Abstract
Exposing Clostridium difficile spores to germinants in a quaternary ammonium matrix was an effective method to reduce environmental contamination by sensitizing the spores, leaving them susceptible to ambient conditions and enhancing killing by acid, high-intensity visible light, and radiation.
Keywords. Clostridium difficile spores, spore contamination, spore germination, surface disinfection, quaternary ammonium compound.
Clostridium difficile is a spore-forming anaerobic bacterium that is the most common cause of healthcare-associated diarrhea in developed countries [1]. Because C difficile is an obligate anaerobe, its spore form is the agent responsible for infection and recurrence of disease [2]. Spores are highly resistant structures consisting of multiple proteinaceous layers that surround a dehydrated core [3]. Prevention of C difficile transmission is challenging in part because spores survive for months on surfaces and are resistant to killing by many commonly used disinfectants such as quaternary ammonium compounds [4, 5]. Sodium hypochlorite (bleach) is a disinfectant with sporicidal activity, but it has several disadvantages, including being corrosive to many materials and irritating to some patients and staff members [6]. Due to these disadvantages, bleach disinfection is often reserved for rooms of patients with known C difficile infection (CDI), whereas quaternary ammonium compounds are often used for everyday disinfection of hospital surfaces. This approach may be problematic because recent studies suggest that spore shedding by asymptomatic carriers of C difficile have the potential to contribute significantly to disease transmission [7]. Consequently, there is a need for development of sporicidal disinfectants that do not adversely affect surfaces and that are nonirritating to staff and patients.
Clostridium difficile spores remain dormant until they are exposed to agents that trigger them to come back to life through the process of germination [8]. Spore germination is defined as the irreversible loss of spore-specific properties and is an essential step required before outgrowth of vegetative cells [9]. Clostridium difficile uses a species-specific mechanism to regulate spore germination that is initiated by the presence of bile salts and amino acids [10, 11]. When exposed to bile salts such as taurocholate, a cascade of events occurs that causes the spore’s cortex layer to deteriorate and eventually leads to rehydration of the core [11]. Because germinated spores become more susceptible to killing by heat and other stressors, induction of germination has been studied as a possible measure to eliminate Bacillus and other Clostridia spp spores from food products (ie, addition of germinants to reduce heat resistance of spores) [12]. Initiation of germination has been shown to increase susceptibility of spores of Bacillus spp (Bacillus subtilis, Bacillus coagulans, and Bacillus cereus) and Clostridium botulinum to killing by radiation and heat [13–17]. We have recently demonstrated that exposing C difficile spores to a germination solution containing amino acids, minerals, and taurocholic acid resulted in initiation of germination in room air and significantly enhanced killing by ultraviolet C (UV-C) radiation and heat [18].
In this study, we tested the hypothesis that the addition of germinants to a quaternary ammonium disinfectant solution would result in sensitization of C difficile spores, leaving them susceptible to killing by ambient room conditions. In addition, we tested whether the addition of germinants to quaternary ammonium solutions would enhance killing by acid, high-intensity blue light, and UV-C radiation. Our findings suggest that this approach may provide a novel strategy to reduce the burden of C difficile spores in the healthcare environment.
METHODS
Spore Strains and Growth Conditions
VA 17 is an epidemic (cdtB+) restriction endonuclease analysis (REA) BI strain, and VA 11 is a nonepidemic (cdtB−) REA J strain; both isolates are toxigenic (tcdA+, tcdB+) strains. American Type Culture Collection (ATCC) 43598 is a toxigenic (tcdA+, tcdB−+) strain classified toxinotype VIII/ribotype 017 from serogroup F. Clostridium difficile cultures were incubated at 37°C for 48 hours in a Whitley MG1000 anaerobic workstation (Microbiology International, Frederick, MD) on prereduced cycloserine-cefoxitin-brucella agar containing 0.1% taurocholic acid and lysozyme 5mg/L [19].
Preparation of Spores
Clostridium difficile spores were prepared as previously described by Perez et al [20] with the following modifications. Spores were harvested from the Clospore media after 2 weeks of incubation in a Whitley MG1000 anaerobic workstation. Vegetative material was removed by density gradient centrifugation in Histodenz (Sigma-Aldrich, St. Louis, MO). Before testing, spore preps were confirmed by phase contrast microscopy and malachite green staining to be >99% dormant, bright-phase spores.
