Abstract
A method for the palladium-catalyzed fluorination of cyclic vinyl triflates has been developed. As with several previous palladium-catalyzed fluorination reactions using fluoride salts, controlling the regioselectivity was nontrivial and presented a challenge in developing a practical synthetic procedure. The addition of triethyl(trifluoromethyl)silane (TESCF3) was found to effectively address this problem and resulted in dramatically improved regioselectivities in this palladium-catalyzed fluorination reaction. This discovery, along with the use of a new biarylphosphine ligand, allowed for the development of an efficient and highly regioselective protocol for the fluorination of vinyl triflates. This method is compatible with a range of sensitive functional groups and provides access to cyclic (five, six, and seven-membered) vinyl fluorides
Keywords: palladium, cross-coupling, fluorination, fluoroalkene
Graphical Abstract
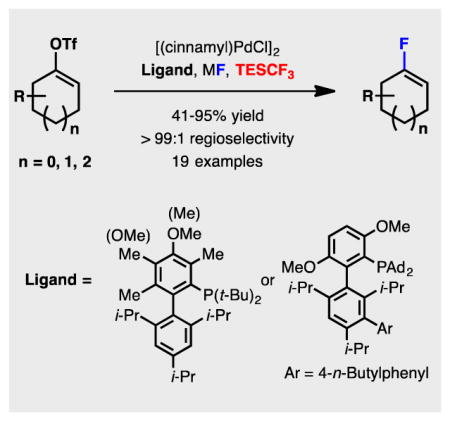
A technique for the palladium-catalyzed fluorination of cyclic vinyl triflates has been developed. The reaction exhibited good functional group tolerance and proceeded efficiently for five, six and seven-membered vinyl triflate substrates, as well as a few acyclic substrates. The addition of triethyl(trifluoromethyl)silane (TESCF3) was found to effectively address the problem of regioisometric side product generation and resulted in dramatically improved regioselectivities in this method.
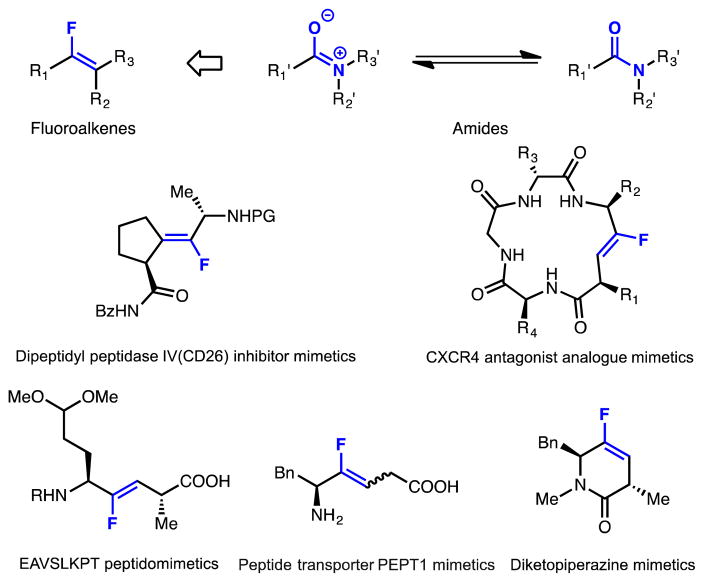
Fluorine-substituted olefins constitute a valuable class of compounds of interest for medicinal chemistry and chemical biology (Figure 1).[1,2] The alkenyl fluoride group resembles an amide linkage in terms of both steric demand and charge distribution,[3] but fluoroalkenes exhibit substantially enhanced stability towards hydrolysis compared to amides. Moreover, in contrast to amides, which often exist as equilibrating s-cis and s-trans rotamers, fluoroalkenes are configurationally stable. As a result, they have been investigated as amide bioisosteres with improved lipophilicity and metabolic stability in pharmaceutical applications and as tools for probing the conformational properties of biologically active amides.[4] In addition to these applications, fluoroalkenes also serve as versatile starting materials for a variety of transformations, including the Diels-Alder reaction,[5a] cyclopropanation,[5b,5c] and epoxidation,[5d] allowing for the construction of other classes of fluorine-containing molecules.
