Abstract
Prostatic neuroendocrine cells (NE) are an integral part of prostate cancer (PCa) that are associated with PCa progression. As the current androgen-deprivation therapy (ADT) with anti-androgens may promote the neuroendocrine PCa (NEPCa) development, and few therapies can effectively suppress NEPCa, understanding the impact of NEPCa on PCa progression may help us to develop better therapies to battle PCa. Here we found NEPCa cells could increase the docetaxel-resistance of their neighboring PCa cells. Mechanism dissection revealed that through secretion of PTHrP, NEPCa cells could alter the p38/MAPK/Hsp27 signals in their neighboring PCa cells that resulted in increased androgen receptor (AR) activity via promoting AR nuclear translocation. The consequences of increased AR function might then increase docetaxel-resistance via increasing p21 expression. In vivo xenograft mice experiments also confirmed NEPCa could increase the docetaxel-resistance of neighboring PCa, and targeting this newly identified PTHrP/p38/Hsp27/AR/p21 signaling pathway with either p38 inhibitor (SB203580) or sh-PTHrP may result in improving/restoring the docetaxel sensitivity to better suppress PCa.
Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed tumors in men in western countries (1). While androgen deprivation therapy (ADT) is effective for some patients, most patients may develop castration resistance with the development of metastatic PCa (2). Docetaxel (Doc) has been used as a standard treatment for these patients with castration resistant PCa (CRPC) (3). However, the therapeutic durability for Doc is limited (average 18 months) with some serious side effects (4, 5).
Neuroendocrine prostate cancer (NEPCa) represents a minor population of PCa, yet NEPCa cells exists in the most advanced stages of PCa (6). Importantly, the population of NEPCa cells might increase during the current ADT with anti-androgen treatments (7). Different from most PAC cells, NEPCa cells express little androgen receptor (AR), thus they are inherently resistant to the ADT treatment. The common markers for these NEPCa cells are Chromogranin (ChrA), neuron-specific enolase (NSE) and synaptophysin (8).
Early studies suggested that NEPCa cells might secrete various factors, including bombesin, parathyroid hormone related protein (PTHrP), adrenomedullin and vascular endothelial growth factor (VEGF) to influence the surrounding PCa progression (9–11). However, the detailed mechanisms, especially their influences on the AR, the key factor for the PCa progression as well as their impact on the efficacy of chemotherapies such as Doc, remain unclear.
In an in vitro system of co-culturing cells and in vivo mouse xenograft studies, we demonstrated that NEPCa could increase the chemo-resistance of neighboring PCa via altering the p38/Hsp27/AR/p21 signals.
Results
NEPCa increased chemo-resistance of neighboring PCa
Early studies suggested that NEPCa is not only resistant to existing therapies in general, its conditioned media (CM) might also enhance the development of castration resistance in its neighboring PCa (CRPC) (12, 13). To further study the impact of NEPCa cells on the chemo-sensitivity on the neighboring PCa cells, we determined the efficacy of Doc on PCa cells before and after their co-culture with the NEPCa cells (Fig. 1A). We found that after co-culture with the NEPCa NCI-H660 cells, CRPC C4-2 cells become more resistant to Doc treatment in a dose-dependent manner ranging from 1 nM to 8 nM (Fig. 1B, left panel) and the viability of these C4-2 cells are increased as gauged by MTT assay. Similar results were also obtained when we replaced C4-2 with CWR22Rv1 cells (Fig. 1B, right panel).
Figure 1.
NEPCa induces chemoresistance of neighboring PCa. C4-2 and CWR22Rv1 cells were co-cultured with or without NCI-H660 or NE1.3 cells in 0.4 μM transwell plates for 72 hours. A. The diagram of co-culture system. 2× 104 prostate adenocarcinoma cells, C4-2/CWR22Rv1, were plated in lower chambers of transwells and 5× 103 neuroendocrine prostate cancer cells, NCI-H660/NE1.3, were plated on the upper insert chambers. After 72 hours, the C4-2/CWR22Rv1 cells and conditioned media were collected. B. MTT assay detection of cell viability. 2× 103 cultured/co-cultured C4-2 and CWR22Rv1 cells were seeded in 48-wells plate. After attachment, cells were treated with Doc of different concentration (0 nM, 1 nM, 2 nM, 4 nM and 8 nM) for 24 or 48 hours. All dose groups are normalized with 0 nM dose group. C. TUNEL assay analysis of cell apoptosis. Cultured/co-cultured cells were plated in 8-wells chamber slide and treated with or without 3 nM Doc for 48 hours and the right panels are the quantitative data for TUNEL. D. Western blot analysis of the expression of cleaved PARP in cultured/co-cultured cells after treating with 3 nM docetaxel for 48 hours. E. MTT assay detection of cultured/co-cultured cell viability after treating with 3 nM Doc for 48 hours. F. Western blot analysis of the expression of cleaved PARP in cultured/co-cultured cells after treating with 3 nM docetaxel for 48 hours. *p < 0.05 and **p < 0.01.
To determine that the viability of these CRPC cells are related to programmed cell death, we applied the TUNEL assay to examine the impact of co-culturing with NEPCa, the results indicated that NCI-H660 cells might protect C4-2 and CWR22Rv1 cells from Doc-induced apoptosis (Fig. 1C). Meanwhile, co-culture with NCI-H660 cells also decreased the level of cleaved PARP, another indicator for the apoptotic process (14) (Fig. 1D).
Importantly, similar results were also obtained when we replaced the NCI-H660 cells with NE1.3 cells, which are LNCaP cells cultured long-term in the absence of androgen (15) and have the characteristics of NEPCa cells (Fig. 1E–F).
Together, results from Fig. 1 suggest that NEPCa may confer a survival advantage to the surrounding PCa through enhancing their resistance to chemotherapy.
NEPCa induced chemo-resistance of surrounding PCa via altering the p38/Hsp27 signals
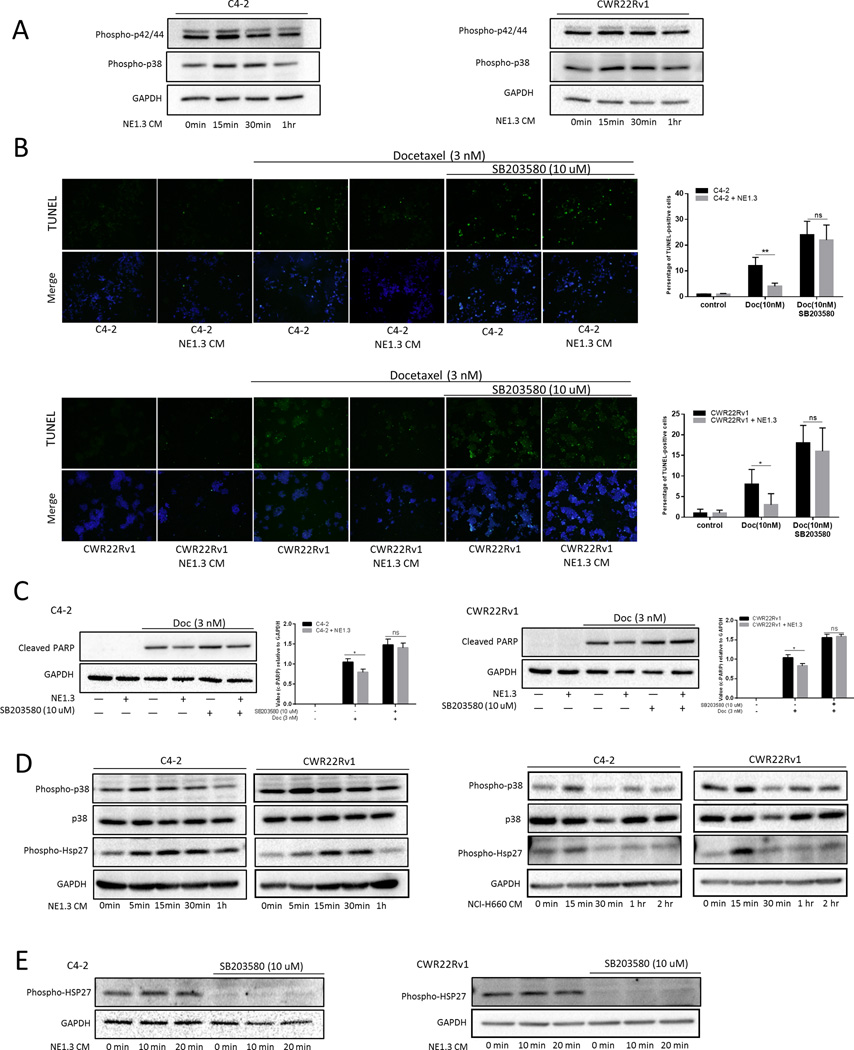
To dissect the molecular mechanism how NEPCa increased chemo-resistance of surrounding PCa, we first screened commonly known signals related to apoptosis and proliferation. We found p42/44/MAPK and p38/MAPK signals could be activated (via phosphorylation) by NEPCa (Fig. 2A). Increasing evidences suggest that p38/MAPK signals may play a crucial role in regulating tumor apoptosis and activation of the p38 kinase has been shown to be essential for tumor survival in response to select chemotherapeutic cancer drugs (16–18). Moreover, pre-incubation of C4-2/CWR22Rv1 cells with 10 μM SB203580, a p38 kinase inhibitor, abolished the NEPCa capacity to increase the chemo-resistance in the neighboring PCa (Fig. 2B–C). Therefore, we focused on the p38/MAPK pathway, and determined its activation through measuring the phosphorylation of p38 (Thr180/Tyr182) with phospho-specific antibodies on a western blot. p38 phosphorylation was increased in a time-dependent manner, with a maximum stimulation at 15 min after treatment with NE1.3 conditioned media (CM) (Fig. 2D). A similar activation can also be detected with NCI-H660 CM (Fig. 2D). These results suggest that NEPCa may increase the surrounding PCa chemo-resistance via altering the p38/MAPK signals.