Reduction of Clostridium difficile Spores on Carriers by Sensitizing with Germinants in a Quaternary Ammonium Matrix
Initial experiments were performed to determine whether C difficile spores would germinate in the presence of a quaternary ammonium disinfectant. Virex II 256 (Sealed Air, Charlotte, NC) containing didecyl dimethyl ammonium chloride (5%–10%) and n-alkyl dimethyl benzyl ammonium chloride (5%–10%) was diluted to the working concentration as directed by manufacturer. Clostridium difficile germinants (10mM taurocholic acid and 100mM l-glycine) were added, and the pH was adjusted to pH 7.5–8.0 with hydrochloric acid. The final concentration of quaternary ammonium was the same for solutions with or without the addition of germinants. Three strains of C difficile spores (described under Spore Strains and Growth Conditions) were inoculated into 1mL water, quaternary ammonium, germinants, or quaternary ammonium with germinants to a final concentration of 6 log10 colony-forming units (CFUs) per mL. After incubation under ambient conditions (~22°C) for 30 minutes, spores were viewed under phase contrast microscopy to determine whether they had changed from bright phase (dormant) to dark phase (germinated). In our experience, spores were fully germinated between 2 and 10 minutes of exposure to germinants (our unpublished data).
Once it was determined that spores germinated in the presence of a quaternary ammonium matrix, stainless steel carriers (1cm2) were inoculated with 6 log10 CFUs of each of the 3 strains of C difficile spores, spores were spread to cover the surface of the carrier, and allowed to air dry for 24 hours. Thirty microliters of water, quaternary ammonium, germinants, or quaternary ammonium with germinants were applied to the inoculated carriers, and the solutions were allowed to air dry. Carriers were processed as described by the American Society for Testing and Materials (ASTM) in “Standard Quantitative Carrier Test Method to Evaluate the Bactericidal, Fungicidal, Mycobactericidal, and Sporicidal Potencies of Liquid Chemicals” after 1 hour, 24 hours, 5 days, 14 days, and 47 days posttreatment [21]. Carriers were processed using Dey Engley neutralizer (Becton, Dickinson and Company, Franklin Lakes, NJ) to assure that any residual quaternary ammonium would not affect viability of cultured spores and subsequent vegetative C difficile. Log10 CFU reduction was determined by comparing recovery of spores from carriers after treatment with solution and recovery of spores from carriers treated with water and processed immediately (T = 0). Each experiment was repeated 3 times.
Reduction of Clostridium difficile Spores on Hospital Surfaces by Sensitizing With Germinants in a Quaternary Ammonium Matrix
A hospital mattress, bedside table, call button, and bedrail were marked with 1- cm2 circles. The circles were inoculated with 7 log10 CFUs of ATCC 43598 C difficile spores, spores were spread to fill the circle, and air dried for 24 hours. Thirty microliters of water, quaternary ammonium, germinants, or quaternary ammonium with germinants were spread inside each inoculated circle. Sterile premoistened swabs were used to sample each surface immediately after application of each solution (baseline control) or 2 hours, 24 hours, and 7 days postapplication of solution. Each circle was swabbed only once; each time point had a distinct circle for sampling. Swabs were transferred to the anaerobic chamber, applied to C difficile Selective Agar plates containing Dey Engley neutralizer, and cultured as described previously. Colony-forming unit recovery was determined using Fotodyne Incorporated TotalLab Quant colony counting software. Log10 CFU reduction was determined by comparing recovery of spores from swabs sampled immediately after solution treatment (T = 0) and recovery of spores from swabs at each time point. Each experiment was repeated 3 times.
Reduction of Sensitized Clostridium difficile Spores by Low pH
Six log10 CFUs of ATCC 43598 spores were inoculated into 1mL of either water, quaternary ammonium, germinants, or quaternary ammonium with germinants. After incubating for 30 minutes under ambient conditions (~22°C), the spore suspensions were split into 2 centrifuge tubes (500 µL in each tube) and washed 3 times by pelleting and resuspended in sterile water. After the final centrifugation, 1 set of treated spores was resuspended in 1mL neutral pH (~6.5) water, and the other set of spores was resuspended in 1mL low pH (2.0 with hydrochloric acid) water and incubated at room temperature for 10 minutes. The spores were washed 3 times by centrifugation and resuspended in pH 7.5 phosphate-buffered saline (PBS). The samples were transferred to the anaerobic chamber, serially diluted, and plated to quantitate viable CFU. Log10 CFU reduction was determined by comparing recovery of spores treated with neutral pH water and recovery of spores treated with pH 2.0 water. The experiment was repeated 3 times.
