 128 µM (Figure 1).
Figure 1.
The inhibition effects of aurofusarin on Lactobacillus spp. and Bifidobacterium spp. Aurofusarin was tested in concentrations between 2 and 128 µM. The IC50 is marked with a red dotted line. The values are normalized mean values from three independent experiments. Ethanol 1% is used as control.
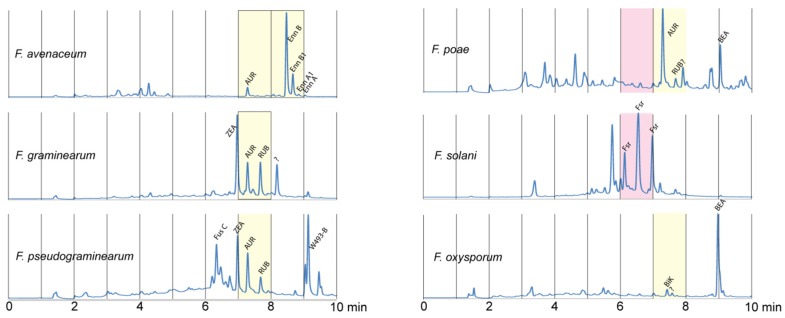
The oCelloScope system was then examined for the potential as a tool for bio-guided identification of antibacterial secondary metabolites from Fusarium. In this test, 14 different Fusarium species were grown on four different media to enable the production of a wide selection of Fusarium secondary metabolites. The species were chosen to cover the phylogenetic diversity [24] and secondary metabolite potential [7] of the Fusarium genus. The resulting extracts were used in a screen against L. acidophilus, E. coli, Staph. aureus and Salm. typhimurium, in which extracts from six Fusarium species exhibited antibacterial activity. The active extracts were separated by HPLC into 10 fractions, which were subsequently screened individually. Two of the resulting fractions (from F. poae and F. solani) contained compounds with an effect against Staph. aureus (Figure 2).
Figure 2.
Isolated fractions with antibacterial effect. Secondary metabolites from each fungus were fractionized by preparative HPLC. Fractions in red showed antibacterial effect against Staph. aureus. Fractions in yellow showed antibacterial effect against L. acidophilus. F. avenaceum was grown on YMA, F. graminearum on PDA, F. poae and F. pseudograminearum on YES and F. oxysporum on rice. AUR: Aurofusarin. Enn: Enniatins. ZEA: Zearalenone. RUB: Rubrofusarin. BEA: Beauvericin. Fus C: Fusarin C. BIK: Bikaverin. ?: unknown.
The active fraction from F. poae did not contain a known compound detectable by the HPLC method, whereas the fraction from F. solani contained various forms of the pigment fusarubin. This is in accordance with previous studies where fusarubins have been shown to inhibit growth of Staph. aureus [25]. Five fractions from F. avenaceum, F. graminearum, F. poae, F. pseudograminearum and F. oxysporum exhibited effects against L. acidophilus. The active fractions against L. acidophilus from F. oxysporum contained the mycelium pigment bikaverin, whereas the active fractions from F. avenaceum, F. graminearum, F. poae and F. pseudograminearum contained the mycelium pigment aurofusarin and the pathway intermediate rubrofusarin. An additional fraction from F. avenaceum, which contained enniatin B, B1, A1 and A, exhibited an effect against L. acidophilus. The majority of the fractions did not inhibit bacterial growth, which is also in agreement with the initial set-up experiment. The inactive fractions contain several of the compounds which did not exhibit antibacterial activity, including zearalenone, fusarin C and trichothecenes (diacetoxyscirpenol, deoxynivalenol, nivalenol, neosolaniol, T-2 toxin). Together these results show that the system is useful as a bio-guided tool to discover compounds with antibacterial activities.