Figure 2.
NEPCa activated p38/MAPK/Hsp27 pathway and p38/MAPK inhibitor can partially reverse NEPCa induced chemoresistance. C4-2 and CWR22Rv1 cells were clotted with or without NE1.3 cells for 72 hours in 6-wells plates and the conditioned media (CM) was collected. A. p44/42 and p38 were phospho-activated by NEPCa. C4-2 and CWR22Rv1 cells were starved with SR media for 24 hours, then treated with CM. Total proteins were analyzed by Western blotting as shown using anti–phospho-p42/44 and anti–phospho-p38 antibodies. B–C. The p38 inhibitor SB203580 (10 μmol/L) can partially reverse NEPC induced chemo-resistance of PCa. We pre-treated C4-2/CWR22Rv1 cells with or without SB203580 for 15 min and subsequently treated with co-culture CM or control media for an additional 24 hours. After that, pre-treated cells were treated with or without 3 nM Doc for 48 hours. TUNEL assay analysis (B) of cell apoptosis and western blot analysis (C) of the expression of cleaved PARP. The right panels are the quantitative data for TUNEL and western-blot. D. PCa/NEPCa co-culture CM phosphorylates p38 and Hsp27 in a time-dependent manner. C4-2 and CWR22Rv1 cells were starved with SR media for 24 hours, then treated with PCa/NEPC co-culture CM for different times (5 min, 15 min, 30 min and 1 hour). Total proteins were analyzed by Western blotting as shown using anti–phospho-p38 and anti–phospho-Hsp27 antibodies. Anti–total p38 was used for control loading. E. Activation of p38 is required for NEPCa induced phosphorylation of Hsp27. We treated C4-2 and CWR22Rv1 cells with or without SB203580 for 15 min and subsequently treated with PCa/NEPCa co-culure CM for different times (0 min, 10 min and 20 min). Western blot analysis of the expression of phosphorylated Hsp27. *p < 0.05, and ns, no statistical differences.
Among many substrates of p38/MAPK, Heat shock protein 27 (Hsp27), as a chaperone of the small heat shock protein group, can be activated through phosphorylation in response to cell stress to prevent apoptosis and to regulate cell differentiation (19, 20). Up-regulation of Hsp27 has been associated with chemo-resistance and radiation resistance (21, 22). p38 kinase can phosphorylate Hsp27 at multiple serines, including Ser15, Ser78 and Ser82 (23). Therefore, we detected the phosphorylation of Hsp27 (Ser78) through phospho-specific antibody and found CM from NEPCa could rapidly induce phosphorylation of Hsp27 (Ser78) in a time-dependent manner (Fig. 2D). Importantly, addition of 10 μM SB203580 inhibited the NE1.3-induced Hsp27 phosphorylation in neighboring PCa, suggesting that activation of p38 is required for NEPCa-increased phosphorylation of Hsp27 (Fig. 2E).
Together, results from Fig. 2 indicated that NEPCa increased the neighboring PCa chemo-resistance likely via the p38/Hsp27 signals.
Androgen receptor in the PCa chemo-resistance induced by NEPCa
It is well documented that activation of AR is related to progression of PCa (24), and it plays an important role as a survival factor in prostate epithelial cells (25). Activation of AR by androgen enhances LNCaP cell survival in the presence of cytotoxic stress (26). More recently, Zoubeidi et al. found that non-nuclear phospho-Hsp27 might displace Hsp90 in the AR complex to chaperone AR into the nucleus with consequent increase of AR activity (27). We therefore examined whether NEPCa-increased p38-Hsp27 signals may also link to the activation of AR.
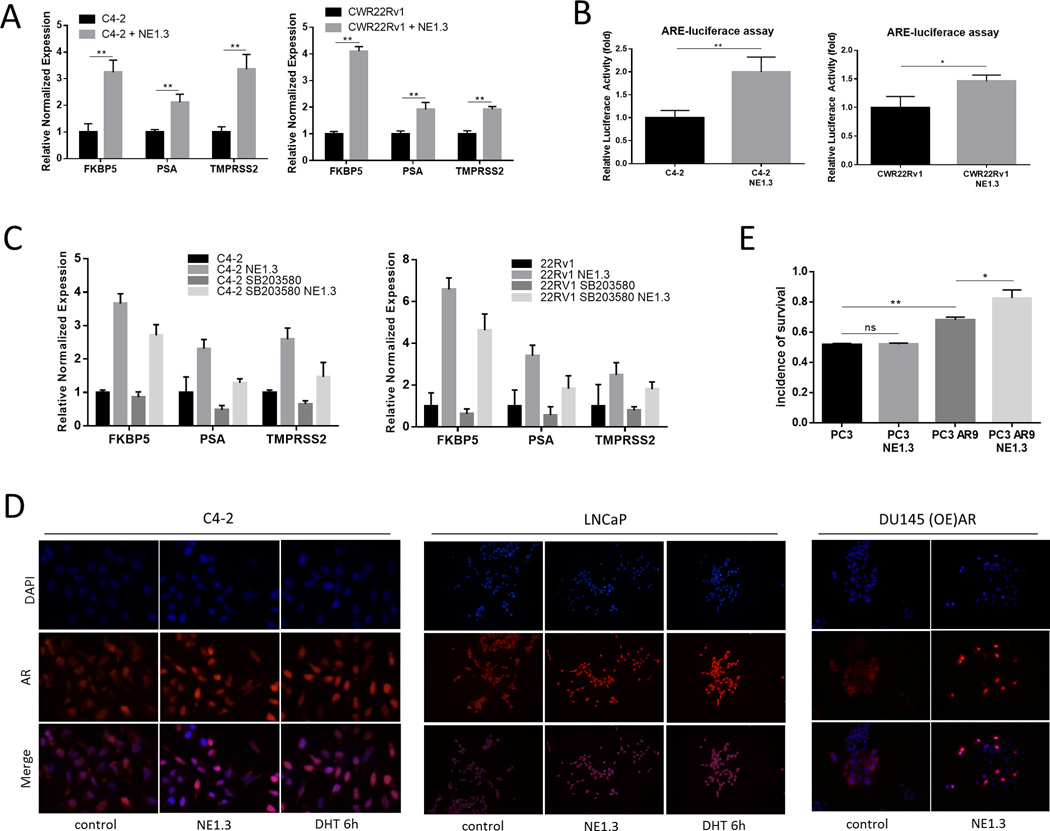
We first detected AR expression at the protein level in the PCa cells after co-culture with NEPCa cells. We found little change of AR expression in C4-2 and CWR22Rv1 cells after co-culture with NE1.3 cells (Fig. S1). However, the transcript level of AR-target genes such as PSA, TMPRSS2 and FKBP5, as detected by quantitative real-time PCR, was increased upon co-culture with NE1.3 cells (Fig. 3A) and NCI-H660 cells (Fig. S2). In addition, ARE-luciferase reporter assay further substantiated the activation of AR (Fig. 3B). Moreover, pre-incubation of C4-2/CWR22Rv1 cells with p38 kinase inhibitor could partially abolished the NEPCa capacity to increase the AR activity in the neighboring PCa (Fig. 3C). Consistent with the increased AR activity, staining with fluorescently labeled anti-AR antibody also confirmed that NEPCa CM could increase AR nuclear translocation (from the cytoplasm) in C4-2 cells (Fig. 3D, left panel). In addition, expression of exogenous AR in AR-negative PCa DU145 cells also demonstrated its nuclear localization in response to NEPCa CM (Fig. 3D, right panel).
Figure 3.