Reduction of Sensitized Clostridium difficile Spores by High-Intensity Blue Light and Ultraviolet C Radiation
Eight log10 CFUs of ATCC 43598 spores were inoculated into 1mL of either water or quaternary ammonium with germinants. After incubating for 30 minutes under ambient conditions (~22°C), the spore suspensions were washed 3 times by pelleting and resuspended in sterile water. Four sets of stainless steel carriers (1cm2) were inoculated with 10 µL of each spore suspension (~6 log10 CFUs), spread to cover the surface of the carrier, and allowed to air dry. One set of carriers was processed immediately (baseline control), the second set was irradiated with UV-C (254nm) light 4 feet from the source (Tru-D Rapid Room Disinfection device; Lumalier, Memphis, TN) for 10 minutes, the third set was placed 2 inches from a high-intensity blue light fixture (Vital Vio) for 24 hours, and the last set was placed under ambient room light for 24 hours. The temperature for each set was monitored and remained between 22°C and 24°C during the duration of treatment. Carriers were processed using the ASTM standard method as described previously [21]. Log10 CFU reduction was calculated by comparing recovery of spores from carriers processed immediately and recovery of spores from carriers treated with room, blue, or UV-C light. The experiment was repeated 3 times.
Bactericidal Activity of Quaternary Ammonium in the Presence of Germinants
Virex II 256 was diluted to working concentration and prepared with or without the addition of germinants (10mM taurocholic acid and 100mM l-glycine). Stainless steel carriers (1cm2) were inoculated with 10 µL of a PBS solution containing isolates from 24-hour cultures of a pulsed-field gel electrophoresis type USA300 methicillin-resistant Staphylococcus aureus (MRSA) and a VanA-type vancomycin-resistant Enterococcus (VRE) (~7 log10 CFUs each). The inoculum was spread to cover the surface of the carrier and allowed to air dry. Thirty microliters of either PBS, Virex II 256, or Virex II 256 containing germinants was applied to carriers and incubated under ambient conditions for 10, 15, 20, or 30 minutes. Carriers were neutralized with 3mL Dey-Engley medium and processed using the ASTM standard method as described previously [21]. Log10 CFU reduction was determined by comparing recovery of spores from carriers after treatment with quaternary ammonium containing solutions and recovery of spores from carriers treated with PBS (control). The experiment was repeated 3 times.
Data Analysis
Data were analyzed with R statistical software (version 3.1.1). Continuous data were analyzed using unpaired t tests.
RESULTS
Sensitizing With Germinants in a Quaternary Ammonium Matrix Results in Reduction of Clostridium difficile Spores on Carriers
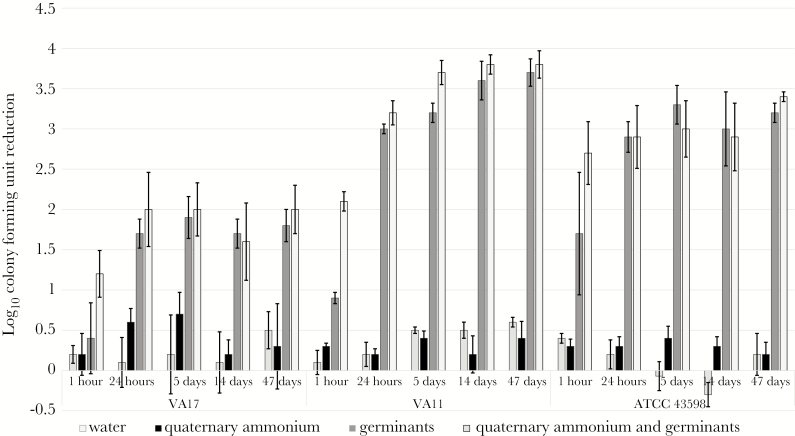
Exposure of dormant C difficile spores to germinants (taurocholic acid and l-glycine) in a quaternary ammonium matrix resulted in initiation of germination in room air. Clostridium difficile spores remained viable on carriers for up to 47 days after exposure to either water or quaternary ammonium and subsequent ambient desiccation. After a single exposure to the quaternary ammonium solution containing germinants, recovery of the 3 spore strains was significantly reduced after 1 hour (1.2 to 2.7 log10 CFU reduction) with a further reduction by 24 hours (2 to 3.2 log10 CFUs) (P < .01 for each comparison to water or quaternary ammonium alone) (Figure 1, Supplementary Data 1). For 2 of the 3 strains, the reduction at 1 hour was significantly greater for the quaternary ammonium solution containing germinants than for germinants alone (P < .01).