Figure 1.
Representative fluoroalkenes as peptidomimetics.
Despite considerable potential, fluoroalkenes are underutilized due to challenges in their synthesis. Current approaches for their preparation generally require multistep functional group manipulations or the use of harsh reaction conditions, which limit the functional group compatibility of these techniques. [6–11] The development of a mild and general method for fluoroalkene synthesis would therefore be highly desirable. Due to the challenge of preparing suitable precursors, cyclic fluoroalkenes are particularly difficult to access using existing methods, and new methods that provide access to these compounds would be especially useful.
In this context, the palladium-catalyzed coupling of cyclic vinyl triflates with simple metal fluoride salts represents a particularly attractive strategy to access fluoroalkenes. In 2009, we reported a palladium(II)-catalyzed fluorination of aryl triflates using a bulky biaryl monophosphine ligand (t-BuBrettPhos).[12a] Since then, more effective catalysts based on AdBrettPhos and HGPhos were developed and the fluorination of aryl bromides was achieved.[12b,12c] However, the generation of regioisomeric side products in these reactions remained an unsolved problem.[12d] Recently, a new fluorinated biaryl phosphine ligand (AlPhos) was developed to improve the rate and regioselectivity in the fluorination of aryl triflates and bromides.[12e] However, as described below, direct application of these new ligands and reaction conditions to cyclic vinyl triflate substrates afforded the corresponding vinyl fluoride products in low yield and as mixtures of regioisomers. Herein, we report the development of a highly regioselective palladium(II)-catalyzed reaction for the fluorination of cyclic vinyl triflates. High regioselectivity was realized through the development of a new biaryl phosphine ligand (L8) and the serendipitous discovery of triethyl(trifluoromethyl)silane (TESCF3) as an additive.
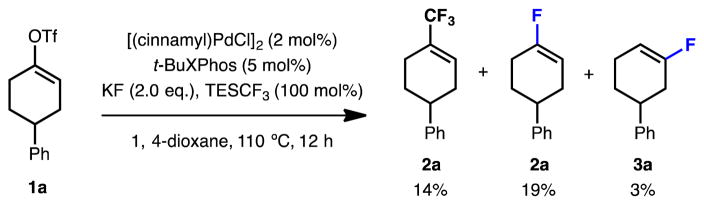
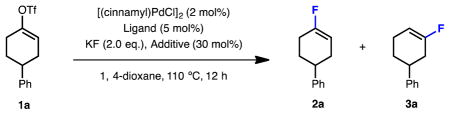
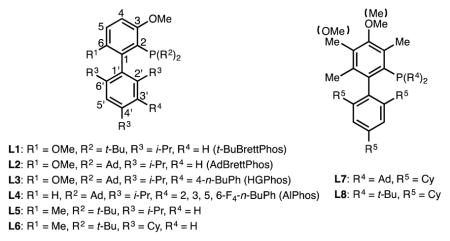
We commenced our study using 4-phenylcyclohexenyl triflate (1a) as the model substrate (Table 1). Preliminary ligand evaluation showed that catalysts based on t-BuBrettPhos (L1), AdBrettPhos (L2), HGPhos (L3) or AlPhos (L4), which were effective ligands for the fluorination of aryl eletrophiles,[12a–c] provided low yields of the desired vinyl fluoride 2a and a substantial amount of the undesired regioisomer 3a (close to 1:1 ratio, entry 1–4). Replacing the 6-methoxyl group of the ligand with a methyl substituent (L5 and L6) led to improved yields, indicating that ligand rigidity may be important in this transformation (entry 5–6).[13] After further evaluation of ligands possessing a trimethylmethoxy-substituted top ring (L7 and L8),[14] a novel biarylphosphine ligand L8 was found to provide 2a in moderate combined yield (58%) though still with poor regioselectivity (1.8:1) (entry 7–8).
Table 1.