The producers of aurofusarin, mainly F. graminearum, F. avenaceum and F. culmorum, are primarily found in temperate climate zones, whereas bikaverin is produced by F. verticillioides, F. fujikuroi and F. subglutinans which are found in subtopic zones. All these Fusarium species are major pathogens of cereals used for human and animal feed. Aurofusarin, causing the characteristic coloring of several Fusarium species, is synthesized by PKS 12 and is a dimeric naphthoquinone. It has previously been shown to affect chicken egg quality and to possess antifungal effects [26,27]. The regulation of aurofusarin remains unknown but nitrogen sources seem to influence the expression in Fusarium graminearum [28]. High levels of both aurofusarin (up to 47 mg/kg) and bikaverin (up to 90 mg/kg) have been reported in cereals [29,30], and aurofusarin has also been found in apples (166 mg/kg) [31]. Here, we show that aurofusarin and bikaverin have an antibacterial effect on strains of Lactobacillus and Bifidobacterium. Bikaverin has previously shown an antibacterial effect against E. coli [32]. Aurofusarin and other naphthoquinones have previously been shown to penetrate the outer membrane of Gram-positive bacteria due to their lipophilic properties [33,34]. Baker et al. tested 22 naphthoquinones, and 15 exhibited antibiotic activities against the Gram-positive Staph. aureus [34]. During our screening, we did not observe any effect against Gram-negative bacteria, but we did observe activity in some fungal extract fractions against Staph. aureus probably due to naphthoquinones. The Lactobacillus and Bifidobacterium strains require anaerobic conditions and this might enhance the effect of aurofusarin on Gram-positive bacteria.
Lactobacillus and Bifidobacterium strains are regarded as having a positive influence on the gut system in humans and other animal species such as swine [35,36]. Inhibition of Lactobacillus and Bifidobacterium might cause a shift towards more harmful bacteria in the gut and thus result in diarrhea or other gut diseases. Diarrhea is a significant problem for pig breeders and is typically caused by bacteria such as E. coli [37]. In theory, a concentration of 50 mg/kg aurofusarin in cereals may result in a concentration of 40–80 µM in the gut system, more than five times the concentration shown to affect Lactobacillus acidophilus in this study. Further investigations of the antibacterial effect of aurofusarin in an animal model could be highly relevant.
3. Conclusions
In this study, we used 17 different secondary metabolites from Fusarium to develop a fast microscopic method for the assessment of antibacterial activity. The system could be used in a bio-guided approach to determine antibacterial fractions in several Fusarium extracts. In our study we observed a strong antibacterial effect of aurofusarin against Lactobacilli, which can have harmful consequences for the gut microbiota.
4. Materials and Methods
4.1. Secondary Metabolites
The following SMs were purchased: antibiotic Y (BioAustralis, Smithfield, Australia), aurofusarin (Adipogen, Epalinges, Switzerland), beauvericin (BioAustralis, Smithfield, Australia), enniatin complex A, A1, B, B1 (BioAustralis, Smithfield, Australia), cyclosporine (Sigma Aldrich, Brøndby, Denmark), diacetoxyscirpenol (Sigma Aldrich, Brøndby, Denmark), deoxynivalenol (Sigma Aldrich, Brøndby, Denmark), fumonisin B1 (BioAustralis, Smithfield, Australia), fusaric acid (Sigma Aldrich, Brøndby, Denmark), moniliformin (Sigma Aldrich, Brøndby, Denmark), nivalenol (Sigma Aldrich, Brøndby, Denmark), neosolaniol (Sigma Aldrich, Brøndby, Denmark), T-2 toxin (Sigma Aldrich, Brøndby, Denmark), zearalenone (Sigma Aldrich, Brøndby, Denmark). Fusarielin A [38], fusarielin H [39] and fusarin C [40] were prepared as described in the respective publications. All metabolites were dissolved in ethanol 96% to a concentration of 51.2 mM.
4.2. Bacterial Strains
Four bacterial species were selected based on differences in morphology and as references to previous studies. The Gram-negative facultative aerobe bacterium, Escherichia coli (ATCC 25922), was chosen as a standard for monitoring the antibacterial effect of fungal compounds. The Salmonella enterica serovar typhimurium 3389-1 (DT12) was chosen as representative of Gram-negative bacteria that cause problems in European agriculture, it was isolated from a clinical case of salmonellosis in pigs (D. L. Baggesen at the Technical University of Denmark, National Veterinary Institute). Staphylococcus aureus (ATCC 29213), a Gram-positive bacterium was chosen to provide a reference to previous studies. Lactobacillus acidophilus (DSMZ 20079) was chosen as a representative of a Gram-positive bacterium found in the guts of humans and monogastric animals. In experiments with aurofusarin, the following bacteria species were used: Lactobacillus acidophilus (20079), Lactobacillus salivarius (20555), Lactobacillus sobrius (16698), Bifidobacterium longum subsp. longum Reuter (20219), Bifidobacterium breve (20213) (All bacteria were obtained from DSMZ, Braunschweig, Germany).