Figure 1.
Clostridium difficile spores on carriers are reduced by sensitizing with germinants in a quaternary ammonium matrix. Six log10 colony-forming units (CFUs) of C difficile (VA17, VA11, and American Type Culture Collection [ATCC] 43598) on steel disk carriers were inoculated with 30 µL water, quaternary ammonium, germinants, or quaternary ammonium containing germinants and allowed to air dry. Carriers were incubated under ambient conditions for up to 47 days. Log10 CFU reduction was determined by comparing recovery of spores from treated carriers and carriers treated with water (T = 0). The means of data from triplicate experiments are presented. Error bars indicate standard error.
Sensitizing with Germinants in a Quaternary Ammonium Matrix Results in Reduction of Clostridium difficile Spores on Hospital Surfaces
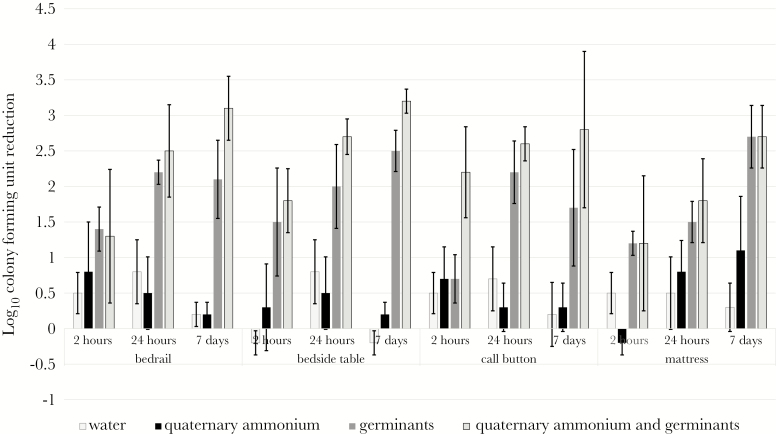
Spores on hospital surfaces treated with water or quaternary ammonium alone were not significantly reduced for up to 1 week after exposure (<1 log10 CFU reduction in recovery). In contrast, by 2 hours after a single exposure to a quaternary ammonium solution containing germinants with subsequent ambient desiccation, recovery of spores on the test surfaces was significantly reduced by ~1–1.5 log10 CFUs in comparison to spores exposed to quaternary ammonium or water alone (P < .01 for each comparison) (Figure 2 Supplementary Data 2). The reduction at 2 hours was significantly greater for the quaternary ammonium solution containing germinants than for germinants alone for the spores on the call button (P < .01), but not on the other surfaces. For each spore strain, further reductions occurred by 24 hours after exposure to the quaternary ammonium solution containing germinants or germinants alone.
Figure 2.
Clostridium difficile spores on hospital surfaces are reduced by sensitizing with germinants in a quaternary ammonium matrix. Seven log10 colony-forming units (CFUs) of C difficile (American Type Culture Collection [ATCC] 43598) applied to common hospital surfaces were inoculated with 30 µL of either water, quaternary ammonium, germinants, or quaternary ammonium containing germinants and allowed to air dry. Swabs were used to recover spores immediately (T = 0), 2 hours, 24 hours, and 7 days after treatment. Log10 CFU reduction was determined by comparing recovery of spores from swabs after treatment and recovery of spores from swabs processed immediately (T = 0). The means of data from triplicate experiments are presented. Error bars indicate standard error.
Sensitized Clostridium difficile Spores Are Killed by Low pH
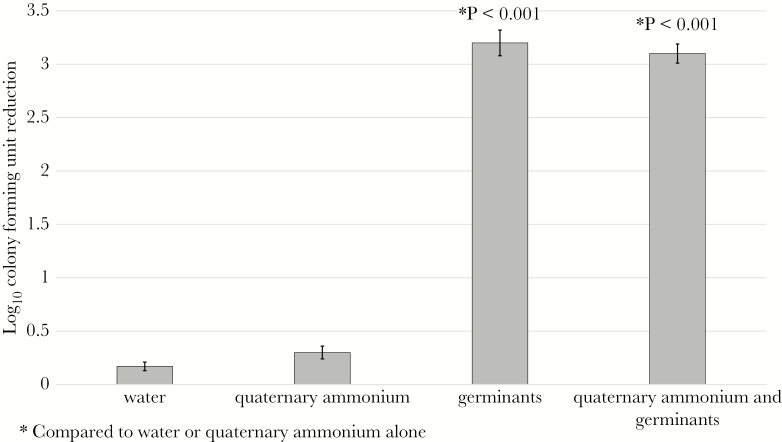
Dormant spores have been previously shown to remain resistant to killing at pH 2.0 [22]. After 30 minutes of incubation in water or quaternary ammonium, C difficile spores remained resistant to killing by subsequent exposure to acidic conditions (pH 2.0) for 10 minutes. In contrast, spores exposed to 30 minutes of incubation in either germinants or quaternary ammonium containing germinants were reduced by >3 log10 CFU when exposed to acidic conditions for 10 minutes (P < .001 for each comparison) (Figure 3 Supplementary Data 3).