Reaction optimization studies.
| |||||
|---|---|---|---|---|---|
| entry | ligand | additive | 2a (%) | 3a (%) | 2a : 3a |
| 1 | L1 | 23 | 21 | 1.1 : 1 | |
| 2 | L2 | 18 | 18 | 1.0 : 1 | |
| 3 | L3 | 11 | 13 | 0.8 : 1 | |
| 4 | L4 | 19 | 19 | 1.0 : 1 | |
| 5 | L5 | 27 | 20 | 1.4 : 1 | |
| 6 | L6 | 37 | 19 | 1.9 : 1 | |
| 7 | L7 | 23 | 20 | 1.2 : 1 | |
| 8 | L8 | 37 | 21 | 1.8 : 1 | |
| 9 | L8 | TMSCF3 | 68 | 2 | 34 : 1 |
| 10 | L8 | TESCF3 | 73 | 1.0 | 73 : 1 |
| 11 | L8 | TIPSCF3 | 47 | 10 | 4.7 : 1 |
| 12 | L6 | TESCF3 | 57 | 5.0 | 11 : 1 |
| 13b | L8 | TESCF3 | 74 | 0.4 | >99 : 1 |
| 14b | L8 | 7 | 4 | 1.8 : 1 | |
Reactions were run at 0.1 mmol scale. Yields were determined by 19F NMR analysis of the crude reaction mixture using 1-fluoronaphthalene as an internal standard.
2-MeTHF, 90 °C.
During our investigation of the palladium-catalyzed trifluoromethylation of vinyl sulfonates,[15b] we made the serendipitous discovery that the corresponding vinyl fluoride was formed as a side product with relatively high regioselectivity (6.3:1, Scheme 1). We hypothesized that the presence of trifluoromethylsilanes, which were used as CF3− sources in trifluoromethylation reactions, might be responsible for the improved regioselectivity of the fluorination process. Indeed, the use of TMSCF3, TESCF3 or TIPSCF3 as substoichiometric additives (30 mol%) drastically improved the regioselectivity (34:1, 73:1, and 4.7:1, respectively) (Table 1, entry 9–11). Although catalyst based on L6 and L8 gave comparable results in the absence of the trifluoromethyl silane additive, the yield and regioselectivity obtained with L8 were considerably higher when TESCF3 (30 mol%) was added (entries 6, 8, 10 and 12). Finally, performing the reaction in 2-MeTHF at 90 °C with L8 as the supporting ligand, along with a substoichiometric amount of TESCF3 afforded the desired product 2a in 74% yield with excellent regioselectivity (>99:1) (entry 13). In addition to this significant improvement in regioselectivity, the addition of the TESCF3 additive also allowed the reaction to be conducted at a lower temperature (entry 13 vs. entry 14).
Scheme 1.
Fluorinated side products generated in the palladium-catalyzed trifluoromethylation reaction.
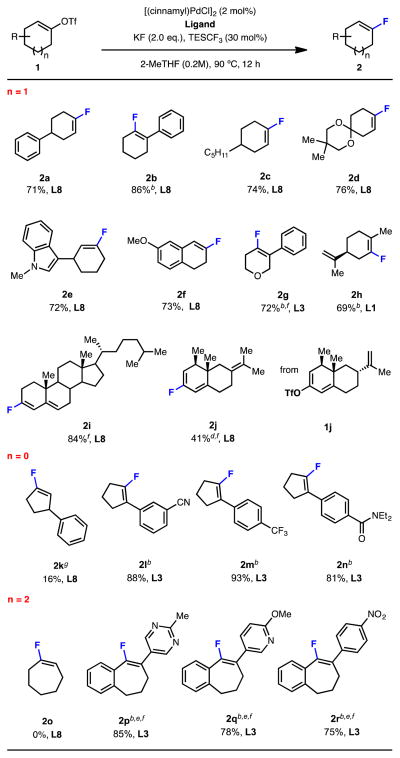
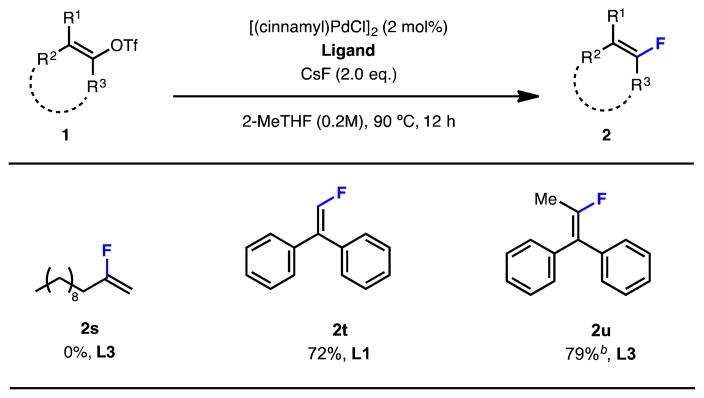
We subsequently examined the substrate scope using these optimized reaction conditions, and found this protocol to be applicable to the fluorination of a variety of 1,2-disubstituted cyclic vinyl triflates (Table 2). In addition, the fluorination of 1,2,2-trisubstituted vinyl triflates, for which regioisomer formation is not an issue,[12d] was possible with L3 as the ligand and CsF as the fluoride source. TESCF3 was not required for these processes. Interestingly, our new ligand (L8) developed for vinyl triflate 1a, did not perform well for 1,2,2-trisubstituted vinyl triflates, which likely stems from its sterically encumbered nature.