4.3. Fungal Strains
Representative strains of fourteen Fusarium species were analyzed in this study, (F. avenaceum IBT 41708, F. cerealis IBT 40125, F. culmorum IBT 2925, F. equiseti IBT 8752, F. graminearum NRRL 31084, F. langsethiae IBT 9951, F. oxysporum IBT8890, F. poae IBT 9999, F. proliferatum IBT 40647, F. pseudograminearum NRRL 1544, F. sambucinum IBT 2524, F. solani FGSC9596, F. sporotrichioides IBT 9948, and F. venenatum IBT 1197) selected from the IBT collection at the Technical University of Denmark, the Agricultural Research Service culture collection (NRRL), at the National Center for Agricultural Utilization Research in Peoria, Illinois, IL, USA or Fungal Genetic Stock Center, Kansas City, MO, USA.
4.4. Culture Media
E. coli and Salm. typhimurium strains were grown aerobically at 37 °C in Luria-Bertani (LB) medium (Merck, Darmstadt, Germany). Staph. aureus was grown aerobically at 37 °C in brain heart infusion (BHI) broth (Merck, Darmstadt, Germany), whereas Lactobacillus acidophilus was grown in Mueller Hinton, MSH broth (Merck, Darmstadt, Germany) at 37 °C. The Bifidobacterium and Lactobacillus strains were grown in anaerobic tubes with MRS-medium (Lactobacillus) and Colon-medium (Bifidobacterium) [41]. All bacteria were grown in 15 mL tubes without shaking. Four different fungal media were used: Yeast Extract Sucrose (YES) medium, Potato Dextrose agar (PDA), Yeast Malt agar (YMA) [42] and Rice medium (30 g of white rice and 50 mL H2O, autoclaved for 15 min at 121 °C).
4.5. Secondary Metabolite Fractionation
All Fusarium species were grown on petri dishes (90 mm) for two weeks in the dark at 25 °C on four different media (YES, YMA, PDA, Rice). Forty plugs (4 mm) were used for extraction from each agar plate with 5 mL extraction solvent (ethyl acetate:dichloromethane:methanol (3:2:1, vol/vol) with 1% formic acid) in an ultra-sonic bath for 45 min. Rice medium (10 g) was extracted with 15 mL extraction solvent. The extracts were then evaporated to dryness and re-dissolved in 1.5 mL ethanol and used for initial antibacterial screen.
Extracts with antibacterial properties were fractionated on an Agilent 1260 semi-preparative HPLC system equipped with a 150 × 10 mm Gemini 5 µm C6-Phenyl 110 Å column (Phenomenex, Torrance, CA, USA) using a flow of 5.000 mL/min and a linear water-acetonitrile gradient, where both were buffered with 50 ppm trifluoroacetic acid. The gradient started at 10% ACN, which was increased to 100% in 10 min and held for 2 min. 100 µL of each extract was injected and the entire 12 min run was divided in 10 fractions. The fractions were evaporated to dryness and re-dissolved in ethanol.