Figure 3.
Sensitized Clostridium difficile spores are killed by low pH. Six log10 colony-forming units (CFUs) of C difficile (American Type Culture Collection [ATCC] 43598) were inoculated into 1mL water, quaternary ammonium, germinants, or quaternary ammonium containing germinants. Spore suspensions were incubated under ambient conditions for 30 minutes, split into 2 sets, and then washed 3 times with water. The treated spores were exposed to pH 2.0 water or neutral pH water for 10 minutes. Log10 CFU reduction was determined by comparing recovery of spores treated with neutral pH water and recovery of spores treated with pH 2.0 water. The means of data from triplicate experiments are presented. Error bars indicate standard error.
Sensitizing Clostridium difficile Spores Enhances Reduction by High-Intensity Blue Light and Ultraviolet C Radiation
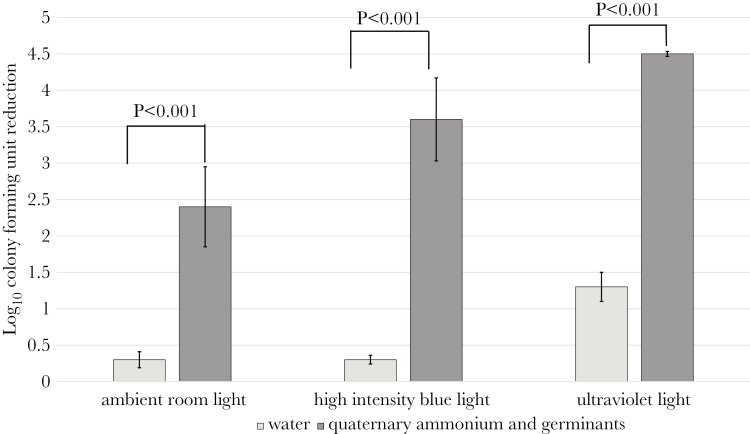
Dormant spores are protected from high-intensity radiation due to the dehydrated state of the vulnerable deoxyribonucleic acid in the spore’s core. Dormant C difficile spores exposed to water under ambient conditions were not reduced by high-intensity blue light after 24 hours. However, when spores were exposed to a quaternary ammonium solution containing germinants, they were significantly reduced by ~2.5 log10 CFUs under ambient conditions after 24 hours, and this reduction was further enhanced by >1 log10 CFU in the presence of high-intensity blue light (P < .001 for each comparison) (Figure 4 Supplementary Data 4). Dormant spores exposed to water were nominally reduced by ~1 log10 CFU after 10 minutes of UV-C radiation. Exposure to quaternary ammonium containing germinants enhanced UV-C spore killing (>3 log10 CFU reduction) (P < .001).
Figure 4.
Sensitizing Clostridium difficile spores enhances reduction by high-intensity blue light and ultraviolet C radiation. Eight log10 colony-forming units (CFUs) of American Type Culture Collection (ATCC) 43598 spores were inoculated into 1mL of either water or quaternary ammonium with germinants. After incubating for 30 minutes under ambient conditions, the spore suspensions were washed 3 times by pelleting and resuspended in sterile water. Treated spores were inoculated onto carriers and either processed immediately, 24 hours after exposure to room lighting, 24 hours after exposure to high-intensity blue light, or after 10 minutes of irradiation with ultraviolet C radiation. Log10 CFU reduction was calculated by comparing recovery of spores from carriers processed immediately and recovery of spores from carriers treated with room, blue, or ultraviolet C light. The means of data from triplicate experiments are presented. Error bars indicate standard error.
