Abstract
Artesunate, an anti-malarial drug, has been repurposed as an anticancer drug due to its induction of cell death via reactive oxygen species (ROS) production. However, the molecular mechanisms regulating cancer cell death and the resistance of cells to artesunate remain unclear. We investigated the molecular mechanisms behind the antitumor effects of artesunate and an approach to overcome artesunate resistance in head and neck cancer (HNC). The effects of artesunate and trigonelline were tested in different HNC cell lines, including three cisplatin-resistant HNC cell lines. The effects of these drugs as well as the inhibition of Keap1, Nrf2, and HO-1 were assessed by cell viability, cell death, glutathione (GSH) and ROS production, protein expression, and mouse tumor xenograft models. Artesunate selectively killed HNC cells but not normal cells. The artesunate sensitivity was relatively low in cisplatin-resistant HNC cells. Artesunate induced ferroptosis in HNC cells by decreasing cellular GSH levels and increasing lipid ROS levels. This effect was blocked by co-incubation with ferrostatin-1 and a trolox pretreatment. Artesunate activated the Nrf2–antioxidant response element (ARE) pathway in HNC cells, which contributed to ferroptosis resistance. The silencing of Keap1, a negative regulator of Nrf2, decreased artesunate sensitivity in HNC cells. Nrf2 genetic silencing or trigonelline reversed the ferroptosis resistance of Keap1-silenced and cisplatin-resistant HNC cells to artesunate in vitro and in vivo. Nrf2–ARE pathway activation contributes to the artesunate resistance of HNC cells, and inhibition of this pathway abolishes ferroptosis-resistant HNC.
Condensed abstract
Our results show the effectiveness and molecular mechanism of artesunate treatment on head and neck cancer (HNC). Artesunate selectively killed HNC cells but not normal cells by inducing an iron-dependent, ROS-accumulated ferroptosis. However, this effect may be suboptimal in some cisplatin-resistant HNCs because of Nrf2–antioxidant response element (ARE) pathway activation. Inhibition of the Nrf2–ARE pathway increased artesunate sensitivity and reversed the ferroptosis resistance in resistant HNC cells.
Abbreviations: HNC, head and neck cancer; GSH, glutathione; TrxR, thioredoxin reductase; Nrf2, nuclear factor erythroid-derived 2-like 2 (NFE2L2); Keap 1, Kelch-like ECH-associated protein 1; ARE, antioxidant response element; HO-1, heme oxygenase 1 (HMOX1); ROS, reactive oxygen species; DCF-DA, 2ʹ,7ʹ-dichlorofluorescein diacetate; MTT, 3-[4]-2,5-diphenyl-2H-tetrazolium bromide; siRNA, short interfering RNA; shRNA, short hairpin RNA
Keywords: Head and neck cancer, Artensunate, Ferroptosis, Nrf2, Reactive oxygen species, Resistance
Graphical abstract
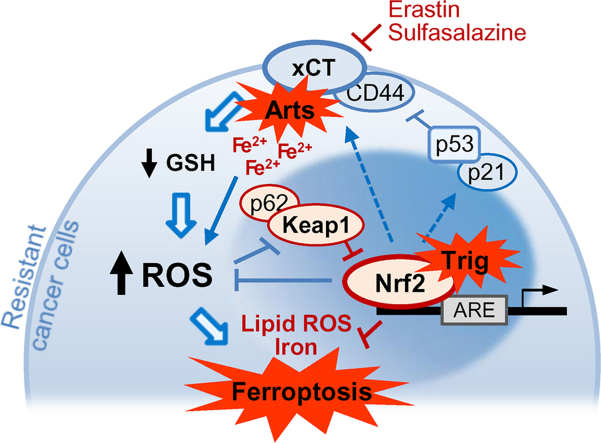
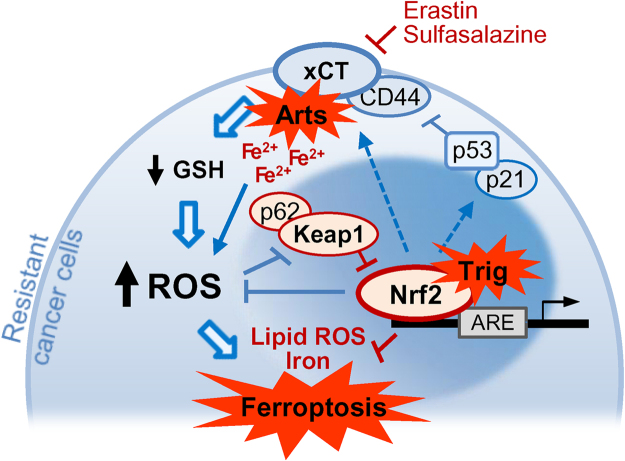
Nrf2 inhibition attenuates artesunate resistance in cisplatin-resistance HNC cells. Artesunate (Arts) selectively kills HNCs but not normal cells via the induction of iron-dependent, ROS-mediated ferroptosis. However, Arts increased Nrf2 expression, which contributed to ferroptosis resistance. Thus, suppression of Nrf2 enhances ferroptosis and causes the death of resistant HNC cells.