4.6. Antibiotic Susceptibility Tests
An overnight culture (0.1 mL) of bacteria was transferred to 8 mL of medium and incubated for 2 h (37 °C) to reach the exponential phase. The OD600 was measured by optical density (UV-3100 PC spectrophotometer; VWR, Herlev, Denmark) and the bacteria were diluted to a concentration of 7.8 × 105 bacteria·mL−1. Beads were added (2 × 104 6-μm·beads/ml, microsphere standard, B-7277; Invitrogen, Naerum, Denmark) in order to focus the microscope. The bacteria were loaded onto an F-base microtiter plate (100 μL/well) (TPP; Sigma-Aldrich, Brondby, Denmark). All pure compounds and fractions of fungal SMs were added as 1 µL in ethanol to each well resulting in a final concentration of 1% ethanol in each sample. Antibiotic susceptibility tests (ASTs) were performed using the oCelloScope detection system (Phillips Bio Cell A/S, Allerød, Denmark). This is a digital time-lapse microscopy scanning through a fluid sample, which generates series of images [43]. Each well was scanned repeatedly every 10 min and 10 pictures are obtained per well. Time-lapse experiments, digital analysis, and image processing were conducted by use of the SESA algorithm as previous described [6,44]. The oCelloScope was placed within an Innova 44 incubator (New Brunswick Scientific) in order to keep the temperature constant at 37 °C. All experiments were done in triplicates. In the experiments with Lactobacillus and Bifidobacterium, we used an anaerobe cabinet to set up the experiment and sealed each well in the plates with oil.
4.7. Digital Analysis
Time-lapse experiments, digital analysis, and image processing were conducted by a custom automation script in MATLAB version 8.0.0.783 (R2012b; The MathWorks, Inc., Natick, MA, USA). The BCA (Background Corrected Absorption) algorithm was developed as an equivalent to OD with increased sensitivity. The algorithm determines the growth kinetics using a background correction mask subtracted from the first scan and partial image histogram summation.
4.8. Statistical Analysis
All data are expressed as mean values. IC50 values are defined as minimum of tested concentrations to inhibit growth by 50%. GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego, CA, USA) was used for statistical analysis.
Acknowledgments
This work was supported by grants from the Danish National Innovation Foundation 137-2012 and from the Danish Agency for Science 4005-00204B and 0602-02612B.
Supplementary Materials
The following are available online at www.mdpi.com/2072-6651/8/12/355/s1, Figure S1: The inhibition effects of 16 different secondary metabolites on L. acidophilus, Figure S2: The inhibition effects of 16 different secondary metabolites on E. coli, Figure S3: The inhibition effects of 16 different secondary metabolites on Staph. aureus, Figure S4: The inhibition effects of 16 different secondary metabolites on S. tryphimurium. Video S1: Growth curve and videos of Staph. aureus grown with or without antibiotic Y. Staph. aureus were incubated in a 96 wells microtiter plate and scanned by oCelloScope every 10 min for 12 h.
Author Contributions
T.E.S., M.F. and J.L.S. conceived and designed the experiments; T.E.S., M.F., A.-M.O.C., S.K.D., N.F.K. and J.L.S. performed the experiments; T.E.S., M.F., A.-M.O.C., S.K.D., N.F.K. and J.L.S. analyzed the data; T.E.S. M.F., H.G. and J.L.S. contributed reagents/materials/analysis tools; T.E.S., M.F., A.-M.O.C., S.K.D., N.F.K., H.G. and J.L.S. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fleming A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenzae. Brit. J. Exp. Pathol. 1929;10:226–236. doi: 10.1093/clinids/2.1.129. [DOI] [Google Scholar]