Androgen receptor roles in the NEPCa-increased surrounding PCa chemo-resistance. A. NE1.3 cells increased AR activity of C4-2 and CWR22Rv1 cells. C4-2 and CWR22Rv1 cells were cultured with or without NE1.3 cells for 72 hours in 6-well plates. Total RNA were analyzed by Q-PCR to show AR regulated genes, PSA, TMPRSS2 and FKBP5. B. ARE-luciferase assay to detect AR activity. C. Activation of p38 is required for NE1.3 cells increased AR activity of C4-2 and CWR22Rv1 cells. We treated C4-2 and CWR22Rv1 cells with or without SB203580 for 15 min and subsequently treated with PCa/NEPCa co-culture CM or 12 hours and total RNA were analyzed by Q-PCR to show AR regulated genes, PSA, TMPRSS2 and FKBP5.D. NE1.3 cells induced AR trans-located into the nucleus in PCa cells. C4-2 (left panels)/ LNCaP (middle panels)/ DU145 overexpressed (OE)-AR cells (right panels) were treated with PCa/NEPC co-culture CM or control media for 8 hours and fixed in methanol for immunofluorescence staining with anti-AR antibodies and DAPI. C4-2/LNCaP cells treated with 10 nM DHT were used as positive control. E. Stable AR overexpressed PC3 cells, PC3 AR9, show more resistance to Doc after co-culture with NE1.3 cells compared to parental PC3 cells. PC3-AR9 cells were cultured with or without NE1.3 cells for 72 hours in 6-well plates and then treated with 3 nM Doc for additonal 48 hours. MTT assay detected the cell viability and normalized with relative cells without treating with Doc. *p < 0.05, **p < 0.01 and ns, no statistical differences.
Importantly, the stably AR overexpressed PC3 cells, PC3 AR9, also revealed more resistance to Doc after co-culturing with NE1.3 cells compared to the parental PC3 cells (Fig. 3E).
Together, results from Fig. 3 suggest that NEPC may increase the neighboring PCa chemo-resistance via increased p38/Hsp27 signals that likely result in increased AR activity via enhanced AR nuclear translocation.
Mechanism dissection how NEPCa-increased p38/Hsp27/AR signals enhance neighboring PCa chemo-resistance
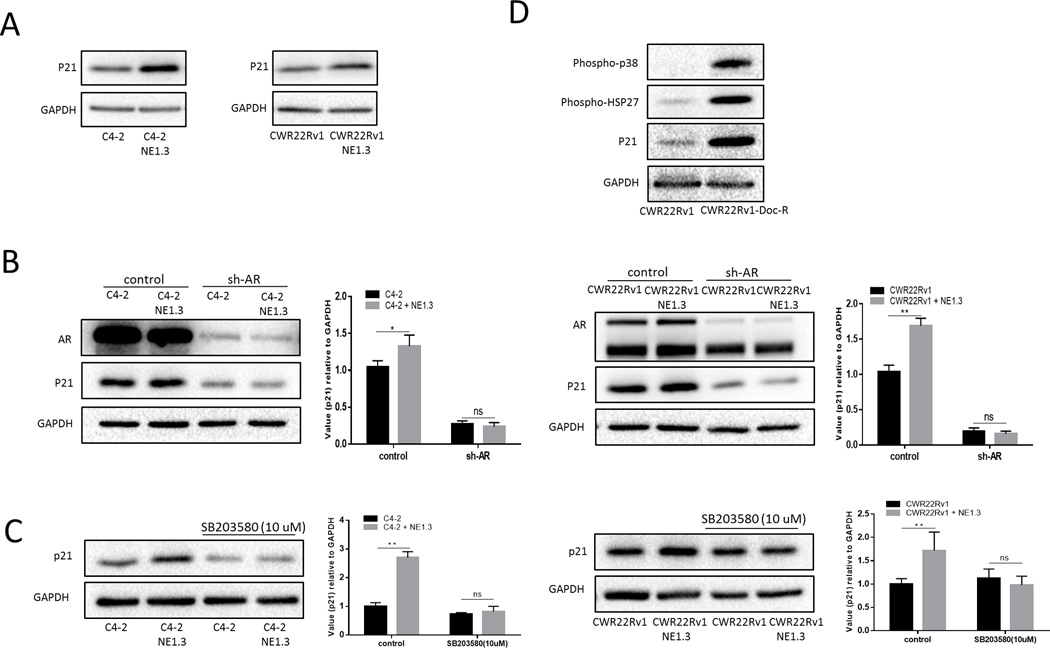
Previously, Lu et al. found that androgen up-regulated the cyclin-dependent kinase inhibitor p21 transcription through an androgen response element (ARE) in its proximal promoter (28), while increased p21 has been linked with chemo-resistance to the anti-microtubule agents in PCa (29, 30). Therefore, we explored the potential linkage of p21 to the newly identified NEPCa-increased p38/Hsp27/AR signals. As shown in Fig. 4A and Fig. S3, co-culture with either NE1.3 or NCI-H660 cells resulted in increasing p21 in C4-2 and CWR22Rv1 cells. Knocking down AR with AR-shRNA partially reversed this NE1.3-cells-increased p21 up-regulation in C4-2 and CWR22Rv1 (Fig. 4B). Importantly, adding p38 inhibitor could also partially reverse induction of p21 in C4-2 and CWR22Rv1 cells in response to NEPCa cells (Fig. 4C).
Figure 4.
NEPCa-increased p38/Hsp27/AR signals enhance neighboring PCa chemo-resistance by upregulating p21. A. NEPCa up-regulated p21 of PCa. C4-2 and CWR22Rv1 cells were cultured with or without NE1.3 cells for 72 hours in 6-well plates. Western blot analysis of the expression of p21 in C4-2 and CWR22Rv1 cells after co-culture with NE1.3 cells for 72 hours. B. Knocking down AR (sh-AR) with lentivirus can partially reverse NEPCa induced p21 of neighboring PCa. Western blot analysis of the expression of p21 in C4-2 (left panel) and CWR22Rv1 (right panel) cells after co-culture with NE1.3 cells. The right panels are the quantitative data for left panel western-blots. C. The p38 inhibitor SB203580 (10 umol/L) can partially reverse NEPCa induced p21. We treated C4-2 and CWR22Rv1 cells with SB203580 for 15 min and then cultured with or without NE1.3 cells for 48 hours. Western blot analysis of the expression of p21 in C4-2 (left panel) and CWR22Rv1 (right panel) cells after co-culture with NE1.3 cells. the right panels are quantitative data for left panel western blots. D. the p38/Hsp27/p21 signals are also activated in Doc-resistant CWR22Rv1 cell line. Western blot analysis of the expression of phospho-p38, phospho-Hsp27 and p21 in CWR22Rv1 cells and Doc resistance CWR22Rv1 cells. *p < 0.05, **p < 0.01 and ns, no statistical differences.
Interestingly, we found that p38/Hsp27/p21 signals are also activated in our Doc-resistant CWR22Rv1 cell line (Fig. 4D), suggesting that increased p38/Hsp2/p21 signaling is also associated with stable chemo-resistance in PCa cells.
Together, results from Fig. 4 and S3 demonstrated that NEPCa might increase the neighboring PCa chemo-resistance via altering the p38/Hsp27/AR/p21 signals.
Parathyroid hormone–related protein (PTHrP) secreted by NEPCa may be the pivotal molecule causing decreased chemo-sensitivity of PCa
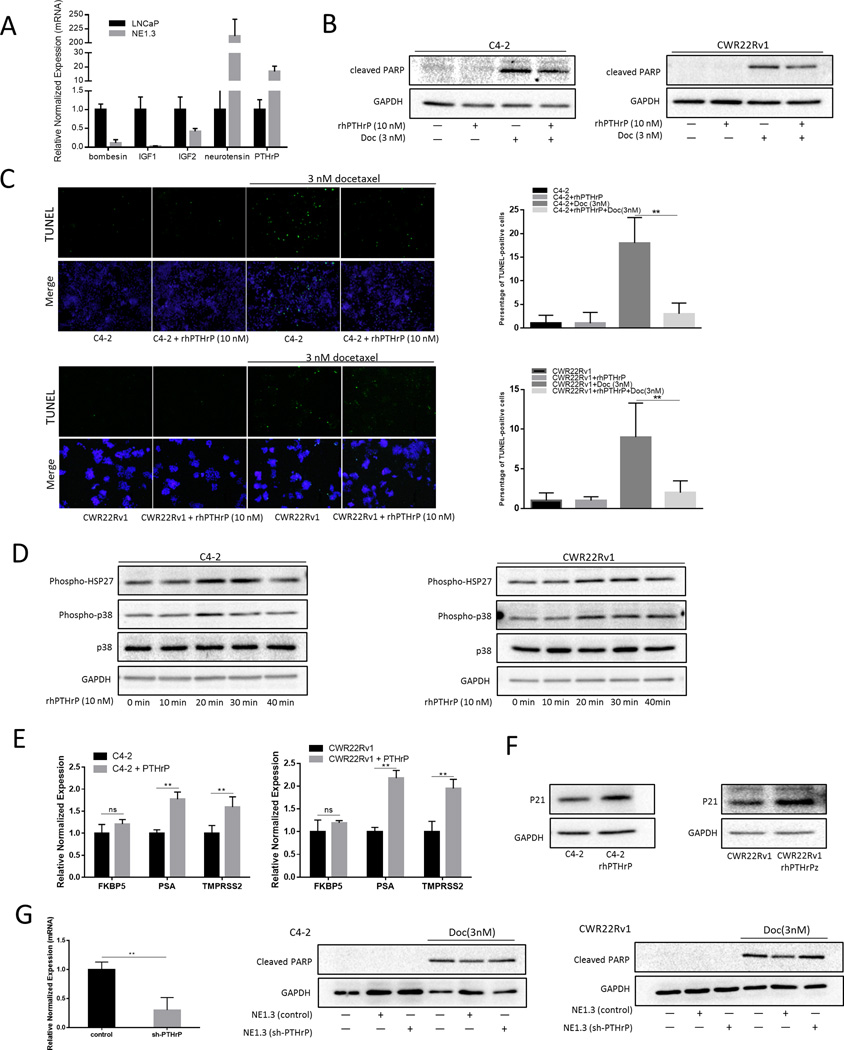
As the identified p38/Hsp27/AR/p21 signals were all in the neighboring PCa cells, we were interested to examine the signals upstream from NEPCa leading to the activation of the p38/Hsp27/AR/p21 signals. Early studies suggested that NEPCa might secrete different kinds of cytokines and peptides, including bombesin, calcitonin, parathyroid hormone-related protein (PTHrP), serotonin and VEGF, to stimulate progression in its neighboring PCa (9–11, 31, 32). We first compared the expression of some cytokines and peptides related to p38/MAPK signals between LNCaP and NE1.3 cells (33–37). Only neurotensin and PTHrP showed a higher expression in NE1.3 cells (Fig. 5A). PTHrP is a key NEPCa-derived peptide-hormone that may have significant impacts on the neighboring PCa progression (38). Indeed, Marin-Aguilar et al. indicated that PTHrP might modulate the colon carcinoma cell proliferation via altering the p38/MAPK signals (33), and DaSilva et al. also found that PTHrP might enhance PCa growth via stabilizing AR (10).
