Abstract
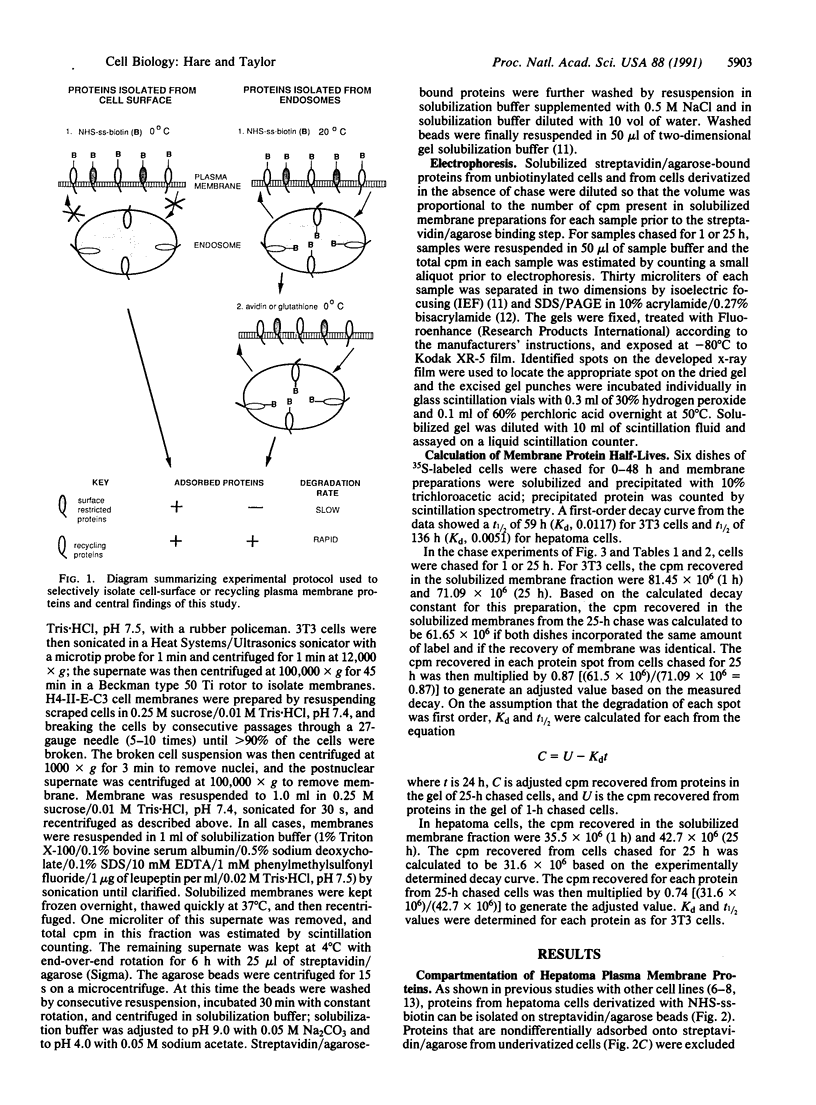
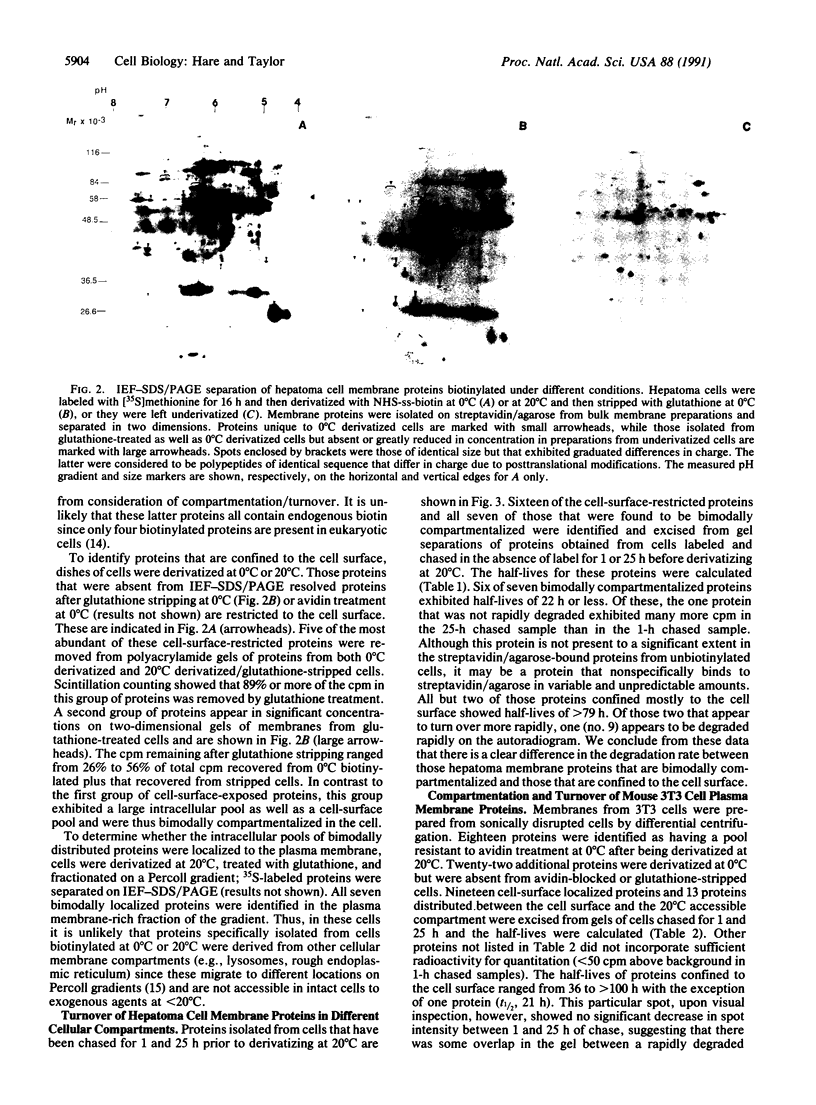
Plasma membrane proteins of intact mouse 3T3 fibroblasts and H4-II-E-C3 hepatoma cells were separated into two groups based on their compartmentation between the cell surface and an intracellular compartment accessible at 20 degrees C but not at 0 degrees C. One group was derivatized at 0 degrees C with sulfosuccinimidyl 2-(biotinamido)ethyl-1,3-dithiopropionate but not at 20 degrees C. The second group was derivatized at 20 degrees C as well as at 0 degrees C. Derivatized proteins were isolated from 35S-labeled cells on streptavidin/agarose and resolved by two-dimensional PAGE. With few exceptions, pulse-chase experiments revealed that those proteins confined exclusively to the cell surface turned over slowly (t1/2, greater than 75 h), while those bimodally compartmentalized between the cell surface and the 20 degrees C accessible compartment were degraded more rapidly (t1/2, less than 31 h). These observations suggest a mechanism to explain the varied metabolic stability of plasma membrane proteins in which the half-life of each protein is determined by the proportion of time spent in the endocytic compartment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajioka R. S., Kaplan J. Intracellular pools of transferrin receptors result from constitutive internalization of unoccupied receptors. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6445–6449. doi: 10.1073/pnas.83.17.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson R. G., Brown M. S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981 May;24(2):493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Baumann H., Doyle D. Turnover of plasma membrane glycoproteins and glycolipids of hepatoma tissue culture cells. J Biol Chem. 1978 Jun 25;253(12):4408–4418. [PubMed] [Google Scholar]
- Bretscher M. S., Lutter R. A new method for detecting endocytosed proteins. EMBO J. 1988 Dec 20;7(13):4087–4092. doi: 10.1002/j.1460-2075.1988.tb03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Schatz G. High resolution one- and two- dimensional electrophoretic analysis of mitochondrial membrane polypeptides. Methods Enzymol. 1979;56:602–613. doi: 10.1016/0076-6879(79)56057-1. [DOI] [PubMed] [Google Scholar]
