Abstract
Background
Data from selected centers show that robotic lobectomy (RL) is safe, effective and has comparable 30-day mortality to video assisted lobectomy (VATS). However, widespread adoption of RL is controversial. We used the STS-GTS-Database to evaluate quality metrics for these two minimally invasive lobectomy techniques.
Methods
A database query for primary clinical stage I or II NSCLC at high volume centers from 2009 to 2013 identified 1,220 RLs and 12,378 VATS. Quality metrics evaluated included operative morbidity, 30-day mortality and nodal upstaging (NU), defined as cN0 to pN1. Multivariable logistic regression was used to evaluate NU.
Results
RL patients were older, less active, less likely to be an ever smoker, and had higher BMI (all p<0.05). They were also more likely to have coronary heart disease or hypertension (all p<0.001) and to have had preoperative mediastinal staging (p<0.0001).
RL operative times were longer (median 186 vs 173 min, p<0.001); all other operative parameters were similar. All postoperative outcomes were similar including complications and 30-day mortality (RL 0.6% vs VATS 0.8%, p=0.4). Median length of stay was 4 days for both, but a higher proportion of RLs stayed < 4 days: 48% vs 39%, p<0.001. NU overall was similar (p=0.6), but with trends favoring VATS in the cT1b group, and RL in the cT2a group.
Conclusions
RL patients had more co-morbidities and RL operative times were longer, but quality outcome measures including complications, hospital stay, 30-day mortality, and NU suggest RL and VATS are equivalent.
Keywords: Lung cancer surgery, Robotics, Thoracoscopy/VATS, Outcomes, Quality
INTRODUCTION
Introduction of minimally invasive techniques to pulmonary lobectomy has revolutionized thoracic surgery. With video assisted thoracoscopic surgery (VATS), anatomic resections are done through small incisions without rib spreading, rather than through a thoracotomy with rib spreading. The benefits of VATS include fewer overall complications, reduced pain, shorter length of stay, better postoperative pulmonary function, lower blood loss, enhanced immune function and a greater ability to deliver adjuvant chemotherapy.[1–5] Several studies showing similar long term survival with VATS compared to thoracotomy have effectively allayed concern that VATS lobectomy might be an inferior oncologic operation.[6,7] Yet, adoption, though increasing in frequency, has grown more slowly than expected based on its demonstrated advantages.[8,9]
Initial reports of robotic lobectomy appeared in 2004 with many early adopters embracing this technologic enhancement in 2008–09. Data from selected centers showed that robotic lobectomy is safe and feasible.[10–15] Early comparisons to thoracotomy demonstrated shorter length of stay and reduced pain.[12,13] Comparisons to VATS lobectomy highlighted similar operative times, nodal harvests and blood loss.[14,16] Data from administrative databases concluded that 30-day mortality was comparable.[17,18] These findings combined with marketing touting 3D vision, wristed movements, magnification and instrument stability have fostered the growing use of robotic lobectomy as an alternative to VATS. However, comparisons to VATS lobectomy have also shown higher costs, making widespread adoption of robotic lobectomy controversial.[19,20]
To address the quality component of this controversy, we used the Society of Thoracic Surgeons – General Thoracic Surgery Database (STS-GTD) to compare outcomes of VATS and robotic lobectomy for early stage non-small cell lung cancer (NSCLC).
PATIENTS METHODS
Patients
The STS-GTD (data versions 2.081 to 2.2, covering from 2009 to 2013) was queried for primary lobectomy operations for lung cancer. Operations without a VATS or robotic designation (33,645) or operations that were coded with both VATS/open (n=303) or robotic/open (n=416) were assumed to be “conversions” and excluded. VATS and robotic cases were excluded if the reporting center had not done at least 20 robotic or 20 VATS cases (75 centers with 1656 cases). Patients with clinical stage I or II NSCLC were included. Cases were also excluded if preoperative chemotherapy (n=539) or radiation therapy (n=346) was reported.
Outcomes
Data from four categories were collected for comparison:
Operative metrics (procedure time, ICU days, length of stay, discharge location),
Operative morbidity (pulmonary, cardiac, transfusion, reoperation for bleeding),
30-day mortality; and,
Nodal upstaging, (cN0 to pN1).