Table 2.
Palladium-catalyzed fluorination of cyclic vinyl triflates.
Isolated yields are reported as an average of two runs on a 1.0 mmol scale.
Reaction conditions: [(cinnamyl)PdCl]2 (2 mol%), ligand (5 mol%), CsF (2.0 eq.), 2-MeTHF, 90 °C, 12 h.
t-BuBrettPhos as the ligand.
In 1,4-dioxane.
In toluene.
110 °C.
130 °C.
1-Cyclohexenyl triflates with substituents at the 4- (2a and 2c), 3- (2e) or 2- (2b) position were all excellent substrates (Table 2, n = 1). Benzofused (2f) and oxygen-containing six-membered cyclic triflates (2g) were compatible as well. Moreover, the method could be used to access fluorinated analogues of biologically active terpene and steroid derivatives (2h, 2i and 2j). In the case of 1j, isomerization of the terminal double bond to the more thermodynamically stable internal position occurs under these reaction conditions.
In general, the fluorination of 1-cyclopentenyl triflates was more difficult, presumably due to the higher energy barrier for C-F reductive elimination from the respective palladium(II) complex (Table 1, n = 0).[16] Thus, vinyl triflate 1k without additional substitution on the double bond provided the desired cyclopentenyl fluoride in low yield. However, substrates possessing an additional substituent at the 2-position reacted efficiently to provide the corresponding cyclic vinyl fluorides in good to excellent yield (2l, 2m and 2n).
Although 1-cycloheptenyl triflate 1o was fully consumed under these conditions, the fluorinated product was not obtained (Table 1, n = 2). GC/MS analysis of the crude reaction mixture indicated the formation of the corresponding alkyne or allene product, implying that the vinyl triflate starting material decomposed through β-hydrogen elimination. Consistent with this hypothesis, seven-membered cyclic vinyl triflates without β-hydrogen atoms were fluorinated in good yields (2p, 2q and 2r).
A variety of functional groups were tolerated in this transformation, including an acetal (2d), a nitrile (2l), a trifluoromethyl group (2m), an amide (2n) and a nitro group (2r). Heterocycles, including an indole (2e), a pyrimidine (2q) and a pyridine (2p) were also compatible.
The fluorination of acyclic 1-substituted vinyl triflates 1s was unsuccessful, presumably as a result of competitive β-hydrogen elimination. More highly substituted vinyl triflates without β-alkenyl hydrogen atoms were successfully fluorinated (1t and 1u).
The formation of side-products is an important factor affecting the synthetic utility of a fluorination reaction due to the challenges often encountered during the separation of fluorinated products from side-products with similar physical properties. Thus, careful analysis of the crude reaction mixture in this fluorination protocol was performed. Generally speaking, the corresponding reduction product was formed in less than 0.5% in all cases while the corresponding vinyl chloride was formed in 2.5%–0.5%. Although the entries in Table 2 could all be purified to >99.5% purity by column chromatography, conditions that would avoid the formation of the vinyl chloride side product would significantly simplify purification of the desired vinyl fluoride.