Figure 5.
Neuroendocrine peptide PTHrP can protect PCa from docetaxel (Doc)-induced apoptosis. A. PTHrP is upregulated in NE1.3 cells have a higher expression of PTHrP compared to parental LNCaP cells. Total RNA were analyzed by Q-PCR to show neuroendocrine-related cytokines and peptides, including bombesin, IGF1, IGF2, PTHrP, and neurotensin. B–C. PTHrP protects PCa from Doc induced apoptosis. C4-2 and CWR22Rv1 cells were treated with or without recombinant human (rh)-PTHrP (10 nM) for 24 hours. Western blot analysis (B) of the expression of cleaved PARP after treating with 3 nM Doc for 48 hours. TUNEL assay analysis (C) of cell apoptosis after treating with or without 3 nM Doc for 48 hours and the right panels are the quantitative data for TUNEL. D. PTHrP phosphorylates p38 kinase and Hsp27 in a time-dependent manner (with maximum at 20 min). C4-2 and CWR22Rv1 cells were serum starved for 24 hours, then treated with rhPTHrP (10 nM) for different times (0 min, 10 min, 20 min, 30 min and 40 min). Total proteins were analyzed by Western blotting as shown using anti–phospho-p38 and anti–phospho-Hsp27 antibodies. E. PTHrP increased AR activity of C4-2 and CWR22Rv1 cells. C4-2 (left panel) and CWR22Rv1 (right panel) cells were treated with or without rh-PTHrP (10 nM) for 12 hours. Total RNA were analyzed by Q-PCR to show AR regulated gene, FKBP5, PSA and TMPRSS2. F. PTHrP upregulates p21 in PCa. C4-2 and CWR22Rv1 cells were serum starved for 24 hours, then treated with rhPTHrP (10 nM) for 48 hours, western blot analysis of the expression of p21 in C4-2 (left panel) and CWR22Rv1 (right panel) cells. G. Knocking down PTHrP partially reversed the NEPC-increased neighboring PCa chemo-resistance. C4-2 and CWR22Rv1 cells were cultured with/without NE1.3 cells with knocked down PTHrP (sh-PTHrP) for 72 hours and western blot analysis of the expression of cleaved PARP after treating with 3 nM Doc for 48 hours. **p < 0.01 and ns, no statistical difference.
Then, we investigated whether PTHrP is one of the key factors released from NEPCa to increase the neighboring PCa chemo-resistance. We found that treatment of C4-2 and CWR22Rv1 cells with PTHrP (10 nM) for 24 hours resulted in a decrease of cleaved PARP induced by treating with Doc, thus a reduced apoptotic induction (Fig. 5B). Similarly TUNEL assays also resulted in reduced apoptosis upon PTHrP treatment in response to Doc treatment (Fig. 5C). Importantly, PTHrP also increased the phosphorylation of p38 and Hsp27 (Fig. 5D), as well as the p21 expression (Fig. 5F), in a time-dependent manner, with a maximum at 20 minutes. The expression of AR downstream genes, including PSA, TMPRSS2 and FKBP5 were also increased after treatment with PTHrP for 12 hours (Fig. 5E). In addition, an interruption approach via lentiviral PTHrP knockdown also partially reversed the NEPCa-increased neighboring PCa chemo-resistance (Fig. 5G).
Together, results from Fig. 5 suggested that NEPCa may function through releasing PTHrP to increase the neighboring PCa chemo-resistance.
NEPCa increased chemo-resistance of neighboring PCa tissues in vivo
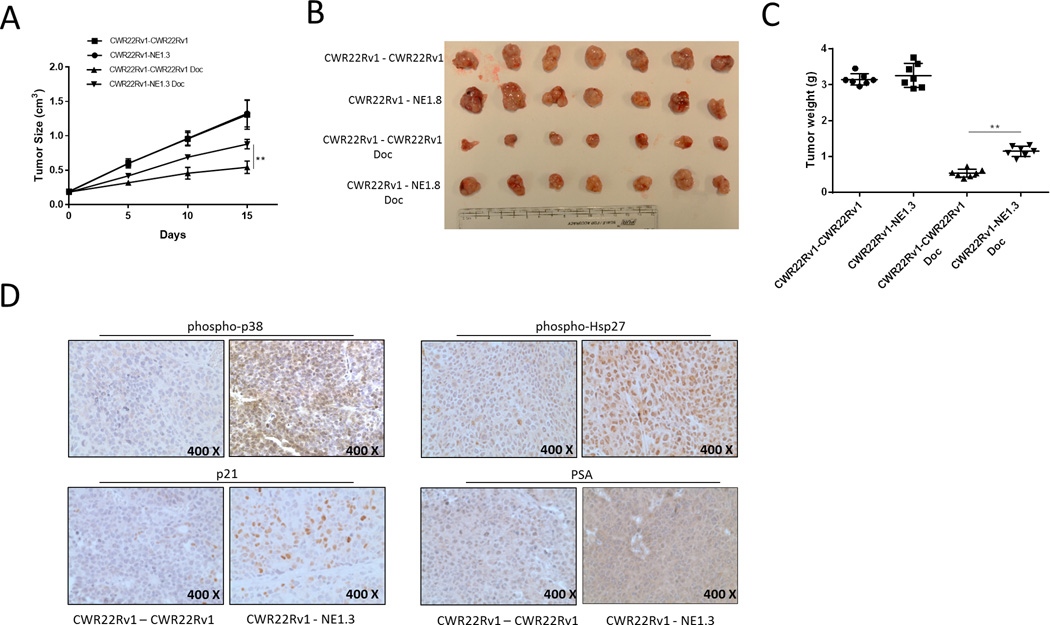
Our results above indicated NEPCa induced chemo-resistance of neighboring PCa likely through p38/Hsp27/AR/p21 signaling pathway in vitro. We sought to determine whether in vivo NEPCa protected PCa from Doc-induced apoptosis. To do so, castrated nude mice were subcutaneously xenografted on left and right flanks either both with CWR22Rv1 cells or with CWR22Rv1 and NE1.3 cells in the two separate locations as it has been shown that NEPCa-released factors can circulate in the vasculature to reach the other side of the mouse (39). After xenograft, when tumors grew to about 0.2 cm3, mice were randomized for intraperitoneal Doc administration at 30 mg/kg every 5 days for 3 treatments or vehicle treatments. The tumor volumes were measured by caliper every 5 days. Mice were sacrificed 3 days after the last Doc administration. The results (Fig. 6A and 6B) showed that after Doc administration, in mice bearing CWR22Rv1 tumors alone, tumor volumes/sizes decreased by about 67% compared to those with vehicle administration. In contrast, in mice bearing the CWR22Rv1 and NE1.3 tumors, the tumor volumes decreased by about 38% compared with those with vehicle administration. After Doc administration, CWR22Rv1 tumor volumes were 1.9 fold larger in mice bearing CWR22Rv1 and NE1.3 tumors than those in mice bearing CWR22Rv1 tumors alone.
Figure 6.
NEPCa increased chemo-resistance of neighboring PCa in the in vivo animal models. Castrated male nude mice (28 mice) were injected subcutaneously with CWR22Rv1 cells (1×106) in the right flank regions. The 14 of these mice were implanted with 1×106 CWR22Rv1 cells (CWR22Rv1-CWR22Rv1) or 1×106 NE1.3 cells (CWR22Rv1-NE1.3) injected subcutaneously in the left flank regions. After xenograft tumors grew to about 0.2 cm3, 7 mice of each group received intraperitoneal (IP) Doc at 30 mg/kg every 5 days for 3 times, with the other 7 mice of each group receiving IP vehicle. A. NE1.3 tumor increased Doc-resistance of CWR22Rv1 tumor. Tumor volumes were measured by caliper every 5 days (n=7). B. Macroscopic appearance of the tumor xenografts (n=7). C. Weights of the xenografts were shown (n=7). D. Representative images of IHC staining for phospho-p38, phospho-Hsp27, p21 and PSA of the CWR22Rv1 tumors in the xenograft model. **p < 0.01.