Statistics
All statistical analyses were approved through the STS-GTD committee and performed with an assigned statistician through the Duke Clinical Research Institute. A total of 80 hours of statistical time was allocated to complete the project. Data between groups were compared using Wilcoxon rank sum test, and the χ2 test for categorical data. A p-value of less than 0.05 was considered significant.
An initial comparative analysis on the patient demographics, clinical and pathologic stage distributions, staging methods, and operative outcomes demonstrated no differences between the two groups. It was then determined that a planned propensity analysis would not add sufficient value to justify the use of limited statistical time, and was aborted. Nodal upstaging (cN0 to pN1 and cN0 to pN2) was also initially assessed and demonstrated potential differences in only the hilar region (cN0 to pN1) but not mediastinal (cN0 to pN2). It was decided to further analyze only hilar nodal upstaging based on this analysis.
The outcome measure of nodal upstaging was initially assessed as a direct comparison by clinical stage using a χ2 test. We then assessed the association of nodal upstaging with operative approach using logistic regression. Based on prior research[9,21], we adjusted for the following pre-specified covariates: female gender, clinical stage/tumor size (cm), BMI (>35 kg/m2 vs ≤35 kg/m2, tumor location (upper, middle, lower), mediastinal staging procedure (EBUS or mediastinoscopy), smoking status (never or past/current smoker) and laterality (left vs right). To account for correlation within the same center, a generalized estimating equation with exchangeable covariance structure was used. Patients were excluded from this analysis if the location of tumor and laterality was not specified or if either clinical or pathologic staging was missing (n=2,325).
A nodal upstaging model was created to further evaluate the effect-modification of the association between nodal upstaging and operative approach. Four potential modifiers were assessed: tumor size (T1a vs T1b vs T2a vs T2b), tumor location (upper, middle, lower), smoking status (ever vs. never) and laterality (left vs. right).
RESULTS
From a total of 52,505 cases, 1,220 robotic lobectomies and 12,378 VATS lobectomies from 140 reporting centers were identified. The number of robotic lobectomies increased each year and the number of centers reporting experience with robotic lobectomy also increased. In the final year, robotic lobectomy accounted for 14% of all minimally invasive lobectomies (Figure 1). Of the 128 centers contributing cases 18 reported both VATS and robotic approaches and four reported only robotic. Four of the 22 centers reporting robotic lobectomies (18%) accounted for 33% of robotic lobectomies (405/1,220), whereas 14 of the 128 centers reporting VATS lobectomies (11%) accounted for 33% of VATS lobectomies (4136/12378).
Figure 1.
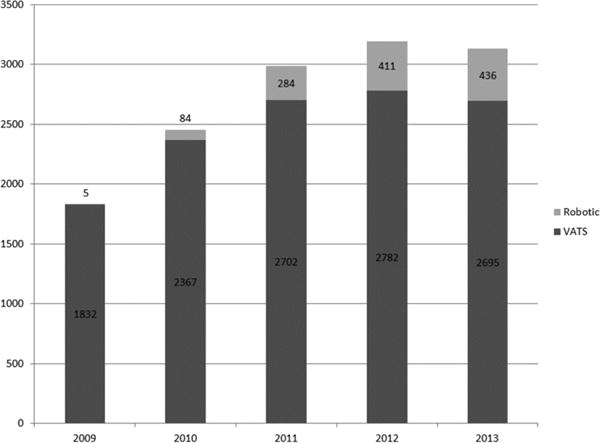
Case volume by surgical approach
Patients in the two groups were similar except that robotic patients were slightly older, less active, less likely to be an ever smoker, and had higher BMI and worse performance status (all p<0.05). Robotic patients were also more likely to have coronary heart disease or hypertension (p<0.001) (Table 1).
Table 1.