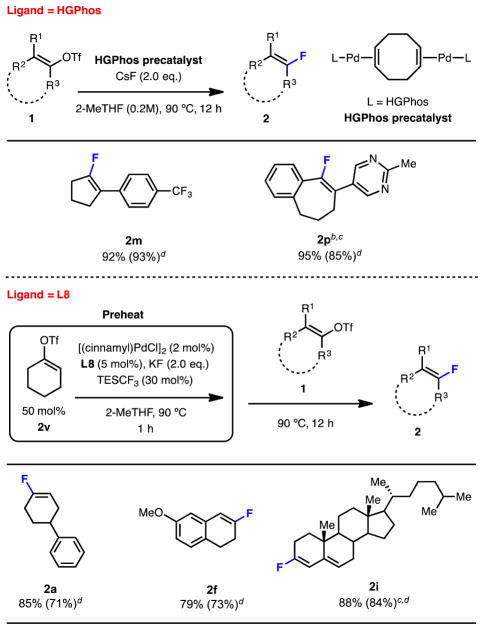
Towards this goal, the use of precatalysts of the form [(1,5-cyclooctadiene)(LPd)2] that activate with minimal generation of reactive or other undesired byproducts were investigated. In the case of vinyl triflates for which regioisomer formation is not a concern, the use of the L3-derived precatalysts of this type in place of L3/[(cinnamyl)PdCl]2 was found to provide comparable or superior yields without formation of vinyl chloride (Table 4, 2m and 2p). However, because of the large size of L8, the corresponding precatalysts based on L8 could not be prepared. Therefore, for fluorination reactions employing L8, a modified reaction protocol employing a “sacrificial” vinyl triflate was developed. We found that preheating the reaction mixture with cyclohexenyl triflate (2v) (50 mol%) as the “sacrificial” vinyl triflate for one hour prior to the addition of the vinyl triflate starting material led to improved yields (Table 4, 2a, 2f, 2i). The volatile fluorination and chlorination products derived from 2v could be easily removed in vacuo. Using this protocol, the corresponding vinyl chloride coming from the vinyl triflate starting material was not detected by GC analysis of the crude reaction mixtures. Moreover, yields obtained using this protocol were generally higher than those obtained previously.
Table 4.
Modified fluorination reaction protocol without the generation of chlorination side products.
Isolated yields are reported as an average of two runs on a 1.0 mmol scale.
In toluene.
110 °C.
Yield when conducted under previous reaction conditions.
In summary, we have developed a method for palladium-catalyzed fluorination of vinyl triflates for the synthesis of cyclic fluoroalkenes. High levels of regiochemical fidelity of this reaction were achieved by employing a new biarylphosphine ligand L8 and TESCF3 as a crucial additive. The reaction exhibited good functional group tolerance and proceeded efficiently for five, six and seven-membered vinyl triflate substrates, as well as a few acyclic substrates. As the synthesis of cyclic vinyl fluorides using existing methods is problematic due to the lack of availability of starting materials and limited functional group compatibility of the existing methods, our palladium-based protocol is complementary to these previously developed processes.[7m,7n,7o] The intriguing “TESCF3 effect” has provided us with a new tool for addressing the problem of the formation of regioisomers in palladium-catalyzed fluorination reactions of vinyl triflates. Studies are undergoing to gain a detailed mechanistic understanding of this phenomenon.
Supplementary Material
Table 3.
Palladium-catalyzed fluorination of six-membered vinyl triflates.
Isolated yields are reported as an average of two runs on a 1.0 mmol scale.
110 °C.
Acknowledgments
We thank the National Institutes of Health (R01GM46059) for financial support. We thank Drs. Yiming Wang and Michael Pirnot for assistance with the preparation of the manuscript. We also thank Dr. Shiliang Shi and Dr. Aaron Sather for helpful discussions. We thank Dr. Yong Zhang and Dr. Jorge Fortanet for preliminary experiments. We thank Dr. Yang Yang for assistance with this manuscript and for carrying out preliminary DFT calculations.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.For reviews, see: Purser S, Moore PR, Swallow S, Gouverneur V. Chem Soc Rev. 2008;37:320. doi: 10.1039/b610213c.Neumann CN, Ritter T. Angew Chem, Int Ed. 2015;54:3216. doi: 10.1002/anie.201410288.Kirk KL. Org Process Res Dev. 2008;12:305.Müller K, Faeh C, Diederich F. Science. 2007;317:1881. doi: 10.1126/science.1131943.