These findings suggested that NEPCa increased Doc-resistance of PCa tissues. Consistently, after Doc administration, mice bearing CWR22Rv1 and NE1.3 tumors showed an increased tumor weight when compared to those bearing CWR22Rv1 tumors alone (Fig. 6C).
To assess the role of NEPCa induced chemo-resistance of neighboring PCa tissues, we detected the expression of molecules that were examined in vitro through IHC such as PSA, phospho-p38, phospho-Hsp27 and p21 in the xenograft tumors, and results were consistent with the findings in vitro, suggesting that molecular mechanisms that we discovered likely also functioned in vivo (Fig. 6D).
Discussion
Overcoming Doc resistance has been a challenge since Doc was first established as a front-line therapy for metastatic CRPCa. It has been found that some molecules are involved in Doc resistance (40–42). However, there was no report indicating that NEPCa, as a component of the PCa tumors, play an important role in Doc resistance. In this study, we first demonstrated NEPC can protect neighboring PCa cells from Doc induced apoptosis and increase the Doc-resistance of their neighboring PCa cells. And our work may provide a potential method to conquer Doc resistance.
Prostatic NE cells are present in all PCa tumors, and are positively correlated with the cancer progression. As normal prostatic NE cells do not divide, there is strong evidence suggesting that transdifferentiation from prostate epithelial cells is the source of these NEPC cells. And this transdifferentiation can be influenced by a variety of means such as androgen ablation (43), hypoxia (44) and a particular tumor microenvironment (TME) (45).
The significance of NEPCa has been regarded as not only a sign for PCa progression, but also an inducer of growth and survival for the neighboring PCa by secreting a variety of cytokines and peptides (9–11, 31, 32). For example, NEPCa secrete several neuropeptides, including bombesin, which can act as a mitogen in PCa tumors via activation of the transcription factor Elk-1 and the immediate early gene c-fos (46). Certain receptors for serotonin may be overexpressed in PCa cells, particularly in high grade tumors, further supporting the hypothesis that NE products may promote androgen-independence of PCa through a paracrine mechanism (47). Bombesin and calcitonin prevent apoptosis of PCa cells in vitro (48, 49). Neuroendocrine differentiation (NED) may promote neovascularization of PCa as NEPCa are the major producers of VEGF (50). In addition, it was seen that contralateral implantation of NEPCa with androgen-sensitive PCa cells resulted in the latter xenograft being resistant to ADT, thus the development of CRPC (39). All these data support the notion that human PCa is heterogeneous in nature, not just for multi-clonal origin for cancer initiation, but also in terms of the TME being composed of multiple kinds of cells helping each other in the tumor ecosystem.
In the present work, we found NEPCa can result in activation of p38/MAPK and Hsp27, which play a significant role in NEPCa-induced chemo-resistance of PCa. P38/MAPK is a well-known sensor of stress stimuli, such as cytokines, irradiation, and heat shock, and is involved in cell differentiation, apoptosis and autophagy (51–53). P38/MAPK has been involved in the progression of CRPC and accumulating evidence implicated an important role of p38/MAPK in activating AR via Hsp27 independent of androgen (27). Hsp27, also known as heat shock protein beta-1 (HspB1), is highly expressed in CRPC. Clinical samples indicated that Hsp27 expression was significantly associated with PCa prognosis, and its overexpression confers resistance to androgen ablation and chemotherapy (21, 22). Previous studies have shown AR stability, nuclear shuttling and transactivation are all related to Hsp27 (27). For example, Razandi et al found Hsp27 is required for sex steroid receptor (e.g. AR) trafficking which leads to kinase activation, and DNA synthesis (54). In another study, the AR cofactor, hic-5/ARA55, was also found able to bind to Hsp27 and may also be involved in up-regulation of AR activity by Hsp27 (55). Recently, in a multicenter Phase II clinical trial for CRPC, OGX-427, an antisense oligo of Hsp27, was found to possibly synergistically enhance Hsp90 efficacy through suppression of cell growth and induction of apoptosis accompanied by decreased expression of AR and PSA, and induction of endoplasmic reticulum stress (56). Here, we showed p38/MAPK activated Hsp27 facilitated AR translocation to the nuclei. Targeting Hsp27 with OGX-427 may be a potential therapy to suppress NEPCa induced chemo-resistance of PCa. We also showed p44/p42/MAPK in PCa cells could be activated by NEPCa NE1.3 cells and some studies also indicated p44/p42/MAPK participated in chemoresistance of Pancreatic Cancer, non-Hodgkin’s lymphoma B cells and prostate cancer (57–59). The roles of p44/p42/MAPK in NEPCa induced docetaxel resistance of neighboring PCa need further exploration.
AR signaling plays critical roles for PCa initiation and progression (24). Activation of AR by androgen enhances LNCaP cell survival in the presence of cytotoxic stress (26). Some studies also indicated interrupting microtubule-dependent trafficking of AR to the nucleus might be one of the potential mechanism of Doc effect (60). And recent data also suggested that enhanced AR activity, particularly expression of AR variants, is correlated with resistance to Doc (41). Clinical data also showed that the cytoplasmic AR in circulating tumor might be associated with patients’ responses to Doc. Consistent with this, clinical studies also found that ADT-naïve metastatic PCa patients have enhanced survival if the initial ADT was coupled with Doc treatment, supporting the notion that increased AR activity reduces the efficacy of Doc or enhances Doc-resistance (61). Our studies strengthen the notion that enhanced AR activity is tightly connected with resistance to the Doc-resistance induced by NEPCa cells.
Recently, Cerasuolo et al. (62) found neuroendocrine transdifferentiation in PCa cell populations, achievable under permanent androgen deprivation conditions, could influence the progression to androgen independence by providing an androgen-like substance from an increased level of cholesterol for the growth of androgen-dependent PCa cells. However, it remains to be determined that this increased cholesterol turns into an increased level of testosterone in this culture condition. On the other hand, it has been demonstrated that growth factors like Insulin-like Growth Factor 1, epidermal growth factor and interleukin-6 could also behave as androgen-like agents by promoting AR phosphorylation and activation(63–65). Here, we also proved that NEPCa activated AR in neighboring PCa cells indirectly via p38/MAPK signal (Fig.3C) with NE1.3 cells that were derived from permanent androgen deprivation as well as the patient-derived NEPCa cell line, NCI-H660 (Fig. 2D). In addition, we provided evidence that PTHrP from NEPCa cells can also impact AR function in neighboring PCa cells.
PTHrP is implicated in cellular calcium transport and smooth muscle cell contractility, and plays crucial roles in cell proliferation and differentiation, especially for endochondral bone formation. PTHrP has also been connected with benign diseases, such as osteoporosis and osteoarthritis, as well as malignancies including most cancers and cancer-associated hypercalcemia. PTHrP is one of the well-known NEPCa derived peptides critical for tumor initiation, growth and metastasis (38). Previous studies showed that PTHrP promoted epithelial-to-mesenchymal transition that is closely connected with metastasis and stemness in PCa (66). It has also been shown that PTHrP enhanced PCa growth via both stabilizing AR and driving a CD11b+Gr1+ cell-mediated positive feedback loop(10, 38). Particularly, it has been associated with skeletal metastases in the late stages of PCa, which frequently resulted in pathological fractures, bone pain and spinal cord compression (67). Our studies indicated a role of PTHrP in protecting PCa from Doc-induced apoptosis, extending its long list of functions in the process of PCa progression, suggesting that it is an important target for the treatment of PCa.
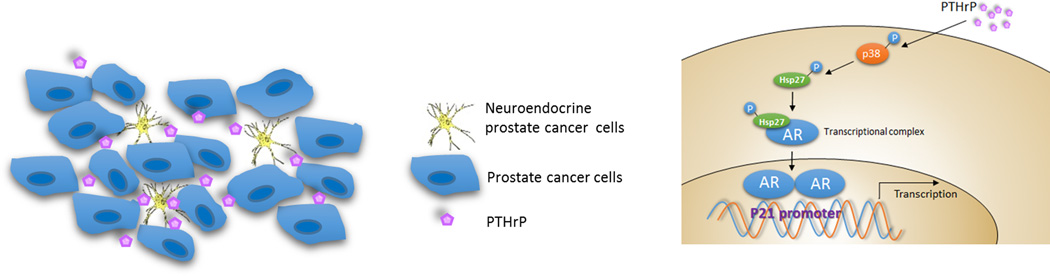
In conclusion, NEPCa development and chemotherapy resistance are frequent occurrences in late stages of PCa. We found that NEPCa could secrete PTHrP to alter the p38/MAPK/Hsp27 signals in their neighboring PCa that resulted in increased AR activity via promoting AR nuclear translocation with a consequent increase of p21 that is linked with an increase of Doc-resistance (Fig. 7). These studies provide foundations to use small molecules targeting this newly identified p38/Hsp27/AR/p21 signaling pathway to restore sensitivity to Doc to better suppress PCa progression.
