Abstract
Coccidioides species are endemic to the southwestern United States and typically cause a mild or asymptomatic primary infection. In some instances, infection can disseminate and involve the central nervous system with meningitis being the most common manifestation. Non-osseous spinal cord involvement is exceedingly rare. We report a case of disseminated coccidioidomycosis in an otherwise healthy 20 year old man with diffuse leptomeningeal enhancement, cerebrospinal fluid findings suggestive of meningitis, and intramedullary spinal cord abscesses. Response to treatment occurred with prolonged systemic liposomal amphotericin B and voriconazole. An extended course of steroids was needed to blunt inflammation.
Keywords: Coccidioides, disseminated coccidioidomycosis, intramedullary spinal cord abscess, meningitis
1. Introduction
The dimorphic fungi Coccidioides immitis and posadasii are endemic to the southwestern United States as well as parts of Mexico and South America [1]. Primary infection is frequently asymptomatic or mild. However, in a small percentage of cases Coccidioides disseminate widely to the skin, meninges, joints, and bones. Infection of the central nervous system often manifests as basilar meningitis [2]. There are few reports of spinal cord involvement with abscesses in the literature [3]. We report a case of disseminated coccidioidomycosis in a 20 year old man presenting with meningitis and intramedullary spinal cord abscesses along with the different management challenges we encountered.
2. Case
A 15 year old HIV negative Pacific Islander man was diagnosed with pulmonary coccidioidomycosis while living in the San Joaquin Valley of California. Despite improving on fluconazole 400 mg daily, he was hospitalized 8 months later for coccidioidal meningitis. Cerebrospinal fluid was notable for a white cell count of 238 cells/mm3 (83% lymphocytes, 6% eosinophils), a protein level of 627 mg/dL (normal range 15–45 mg/dL), a glucose level of 9 mg/dL (normal range 40–70 mg/dL), and Coccidioides complement fixing antibody titer of 1:64. The fluconazole dose was increased to 1200 mg daily. Hydrocephalus was present but he did not require a shunt. Due to improvement in symptoms and gastrointestinal intolerance from fluconazole, he discontinued the medication. Two months later he presented with seizures. Cerebrospinal fluid was again abnormal with a lymphocytic pleocytosis, elevated protein, and Coccidioides complement fixing antibody titer of 1:128. He was started on levetiracetam and phenytoin and continued on fluconazole 1200 mg daily. There were no concerns for major drug interactions with this regimen. He remained clinically stable for the following 2 years during which time the anti-epileptics were eventually stopped and fluconazole was decreased to a better-tolerated dose of 400 mg twice daily. On 3 subsequent occasions he presented with seizures and meningitis in the setting of medication non-adherence. His resulting neurologic deficits from these episodes included seventh cranial nerve palsy with left-sided facial weakness, inability to completely close his left eye, left-sided hearing loss, difficulty with balance, and moderate neurocognitive disorder.
Now 20 years of age, he presented to our care 1 month after the most recent seizure having since resumed fluconazole 400 mg twice daily (day 0). He reported a several month history of progressive proximal right upper extremity weakness in addition to 1 month of urinary retention and occasional incontinence. These new deficits were appreciated on physical exam with findings of significantly diminished proximal right upper extremity strength, markedly decreased rectal tone, and bilateral clonus.
Magnetic resonance imaging (MRI) revealed extensive leptomeningeal enhancement of the brain and spine ( Fig. 1). There was intramedullary extension of disease at the cervicomedullary junction as well as in the cervical and thoracic spine; this was most notable in the cervical spine where there was a 3.2 cm rim-enhancing intramedullary abscess with exuberant surrounding edema. Hydrocephalus was not present. Lumbar puncture could not safely be performed due to concern for spinal cord herniation given the degree of spinal cord edema [4]. Neurosurgical intervention for drainage was also considered too high risk and unlikely to be of greater benefit than medical management alone. Serum Coccidioides antibody by complement fixation was 1:16.