- 2.Bugni T.S., Harper M.K., McCulloch M.W.B., Reppart J., Ireland C.M. Fractionated marine invertebrate extract libraries for drug discovery. Molecules. 2008;13:1372–1383. doi: 10.3390/molecules13061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang G., Mayhudin N.A., Mitova M.I., Sun L., van der Sar S., Blunt J.W., Cole A.L.J., Ellis G., Laatsch H., Munro M.H.G. Evolving trends in the dereplication of natural product extracts: New methodology for rapid, small-scale investigation of natural product extracts. J. Nat. Prod. 2008;71:1595–1599. doi: 10.1021/np8002222. [DOI] [PubMed] [Google Scholar]
- 4.Månsson M., Phipps R.K., Gram L., Munro M.H.G., Larsen T.O., Nielsen K.F. Explorative solid-phase extraction (E-SPE) for accelerated microbial natural product discovery, dereplication, and purification. J. Nat. Prod. 2010;73:1126–1132. doi: 10.1021/np100151y. [DOI] [PubMed] [Google Scholar]
- 5.Bladt T.T., Durr C., Knudsen P.B., Kildgaard S., Frisvad J.C., Gotfredsen C.H., Seiffert M., Larsen T.O. Bio-activity and dereplication-based discovery of ophiobolins and other fungal secondary metabolites targeting leukemia cells. Molecules. 2013;18:14629–14650. doi: 10.3390/molecules181214629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fredborg M., Andersen K.R., Jørgensen E., Droce A., Olesen T., Jensen B.B., Rosenvinge F.S., Sondergaard T.E. Real-time optical antimicrobial susceptibility testing. J. Clin. Microbiol. 2013;51:2047–2053. doi: 10.1128/JCM.00440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen F.T., Gardiner D.M., Lysøe E., Fuertes P.R., Tudzynski B., Wiemann P., Sondergaard T.E., Giese H., Brodersen D.E., Sørensen J.L. An update to polyketide synthase and non-ribosomal synthetase genes and nomenclature in Fusarium. Fungal Genet. Biol. 2015;75:20–29. doi: 10.1016/j.fgb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Bushnell G.W., Li Y.L., Poulton G.A. Pyrones. X. Lateropyrone, a new antibiotic from the fungus Fusarium lateritium Nees. Can. J. Chem. 1984;62:2101–2106. doi: 10.1139/v84-358. [DOI] [Google Scholar]
- 9.Castlebury L.A., Sutherland J.B., Tanner L.A., Henderson A.L., Cerniglia C.E. Use of a bioassay to evaluate the toxicity of beauvericin to bacteria. World J. Microbiol. Biotech. 1999;15:131–133. doi: 10.1023/A:1008895421989. [DOI] [Google Scholar]
- 10.Golinski P., Wnuk S., Chelkowski J., Visconti A., Schollenberger M. Antibiotic Y: Biosynthesis by Fusarium avenaceum (Corda ex Fries) Sacc., isolation, and some physicochemical and biological properties. Appl. Environ. Microbiol. 1986;51:743–745. doi: 10.1128/aem.51.4.743-745.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamill R.L., Higgens C.E., Boaz H.E., Gorman M. The structure of beauvericin, a new depsipeptide antibiotic toxic to Artemia salina. Tetrahedron Lett. 1969:4255–4258. doi: 10.1016/S0040-4039(01)88668-8. [DOI] [Google Scholar]
- 12.Meca G., Sospedra I., Adela Valero M., Manes J., Font G., Jose Ruiz M. Antibacterial activity of the enniatin B, produced by Fusarium tricinctum in liquid culture, and cytotoxic effects on Caco-2 cells. Toxicol. Mech. Meth. 2011;21:503–512. doi: 10.3109/15376516.2011.556202. [DOI] [PubMed] [Google Scholar]
- 13.Meca G., Sospedra I., Soriano J.M., Ritieni A., Moretti A., Manes J. Antibacterial effect of the bioactive compound beauvericin produced by Fusarium proliferatum on solid medium of wheat. Toxicon. 2010;56:349–354. doi: 10.1016/j.toxicon.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Nilanonta C., Isaka M., Chanphen R., Thong-Orn N., Tanticharoen M., Thebtaranonth Y. Unusual enniatins produced by the insect pathogenic fungus Verticillium hemipterigenum: Isolation and studies on precursor-directed biosynthesis. Tetrahedron. 2003;59:1015–1020. doi: 10.1016/S0040-4020(02)01631-9. [DOI] [Google Scholar]