Figure 7.
Mechanisms and regulatory pathways of NEPCa-promoted neighboring PCa chemo-resistance. NEPCa secreted PTHrP can activate p38/MAPK/Hsp27 pathway, then promote AR translocation and upregulate p21.
Materials and Methods
Cell lines
The CWR22Rv1, DU145 and NCI-H660 cell lines were purchased from the American Type Culture Collection. We stably transfected human PCa PC3 cells with AR cDNA and named this cell line as PC3-AR9 (68). CWR22Rv1, DU145 and PC3-AR9 cells were cultured in RPMI 1640 with 10% FBS, penicillin (25 units/ml) and streptomycin (25 g/ml). Doc-resistant clone, CWR22Rv1-doc-R was selected by culturing cells with Doc in a dose-escalation manner. After sensitive clones were no longer present and surviving cells repopulated the dish, the concentration of Doc was increased gradually. CWR22Rv1-Doc-R cells were further exposed to 5 nM Doc. NCI-H660 cells were maintained in HITES media supplemented with 5% FBS. The C4-2 cell line was a gift from Dr. Jer-Tsong Hsieh (Southwestern Medical Center) and grown in RPMI-1640 media as above. The NE1.3 cells were a gift from Dr. Lin Ming-Fong (University of Nebraska Medical Center) and were maintained in an SR media (Phenol Red free RPMI 1640 supplemented with 5% charcoal/dextran-treated, heat-inactivated FBS, 1% glutamine, and 0.5% gentamicin). All cell lines were cultured in a 5% (v/v) CO2 humidified incubator at 37°C Both C4-2 and CWR22Rv1 cells did not show NE differentiation in SR media for 72 hours.
Reagents and Materials
GAPDH (6c5) and AR (N-20) antibodies were purchased from Santa Cruz Biotechnology. Phospho-p38 (Thr180/Tyr182) antibody was from Cell Signaling Technology. AR antibody for IP was from Millipore. The p38 and Phospho-Hsp27 (ser78) antibody were purchased from One World Lab. Anti-mouse/rabbit second antibody for Western Blot was from Invitrogen. The p38 inhibitor (SB-203580) was from Apexbio. Doc was from Fisher Scientific.
Cell Proliferation Assay
Cells were seeded in 48-well plates (2×103 cells/200 µl media/well) and incubated overnight for attachment. Then they were treated with indicated doses of drugs in normal media for 24 or 48 hours. After treatment, the media was replaced with MTT (0.5 mg/ml) at 37°C for 45 min. After removal of media, the blue crystals were dissolved with 200 µl dimethyl sulfoxide (DMSO)/well, and absorbance at 570 nm was measured.
Fluorescent in Situ Detection of DNA Fragmentation (TUNEL)
Apoptotic cell death was determined using TUNEL staining with an In Situ Cell Death Detection Kit (Roche Molecular Biochemicals), following the manufacturer's protocol. TUNEL–positive cells were calculated as the number of positive cells divided by the total number of cells/field in 10 random fields at 40× magnification.
Immunofluorescence Staining
C4-2, CWR22Rv1 and DU145 cells (3×103 cells/500 µl media/chamber) were seeded on the chamber slides. After indicated treatments, cells were fixed in 4% neutral buffered para-formaldehyde and blocked in PBS containing 5% BSA for 1 hour at room temperature in a humidified chamber. Sections were washed and incubated with antibodies specific for AR (N-20) overnight at 4°C, and then with goat anti-rabbit IgG (Alexa Fluor 594, Invitrogen) for 1 hour in the dark at room temperature. Slides were mounted by adding DAPI-Fluoromount-G (Southern Biotech) and examined with a Zeiss axiophot photomicroscope (Carl Zeiss, Oberkochen, Germany).
Luciferase Reporter Assays
Cells were plated in 24-well plates and transfected with androgen response element (ARE)-luciferase pGL3 and pRL-TK-luciferase plasmid using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions. After indicated treatments, cells were lysed and the luciferase activity was detected by the Dual-luciferase Assay (Promega) and pRL-TK-luciferase was used as the internal control. Each sample was normalized by pRL-TK-luciferase activity, and data were presented as mean ± SE from at least three independent experiments.
Lentivirus package and transfection
The pLVTHM-shPTHrP or pLKO.1-shAR, the psAX2 packaging plasmid, and pMD2G envelope plasmid, were then transfected into 293T cells using the standard calcium chloride transfection method for 48 hours to get the lentivirus soup. The lentivirus soup were collected and concentrated by density gradient centrifugation, then frozen at −80 °C for later use. The pSuperior–ARsiRNA targeting human AR mRNA sequence is 5’-gtggccgcagcaaggggctg-3’, the pSuperior-PTHrPsiRNA targeting human PTHrP mRNA sequence is 5’- gctcacagattgaggtaataa-3’.
RNA Extraction and Quantitative Real-Time PCR Analysis
Total RNAs were isolated using Trizol reagent (Invitrogen) and 1 µg of total RNA was subjected to reverse transcription using Superscript III transcriptase (Invitrogen). Quantitative real-time PCR (qRT-PCR) was conducted using a Bio-Rad CFX96 system with SYBR green to determine the mRNA expression level of a gene of interest. Expression levels were normalized to the expression of GAPDH RNA.
Western Blot Analysis
The expressions of specific genes were determined by the Western blot according to a previous study (69). Briefly, cells were lysed in RIPA buffer and proteins (20 µg) were separated on 8–10% SDS/PAGE gel and then transferred onto PVDF membranes (Millipore). After blocking membranes, they were incubated with appropriate dilutions (1:1000) of specific primary antibodies, the blots were incubated with HRP-conjugated secondary antibodies and visualized using ECL system (Thermo Fisher Scientific).
In Vivo Subcutaneously Implantation and Docetaxel Administration Studies
Six-eight weeks old male nude mice were castrated and used to create the animal model. CWR22Rv1 cells (1×106 suspended in 200 µl Matrigel) were injected subcutaneously in the right flank region of all the 28 mice. In the control group, CWR22Rv1 cells (1×106 suspended in 200 µl Matrigel) were also injected subcutaneously in the left flank region of 14 mice (CWR22Rv1-CWR22Rv1), while NE1.3 cells (1×106 suspended in 200µl Matrigel) were injected into the left flank of the other 14 mice (CWR22Rv1-NE1.3). After xenograft tumors grew to about 0.2 cm3, 7 of the CWR22Rv1-CWR22Rv1 mice and 7 of the CWR22Rv1-NE1.3 mice were randomized to intraperitoneal Doc administration at 30 mg/kg every 5 days for 3 treatments and the 7 remaining mice of each group treated with vehicle. The mice were monitored and tumor volumes were measured by caliper (0.5236 × length × width) every 5 days. Mice were sacrificed 3 days after the last Doc administration, and subcutaneous tumors were harvested for IHC studies. All the animal experiments were performed in accordance with the guidelines of the University of Rochester Medical Center Animal Care and Use Committee for animal experiments.
Immunohistochemistry (IHC)
Prostate tumor xenografts were fixed in 10% (v/v) formaldehyde in PBS, embedded in paraffin and cut into 5 µm sections. Prostate cancer sections were deparaffinized in xylene solution and rehydrated using gradient ethanol concentrations. Antigen retrieval were preformed using EDTA at 98°C for 10 minutes. Then slides were incubated with H2O2 to block the endogenous peroxidase, and incubated with the primary antibody at 4 °C overnight. After rinsing with Tris-buffered saline, the slides were incubated for 1 hour with biotin-conjugated secondary antibody, washed, and then incubated with enzyme conjugate horseradish peroxidase (HRP)-streptavidin. Freshly prepared DAB (Zymed, South San Francisco, CA) was used as substrate to detect HRP. Finally, slides were counter-stained with hematoxylin and mounted with aqueous mounting media. Positive cells were calculated as the number of immunopositive cells × 100% divided by total number of cells/field in 10 random fields at 400 × magnification.
Statistics
All statistical analyses were carried out with SPSS 19.0 (SPSS Inc, Chicago, IL). The data values were presented as the mean ± SD. Differences in mean values between two groups were analyzed by two-tailed Student’s t test and the mean values of more than two groups were compared with one way ANOVA. p ≤ 0.05 was considered statistically significant.
Supplementary Material
NEPCamay not regulate AR expression in PCa. C4-2 and CWR22Rv1 cells were co-cultured with or without NE1.3 cells for 72 hours in 6-well plates. Western blot analysis of the expression of AR in C4-2(left panel) and CWR22Rv1(right panel) cells.
NEPCa could increase AR activity of surrounding PCa.C4-2 and CWR22Rv1 cells were co-cultured with or without NCI-H660cells for 72 hours in 6-well plates. Total RNA of C4-2 (left panel) and CWR222Rv1 (right panel) cells were analyzed by Q-PCR to show AR regulated genes, PSA, TMPRSS2 and FKBP5.