Patient Characteristics
| Overall N=13598 |
Robotic N=1220 |
VATS N=12378 |
P-Value | |
|---|---|---|---|---|
| Age (Median, IQR*) | 68.0 (61.0, 75.0) | 69.0 (61.0, 75.0) | 68.0 (61.0, 75.0) | 0.0253 |
| Gender | ||||
| Male | 5,857 (43.1%) | 527 (43.2%) | 5,330 (43.1%) | 0.9306 |
| Female | 7,739 (56.9%) | 693 (56.8%) | 7,046 (56.9%) | · |
| BMI (kg/m2) | ||||
| <35 | 11,949 (87.9%) | 1,074 (88.0%) | 10,875 (87.9%) | 0.0191 |
| >=35 | 1,245 (9.2%) | 137 (11.2%) | 1,108 (9.0%) | · |
| American Society of Anesthesiologist Risk Class | ||||
| I | 57 (0.4%) | 7 (0.6%) | 50 (0.4%) | 0.1218 |
| II | 2,528 (18.6%) | 197 (16.1%) | 2,331 (18.8%) | · |
| III | 10,037 (73.8%) | 932 (76.4%) | 9,105 (73.6%) | · |
| IV | 969 (7.1%) | 84 (6.9%) | 885 (7.1%) | · |
| V | 3 (0.0%) | 0 (0.0%) | 3 (0.0%) | · |
| Zubrod Score | ||||
| Normal activity, no symptoms | 6,579 (48.4%) | 501 (41.1%) | 6,078 (49.1%) | <.0001 |
| Symptoms but fully ambulatory | 6,522 (48.0%) | 657 (53.9%) | 5,865 (47.4%) | · |
| Symptoms but in bed less than 50% of the time | 407 (3.0%) | 47 (3.9%) | 360 (2.9%) | · |
| Symptoms but in bed >50% but less than 100% | 65 (0.5%) | 12 (1.0%) | 53 (0.4%) | · |
| Bedridden | 13 (0.1%) | 3 (0.2%) | 10 (0.1%) | · |
| Moribund | 2 (0.0%) | 0 (0.0%) | 2 (0.0%) | · |
| Coronary Artery Disease | 2,829 (20.8%) | 304 (24.9%) | 2,525 (20.4%) | 0.0009 |
| Congestive Heart Failure | 344 (2.5%) | 41 (3.4%) | 303 (2.4%) | 0.0703 |
| Hypertension | 8,244 (60.6%) | 822 (67.4%) | 7,422 (60.0%) | <.0001 |
| Diabetes Mellitus | 2,344 (17.2%) | 233 (19.1%) | 2,111 (17.1%) | 0.1475 |
| Chronic Obstructive Pulmonary Disease | 4,497 (33.1%) | 425 (34.8%) | 4,072 (32.9%) | 0.4393 |
| Peripheral Vascular Disease | 1,246 (9.2%) | 99 (8.1%) | 1,147 (9.3%) | 0.1240 |
| Preoperative Dialysis | 79 (0.6%) | 9 (0.7%) | 70 (0.6%) | 0.4940 |
| DLCO % Predicted (Median, IQR) | 74.0 (60.0, 89.0) | 75.0 (61.0, 89.0) | 74.0 (60.0, 89.0) | 0.0959 |
| FEV1 % Predicted (Median, IQR) | 84.0 (70.0, 98.0) | 84.0 (72.0, 98.0) | 84.0 (70.0, 98.0) | 0.6015 |
| Ever Smoked Cigarettes | 11,339 (83.4%) | 985 (80.7%) | 10,354 (83.6%) | 0.0087 |
IQR – Interquartile range
The majority of patients in both groups had tumors that were cT1a or cT1b, and right-sided, with a predilection for the upper lobe on both sides. Robotic patients were more likely to have had preoperative mediastinal staging (EBUS or mediastinoscopy as a primary or secondary procedure or EUS, EBUS, PET/CT and/or mediastinoscopy prior to treatment) (p<0.0001) (Table 2).
Table 2.