- 15.Nilanonta C., Isaka M., Kittakoop P., Palittapongarnpim P., Kamchonwongpaisan S., Pittayakhajonwut D., Tanticharoen M., Thebtaranonth Y. Antimycobacterial and antiplasmodial cyclodepsipeptides from the insect pathogenic fungus Paecilomyces tenuipes BCC 1614. Planta Medica. 2000;66:756–758. doi: 10.1055/s-2000-9776. [DOI] [PubMed] [Google Scholar]
- 16.Sebastia N., Meca G., Miguel Soriano J., Manes J. Antibacterial effects of enniatins J1 and J3 on pathogenic and lactic acid bacteria. Food Chem. Toxicol. 2011;49:2710–2717. doi: 10.1016/j.fct.2011.06.070. [DOI] [PubMed] [Google Scholar]
- 17.Shenyakin M.M., Ovchinnikov Y.A., Ivanov V.T., Evstratov A.V. Topochemical approach in studies of the structure-activity relation: Enantio-enniatin B. Nature. 1967;213:412–413. doi: 10.1038/213412a0. [DOI] [PubMed] [Google Scholar]
- 18.Tomoda H., Nishida H., Huang X.H., Masuma R., Kim Y.K., Omura S. New cyclodepsipeptides, enniatins D, E and F produced by Fusarium sp. FO-1305. J. Antibiot. 1992;45:1207–1215. doi: 10.7164/antibiotics.45.1207. [DOI] [PubMed] [Google Scholar]
- 19.Son S.W., Kim H.Y., Choi G.J., Lim H.K., Jang K.S., Lee S.O., Lee S., Sung N.D., Kim J.C. Bikaverin and fusaric acid from Fusarium oxysporum show antioomycete activity against Phytophthora infestans. J. Appl. Microbiol. 2008;104:692–698. doi: 10.1111/j.1365-2672.2007.03581.x. [DOI] [PubMed] [Google Scholar]
- 20.Supothina S., Isaka M., Kirtikara K., Tanticharoen M., Thebtaranonth Y. Enniatin production by the entomopathogenic fungus Verticillium hemipterigenum BCC 1449. J. Antibiot. 2004;57:732–738. doi: 10.7164/antibiotics.57.732. [DOI] [PubMed] [Google Scholar]
- 21.May H.D., Wu Q.Z., Blake C.K. Effects of the Fusarium spp. mycotoxins fusaric acid and deoxynivalenol on the growth of Ruminococcus albus and Methanobrevibacter ruminantium. Can. J. Microbiol. 2000;46:692–699. doi: 10.1139/cjm-46-8-692. [DOI] [PubMed] [Google Scholar]
- 22.Vesonder R.F., Ellis J.J., Rohwedder W.K. Elaboration of vomitoxin and zearalenone by Fusarium isolates and the biological activity of Fusarium-produced toxins. Appl. Environ. Microbiol. 1981;42:1132–1134. doi: 10.1128/aem.42.6.1132-1134.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szkaradkiewicz A., Wal M. Effect of cyclosporin on uropathogenic Escherichia coli adherence to human endothelial cells. Int. J. Antimicrob. Agents. 2001;18:89–91. doi: 10.1016/S0924-8579(01)00341-7. [DOI] [PubMed] [Google Scholar]
- 24.O’Donnell K., Rooney A.P., Proctor R.H., Brown D.W., McCormick S.P., Ward T.J., Frandsen R.J.N., Lysøe E., Rehner S.A., Aoki T., et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013;52:20–31. doi: 10.1016/j.fgb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Arnstein H.R.V., Cook A.H., Lacey M.S. Production of antibiotics by fungi. Part II: Production by Fusarium javanicum and other fusaria. Br. J. Exp. Pathol. 1946;27:349–355. [PMC free article] [PubMed] [Google Scholar]
- 26.Dvorska J. Effects of aurofusarin, a dimeric naphthoquinone metabolite of Fusarium graminearum, on fatty acid profiles and antioxidant composition of quail eggs. Toxicology. 2001;164:176. [Google Scholar]
- 27.Dvorska J.E., Surai P.F., Speake B.K., Sparks N.H.C. Effect of the mycotoxin aurofusarin on the antioxidant composition and fatty acid profile of quail eggs. Br. Poult. Sci. 2001;42:643–649. doi: 10.1080/00071660120088470. [DOI] [PubMed] [Google Scholar]
- 28.Sørensen J.L., Nielsen K.F., Sondergaard T.E. Redirection of pigment biosynthesis to isocoumarins in Fusarium. Fungal Genet. Biol. 2012;49:613–618. doi: 10.1016/j.fgb.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Busman M., Butchko R.A.E., Proctor R.H. LC-MS/MS method for the determination of the fungal pigment bikaverin in maize kernels as an indicator of ear rot. Food Addit. Contam. Part A-Chem. 2012;29:1736–1742. doi: 10.1080/19440049.2012.704528. [DOI] [PubMed] [Google Scholar]