NEPCaup-regulated p21 of PCa. C4-2 and CWR22Rv1 cells were co-cultured with or without NCI-H660 cells for 48 hours in 6-well plates. Western blot analysis of the expression of p21 in C4-2 (left panel) and CWR22Rv1 (right panel) cells.
Acknowledgments
This work was supported by NIH grants (CA155477 and CA156700), George Whipple Professorship Endowment and Taiwan Department of Health Clinical Trial, Research Center of Excellence (DOH99-TD-B-111-004 to China Medical University, Taichung, Taiwan) and China 973 Program (2012CB518305). We thank Karen Wolf for help preparing the manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Reference
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32(49):5501–5511. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pienta KJ, Smith DC. Advances in prostate cancer chemotherapy: a new era begins. CA Cancer J Clin. 2005;55(5):300–318. doi: 10.3322/canjclin.55.5.300. quiz 23–5. [DOI] [PubMed] [Google Scholar]
- 4.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(2):242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Terry S, Beltran H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front Oncol. 2014;4:60. doi: 10.3389/fonc.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan TC, Veeramani S, Lin FF, Kondrikou D, Zelivianski S, Igawa T, et al. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocrine-related cancer. 2006;13(1):151–167. doi: 10.1677/erc.1.01043. [DOI] [PubMed] [Google Scholar]
- 8.Abrahamsson PA. Neuroendocrine cells in tumour growth of the prostate. Endocrine-related cancer. 1999;6(4):503–519. doi: 10.1677/erc.0.0060503. [DOI] [PubMed] [Google Scholar]
- 9.Levine L, Lucci JA, 3rd, Pazdrak B, Cheng JZ, Guo YS, Townsend CM, Jr, et al. Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer research. 2003;63(13):3495–3502. [PubMed] [Google Scholar]
- 10.DaSilva J, Gioeli D, Weber MJ, Parsons SJ. The neuroendocrine-derived peptide parathyroid hormone-related protein promotes prostate cancer cell growth by stabilizing the androgen receptor. Cancer research. 2009;69(18):7402–7411. doi: 10.1158/0008-5472.CAN-08-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conn EM, Botkjaer KA, Kupriyanova TA, Andreasen PA, Deryugina EI, Quigley JP. Comparative analysis of metastasis variants derived from human prostate carcinoma cells: roles in intravasation of VEGF-mediated angiogenesis and uPA-mediated invasion. The American journal of pathology. 2009;175(4):1638–1652. doi: 10.2353/ajpath.2009.090384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DaSilva JO, Amorino GP, Casarez EV, Pemberton B, Parsons SJ. Neuroendocrine-derived peptides promote prostate cancer cell survival through activation of IGF-1R signaling. The Prostate. 2013;73(8):801–812. doi: 10.1002/pros.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint Jean L, et al. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer research. 2008;68(16):6762–6769. doi: 10.1158/0008-5472.CAN-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ZQ, Stingl L, Morrison C, Jantsch M, Los M, Schulze-Osthoff K, et al. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 1997;11(18):2347–2358. doi: 10.1101/gad.11.18.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang XQ, Kondrikov D, Yuan TC, Lin FF, Hansen J, Lin MF. Receptor protein tyrosine phosphatase alpha signaling is involved in androgen depletion-induced neuroendocrine differentiation of androgen-sensitive LNCaP human prostate cancer cells. Oncogene. 2003;22(43):6704–6716. doi: 10.1038/sj.onc.1206764. [DOI] [PubMed] [Google Scholar]
- 16.Chung LY, Tang SJ, Sun GH, Chou TY, Yeh TS, Yu SL, et al. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(15):4037–4047. doi: 10.1158/1078-0432.CCR-11-3348. [DOI] [PubMed] [Google Scholar]
- 17.Small GW, Shi YY, Higgins LS, Orlowski RZ. Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer research. 2007;67(9):4459–4466. doi: 10.1158/0008-5472.CAN-06-2644. [DOI] [PubMed] [Google Scholar]
- 18.Marengo B, De Ciucis CG, Ricciarelli R, Furfaro AL, Colla R, Canepa E, et al. p38MAPK inhibition: a new combined approach to reduce neuroblastoma resistance under etoposide treatment. Cell death & disease. 2013;4:e589. doi: 10.1038/cddis.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2(9):645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 20.O'Shaughnessy RF, Welti JC, Cooke JC, Avilion AA, Monks B, Birnbaum MJ, et al. AKT-dependent HspB1 (Hsp27) activity in epidermal differentiation. The Journal of biological chemistry. 2007;282(23):17297–17305. doi: 10.1074/jbc.M610386200. [DOI] [PubMed] [Google Scholar]
- 21.Hsu HS, Lin JH, Huang WC, Hsu TW, Su K, Chiou SH, et al. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer. 2011;117(7):1516–1528. doi: 10.1002/cncr.25599. [DOI] [PubMed] [Google Scholar]
- 22.Hadchity E, Aloy MT, Paulin C, Armandy E, Watkin E, Rousson R, et al. Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Mol Ther. 2009;17(8):1387–1394. doi: 10.1038/mt.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L, Chen S, Bergan RC. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene. 2006;25(21):2987–2998. doi: 10.1038/sj.onc.1209337. [DOI] [PubMed] [Google Scholar]
- 24.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19(20):5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin J, Adam RM, Santiestevan E, Freeman MR. The phosphatidylinositol 3'-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer research. 1999;59(12):2891–2897. [PubMed] [Google Scholar]
- 26.Berchem GJ, Bosseler M, Sugars LY, Voeller HJ, Zeitlin S, Gelmann EP. Androgens induce resistance to bcl-2-mediated apoptosis in LNCaP prostate cancer cells. Cancer research. 1995;55(4):735–738. [PubMed] [Google Scholar]
- 27.Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P, et al. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer research. 2007;67(21):10455–10465. doi: 10.1158/0008-5472.CAN-07-2057. [DOI] [PubMed] [Google Scholar]
- 28.Lu S, Jenster G, Epner DE. Androgen induction of cyclin-dependent kinase inhibitor p21 gene: role of androgen receptor and transcription factor Sp1 complex. Mol Endocrinol. 2000;14(5):753–760. doi: 10.1210/mend.14.5.0461. [DOI] [PubMed] [Google Scholar]
- 29.Geng H, Rademacher BL, Pittsenbarger J, Huang CY, Harvey CT, Lafortune MC, et al. ID1 enhances docetaxel cytotoxicity in prostate cancer cells through inhibition of p21. Cancer research. 2010;70(8):3239–3248. doi: 10.1158/0008-5472.CAN-09-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhardwaj A, Srivastava SK, Singh S, Arora S, Tyagi N, Andrews J, et al. CXCL12/CXCR4 signaling counteracts docetaxel-induced microtubule stabilization via p21-activated kinase 4-dependent activation of LIM domain kinase 1. Oncotarget. 2014;5(22):11490–11500. doi: 10.18632/oncotarget.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah GV, Thomas S, Muralidharan A, Liu Y, Hermonat PL, Williams J, et al. Calcitonin promotes in vivo metastasis of prostate cancer cells by altering cell signaling, adhesion, and inflammatory pathways. Endocrine-related cancer. 2008;15(4):953–964. doi: 10.1677/ERC-08-0136. [DOI] [PubMed] [Google Scholar]
- 32.Dizeyi N, Hedlund P, Bjartell A, Tinzl M, Austild-Tasken K, Abrahamsson PA. Serotonin activates MAP kinase and PI3K/Akt signaling pathways in prostate cancer cell lines. Urol Oncol. 2011;29(4):436–445. doi: 10.1016/j.urolonc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Marin-Aguilera M, Codony-Servat J, Reig O, Lozano JJ, Fernandez PL, Pereira MV, et al. Epithelial-to-mesenchymal transition mediates docetaxel resistance and high risk of relapse in prostate cancer. Mol Cancer Ther. 2014;13(5):1270–1284. doi: 10.1158/1535-7163.MCT-13-0775. [DOI] [PubMed] [Google Scholar]
- 34.Yuan J, Rozengurt EPKD. PKD2, and p38 MAPK mediate Hsp27 serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J Cell Biochem. 2008;103(2):648–662. doi: 10.1002/jcb.21439. [DOI] [PubMed] [Google Scholar]
- 35.Heron-Milhavet L, LeRoith D. Insulin-like growth factor I induces MDM2-dependent degradation of p53 via the p38 MAPK pathway in response to DNA damage. The Journal of biological chemistry. 2002;277(18):15600–15606. doi: 10.1074/jbc.M111142200. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Song G, Gao H, Farmer JL, Satterfield MC, Burghardt RC, et al. Insulin-like growth factor II activates phosphatidylinositol 3-kinase-protooncogenic protein kinase 1 and mitogen-activated protein kinase cell Signaling pathways, and stimulates migration of ovine trophectoderm cells. Endocrinology. 2008;149(6):3085–3094. doi: 10.1210/en.2007-1367. [DOI] [PubMed] [Google Scholar]