- Casciola L. A., Grant K. I., Gevers W., Coetzee G. A., van der Westhuyzen D. R. Low-density-lipoprotein receptors in human fibroblasts are not degraded in lysosomes. Biochem J. 1989 Sep 1;262(2):681–683. doi: 10.1042/bj2620681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Chu F. F., Doyle D. Turnover of plasma membrane proteins in rat hepatoma cells and primary cultures of rat hepatocytes. J Biol Chem. 1985 Mar 10;260(5):3097–3107. [PubMed] [Google Scholar]
- Collawn J. F., Stangel M., Kuhn L. A., Esekogwu V., Jing S. Q., Trowbridge I. S., Tainer J. A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990 Nov 30;63(5):1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L., Aronson N. N., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Griffiths G., Howell K. E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989 Apr;108(4):1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. F. Compartmentation and turnover of the low density lipoprotein receptor in skin fibroblasts. J Biol Chem. 1990 Dec 15;265(35):21758–21763. [PubMed] [Google Scholar]
- Hare J. F. Dissection of membrane protein degradation mechanisms by reversible inhibitors. J Biol Chem. 1988 Jun 25;263(18):8759–8764. [PubMed] [Google Scholar]
- Hare J. F., Lee E. Metabolic behavior of cell surface biotinylated proteins. Biochemistry. 1989 Jan 24;28(2):574–580. doi: 10.1021/bi00428a024. [DOI] [PubMed] [Google Scholar]
- Hare J. F. Mechanisms of membrane protein turnover. Biochim Biophys Acta. 1990 Feb 28;1031(1):71–90. doi: 10.1016/0304-4157(90)90003-u. [DOI] [PubMed] [Google Scholar]
- Hare J. F. Turnover and compartmentation of gp70/p15 E in psi 2 cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):596–603. doi: 10.1016/0006-291x(89)92641-7. [DOI] [PubMed] [Google Scholar]
- Johnson K. F., Chan W., Kornfeld S. Cation-dependent mannose 6-phosphate receptor contains two internalization signals in its cytoplasmic domain. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10010–10014. doi: 10.1073/pnas.87.24.