Highlights
-
•
Artesunate selectively killed cancer cells by inducing ferroptosis.
-
•
This was suboptimal in some cisplatin-resistant HNC because of Nrf2–ARE pathway activation.
-
•
Keap 1 silencing induced Nrf2 activation and decreased artesunate sensitivity in HNC cells.
-
•
Activation of the Nrf2–ARE pathway contributed to the ferroptosis resistance of HNC cells.
-
•
Nrf2 silencing or trigonelline increased artesunate sensitivity and reversed ferroptosis resistance.
1. Introduction
Head and neck cancer (HNC) is the sixth most common type of cancer worldwide, accounting for an estimated 650,000 new cancer cases and 350,000 cancer deaths every year [1]. Squamous cell carcinoma consists of more than 90% of HNCs that arise in the essential organs responsible of respiration, swallowing, articulation and speech, including the oral/nasal cavity, larynx, and pharynx. HNC is commonly treated with the multimodal approach of surgery, radiotherapy, and systemic chemotherapy. Radiotherapy and chemotherapy are typically used to preserve the morphology and functionality of HNC-affected organs [2], [3], [4]. Cisplatin is used as a first-line chemotherapeutic agent in combination with radiotherapy in an organ preserving protocol for HNC [5]. However, cisplatin is commonly associated with acquired resistance and the toxicity of various organs in clinical settings, which leads to a failure in cancer patient management [6]. Therefore, a new approach to circumventing cisplatin resistance and toxicity is very urgent for the improved treatment of HNC [7]. Recently, the induction of regulated nonapoptotic cell death was presented as a useful strategy to eliminate cancer cells resistant to drug-induced apoptosis [8].
Artesunate is a water-soluble semi-synthetic derivative of artemisinin and a first-line treatment for malaria [9]. Artesunate has been repurposed as an anticancer drug that induces cell death by reactive oxygen species (ROS) production [10], [11], [12], [13]. Artemisinins enhanced anticancer toxicity by utilizing ferrous iron to generate radicals that could kill cancer cells [14], [15]. Artesunate also induces lysosomal ROS-mediated mitochondrial apoptosis and targets the DNA damage and repair systems in conventional chemotherapeutic drug-resistant cancer cells [16], [17], [18], [19], [20]. Furthermore, a recent report suggested that artesunate induced iron-dependent, ROS-producing mitochondrial stress and non-caspase apoptosis [21]. Recently, artesunate was identified as a specific activator of a novel iron-dependent, caspase-independent, nonapoptotic ferroptosis that killed resistant cancer cells with oncogenic KRAS reprogramming [22]. Artesunate has been tested for its anticancer effects on various types of human malignancies but rarely using HNC cells [12], [14], [16], [17], [18], [19], [21], [23], [24], [25].
Ferroptosis is a new form of regulated cell death that is distinct from apoptosis, necroptosis, and autophagic cell death at morphological, biochemical, and genetic levels [26]. In addition, the inhibition of cystine/glutamate antiporter (xCT), a key molecule related to ferroptosis, may induce the eradication of cancer cell resistance to conventional radiotherapy or chemotherapy [27]. Recently, activation of the p62- Keap 1 (Kelch-like ECH-associated protein 1)-Nrf2 (nuclear factor erythroid 2-related factor 2) pathway was shown to determine the therapeutic response to ferroptosis-targeted therapies in cancer cells [28].
Our prior study suggested that cisplatin resistance in HNCs can be overcome by the induction of ferroptosis [29]. In addition, artesunate downregulates RAD51 and increases γH2AX formation, which results in sensitizing ovarian cancer cells to cisplatin [20]. However, the molecular mechanisms regulating cancer cell death and artesunate resistance remain unclear. The resistance of cancer cells to artesunate-induced ferroptosis has been largely unstudied in human cancers, including HNCs. Investigations on this topic will lead to a better molecular and therapeutic understanding of how artesunate overcomes HNC resistance to conventional chemotherapy. Here we investigated the molecular mechanisms of artesunate-based antitumor effects and resistance and identified an approach for overcoming HNC resistance. Activation of the Nrf2-antioxidant response element (ARE) pathway contributed to the ferroptosis resistance of cisplatin-resistant HNC cells and a combined therapy targeting the Nrf2-ARE pathway with artesunate eliminated HNC resistance in vitro and in vivo.
2. Materials and methods
2.1. Cell lines
HNC cell lines (HN2–10) and SNU cell lines were purchased from the Korea Cell Line Bank (Seoul, Republic of Korea) and authenticated by short tandem repeat-based DNA fingerprinting and multiplex polymerase chain reaction (PCR). The cells were cultured in Eagle's minimum essential medium or Roswell Park Memorial Institute (RPMI) 1640 (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum at 37 °C in a humidified atmosphere containing 5% CO2. Normal oral keratinocytes or fibroblasts were obtained from patients undergoing oral surgery and used for in vitro assays. Three cisplatin-resistant HNC cell lines (HN3-cisR, HN4-cisR, and HN9-cisR) were developed from the parental HN3, HN4, and HN9 cells, respectively, by subjecting them to continuous exposure of increasing cisplatin concentrations [30]. The half-maximal inhibitory concentrations (IC50) of cisplatin (Sigma-Aldrich, St. Louis, MO, USA) in the parental (2.2–3.5 µM) and cisplatin-resistant (25.5–38.9 µM) HNC cells were determined using cell viability assays.