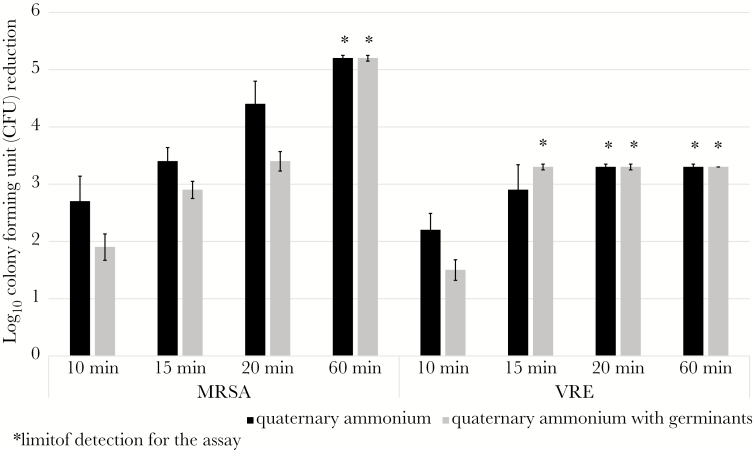
Bactericidal Activity of Quaternary Ammonium in the Presence of Germinants
The addition of germinants reduced the killing efficacy of quaternary ammonium against MRSA and VRE by ~1 log10 CFU after 10 minutes of exposure (Figure 5 Supplementary Data 5). For VRE, quaternary ammonium with or without germinants had equal killing efficacy after 15 minutes of exposure. However, killing of MRSA was reduced by ~1 log10 CFU in the presence of germinants for exposures up to 20 minutes. After 30 minutes of exposure, quaternary ammonium with or without germinants had equal killing efficacy against MRSA.
Figure 5.
Bactericidal activity of quaternary ammonium in the presence of germinants. Seven log10 colony-forming units (CFUs) of pulsed-field gel electrophoresis type USA300 methicillin-resistant Staphylococcus aureus (MRSA) and VanA-type vancomycin-resistant Enterococcus (VRE) on steel disk carriers were exposed to phosphate-buffered saline ([PBS] control), quaternary ammonium, or quaternary ammonium containing germinants. Carriers were incubated for 10, 15, 20, and 30 minutes under ambient conditions. Log10 CFU reduction was determined by comparing recovery of spores from PBS-treated carriers and carriers treated with solutions containing quaternary ammonium. The means of data from triplicate experiments are presented. Error bars indicate standard error.
DISCUSSION
We found that exposure of dormant C difficile spores to germinants in a quaternary ammonium matrix resulted in initiation of germination in room air. After a single exposure, recovery of spores on steel carriers was reduced by 1.2 to 2.7 log10 CFU after 1 hour and by 2 to 3.2 log10 CFU after 24 hours. Similar results were obtained on the porous and textured surfaces commonly found in hospitals; however, there was more variability in reduction due to the type of surface tested (ie, call buttons had a bumpy plastic surface and the mattress had a smooth vinyl surface). Moreover, germinated spores exhibited enhanced killing by acid (pH 2), by blue light at 405nm after 24 hours of exposure, and by UV-C radiation. The addition of germinants delayed the bactericidal activity of quaternary ammonium within the first 10 minutes of contact time; however, similar activity was achieved after 15 minutes of contact time, suggesting that longer contact time is required for germinant containing solutions. These findings suggest that the addition of germinants to quaternary ammonium-based disinfectants could represent a novel strategy to reduce the burden of C difficile spores on hospital surfaces and to enhance killing by technologies such as blue light and radiation.
Triggering germination has been studied as a means to enhance killing of spores of Bacillus spp and of other Clostridia spp [12–17], due in part to the detailed elucidation of the germination process in these species [9]. For Bacillus and other Clostridia spp, germination consists of 2 distinct constitutive stages [23]. Stage I is composed of 3 processes: (1) release of H+, monovalent cations and Zn2+ from the spore core (increasing core pH from ~6.5 to 7.7); (2) release of dipicolonic acid and Ca2+ from the spore core; and (3) increase in the spore core’s hydration [23]. Stage II is composed of 2 processes: (1) hydrolysis of the spore’s cortex, and (2) swelling of the spore core due to further increase in hydration and expansion of the germ cell wall [23]. In contrast, recent studies have revealed that C difficile has a species-specific germination process that is triggered by bile salts and glycine [10]. Unlike Bacillus and other Clostridia spp that use inner membrane germinant receptors to sense small molecule nutrients, C difficile uses a subtilisin-like serine protease as a germinant receptor to sense bile salts [24]. As a result of these differences, degradation of the thick protective cortex layer (Stage II germination) precedes release of dipicolinic acid from the core (Stage I) [11].