Baseline Tumor Characteristics
| Overall | Robotic N=1220 |
VATS N=12378 |
P-Value | |
|---|---|---|---|---|
| Clinical Stage | ||||
| cT1aN0 | 6,095 (44.8%) | 515 (42.2%) | 5,580 (45.1%) | 0.2325 |
| cT1bN0 | 3,481 (25.6%) | 328 (26.9%) | 3,153 (25.5%) | · |
| cT2aN0 | 2,655 (19.5%) | 269 (22.0%) | 2,386 (19.3%) | · |
| cT2bN0 | 636 (4.7%) | 51 (4.2%) | 585 (4.7%) | · |
| cT1aN1 | 196 (1.4%) | 16 (1.3%) | 180 (1.5%) | · |
| cT1bN1 | 196 (1.4%) | 15 (1.2%) | 181 (1.5%) | · |
| cT2aN1 | 240 (1.8%) | 18 (1.5%) | 222 (1.8%) | · |
| cT2bN1 | 99 (0.7%) | 8 (0.7%) | 91 (0.7%) | · |
| Laterality | ||||
| Right | 7,975 (58.6%) | 757 (62.0%) | 7,218 (58.3%) | 0.3583 |
| Left | 5,087 (37.4%) | 439 (36.0%) | 4,648 (37.6%) | · |
| Bilateral | 19 (0.1%) | 1 (0.1%) | 18 (0.1%) | · |
| Tumor Location – Lobe | ||||
| Upper | 8,053 (59.2%) | 717 (58.8%) | 7,336 (59.3%) | 0.9220 |
| Middle | 948 (7.0%) | 87 (7.1%) | 861 (7.0%) | · |
| Lower | 4,458 (32.8%) | 405 (33.2%) | 4,053 (32.7%) | · |
| Mediastinal Staging Done1 | 11,521 (84.7%) | 1,126 (92.3%) | 10,395 (84.0%) | <.0001 |
Defined as at least one of (a) Primary or secondary procedure including endobronchial ultrasound during bronchoscopic diagnostic or therapeutic interventions, mediastinoscopy with or without biopsy or (b) pre-treatment including EUS, EBUS, PET/CT, and mediastinoscopy
Median operative times for robotic lobectomy were longer (186 minutes, IQR=145–247) compared to VATS lobectomy (173 min, IQR=130–222, p<0.001), but all other outcomes in this category were similar. Use of blood products intraoperatively (1%) and intensive care utilization after surgery was rare (1%) for both groups. The incidence of unexpected return to the operating room was similar, occurring in 41 (3.4%) robotic and 438 (3.5%) VATS cases (p=0.74). Bleeding was the most common reason for return to the operating room in both groups, accounting for 29.6% (n=56) of returns in the VATS group and 14.8% (n=4) of returns in the robotic group (p=0.35). There were no statistically significant differences in any postoperative complications between the two groups, although the incidence of BPF was twice as high in the robotic group (0.6% vs. 0.3%, p=0.08). (Table 3)
Table 3.
Post Operative Morbidity
| Overall N=13598 |
Robotic N=1220 |
VATS N=12378 |
P-Value | |
|---|---|---|---|---|
| Air Leak > 5 Days | 1,334 (9.8%) | 122 (10.0%) | 1,212 (9.8%) | 0.8135 |
| Atelectasis Requiring Bronchoscopy | 391 (2.9%) | 31 (2.5%) | 360 (2.9%) | 0.4625 |
| Pneumonia | 442 (3.3%) | 33 (2.7%) | 409 (3.3%) | 0.2596 |
| Adult Respiratory Distress Syndrome | 61 (0.4%) | 2 (0.2%) | 59 (0.5%) | 0.1187 |
| Respiratory Failure1 | 119 (1.9%) | 16 (1.9%) | 103 (1.9%) | 0.9971 |
| Bronchopleural Fistula | 42 (0.3%) | 7 (0.6%) | 35 (0.3%) | 0.0808 |
| Pulmonary Embolus | 62 (0.5%) | 4 (0.3%) | 58 (0.5%) | 0.4858 |
| Pneumothorax requiring CT Reinsertion | 474 (3.5%) | 51 (4.2%) | 423 (3.4%) | 0.1697 |
| Initial Ventilatory Support > 48 Hours | 50 (0.4%) | 6 (0.5%) | 44 (0.4%) | 0.4535 |
| Reintubation | 308 (2.3%) | 25 (2.0%) | 283 (2.3%) | 0.5928 |
| Tracheostomy | 99 (0.7%) | 9 (0.7%) | 90 (0.7%) | 0.9688 |
| Atrial Arrhythmia Requiring Treatment | 1,346 (9.9%) | 125 (10.2%) | 1,221 (9.9%) | 0.6840 |
| Deep Venous Thrombosis | 52 (0.4%) | 5 (0.4%) | 47 (0.4%) | 0.8722 |
| Empyema Requiring Treatment | 50 (0.4%) | 6 (0.5%) | 44 (0.4%) | 0.4546 |
| Chylothorax Requiring Medical Intervention | 64 (0.5%) | 4 (0.3%) | 60 (0.5%) | 0.4435 |
| Unexpected Admission to ICU | 452 (3.3%) | 36 (3.0%) | 416 (3.4%) | 0.4547 |
| Myocardial Infarction | 42 (0.3%) | 5 (0.4%) | 37 (0.3%) | 0.5069 |
| Recurrent Laryngeal Nerve Paresis/Paralysis | 26 (0.2%) | 2 (0.2%) | 24 (0.2%) | 0.8177 |
| Required Reoperation for Bleeding2 | 65 (0.9%) | 3 (0.8%) | 62 (0.9%) | 0.8449 |
Median length of stay was four days in both groups but a higher proportion of robotic patients were discharged sooner (IQR=2–5 days for robotic lobectomy and 3–6 days for VATS lobectomy (p<0.001)): More robotic patients were discharged in less than four days (48% vs 39% (p<0.001)), and more VATS patients stayed between 4 – 7 days (34.8% vs 38.6%) and more than 7 days (16.9% vs 20.3%). In hospital mortality for robotic lobectomy and VATS lobectomy were 0.3% and 0.6% respectively (p=0.18) and 30-day mortality was 0.6% and 0.8% respectively (p=0.42). Most patients in both groups (94.5% vs 92.5%, p=0.82) were discharged home.