2.2. Cell viability assays
Cell viability after exposure to artesunate (1–100 μM, Sigma-Aldrich) and/or trigonelline (100 μM, Sigma-Aldrich) with or without Nrf2 genetic inhibition and activation was assessed using MTT, trypan blue exclusion, and clonogenic assays. For the MTT assay, cells were incubated with the tetrazolium compound MTT (3-[4]-2,5-diphenyl-2H-tetrazolium bromide; Sigma-Aldrich) for 4 h, followed by a solubilization buffer for 2 h. Next, the absorbance was measured at 570 nm using a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The trypan blue exclusion assay was performed with 0.4% trypan blue staining, and cells were counted using a hemocytometer. The clonogenic assay was performed with a 0.5% crystal violet solution and quantification of the number of colonies (>50 cells) after 14 days in culture. A cell death assay was performed using annexin V and propidium iodide (PI) (Sigma-Aldrich) staining. Positively stained cells were counted using flow cytometry and Cell Quest Pro software (BD Biosciences, Franklin Lakes, NJ, USA). All assays were performed with triplicate samples and repeated three times. The combination index (CI) of drug interaction was calculated using the Chou–Talalay method with a CI<1 defined as a synergistic interaction (ComboSyn, Inc., Paramus, NJ, USA) [31].
2.3. Glutathione synthesis and ROS measurement
Cellular GSH levels were measured in the lysates of cells exposed to different drugs for 24 h using a GSH colorimetric detection kit (BioVision Inc., Milpitas, CA, USA). Cellular ROS generation in the supernatant of HNC cell lysates treated for 24 h was measured by adding 10 µM 2ʹ,7ʹ-dichlorofluorescein diacetate (DCF-DA) (cytosolic ROS; Enzo Life Sciences, Farmingdale, NY, USA) or 2 µM C11-BODIPY C11 (lipid peroxidation; Thermo Fisher Scientific) for 30 min at 37 °C. The ROS levels were analyzed using a FACSCalibur flow cytometer equipped with CellQuest Pro (BD Biosciences).
2.4. RNA interference and gene transfection
For the silencing of the KEAP1 gene, cisplatin-sensitive HN9 cells were seeded. For the silencing of NFE2L2 and HMOX1 genes, cisplatin-resistant HN3- and HN9-cisR cells were seeded. Cells were transfected 24 h later with 10 nM small interfering RNA (siRNA) or scrambled control siRNA (Integrated DNA Technologies, Coralville, IA, USA). For stable KEAP1 or NFE2L2 knockdown, the HN9-cisR cells were transfected with a lentiviral vector containing small hairpin RNA (shRNA) directed against KEAP1 or NFE2L2 or control shRNA (Transomic, Huntsville, AL, USA). Cells with a stable transfection were selected using 2 µg/mL puromycin (Sigma-Aldrich). The siRNA or shRNA-induced gene silencing was confirmed using Western blotting and reverse transcription–quantitative PCR (RT–qPCR) from 1 to 2 µg of total RNA from each sample using the SuperScript® III RT-PCR system (Thermo Fisher Scientific).
2.5. Western blotting
Cells were plated, grown to 70% confluence, and then subjected to treatment with the indicated drugs. Cells were lysed at 4 °C in a radioimmunoprecipitation assay (RIPA) lysis buffer (Thermo Fisher Scientific). A total of 50 µg of protein was resolved by SDS-PAGE on 10–12% gels, transferred to nitrocellulose or polyvinylidene difluoride membranes, and probed with primary and secondary antibodies. The following primary antibodies were used: Nrf2, Keap1, NQO1, HO-1 (Abcam, Cambridge, UK), xCT, p53, RAD51, and CD44 (Cell Signaling Technology, Danvers, MA). β-actin (Sigma-Aldrich) was used as a loading control. All of the antibodies were diluted between 1:500 and 1:5000.
2.6. Nrf2 transcriptional activity assay
The transcriptional activity of Nrf2 was assayed using a Cignal Antioxidant Response Reporter kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions.
2.7. Preclinical studies
All animal procedures were approved by the Institutional Animal Care and Use Committee of our institution. Six-week-old athymic BALB/c male nude mice (nu/nu) were purchased from Central Lab Animal Inc. (Seoul, Republic of Korea). HN9 cells (5×106) with or without a control or KEAP1 shRNA transfection were injected subcutaneously into each flank. The mice with palpable nodules after the cell injections underwent a vehicle, artesunate, or trigonelline treatment. After euthanasia, the tumors were extracted and compared among groups. In another set of preclinical experiments, HN9-cisR cells (5×106) with or without a control or NFE2L2 shRNA transfection were injected subcutaneously into the flank of nude mice. Beginning on the day that gross nodules from tumor implants were detected, the mice were subjected to six different treatments: vehicle, artesunate (50 mg/kg daily, oral administration), or trigonelline (50 mg/kg daily, oral administration) with control or NFE2L2 shRNA transfections. After euthanasia, the tumors were isolated and analyzed for cellular GSH measurements and immunofluorescence staining of γH2AX formation. Arbitrary fluorescence units (AFU) were compared between the differently treated tumors. Two-tailed Mann–Whitney U tests were used to compare the statistical differences between the treatment groups.