These differences between C difficile and other spore-forming bacteria have important implications for utilization of germination as means to enhance killing of C difficile spores. Research is needed to identify and optimize chemical germinants that target C difficile’s unique mode of germination. In addition, studies are needed to identify stressors that enhance killing of germinated C difficile spores in room air. It is likely that germinated spores are killed on surfaces in room air due to desiccation and exposure to oxygen, but other factors may contribute. Notably, at 1 hour, spores were reduced more by exposure to quaternary ammonium compounds plus germinants than by exposure to germinants alone, suggesting that the combination enhanced killing. Wheeldon et al [25] recently reported that exposure of C difficile spores to taurocholate under aerobic conditions resulted in enhanced killing on copper surfaces [25]. In conjunction with our findings, such reports should stimulate additional research to identify other stressors that enhance killing of germinated spores.
Our findings have important practical applications. First, daily disinfection of non-CDI rooms is typically performed with nonsporicidal agents such as quaternary ammonium compounds. Because spore contamination is not uncommon in non-CDI rooms, the addition of germinants to daily disinfection may reduce hidden reservoirs of contamination [7]. Second, spores that are sensitized by germinants could potentially have a decreased chance of infecting susceptible patients because they may not survive the low pH of normal stomach acid. Further testing in an animal model of C difficile colonization is needed to test this hypothesis. Third, our findings suggest that novel lighting fixtures that emit high-intensity visible light could be effective in reducing dormant C difficile spores that are exposed to germinants [26]. Spores exposed to germinants change from phase bright (reflecting visible light) to phase dark (visible light can pass through), potentially allowing high-intensity, visible light to reach its target. Finally, our findings suggest that the time required for UV-C devices to achieve a 3 log10 CFU reduction of spores on surfaces could be reduced from 45 minutes to 10 minutes with the application of quaternary ammonium solution containing germinants [18, 27]. Because precleaning of surfaces is recommended before running UV-C devices, the addition of germinants could increase the feasibility of UV-C use in hospital settings by reducing the time required for device operation.
CONCLUSIONS
Our study has some limitations. First, only 3 strains of C difficile were studied. More studies are necessary to determine whether this strategy is effective against a broader range of strains. Second, the studies on hospital surfaces involved inoculation of spores onto surfaces rather than spore contamination from patients. Additional studies are needed on native C difficile contamination. Third, it is possible that factors such as organic matter might reduce the efficacy of the germinants in the quaternary ammonium solutions. Fourth, the presence of germinants reduced the efficacy of quaternary ammonium against vegetative bacteria. Longer exposures were necessary to achieve optimal killing of MRSA and VRE. Additional studies are needed to develop formulations with rapid, high-level bactericidal activity. Finally, as previously described by Gould et al [28], a persistent “superdormant” fraction of spores remains unaltered by exposure to germinants. More recently, Ghosh and Setlow [29] isolated superdormant spores of B subtilis and Bacillus megaterium and found that superdormant spores require an increased signal for triggering spore germination compared with most spores in populations. Further studies are therefore needed to optimize germinants for C difficile to further enhance killing by triggering germination in the superdormant fraction of spores.
Supplementary Data
Supplementary material is available at Open Forum Infectious Diseases online.
Acknowledgments
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This study was funded by a Merit Review Grant from the Department of Veterans Affairs (to C. J. D.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005; 353:2442–9. [DOI] [PubMed] [Google Scholar]
- 2. Deakin LJ, Clare S, Fagan RP, et al. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect Immun 2012; 80:2704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paredes-Sabja D, Shen A, Sorg JA. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol 2014; 22: 406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gerding DN, Muto CA, Owens RC., Jr Measures to control and prevent Clostridium difficile infection. Clin Infect Dis 2008; 46(Suppl 1):S43–9. [DOI] [PubMed] [Google Scholar]
- 5. Kelly CP, LaMont JT. Clostridium difficile–more difficult than ever. N Engl J Med 2008; 359:1932–40. [DOI] [PubMed] [Google Scholar]