The overall rates of nodal upstaging were 8.44% for robotic lobectomy and 7.96% for VATS lobectomy, and were similar in both groups at each clinical stage. (Table 4) Nodal upstaging was more likely with more advanced clinical T stage in both groups. When the association between nodal upstaging and operative approach was tested using logistic regression and the seven pre specified co-variates, no association was identified with operative approach. However, statistically significant associations with nodal upstaging were found for clinical stage, female gender, laterality and mediastinal staging procedure. (Table 5)
Table 4.
Pathological nodal upstaging overall and stratified by clinical staging
| Clinical Stage | Proportion of Cases Upstaged to pN1 (Number Upstaged/Total Cases (%)) |
P-value | ||
|---|---|---|---|---|
| Overall | Robotic | VATS | ||
| cT1aN0 | 322/5,412 (5.95) | 29/471 (6.16) | 293/4,941 (5.93) | 0.8422 |
| cT1bN0 | 257/3,008 (8.54) | 19/293 (6.48) | 238/2,715 (8.77) | 0.1844 |
| cT2aN0 | 254/2,307 (11.01) | 34/244 (13.93) | 220/2,063 (10.66) | 0.1228 |
| cT2bN0 | 69 /546 (12.64) | 7/47 (14.89) | 62/499 (12.42) | 0.6263 |
|
| ||||
| Total | 902/11,273 (8.00) | 89/1,055 (8.44) | 813/10,218 (7.96) | 0.5847 |
Table 5.
OR and 95% CI of VATS on nodal upstaging after adjustment
| Risk Factor |
Unadjusted OR (95% CI) |
Unadjusted P |
Adjusted OR (95% CI) |
Adjusted P |
|---|---|---|---|---|
| Robotic vs. VATs | 1.10 (0.89,1.36) | 0.3749 | 1.04 (0.85,1.27) | 0.7240 |
| Female vs. Male | 0.80 (0.68,0.94) | 0.0077 | 0.85 (0.72,0.99) | 0.0431 |
| Clinical Stage (T1b vs. T1a) | 1.09 (0.96,1.25) | 0.1866 | 1.44 (1.23,1.68) | <.0001 |
| Clinical Stage (T2a vs. T1a) | 1.59 (1.36,1.85) | <.0001 | 1.89 (1.59,2.24) | <.0001 |
| Clinical Stage (T2b vs. T1a) | 1.73 (1.35,2.22) | <.0001 | 2.18 (1.69,2.81) | <.0001 |
| BMI > 35 vs. <= 35 kg/m2 | 1.18 (0.94,1.48) | 0.1644 | 1.20 (0.95,1.52) | 0.1239 |
| Tumor Location (Lower vs. Upper) | 1.17 (1.01,1.36) | 0.0379 | 1.16 (0.99,1.34) | 0.0597 |
| Tumor Location (Middle vs. Upper) | 0.79 (0.59,1.06) | 0.1234 | 0.95 (0.70,1.28) | 0.7280 |
| Mediastinal Staging Procedure | 2.00 (1.52,2.61) | <.0001 | 1.97 (1.50,2.57) | <.0001 |
| Smoking Status (Past or Current Smoker vs. Never Smoker) | 1.23 (0.98,1.55) | 0.0685 | 1.21 (0.96,1.51) | 0.1005 |
| Laterality (Left vs. Right) | 1.31 (1.16,1.49) | <.0001 | 1.29 (1.13,1.47) | 0.0002 |
Creation of a nodal upstaging model confirmed significant interaction between tumor size (advancing cT stage) and nodal upstaging (p=0.003). However, the association between operative approach and nodal upstaging by each specific T stage strata (T1a, T1b, T2a, T2b) produced mixed results, with trends favoring VATS lobectomy in the cT1b group, and robotic lobectomy in the cT2a group (Table 6).