3. Results
3.1. Artesunate selectively kills cancer cells while preserving normal cells
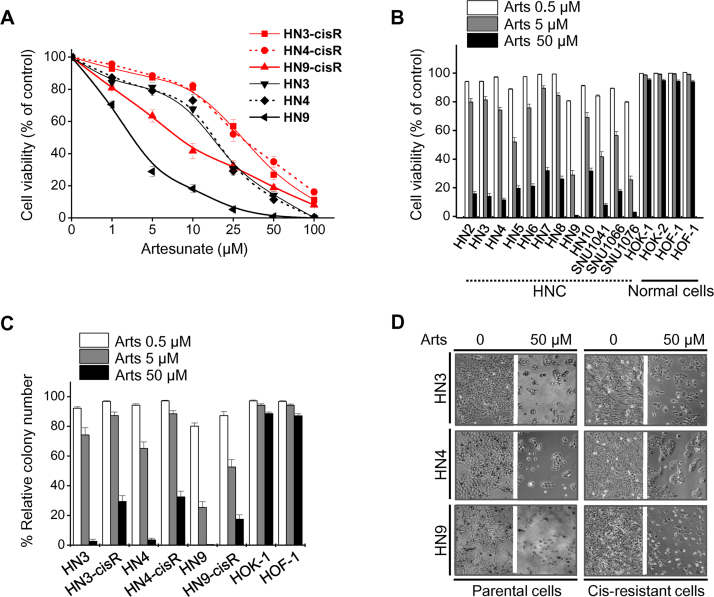
Artesunate decreased the viability of both cisplatin-sensitive (HN3, HN4, and HN9) and cisplatin-resistant (HN3-cisR, HN4-cisR, and HN9-cisR) HNC cells in a dose-dependent manner (Fig. 1A). HN9 was more sensitive to artesunate than HN3 or HN4; however, all cells were killed by 100 μM artesunate. All three cisplatin-resistant HNC cell lines showed relatively lower artesunate sensitivity when compared with their parental cisplatin-sensitive cell lines. The cell viability of HNC cell lines and normal cells were assessed to determine artesunate sensitivity. The viability of most HNC cell lines significantly decreased, while the majority of normal human oral keratinocytes (HOK) and oral fibroblasts (HOF) survived treatment with 50 μM artesunate (Fig. 1B). Colony forming ability was significantly decreased in cisplatin-sensitive HNC cells and preserved in normal cells when compared with cisplatin-resistant HNC cells (Fig. 1C). The representative microphotographs of cell growth inhibition by artesunate are shown in Fig. 1D.
Fig. 1.
Artesunate selectively kills head and neck cancer (HNC) cells but not normal cells. (A, B) Cell viability was assessed after exposure to different concentrations of artesunate (Arts) in normal cells (HOK and HOF) and HNC cells, including cisplatin (cis)-resistant cells (HN3-cisR, HN4-cisR, and HN9-cisR) and their parental cells (HN3, HN4, and HN9). (C) Clonogenic assays of HNC cells and normal cells exposed to different concentrations of Arts were performed in triplicate. (D) Microphotographs show cis-sensitive (parental) and -resistant HNC cells after exposure to vehicle or 50 μM Arts for 72 h.
3.2. Artesunate induces ferroptotic cell death in HNC cells
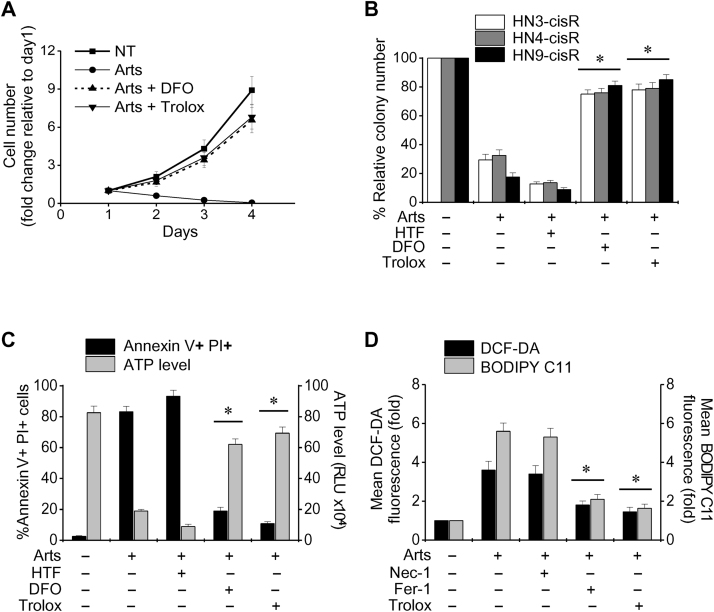
Artesunate significantly decreased the numbers of cells and colonies (all P<0.001) (Fig. 2A and B). The cell viability (from annexin V–PI staining and ATP measurements) was significantly decreased in HNC cells (P<0.001) (Fig. 2C). The viability further decreased with the addition of holo-transferrin (20 μg/mL), whereas this effect was markedly blocked by pretreatment with the iron chelator deferoxamine (100 mM) or the antioxidant trolox (0.5 mM) (P<0.01). Furthermore, the total ROS and lipid ROS levels (from DCF-DA and BODIPY C11 measurements) significantly increased in artesunate-treated HNC cells, and this effect was markedly blocked by the co-incubation of HNC cells with ferrostatin-1 (20 μM) or trolox (0.5 mM) but not necrostatin-1 (20 μM) (Fig. 2D). Taken together, our findings suggested that artesunate induced ferroptotic cell death in HNC cells.