- 2.(a) Welch J, Lin J. Tetrahedron. 1996;52:291. [Google Scholar]; (b) Narumi T, Hayashi R, Tomita K, Kobayashi K, Tanahara N, Ohno H, Naito T, Kodama E, Matsuoka M, Oishi S, Fujii N. Org Biomol Chem. 2010;8:616. doi: 10.1039/b917236j. [DOI] [PubMed] [Google Scholar]; (c) Lamy C, Hofmann J, Parrot-Lopez H, Goekjian P. Tetrahedron Lett. 2007;48:6177. [Google Scholar]; (d) Niida A, Tomita K, Mizumono M, Tanigaki H, Terada T, Oishi S, Otaka A, Inui KI, Fuji N. Org Lett. 2006;8:613. doi: 10.1021/ol052781k. [DOI] [PubMed] [Google Scholar]; (e) Niida A, Mizumoto M, Narumi T, Inokuchi E, Oishi S, Ohno H, Otaka A, Kitaura K, Fujii N. J Org Chem. 2006;71:4118. doi: 10.1021/jo060202z. [DOI] [PubMed] [Google Scholar]
- 3.(a) Abraham RJ, Ellison SLR, Schonholzer P, Thomas WA. Tetrahedron. 1986;42:2101. [Google Scholar]; (b) Urban J, Tillman B, Cronin WA. J Phys Chem A. 2006;110:11120. doi: 10.1021/jp062881n. [DOI] [PubMed] [Google Scholar]
- 4.Couve-Bonnaire S, Cahard D, Pannecoucke X. Org Biomol Chem. 2007;5:1151. doi: 10.1039/b701559c. [DOI] [PubMed] [Google Scholar]
- 5.(a) Ernet T, Maulitz AH, Wurthwein E-U, Haufe GJ. Chem Soc, Perkin Trans 1. 2001:1929. [Google Scholar]; (b) Meyer OGJ, Frohlich R, Haufe G. Synthesis. 2000;10:1479. [Google Scholar]; (c) Wong OA, Shi YJ. Org Chem. 2009;74:8377. doi: 10.1021/jo901553t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For reviews, see: Van Steenis JH, Van der Gen A. J Chem Soc Perkin Trans. 2002;1:2117.Landelle G, Bergeron M, Turcotte-Savard MO, Paquin JF. Chem Soc Rev. 2011;40:2867. doi: 10.1039/c0cs00201a.Burton DJ, Yang ZY, Qiu W. Chem Rev. 1996;96:1641. doi: 10.1021/cr941140s.
- 7.Representative fluoroalkene synthesis from fluorinated precursors: Patrick TB, Nadji S. J Fluorine Chem. 1990;49:147.Narumi T, Tomita K, Inokuchi E, Kobayashi K, Oishi S, Ohno H, Fujii N. Org Lett. 2007;9:3465. doi: 10.1021/ol701627v.Zhang H, Zhou CB, Chen QY, Xiao JC, Hong R. Org Lett. 2011;13:560. doi: 10.1021/ol102645g.Hassan A, Montgomery TP, Krische MJ. Chem Commun. 2012;48:4692. doi: 10.1039/c2cc31743e.Prakash GKS, Chacko S, Vaghoo H, Shao N, Gurung L, Mathew T, Olah GA. Org Lett. 2009;11:1127. doi: 10.1021/ol8029627.Zajc B, Kake S. Org Lett. 2006;8:4457. doi: 10.1021/ol0616236.Van Steenis JH, Van der Gen A. Eur J Org Chem. 2001:897.Asakura N, Usuki Y, Iio H. J Fluorine Chem. 2003;124:81.Pigeon X, Bergeron M, Barabé F, Dubé P, Frost HN, Paquin JF. Angew Chem, Int Ed. 2010;49:1123. doi: 10.1002/anie.200904747.Ichikawa J, Miyazaki S, Fujiwara M, Minami T. J Org Chem. 1995;60:2320.Thornbury RT, Toste FD. Angew Chem, Int Ed. 2016;55:11629. doi: 10.1002/anie.201605651.