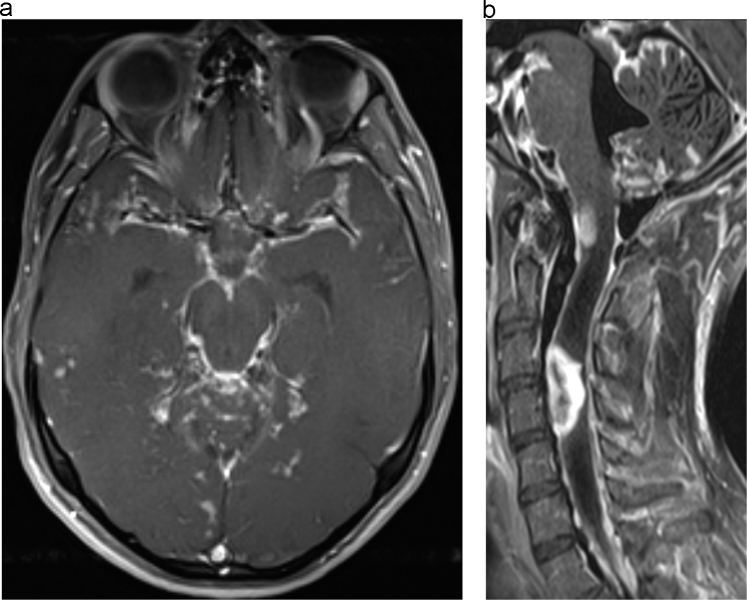
Fig. 1.
A: Postcontrast axial T1 weighted fat saturated images of the brain obtained at the initial presentation to our care demonstrate extensive leptomeningeal enhancement involving the basilar cisterns, cerebellar folia, and sylvian fissures. B: Postcontrast sagittal T1 weighted fat saturated images of the cervical spine obtained at the initial presentation to our care demonstrate extensive nodular leptomeningeal enhancement of the cervical spine as well as two large intramedullary enhancing lesions. Marked T1 hypointensity about the intramedullary lesion in the cervical spine is compatible with surrounding spinal cord edema. Brainstem and cerebellar leptomeningeal enhancement is also redemonstrated.
The patient was started on liposomal amphotericin B at 3 mg/kg daily in addition to a dexamethasone taper. Fluconazole 400 mg twice daily was also continued. On this regimen, his arm weakness gradually improved. Upon cessation of steroids however, both neurologic symptoms as well as MRI findings worsened. In addition to resumption of steroids, amphotericin was increased to 5 mg/kg (day 44) and fluconazole was empirically changed to voriconazole 200 mg twice daily (day 58) due to concern for refractory disease. Repeat spine MRI (day 154) demonstrated marked improvement in leptomeningeal enhancement and decrease in intramedullary lesions and associated spinal cord edema (Fig. 2). Repeat serum complement fixation titer decreased to 1:4 (day 93). Further attempts at weaning steroids have been limited by recrudescence of radiologic findings though neurologic deficits have remained stable. The patient continues on liposomal amphotericin at a better tolerated dose of 3 mg/kg daily, voriconazole 150 mg twice daily, dexamethasone 1 mg daily, and levetiracetam. Recent voriconazole trough levels have ranged from 1.9 to 3.7 μg/mL. His clinical course has been complicated by sequelae of long-term steroid and amphotericin administration that include chronic kidney disease, potassium and magnesium wasting, and Cushingoid features. Despite the morbidity associated with therapy, he remains clinically improved with observed radiographic improvement and a serum complement fixation titer of 1:2 (day 261).
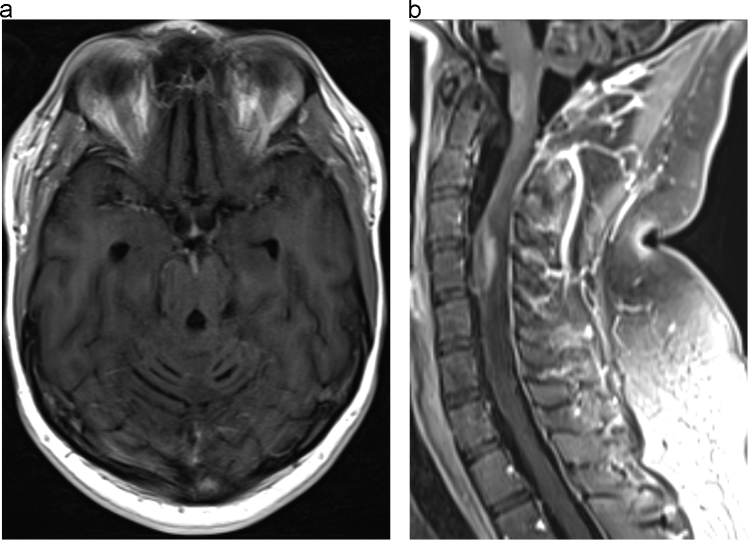
Fig. 2.
A: Postcontrast axial T1 weighted images of the brain without fat suppression obtained five months after therapy demonstrate near complete resolution of the previously seen intracranial leptomeningeal enhancement. B: Postcontrast sagittal T1 weighted images of the cervical spine with fat suppression obtained five months after therapy demonstrate marked improvement in the previously seen leptomeningeal enhancement and improvement in the intramedullary lesions.