Fig. 2.
Artesunate induces iron-dependent, ROS-mediated ferroptosis in HNC cells. (A) Change in the number of cis-sensitive HN9 cells with treated with 50 μM Arts at different treatment days. The cells received no pretreatment or pretreatment with holo-transferrin (HTF, 20 μg/mL), the iron chelator deferoxamine (DFO, 100 mM) or the antioxidant trolox (0.5 mM). The error bars represent the standard error from three independent experiments each performed in triplicate. (B) Colony forming assay for cis-resistant HNC cells treated with 50 μM Arts for 72 h. (C) Cell death was determined with annexin V and PI staining and flow cytometry. Cell viability was measured by cellular ATP levels in HN9 cells exposed to 50 μM Arts for 72 h. The cells were pretreated with HTF, DFO, or trolox. (D) Elevation of total ROS (DCF-DA) and lipid (BODIPY C11) ROS in HN9 cells exposed to 50 μM Arts for 24 h with or without necrostatin-1 (Nec-1, 20 μM), ferrostatin-1 (Fer-1, 20 μM), and trolox (0.5 mM). The error bars represent the standard error from three replicates. *P<0.01 relative to Arts alone.
3.3. Nrf2 activation contributes to the resistance of HNCs to artesunate-induced ferroptosis
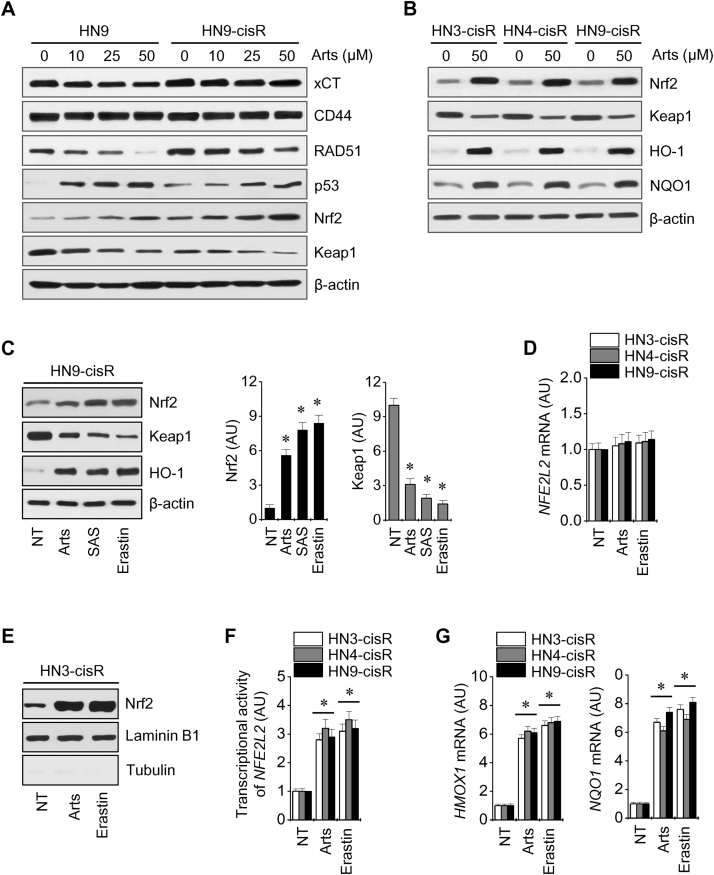
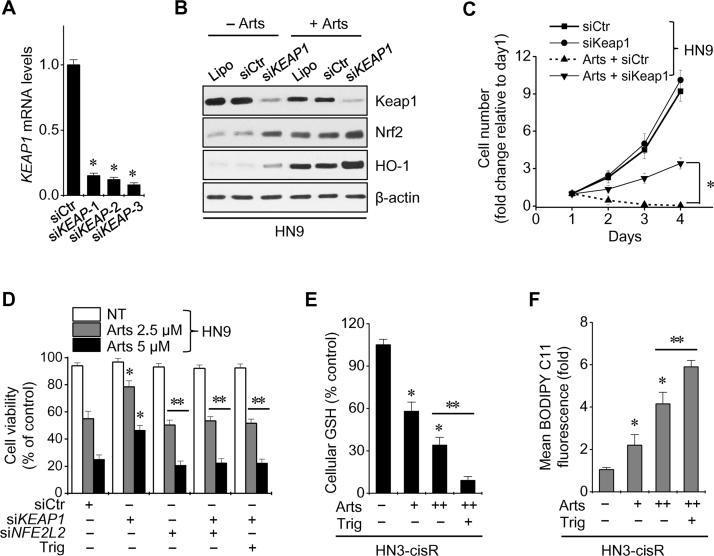
Artesunate decreased the levels of xCT, RAD51, and Keap1 proteins, but increased the levels of p53 and Nrf2 proteins in HN9 and HN9-cisR cells (Fig. 3A). Basal Nrf2 levels were higher in cisplatin-resistant HNC cells when compared with cisplatin-sensitive cells, and Nrf2, its ARE, HO-1, and NQO1 were activated in HNC cells after exposure to artesunate (Fig. 3B). Artesunate activated Nrf2 by inhibiting Keap1, as shown by the ferroptosis inducers erastin and sulfasalazine (Fig. 3C). However, the Nrf2 mRNA levels were unchanged in three cisplatin-resistant HNC cells exposed to artesunate or erastin (Fig. 3D). The expression of Nrf2 in the nuclear extracts of HNC cells treated with artesunate or erastin was markedly increased (Fig. 3E). Artesunate and erastin also induced the transcriptional activity of the Nrf2 promoter-luciferase reporter, ARE, HO-1, and NQO1 (Fig. 3F and G). The silencing of the KEAP1 gene activated the Nrf2-ARE pathway and subsequently increased the number of artesunate-treated sensitive HN9 cells (Fig. 4A–C). The decreased sensitivity of Keap1-silenced HN9 cells to artesunate was reversed by a combined treatment including Nrf2 silencing or use of the Nrf2 inhibitor trigonelline (100 μM) (Fig. 4D). Artesunate significantly decreased cellular glutathione (GSH) levels and caused lipid ROS accumulation, and this effect was significantly augmented by the co-incubation of trigonelline in HN3-cisR cells (P<0.05) (Fig. 4E and F).