- 37.Guo YS, Hellmich MR, Wen XD, Townsend CM., Jr Activator protein-1 transcription factor mediates bombesin-stimulated cyclooxygenase-2 expression in intestinal epithelial cells. The Journal of biological chemistry. 2001;276(25):22941–22947. doi: 10.1074/jbc.M101801200. [DOI] [PubMed] [Google Scholar]
- 38.Park SI, Lee C, Sadler WD, Koh AJ, Jones J, Seo JW, et al. Parathyroid hormone-related protein drives a CD11b+Gr1+ cell-mediated positive feedback loop to support prostate cancer growth. Cancer research. 2013;73(22):6574–6583. doi: 10.1158/0008-5472.CAN-12-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin RJ, Wang Y, Masumori N, Ishii K, Tsukamoto T, Shappell SB, et al. NE-10 neuroendocrine cancer promotes the LNCaP xenograft growth in castrated mice. Cancer research. 2004;64(15):5489–5495. doi: 10.1158/0008-5472.CAN-03-3117. [DOI] [PubMed] [Google Scholar]
- 40.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22(3):373–388. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thadani-Mulero M, Portella L, Sun S, Sung M, Matov A, Vessella RL, et al. Androgen receptor splice variants determine taxane sensitivity in prostate cancer. Cancer research. 2014;74(8):2270–2282. doi: 10.1158/0008-5472.CAN-13-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Liu C, Nadiminty N, Lou W, Tummala R, Evans CP, et al. Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer. Mol Cancer Ther. 2013;12(9):1829–1836. doi: 10.1158/1535-7163.MCT-13-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. European urology. 2004;45(5):586–592. doi: 10.1016/j.eururo.2003.11.032. discussion 92. [DOI] [PubMed] [Google Scholar]
- 44.Qi J, Nakayama K, Cardiff RD, Borowsky AD, Kaul K, Williams R, et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell. 2010;18(1):23–38. doi: 10.1016/j.ccr.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dang Q, Li L, Xie H, He D, Chen J, Song W, et al. Anti-androgen enzalutamide enhances prostate cancer neuroendocrine (NE) differentiation via altering the infiltrated mast cells --> androgen receptor (AR) --> miRNA32 signals. Mol Oncol. 2015 doi: 10.1016/j.molonc.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao D, Qu X, Weber HC. GRP receptor-mediated immediate early gene expression and transcription factor Elk-1 activation in prostate cancer cells. Regul Pept. 2002;109(1–3):141–148. doi: 10.1016/s0167-0115(02)00197-0. [DOI] [PubMed] [Google Scholar]
- 47.Dizeyi N, Bjartell A, Nilsson E, Hansson J, Gadaleanu V, Cross N, et al. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. The Prostate. 2004;59(3):328–336. doi: 10.1002/pros.10374. [DOI] [PubMed] [Google Scholar]
- 48.Salido M, Vilches J, Roomans GM. Changes in elemental concentrations in LNCaP cells are associated with a protective effect of neuropeptides on etoposide-induced apoptosis. Cell Biol Int. 2004;28(5):397–402. doi: 10.1016/j.cellbi.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Vilches J, Salido M, Fernandez-Segura E, Roomans GM. Neuropeptides, apoptosis and ion changes in prostate cancer. Methods of study and recent developments. Histol Histopathol. 2004;19(3):951–961. doi: 10.14670/HH-19.951. [DOI] [PubMed] [Google Scholar]
- 50.Harper ME, Glynne-Jones E, Goddard L, Thurston VJ, Griffiths K. Vascular endothelial growth factor (VEGF) expression in prostatic tumours and its relationship to neuroendocrine cells. Br J Cancer. 1996;74(6):910–916. doi: 10.1038/bjc.1996.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O, et al. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature. 2001;411(6833):102–107. doi: 10.1038/35075107. [DOI] [PubMed] [Google Scholar]
- 52.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 53.Corcelle E, Djerbi N, Mari M, Nebout M, Fiorini C, Fenichel P, et al. Control of the autophagy maturation step by the MAPK ERK and p38: lessons from environmental carcinogens. Autophagy. 2007;3(1):57–59. doi: 10.4161/auto.3424. [DOI] [PubMed] [Google Scholar]
- 54.Razandi M, Pedram A, Levin ER. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol Cell Biol. 2010;30(13):3249–3261. doi: 10.1128/MCB.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia Y, Ransom RF, Shibanuma M, Liu C, Welsh MJ, Smoyer WE. Identification and characterization of hic-5/ARA55 as an hsp27 binding protein. The Journal of biological chemistry. 2001;276(43):39911–39918. doi: 10.1074/jbc.M103510200. [DOI] [PubMed] [Google Scholar]
- 56.Lamoureux F, Thomas C, Yin MJ, Fazli L, Zoubeidi A, Gleave ME. Suppression of heat shock protein 27 using OGX-427 induces endoplasmic reticulum stress and potentiates heat shock protein 90 inhibitors to delay castrate-resistant prostate cancer. European urology. 2014;66(1):145–155. doi: 10.1016/j.eururo.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Y, Shen S, Guo J, Chen H, Greenblatt DY, Kleeff J, et al. Mitogen-activated protein kinases and chemoresistance in pancreatic cancer cells. The Journal of surgical research. 2006;136(2):325–335. doi: 10.1016/j.jss.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 58.Jazirehi AR, Vega MI, Chatterjee D, Goodglick L, Bonavida B. Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway, Bcl-xL down-regulation, and chemosensitization of non-Hodgkin's lymphoma B cells by Rituximab. Cancer research. 2004;64(19):7117–7126. doi: 10.1158/0008-5472.CAN-03-3500. [DOI] [PubMed] [Google Scholar]
- 59.Zelivianski S, Spellman M, Kellerman M, Kakitelashvilli V, Zhou XW, Lugo E, et al. ERK inhibitor PD98059 enhances docetaxel-induced apoptosis of androgen-independent human prostate cancer cells. Int J Cancer. 2003;107(3):478–485. doi: 10.1002/ijc.11413. [DOI] [PubMed] [Google Scholar]
- 60.Thadani-Mulero M, Nanus DM, Giannakakou P. Androgen receptor on the move: boarding the microtubule expressway to the nucleus. Cancer research. 2012;72(18):4611–4615. doi: 10.1158/0008-5472.CAN-12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fizazi K, Faivre L, Lesaunier F, Delva R, Gravis G, Rolland F, et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol. 2015 doi: 10.1016/S1470-2045(15)00011-X. [DOI] [PubMed] [Google Scholar]
- 62.Cerasuolo M, Paris D, Iannotti FA, Melck D, Verde R, Mazzarella E, et al. Neuroendocrine Transdifferentiation in Human Prostate Cancer Cells: An Integrated Approach. Cancer research. 2015;75(15):2975–2986. doi: 10.1158/0008-5472.CAN-14-3830. [DOI] [PubMed] [Google Scholar]
- 63.Fan W, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. The Journal of biological chemistry. 2007;282(10):7329–7338. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- 64.Leotoing L, Manin M, Monte D, Baron S, Communal Y, Lours C, et al. Crosstalk between androgen receptor and epidermal growth factor receptor-signalling pathways: a molecular switch for epithelial cell differentiation. Journal of molecular endocrinology. 2007;39(2):151–162. doi: 10.1677/JME-07-0021. [DOI] [PubMed] [Google Scholar]
- 65.Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, et al. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer research. 1998;58(20):4640–4645. [PubMed] [Google Scholar]
- 66.Ongkeko WM, Burton D, Kiang A, Abhold E, Kuo SZ, Rahimy E, et al. Parathyroid hormone related-protein promotes epithelial-to-mesenchymal transition in prostate cancer. PloS one. 2014;9(1):e85803. doi: 10.1371/journal.pone.0085803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao J, Li X, Koh AJ, Berry JE, Thudi N, Rosol TJ, et al. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int J Cancer. 2008;123(10):2267–2278. doi: 10.1002/ijc.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci U S A. 2008;105(34):12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu S, Li L, Yeh S, Cui Y, Li X, Chang HC, et al. Infiltrating T cells promote prostate cancer metastasis via modulation of FGF11-->miRNA-541-->androgen receptor (AR)-->MMP9 signaling. Mol Oncol. 2015;9(1):44–57. doi: 10.1016/j.molonc.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NEPCamay not regulate AR expression in PCa. C4-2 and CWR22Rv1 cells were co-cultured with or without NE1.3 cells for 72 hours in 6-well plates. Western blot analysis of the expression of AR in C4-2(left panel) and CWR22Rv1(right panel) cells.
NEPCa could increase AR activity of surrounding PCa.C4-2 and CWR22Rv1 cells were co-cultured with or without NCI-H660cells for 72 hours in 6-well plates. Total RNA of C4-2 (left panel) and CWR222Rv1 (right panel) cells were analyzed by Q-PCR to show AR regulated genes, PSA, TMPRSS2 and FKBP5.
NEPCaup-regulated p21 of PCa. C4-2 and CWR22Rv1 cells were co-cultured with or without NCI-H660 cells for 48 hours in 6-well plates. Western blot analysis of the expression of p21 in C4-2 (left panel) and CWR22Rv1 (right panel) cells.