- 30.Uhlig S., Eriksen G.S., Hofgaard I.S., Krska R., Beltran E., Sulyok M. Faces of a changing climate: Semi-quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins. 2013;5:1682–1697. doi: 10.3390/toxins5101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sørensen J.L., Phipps R.K., Nielsen K.F., Schroers H.J., Frank J., Thrane U. Analysis of Fusarium avenaceum metabolites produced during wet apple core rot. J. Agric. Food Chem. 2009;57:1632–1639. doi: 10.1021/jf802926u. [DOI] [PubMed] [Google Scholar]
- 32.Deshmukh R., Mathew A., Purohit H.J. Characterization of antibacterial activity of bikaverin from Fusarium sp. HKF15. J. Biosci. Bioeng. 2014;117:443–448. doi: 10.1016/j.jbiosc.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Medentsev A.G., Akimenko V.K. Naphthoquinone metabolites of the fungi. Phytochemistry. 1998;47:935–959. doi: 10.1016/S0031-9422(98)80053-8. [DOI] [PubMed] [Google Scholar]
- 34.Baker R.A., Tatum J.H., Nemec S., Jr. Antimicrobial activity of naphthoquinones from Fusaria. Mycopathologia. 1990;111:9–15. doi: 10.1007/BF02277294. [DOI] [PubMed] [Google Scholar]
- 35.Brown M. Modes of action of probiotics: Recent developments. J. Anim. Vet. Adv. 2011;10:1895–1900. doi: 10.3923/javaa.2011.1895.1900. [DOI] [Google Scholar]
- 36.Ohashi Y., Ushida K. Health-beneficial effects of probiotics: Its mode of action. Anim. Sci. J. 2009;80:361–371. doi: 10.1111/j.1740-0929.2009.00645.x. [DOI] [PubMed] [Google Scholar]
- 37.Fairbrother J.M., Nadeau E., Gyles C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005;6:17–39. doi: 10.1079/AHR2005105. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi H., Sunaga R., Furihata K., Morisaki N., Iwasaki S. Isolation and structures of an antifungal antibiotic, Fusarielin A, and related compounds produced by a Fusarium sp. J. Antibiot. 1995;48:42–52. doi: 10.7164/antibiotics.48.42. [DOI] [PubMed] [Google Scholar]
- 39.Sørensen J.L., Hansen F.T., Sondergaard T.E., Staerk D., Lee T.V., Wimmer R., Klitgaard L.G., Purup S., Giese H., Frandsen R.J. Production of novel fusarielins by ectopic activation of the polyketide synthase 9 cluster in Fusarium graminearum. Environ. Microbiol. 2012;14:1159–1170. doi: 10.1111/j.1462-2920.2011.02696.x. [DOI] [PubMed] [Google Scholar]
- 40.Sondergaard T.E., Hansen F.T., Purup S., Nielsen A.K., Bonefeld-Jorgensen E.C., Giese H., Sørensen J.L. Fusarin C acts like an estrogenic agonist and stimulates breast cancer cells in vitro. Toxicol. Lett. 2011;205:116–121. doi: 10.1016/j.toxlet.2011.05.1029. [DOI] [PubMed] [Google Scholar]
- 41.Holdeman L.V., Cato E.P., Moore W.E.C. Anaerobe Laboratory Manual. 4th ed. Virginia Polytechnic Institute and State University; Blacksburg, VA, USA: 1977. [Google Scholar]
- 42.Frisvad J.C. Media and growth conditions for induction of secondary metabolite production. Meth. Mol. Biol. 2012;944:47–58. doi: 10.1007/978-1-62703-122-6_3. [DOI] [PubMed] [Google Scholar]
- 43.Fredborg M., Rosenvinge F.S., Spillum E., Kroghsbo S., Wang M., Sondergaard T.E. Automated image analysis for quantification of filamentous bacteria. BMC Microbiol. 2015;15:255. doi: 10.1186/s12866-015-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uggerhoj L.E., Poulsen T.J., Munk J.K., Fredborg M., Sondergaard T.E., Frimodt-Moller N., Hansen P.R., Wimmer R. Rational design of alpha-helical antimicrobial peptides: Do’s and don’ts. ChemBioChem. 2015;16:242–253. doi: 10.1002/cbic.201402581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.