Fig. 3.
Artesunate increases Nrf2 expression levels during ferroptosis in HNC cells. (A, B) Western blot analyses of cis-sensitive HN9 and three cis-resistant HNC cells exposed to Arts for 24 h. β-actin was used as a loading control. (C) Change in Nrf2, Keap1, and HO-1 protein levels after exposure to dimethyl sulfoxide as a control (NT), 50 μM Arts, 20 μM erastin, or 2 mM sulfasalazine (SAS) in HN9-cisR cells. *P<0.01 relative to control. (D) Nrf2 mRNA levels were assessed in three types of cisplatin-resistant HNC cells exposed to 50 μM Arts or 20 μM erastin for 24 h. (E, F) Expression of Nrf2 in nuclear extracts and the transcriptional activity of Nrf2 in HN3-cisR cells treated with 50 μM Arts or 20 μM erastin for 24 h. (G) Changes in HO-1 and NQO1 mRNA levels in cis-resistant HNC cells exposed to 50 μM Arts or 20 μM erastin for 24 h. The error bars represent the standard error from three replicates. * P<0.01 relative to NT control.
Fig. 4.
Nrf2 activation contributes to the resistance of HNC ferroptosis. (A-D) Cis-sensitive HN9 cells were transfected with siRNA control (siCtr), siKeap1, or siNFE2L2. The mRNA (A), proteins (B), cell numbers (C), and cell viability (D) were measured after transfection with or without Arts. (E, F) Cellular GSH and lipid ROS levels were measured in HN3-cisR cells exposed to 25 or 50 μM Arts with or without trigonelline (Trig; 100 μM). The error bars represent the standard error from three replicates. *P<0.05 relative to control; *P<0.01 between the groups.
3.4. Inhibition of the Nrf2-ARE pathway reversed resistance to artesunate-induced ferroptosis
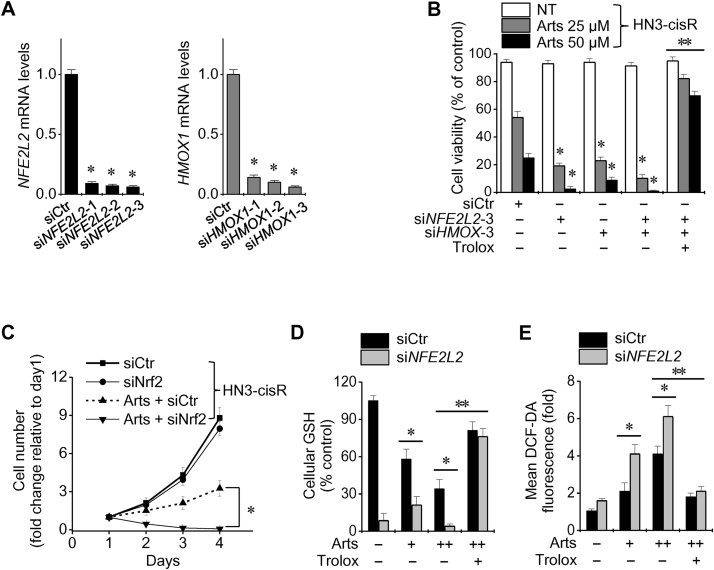
Silencing of Nrf2 and/or HO-1 genes decreased the cell viability of cisplatin-resistant HNC cells more than siRNA control cells after exposure to artesunate (Fig. 5A and B). In HN3-cisR cells transfected with siNrf2, the cell viability and cellular GSH level significantly decreased and the cellular ROS levels significantly increased when compared with the siRNA control. These effects were blocked by pretreatment with the antioxidant trolox (P<0.01) (Fig. 5C–E).