Table 6.
Association between operative approach and nodal upstaging (cN0 to pN1) stratified by clinical T stage
| Strata | Risk Factor | Adjusted OR (95% CI) | Adjusted P |
|---|---|---|---|
| T1a | Robotic vs. VATs | 1.01 (0.69,1.48) | 0.9652 |
| T1b | Robotic vs. VATs | 0.69 (0.48,1.01) | 0.0536 |
| T2a | Robotic vs. VATs | 1.30 (0.95,1.78) | 0.0973 |
| T2b | Robotic vs. VATs | 1.16 (0.46,2.92) | 0.7592 |
COMMENT
The primary finding in this analysis is that there are no significant differences in perioperative outcomes between robotic lobectomy and VATS lobectomy. We also found no difference in nodal upstaging between the two procedures. Overall, these results are similar to the single institution comparative studies of robotic lobectomy vs VATS[14,16], to the multi-institutional comparative studies against the STS-GTD[18,22], to other administrative database comparisons[17], and to a recent meta-analysis[23].
Longer operating times for robotic lobectomy were found in the present study, confirming the findings of several other studies.[16,22] This probably reflects challenges in the early experience with the robot such as docking, refining port placement and surgical technique, and troubleshooting. Time expended in these efforts should decrease with increasing experience[12,13,24], but this study included only robotic lobectomies from centers that had done at least 20 and therefore should be facile with the procedure. These differences may also be related to surgeon background with minimally invasive techniques, training on the robot, whether transition is from open to robotic or VATS to robotic, and utilization of two surgeons during the initial cases.[12–14] Further technological developments and more experience with the robot are likely to narrow the gap but there is a fixed time cost with robotics particularly docking that is not a component of the VATS lobectomy.
Although median length of stay was similar between the groups, a significantly higher proportion of patients in the robotic group were discharged in less than four days. It is unclear why two minimally invasive approaches might result in different lengths of stay given that the drivers of length of stay such as chest tube duration, air leak, and atrial fibrillation are similar between the groups in this and other studies. One explanation may be that there are inherent benefits with the robotic technique in terms of patient comfort[14] and mobility that make the subjective assessment of readiness for discharge happen faster. Another is that early adopters of robotics may be from higher volume programs with dedicated postoperative protocols focused on early discharge.
The significant differences in mediastinal staging and presence of comorbidities seen in this study suggest that there are differences in the practice patterns of the surgeons contributing cases. With only 22 groups contributing robotic cases, and one third of cases coming from only four centers, the robotic surgeons are probably a more homogeneous, conscientious collection compared to the large variety of surgeons and groups performing VATS. All of these findings fit together to explain why there might be differences in a retrospective review related to practice patterns and selection bias that would disappear in a prospective study.
One interesting observation was that although the rates of return to surgery were similar, proportionately more patients in the VATS group returned for bleeding than did patients in the robotic group. Although this difference was not statistically significant, it does reflect similar findings in other studies[17,18] and prompts speculation about why this might be. Perhaps the greater dexterity of the robotic instruments and use of bipolar cautery result in better hemostasis than the suction tip or cotton tipped blunt dissections often used during VATS lobectomy.
We used the rate of hilar (N1) nodal upstaging as a surrogate measure of the oncologic quality of the operation. We excluded N2 upstaging for two reasons: our preliminary analysis showed no difference and a prior STS-GTD analysis demonstrated no difference in mediastinal nodal upstaging between open and VATS cases but did highlight differences in the completeness of hilar upstaging favoring open surgery[9]. Though our rates of hilar upstaging were similar, it’s important to recognize that nodal upstaging is, not only significantly influenced by tumor size[21], but also by female gender, laterality and mediastinal staging procedure. This suggests that use of this metric requires that future studies account for these factors in their assessment.