3. Discussion
In a small number of patients with coccidioidomycosis, infection can disseminate widely. The central nervous system is a common site of extrapulmonary infection which typically manifests as basilar meningitis. Most of the literature that does describe spinal disease is focused on osseus involvement [5]. In rare instances, spinal cord involvement can occur.
Bañuelos et al. [6] described 2 such cases in 1996. In the first, a 21 year old diabetic man was diagnosed with culture and histopathology-proven C. immitis brain abscess with an associated thoracic spinal cord lesion. In the second case, a 42 year old man developed C. immitis meningitis with an intradural extramedullary lesion extending from C4 through the mesencephalon. Mischel et al. [7] described another case in which a 30 year old man with AIDS and Kaposi's sarcoma was found to have disseminated coccidioidomycosis, evidenced by autopsy findings of abundant C. immitis spherules in the lungs, meninges, brain parenchyma, spinal cord, and nerve roots. More recently, Lammering et al. [8] described imaging findings in a series of patients with proven coccidioidal CNS meningitis seen at a tertiary referral center. Of the 22 patients who had spinal imaging, 19 (86%) had concurrent intraspinal disease. While the majority of these 19 had spinal leptomeningeal enhancement, 7 had intramedullary involvement as seen in our patient suggesting that this complication may be more common than generally appreciated. Finally, Tan et al. [3] described a 55 year old man who presented with rapidly progressive quadriparesis in the setting of disseminated coccidioidomycosis with cervical intramedullary involvement. Cases reports of spinal cord involvement of Coccidioides, particularly intramedullary involvement, are few and illustrate overall poor neurologic and survival outcomes.
Successful treatment of central nervous system coccidioidomycosis can be challenging. Current clinical practice guidelines address treatment of meningitis but there is limited data to guide management of spinal cord or refractory disease [9]. High dose fluconazole remains the primary treatment for meningitis while growing evidence supports the successful use of voriconazole and posaconazole for clinical failures [10], [11]. Intrathecal amphotericin has been used for coccidioidal meningitis though toxicity is significant. Furthermore, benefit for treatment of brain abscesses, particularly where the meninges are not involved, is less clear. Animal model studies suggest that treatment with systemic liposomal amphotericin leads to effective clearance of infection from the brain and spinal cord [12]. Further support for the use of systemic amphotericin for brain abscesses can be derived from case reports describing successful application of this treatment approach [6]. Echinocandins when used alone do not appear to have therapeutic efficacy against coccidioidomycosis [13], [14]. One pediatric case series did describe successful salvage treatment of refractory disease with combination caspofungin and voriconazole though the benefit could have been due to voriconazole alone [15]. Finally, practice guidelines describe the use of steroids as limited to anecdotal experience in select cases of meningitis-related complications including cerebral vasculitis, cranial neuropathy, and arachnoiditis [9].
Our case highlights not only a rare clinical presentation of disseminated coccidioidomycosis with intramedullary spinal cord involvement but the potential for treatment to mitigate disease morbidity and mortality. Our patient appeared to have progressive disease despite use of fluconazole for several years, though medication non-compliance likely contributed in part to this. We did not have an isolate to test for antifungal susceptibility and chose to switch from fluconazole to voriconazole based on clinical failure. Voriconazole has the advantages of high oral bioavailability, good central nervous system penetration, and ability for serum concentration monitoring. Given the extent of spinal cord disease and inflammation as well as potential for paralysis, we also expanded therapy to include systemic liposomal amphotericin and dexamethasone. Though overall prognosis remains uncertain, this approach has led to a period of clinical and radiographic stability.
In summary, this case illustrates several important lessons. For one, spinal cord coccidioidomycosis with intramedullary involvement may be a more common manifestation of disseminated infection than previously appreciated. Secondly, combination therapy with systemic liposomal amphotericin and voriconazole should be considered in refractory cases. Steroids can be a helpful adjunct in the management of extensive spinal cord inflammation. Though long-term use of all of these therapies is limited by toxicities, an aggressive approach is needed to achieve stability.
Conflict of Interest
The authors do not have any conflicts of interest to disclose.
Acknowledgements
The authors thank Dr. Ferric Fang along with numerous other providers for their assistance in the management of this challenging case.