Fig. 5.
Nrf2 inhibition reversed the resistance to artesunate. (A, B) Silencing of Nrf2 and HO-1 genes was examined using an siRNA control (siCtr) and siRNA against NFE2L2 and HMOX1. *P<0.01 relative to siCtr, **P<0.01 relative to siNFE2L2 transfection. (C–E) Changes in cell number, cellular GSH, and total ROS levels of HN3-cisR cells exposed to 25 or 50 μM Arts, trolox (0.5 mM) or the combinations. *P<0.05 and **P<0.01 compared between groups.
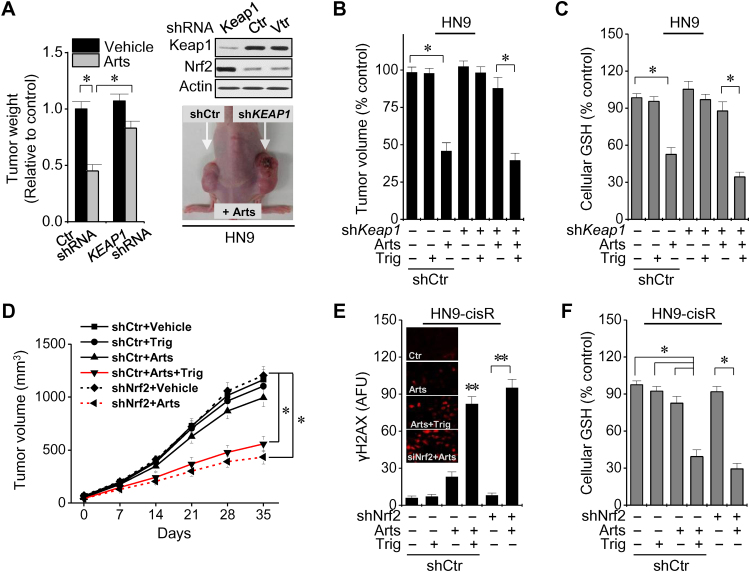
The in vivo effects of Nrf2 genetic or pharmacological silencing in HNC cells were examined. Artesunate-induced inhibition of tumor growth was reduced in nude mice treated with Keap1 shRNA-transfected HN9 cells when compared with shRNA control mice (Fig. 6A). However, co-treatment with trigonelline significantly decreased in vivo tumor growth and cellular GSH levels in mice that received Keap1-silenced, Nrf2-activated HN9 cells (Fig. 6B and C). The tumor volumes were markedly lower in nude mice treated with shNrf2-transfected HN9-cisR cells or trigonelline (Fig. 6D). Cellular GSH levels greatly deceased and γH2AX formation greatly increased in the tumors of mice co-treated with artesunate and shNrf2 transfection or trigonelline when compared with shRNA control mice or mice treated with artesunate or trigonelline alone (P<0.05) (Fig. 6E and F). Therefore, artesunate induced artesunate sensitivity in resistant HNC cells in vivo via γH2AX formation and GSH depletion.
Fig. 6.
Nrf2 genetic or pharmacological silencing sensitizes resistant HNC cells to Arts. (A) Arts sensitivity was increased by Nrf2 genetic inhibition in HN9 cells after Keap1 shRNA transection in nude mice. *P<0.05 versus the shRNA control. (B, C) Measurement of tumor growth and cellular GSH levels of tumors harvested in nude mice implanted with HN9 cells with control or Keap1 shRNA transfection. *P<0.05 compared between two groups. (D) In vivo growth of HN3-cisR cells with control or Nrf2 shRNA and the daily oral administration of 50 mg/kg Arts or trigonelline (Trig). (E, F) Immunofluorescence staining of γH2AX formation (red color) and cellular GSH measurement in control or Nrf2 shRNA-transfected HN9-cisR tumors after treatment with Arts or Trig. Arbitrary fluorescence units (AFU) were compared between groups. The error bars represent the standard error. *P<0.05 compared between two groups; ** P<0.01 relative to control or compared between two groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The present study examined if artesunate effectively eliminated HNCs in vitro and in vivo. Artesunate selectively killed HNC cells by inducing an iron-dependent, ROS-accumulated ferroptosis. However, this may be suboptimal in some cisplatin-resistant HNCs because of activation of the Nrf2–ARE pathway. Silencing of Keap1, a negative regulator of Nrf2, induced activation of Nrf2-ARE pathway and decreased artesunate sensitivity in HNC cells, thus producing ferroptosis-resistant HNC cells. Nrf2 genetic silencing or trigonelline reversed the ferroptosis resistance of Keap1-silenced and cisplatin-resistant HNC cells. Therefore, treatment with artesunate in combination with the targeting of the Nrf2-ARE pathway effectively eliminated resistant HNC cells in vitro and in vivo. This study is the first to report the molecular mechanisms of artesunate-induced HNC cell death and resistance as well as an effective method to overcome ferroptosis resistance.