There are several limitations to our study. First, although cases with both a VATS/robotic code and an open code were labeled “conversions”, it was impossible to confirm this fact and to determine if this was planned, unplanned or coding error. In the versions of the database used there is no field to denote a conversion and why it occurred. Additionally, a planned conversion during the learning curve was suggested as good practice.[13] Second, the addition of a field denoting a robotic case was added in 2009 and as such robotic cases recorded at and just after this change may be included in the VATS group. Lastly, exclusion of certain analyses was required to manage the study within the allocated statistical hours.
Interest in robotic lobectomy has clearly increased. Is this change a consequence of surgeons adopting robotic lobectomy as their transition from open to minimally invasive lobectomy, or does it reflect minimally invasive surgeons choosing robotic lobectomy instead of VATS lobectomy? An analysis of a State Inpatient Database from 2008 to 2010 in eight states [17] also found growing use of robotics and suggested that the former is more likely. Robotic lobectomies as a proportion of all lobectomies (VATS, Open and Robotic) rose from 0.2% to 1.2% and finally to 3.4%. Given the benefits of minimally invasive lobectomy and the persisting limited use of VATS lobectomy, the true value of robotics may be in further increasing the proportion of patients benefitting from a minimally invasive approach to lobectomy.
In conclusion, robotic and VATS approaches to lobectomy were equivalent in all objective measures of quality, including postoperative complications, length of stay, 30-day mortality, and nodal upstaging. While robotic lobectomy had slightly longer operative times, it appears to be an acceptable alternative to VATS for the surgical management of early stage lung cancer. Prospective studies to define the role of robotic technology for major pulmonary resection are warranted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Fifty-second Annual Meeting of the Society of Thoracic Surgeons, Phoenix, AZ, Jan 23–27, 2016.
References
- 1.Paul S, Altorki NK, Sheng S, Lee PC, Harpole DH, Onaitis MW, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. American Association for Thoracic Surgery. 2010 Feb;139(2):366–78. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 2.Nakata M, Saeki H, Yokoyama N, Kurita A, Takiyama W, Takashima S. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 2000 Sep;70(3):938–41. doi: 10.1016/s0003-4975(00)01513-7. [DOI] [PubMed] [Google Scholar]
- 3.Whitson BA, D’Cunha J, Andrade RS, Kelly RF, Groth SS, Wu B, et al. Thoracoscopic versus thoracotomy approaches to lobectomy: differential impairment of cellular immunity. Ann Thorac Surg. 2008 Dec;86(6):1735–44. doi: 10.1016/j.athoracsur.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Yim AP, Wan S, Lee TW, Arifi AA. VATS Lobectomy Reduces Cytokine Responses Compared With Conventional Surgery. Ann Thorac Surg. 2000 Jul;70(1):243–7. doi: 10.1016/s0003-4975(00)01258-3. [DOI] [PubMed] [Google Scholar]
- 5.Lee JG, Cho BC, Bae MK, Lee CY, Park IK, Kim DJ, et al. Thoracoscopic lobectomy is associated with superior compliance with adjuvant chemotherapy in lung cancer. Ann Thorac Surg. 2011 Feb;91(2):344–8. doi: 10.1016/j.athoracsur.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Licht PB, Jørgensen OD, Ladegaard L, Jakobsen E. A National Study of Nodal Upstaging After Thoracoscopic Versus Open Lobectomy for Clinical Stage I Lung Cancer. Ann Thorac Surg. 2013 May 16; doi: 10.1016/j.athoracsur.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Cao C, Zhu Z-H, Yan TD, Wang Q, Jiang G, Liu L, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardio-Thoracic Surg. 2013 Nov;44(5):849–54. doi: 10.1093/ejcts/ezt406. [DOI] [PubMed] [Google Scholar]