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K. F., Brush H. A., Krieger M. Unusual forms of low density lipoprotein receptors in hamster cell mutants with defects in the receptor structural gene. J Cell Biol. 1986 May;102(5):1567–1575. doi: 10.1083/jcb.102.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand J. N., Kent C. A one-step technique for the subcellular fractionation of total cell homogenates. Anal Biochem. 1986 Nov 15;159(1):157–162. doi: 10.1016/0003-2697(86)90321-0. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Oei J., Saunders M., Gravel R. [3H]biotin-labeled proteins in cultured human skin fibroblasts from patients with pyruvate carboxylase deficiency. J Biol Chem. 1983 May 25;258(10):6660–6664. [PubMed] [Google Scholar]
- Sahagian G. G., Neufeld E. F. Biosynthesis and turnover of the mannose 6-phosphate receptor in cultured Chinese hamster ovary cells. J Biol Chem. 1983 Jun 10;258(11):7121–7128. [PubMed] [Google Scholar]
- Sargiacomo M., Lisanti M., Graeve L., Le Bivic A., Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989 Mar;107(3):277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Schmid S. L., Carter L. L. ATP is required for receptor-mediated endocytosis in intact cells. J Cell Biol. 1990 Dec;111(6 Pt 1):2307–2318. doi: 10.1083/jcb.111.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Lodish H. F. Kinetics of internalization and recycling of the asialoglycoprotein receptor in a hepatoma cell line. J Biol Chem. 1982 Apr 25;257(8):4230–4237. [PubMed] [Google Scholar]
- Sullivan P. C., Ferris A. L., Storrie B. Effects of temperature, pH elevators, and energy production inhibitors on horseradish peroxidase transport through endocytic vesicles. J Cell Physiol. 1987 Apr;131(1):58–63. doi: 10.1002/jcp.1041310110. [DOI] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. Temperature dependence of endocytosis mediated by the asialoglycoprotein receptor in isolated rat hepatocytes. Evidence for two potentially rate-limiting steps. J Biol Chem. 1981 Mar 25;256(6):2615–2617. [PubMed] [Google Scholar]
- Yoshimura A., Seguchi T., Yoshida T., Shite S., Waki M., Kuwano M. Novel feature of metabolism of low density lipoprotein receptor in a mouse macrophage-like cell line, J774.1. J Biol Chem. 1988 Aug 25;263(24):11935–11942. [PubMed] [Google Scholar]