- 8.Deoxyfluorination of ketones: Sano K, Fukuhara T, Hara S. J Fluorine Chem. 2009;130:708.Biedermann D, Sarek J, Klinot J, Hajduch M, Dzubak P. Synthesis. 2005:1157.
- 9.Fluoroalkene synthesis by olefin metathesis: Salim S, Bellingham RK, Satcharoen V, Brown RCD. Org Lett. 2003;5:3403. doi: 10.1021/ol035065w.Marhold M, Buer A, Hiemstra H, van Maarseveen JH, Haufe G. Tetrahedron Lett. 2004;45:57.Nguyen TT, Koh MJ, Shen X, Romiti F, Schrock RR, Hoveyda AH. Science. 2016;352:569. doi: 10.1126/science.aaf4622.
- 10.Hydrofluorination of alkynes: Akana JA, Bhattacharyya KX, Mueller P, Sadighi JP. J Am Chem Soc. 2007;129:7736. doi: 10.1021/ja0723784.Gorske BC, Mbofana CT, Miller SJ. Org Lett. 2009;11:4318. doi: 10.1021/ol9016782.Okoromoba O, Han J, Hammond G, Xu B. J Am Chem Soc. 2014;136:14381. doi: 10.1021/ja508369z.Nguyen TH, Abarbri M, Guilloteau D, Mavel S, Emond P. Tetrahedron. 2011;67:3434.Alonso P, Pardo P, Fañanás FJ, Rodríguez F. Chem Commun. 2014;50:14364. doi: 10.1039/c4cc07376b.
- 11.Electrophilic fluorination of alkenylmetal species: M = Li, see: Kerr WJ, Morrison AJ, Pazicky M, Weber T. Org Lett. 2012;14:2250. doi: 10.1021/ol300652k.Yang MH, Matikonda SS, Altman RA. Org Lett. 2013;15:3894. doi: 10.1021/ol401637n.M = Sn, see: Tius MA, Kawakami JK. Tetrahedron. 1995;51:3997.M = Si, see: Greedy B, Gourverneur V. Chem Commun. 2001:233.M = B, see: Petasis NA, Yudin AK, Zavialov IA, Prakash GKS, Olah GA. Synlett. 1997:606.Furuya T, Ritter T. Org Lett. 2009;11:2860. doi: 10.1021/ol901113t.Ye Y, Schimler SD, Hanley PS, Sanford MS. J Am Chem Soc. 2013;135:16292. doi: 10.1021/ja408607r.
- 12.(a) Watson DA, Su M, Teverovskiy G, Zhang Y, García-Fortanet J, Kinzel T, Buchwald SL. Science. 2009;325:1661. doi: 10.1126/science.1178239. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee HG, Milner PJ, Buchwald SL. Org Lett. 2013;15:5602. doi: 10.1021/ol402859k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee HG, Milner PJ, Buchwald SL. J Am Chem Soc. 2014;136:3792. doi: 10.1021/ja5009739. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Milner PJ, Kinzel T, Zhang Y, Buchwald SL. J Am Chem Soc. 2014;26:2183. doi: 10.1021/ja509144r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sather AC, Lee HG, Rose VYDL, Yang Y, Müller P, Buchwald SL. J Am Chem Soc. 2015;137:13433. doi: 10.1021/jacs.5b09308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Fors BP, Buchwald SL. Angew Chem, Int Ed. 2011;50:9943. doi: 10.1002/anie.201104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueda S, Ali S, Fors BP, Buchwald SL. J Org Chem. 2012;77:2543. doi: 10.1021/jo202537e. Ligands (such as L7 and L8) with the trimethylmethoxy-substituted top ring exist as two isomers in a ratio close to 1:0.97. These ligands are used as surrogates for the ligands with the tetramethyl-substituted top ring, since they can be prepared from a more readily available precursor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Cho EJ, Senecal TD, Kinzel T, Zhang Y, Watson DA, Buchwald SL. Science. 2010;328:1679. doi: 10.1126/science.1190524. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cho EJ, Buchwald SL. Org Lett. 2011;13:6552. doi: 10.1021/ol202885w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milner PJ, Yang Y, Buchwald SL. Organometallics. 2015;34:4775. doi: 10.1021/acs.organomet.5b00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.