- 8.Gopaldas RR, Bakaeen FG, Dao TK, Walsh GL, Swisher SG, Chu D. Video-assisted thoracoscopic versus open thoracotomy lobectomy in a cohort of 13,619 patients. Ann Thorac Surg. 2010 May;89(5):1563–70. doi: 10.1016/j.athoracsur.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Boffa DJ, Kosinski AS, Paul S, Mitchell JD, Onaitis M. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg. 2012 Aug 26;94(2):347–53. doi: 10.1016/j.athoracsur.2012.04.059. discussion 353. [DOI] [PubMed] [Google Scholar]
- 10.Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg. 2006 Jan;131(1):54–9. doi: 10.1016/j.jtcvs.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary Resection Using a Total Endoscopic Robotic Video-Assisted Approach. Semin Thorac Cardiovasc Surg. 2011 Jan;23(1):36–42. doi: 10.1053/j.semtcvs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Veronesi G, Galetta D, Maisonneuve P, Melfi F, Schmid RA, Borri A, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg. 2010 Jul;140(1):19–25. doi: 10.1016/j.jtcvs.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Cerfolio RJ, Bryant AS, Skylizard L, Minnich DJ. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg. 2011 Oct;142(4):740–6. doi: 10.1016/j.jtcvs.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Louie BE, Farivar AS, Aye RW, Vallières E. Early Experience With Robotic Lung Resection Results in Similar Operative Outcomes and Morbidity When Compared With Matched Video-Assisted Thoracoscopic Surgery Cases. Ann Thorac Surg. 2012 Mar 20;93(5):1598–605. doi: 10.1016/j.athoracsur.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 15.Gharagozloo F, Margolis M, Tempesta B, Strother E, Najam F. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg. 2009 Aug;88(2):380–4. doi: 10.1016/j.athoracsur.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 16.Jang H-J, Lee H-S, Park SY, Zo JI. Comparison of the Early Robot-Assisted Lobectomy Experience to Video-Assisted Thoracic Surgery Lobectomy for Lung Cancer. Innovations (Phila) 2011 Sep;6(5):305–10. doi: 10.1097/IMI.0b013e3182378b4c. [DOI] [PubMed] [Google Scholar]
- 17.Kent M, Wang T, Whyte R, Curran T, Flores R, Gangadharan S. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg. 2014 Jan;97(1):236–42. doi: 10.1016/j.athoracsur.2013.07.117. discussion 242–4. [DOI] [PubMed] [Google Scholar]
- 18.Farivar AS, Cerfolio RJ, Vallières E, Knight AW, Bryant A, Lingala V, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the society of thoracic surgeons database. Innovations (Phila) 2014;9(1):10–5. doi: 10.1097/IMI.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 19.Deen SA, Wilson JL, Wilshire CL, Vallières E, Farivar AS, Aye RW, et al. Defining the Cost of Care for Lobectomy and Segmentectomy: A Comparison of Open, Video-Assisted Thoracoscopic, and Robotic Approaches. Ann Thorac Surg. 2014 Jan 27;97(3):1000–7. doi: 10.1016/j.athoracsur.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Swanson SJ, Miller DL, McKenna RJ, Howington J, Marshall MB, Yoo AC, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier) J Thorac Cardiovasc Surg. 2014 Mar;147(3):929–37. doi: 10.1016/j.jtcvs.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JL, Louie BE, Cerfolio RJ, Park BJ, Vallières E, Aye RW, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg. 2014 Jun;97(6):1901–6. doi: 10.1016/j.athoracsur.2014.01.064. discussion 1906–7. [DOI] [PubMed] [Google Scholar]
- 22.Adams RD, Bolton WD, Stephenson JE, Henry G, Robbins ET, Sommers E. Initial multicenter community robotic lobectomy experience: comparisons to a national database. Ann Thorac Surg. 2014 Jun;97(6):1893–8. doi: 10.1016/j.athoracsur.2014.02.043. discussion 1899–900. [DOI] [PubMed] [Google Scholar]
- 23.Ye X, Xie L, Chen G, Tang J-M, Ben X-S. Robotic thoracic surgery versus video-assisted thoracic surgery for lung cancer: a meta-analysis. Interact Cardiovasc Thorac Surg. 2015;21(4):409–14. doi: 10.1093/icvts/ivv155. [DOI] [PubMed] [Google Scholar]
- 24.Veronesi G, Agoglia BG, Melfi F, Maisonneuve P, Bertolotti R, Bianchi PP, et al. Experience with robotic lobectomy for lung cancer. Innovations (Phila) 2011 Nov;6(6):355–60. doi: 10.1097/IMI.0b013e3182490093. [DOI] [PubMed] [Google Scholar]