- 6. Barbut F, Menuet D, Verachten M, Girou E. Comparison of the efficacy of a hydrogen peroxide dry-mist disinfection system and sodium hypochlorite solution for eradication of Clostridium difficile spores. Infect Control Hosp Epidemiol 2009; 30:507–14. [DOI] [PubMed] [Google Scholar]
- 7. Riggs MM, Sethi AK, Zabarsky TF, et al. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis 2007; 45:992–8. [DOI] [PubMed] [Google Scholar]
- 8. Gould GW. Symposium on bacterial spores: IV. Germination and the problem of dormancy. J Appl Bacteriol 1970; 33:34–49. [DOI] [PubMed] [Google Scholar]
- 9. Paidhungat M, Setlow P. Spore germination and outgrowth. In: Sonenshein AL, Hoch JA, Losick R, ed. Bacillus subtilis and Its Closest Relatives: From Genes to Cells. Washington, DC: ASM Press; 2002: pp 537–49. [Google Scholar]
- 10. Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 2008; 190:2505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Francis MB, Allen CA, Sorg JA. Spore cortex hydrolysis precedes dipicolinic acid release during Clostridium difficile spore germination. J Bacteriol 2015; 197:2276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akhtar S, Paredes-Sabja D, Torres JA, Sarker MR. Strategy to inactivate Clostridium perfringens spores in meat products. Food Microbiol 2009; 26:272–7. [DOI] [PubMed] [Google Scholar]
- 13. Durban E, Goodnow R, Grecz N. Changes in resistance to radiation and heat during sporulation and germination of Clostridium botulinum 33A. J Bacteriol 1970; 102:590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gould GW, Jones A, Wrighton C. Limitations of the initiation of germination of bacterial spores as a spore control procedure. J Appl Bacteriol 1968; 31:357–66. [DOI] [PubMed] [Google Scholar]
- 15. Munakata N. Ultraviolet sensitivity of Bacillus subtilis spores upon germination and outgrowth. J Bacteriol 1974; 120:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stuy JH. Studies on the mechanism of radiation in activation of micro-organisms. III. Inactivation of germinating spores of Bacillus cereus . Biochim Biophys Acta 1956; 22:241–6. [DOI] [PubMed] [Google Scholar]
- 17. Stuy JH. Studies on the radiation inactivation of micro-organisms. IV. Photoreactivation of the intermediate stages in the transformation of Bacillus cereus spores into vegetative cells. Antonie Van Leeuwenhoek 1956; 22:337–49. [DOI] [PubMed] [Google Scholar]
- 18. Nerandzic MM, Donskey CJ. Triggering germination represents a novel strategy to enhance killing of Clostridium difficile spores. PLoS One 2010; 5:e12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nerandzic MM, Donskey CJ. Effective and reduced-cost modified selective medium for isolation of Clostridium difficile . J Clin Microbiol 2009; 47:397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perez J, Springthorpe VS, Sattar SA. Clospore: a liquid medium for producing high titers of semi-purified spores of Clostridium difficile . J AOAC Int 2011; 94:618–26. [PubMed] [Google Scholar]
- 21. American Society for Testing and Materials. Standard Quantitative Carrier Test Method to Evaluate the Bactericidal, Fungicidal, Mycobactericidal, and Sporicidal Potencies of Liquid Chemicals. (Method E 2111–12). West Conshohocken, PA: American Society for Testing and Materials; 2012. [Google Scholar]
- 22. Nerandzic MM, Sunkesula VC, Thriveen SC, et al. Unlocking the sporicidal potential of ethanol: induced sporicidal activity of ethanol against Clostridium difficile and Bacillus spores under altered physical and chemical conditions. PLoS One 2015; 10:e0132805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Setlow P. Spore germination. Curr Opin Microbiol 2003; 6:550–6. [DOI] [PubMed] [Google Scholar]
- 24. Fimlaid KA, Jensen O, Donnelly ML, et al. Identification of a novel lipoprotein regulator of Clostridium difficile spore germination. PLoS Pathog 2015; 11:e1005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wheeldon LJ, Worthington T, Lambert PA, et al. Antimicrobial efficacy of copper surfaces against spores and vegetative cells of Clostridium difficile: the germination theory. J Antimicrob Chemother 2008; 62:522–5. [DOI] [PubMed] [Google Scholar]
- 26. Maclean M, Murdoch LE, MacGregor SJ, Anderson JG. Sporicidal effects of high-intensity 405nm visible light on endospore-forming bacteria. Photochem Photobiol 2013; 89:120–6. [DOI] [PubMed] [Google Scholar]
- 27. Nerandzic MM, Cadnum JL, Pultz MJ, Donskey CJ. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect Dis 2010; 10:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gould GW, Jones A, Wrighton C. Limitations of the initiation of germination of bacterial spores as a spore control procedure. J Appl Bacteriol 1968; 31:357–66. [DOI] [PubMed] [Google Scholar]
- 29. Ghosh S, Setlow P. Isolation and characterization of superdormant spores of Bacillus species. J Bacteriol 2009; 191:1787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.