References
- 1.Laniado-Laborin R. Expanding understanding of epidemiology of coccidioidomycosis in the Western hemisphere. Ann. N. Y Acad. Sci. 2007;1111:19–34. doi: 10.1196/annals.1406.004. [DOI] [PubMed] [Google Scholar]
- 2.Welsh O., Vera-Cabrera L., Rendon A., Gonzalez G., Bonifaz A. Coccidioidomycosis. Clin. Dermatol. 2012;30:573–591. doi: 10.1016/j.clindermatol.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Tan L.A., Kasliwal M.K., Nag S., O’Toole J.E., Traynelis V.C. Rapidly progressive quadriparesis heralding disseminated coccidioidomycosis in an immunocompetent patient. J. Clin. Neurosci. 2014;21:1049–1051. doi: 10.1016/j.jocn.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 4.Hollis P.H., Malis L.I., Zappulla R.A. Neurological deterioration after lumbar puncture below complete spinal subarachnoid block. J. Neurosurg. 1986;64:253–256. doi: 10.3171/jns.1986.64.2.0253. [DOI] [PubMed] [Google Scholar]
- 5.Szeyko L.A., Taljanovic M.S., Dzioba R.B., Rapiejko J.L., Adam R.D. Vertebral coccidioidomycosis: presentation and multidisciplinary management. Am. J. Med. 2012;125:304–314. doi: 10.1016/j.amjmed.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Bañuelos A.F., Williams P.L., Johnson R.H., Bibi S., Fredricks D.N., Gilroy S.A. Central nervous system abscesses due to Coccidioides species. Clin. Infect. Dis. 1996;22:240–250. doi: 10.1093/clinids/22.2.240. [DOI] [PubMed] [Google Scholar]
- 7.Mischel P.S., Vinters H.V. Coccidioidomycosis of the central nervous system: neuropathological and vasculopathic manifestations and clinical correlates. Clin. Infect. Dis. 1995;20:400–405. doi: 10.1093/clinids/20.2.400. [DOI] [PubMed] [Google Scholar]
- 8.Lammering J.C., Iv M., Gupta N., Pandit R., Patel M.R. Imaging spectrum of CNS coccidioidomycosis: prevalence and significance of concurrent brain and spinal disease. AJR Am. J. Roentgenol. 2013;200:1334–1346. doi: 10.2214/AJR.12.9264. [DOI] [PubMed] [Google Scholar]
- 9.Galgiani J.N., Ampel N.M., Blair J.E., Catanzaro A., Geertsma F., Hoover S.E. Infectious disease society of America (IDSA) clinical practice guidelines for the treatment of coccidioidomycosis. Clin. Infect. Dis. 2016;2016(63):e112–e146. doi: 10.1093/cid/ciw360. [DOI] [PubMed] [Google Scholar]
- 10.Kim M.M., Vikram H.R., Kusne S., Seville M.T., Blair J.E. Treatment of refractory coccidioidomycosis with voriconazole or posaconazole. Clin. Infect. Dis. 2011;53:1060–1066. doi: 10.1093/cid/cir642. [DOI] [PubMed] [Google Scholar]
- 11.Schein R., Homans J., Larsen R.A., Neely M. Posaconazole for chronic refractory coccidioidal meningitis. Clin. Infect. Dis. 2011;53:1252–1254. doi: 10.1093/cid/cir734. [DOI] [PubMed] [Google Scholar]
- 12.Clemons K.V., Sobel R.A., Williams P.L., Pappagianis D., Stevens D.A. Efficacy of intravenous liposomal amphotericin B (AmBisome) against coccidioidal meningitis in rabbits. Antimicrob. Agents Chemother. 2002;46:2420–2426. doi: 10.1128/AAC.46.8.2420-2426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsue G., Napier J.T., Prince R.A., Chi J., Hospenthal D.R. Treatment of meningeal coccidioidomycosis with caspofungin. J. Antimicrob. Chemother. 2004;54:292–294. doi: 10.1093/jac/dkh306. [DOI] [PubMed] [Google Scholar]
- 14.González G.M., González G., Najvar L.K., Graybill J.R. Therapeutic efficacy of caspofungin alone and in combination with amphotericin B deoxycholate for coccidioidomycosis in a mouse model. J. Antimicrob. Chemother. 2007;60:1341–1346. doi: 10.1093/jac/dkm383. [DOI] [PubMed] [Google Scholar]
- 15.Levy E.R., McCarty J.M., Shane A.L., Weintrub P.S. Treatment of pediatric refractory coccidioidomycosis with combination voriconazole and caspofungin: a retrospective case series. Clin. Infect. Dis. 2013;56:1573–1578. doi: 10.1093/cid/cit113. [DOI] [PubMed] [Google Scholar]