Artesunate is an anti-malarial agent used in preference to quinine as the first-line treatment for severe malaria in endemic areas, e.g., Plasmodium falciparum malaria [32]. Artesunate is a semi-synthetic, water-soluble derivative of artemisinins, a group of drugs discovered in the 1970s by Tu Youyou, one of the 2015 Nobel Prize winners in Physiology or Medicine [33]. Artemisinin was isolated from qinghao (Artemisia annua L or sweet wormwood) and used as an herbal medicine in Chinese traditional medicine for at least 2000 years [34]. The death of parasites is caused by the activation of heme and iron oxide, which resulting in the generation of free radicals that damage susceptible proteins [35].
Early research assessed the anticancer effects of artemisinin and its derivatives in hepatocellular carcinoma and other cancer types [36]. ROS is released when the peroxide lactone group in the drug structure contacts the high iron concentrations common in cancer cells. Thus, mitochondrial apoptosis occurs from the iron-dependent generation of cytotoxic oxidative stress [14], [15], [16], [17]. Artesunate also induces oxidative DNA damage, sustained DNA double-strand breaks, and the damage response in cancer cells [18], [19], [20]. These effects were clearly observed in cisplatin-sensitive and -resistant HNC cells in our study. However, the pattern of cell death induced by artesunate appeared to be a novel form of regulated cell death, i.e., ferroptosis, based on its iron-dependence, ROS production, and caspase-independent cell death. Recent two reports also identified artesunate as a specific activator of ferroptosis, thus highlighting its potential to overcoming the resistance of cancer cells [21], [22].
The current study showed that artesunate induced ferroptosis in HNC cells via cellular glutathione depletion and ROS accumulation. The effects were abrogated by enhanced cellular iron chelation and ROS scavenging. Iron is an essential dietary nutrient that has increased concentrations in cancer cells to support cell growth. Noncancer cells are more susceptible to iron addition than iron depletion [37]. Iron cycles between oxidized and reduced forms, thus contributing to free radical formation and the modulation of cell death with ROS [38]. Ferroptosis is a recently characterized form of iron-dependent, nonapoptotic cell death with distinct morphological and mechanistic characteristics [26]. Ferroptosis causes cell death through the iron-mediated accumulation of lipid ROS and interference of cellular integrity and membrane fluidity and permeability [38]. Our work as well as previous observations identified artesunate as a specific inducer of ferroptosis in cancer cells with distinct cell death patterns, namely glutathione depletion and lipid ROS accumulation. Similar to reports on erastin and sulfasalazine, artesunate-based effects may the result of the activation of ferrous iron and blocking cystine-glutamate transporters [26]. However, our study further showed that artesunate sensitivity decreased in some cisplatin-resistant HNCs because of Nrf2–ARE pathway activation. In fact, this pathway was also shown to decrease artesunate sensitivity in sensitive HNC cells, thus contributing to the ferroptosis resistance of HNC cells.
Nrf2 is the central player for the regulation of antioxidant molecules in cells [39]. Indeed, Nrf2 is an oncogenic transcriptional factor that plays a key role against environmental or intracellular stress and controls the abundant cellular antioxidant systems responsible for GSH production in cancer cells [40]. Cancer cells frequently show Nrf2 overexpression, which is linked to increased resistance to anticancer therapies and poor survival outcomes in cancer patients [41]. Because Nrf2 is constantly degraded by the proteasomal activity of Keap1, the inhibition of Keap1 can activate the Nrf2-ARE pathway upon oxidative and electrophilic stresses [42]. The sensitivity of cancer cells to ferroptosis-targeted therapies is determined by the activation of p62-Keap1-Nrf2 antioxidant signaling pathway. This pathway is a key negative regulator of ferroptosis in hepatocellular carcinoma cells [28]. Inhibition of p62, the ARE of HO-1, NQO1, and ferritin heavy chain-1 significantly enhanced the antitumor activity of ferroptosis inducers, e.g., erastin, sulfasalazine, and sorafenib, in hepatoma cells [28]. P62 knockdown promotes the accumulation of Keap1 protein and enhanced Keap1-mediated Nrf2 degradation, suggesting that the interaction p62-Keap1 is responsible for Nrf2 expression during ferroptosis [28]. In the present study, the ferroptosis induced by artesunate was suboptimal in some resistant HNC cells because of activation of the Nrf2–ARE pathway. The cisplatin-resistant cancer cell lines showed less susceptibility to artesunate-induced ferroptotic cell death. Furthermore, genetic inhibition of Nrf2 and/or HO-1 or treatment with trigonelline enhanced growth suppression, ROS accumulation, and ferroptotic cell death when co-administered with artesunate. The inhibition of Nrf2 combined with ferroptosis inducers effectively killed resistant HNC cells (Fig. 7).
Fig. 7.
Nrf2 inhibition attenuates artesunate resistance in cis-resistance HNC cells. Artesunate (Arts) selectively kills HNCs but not normal cells via the induction of iron-dependent, ROS-mediated ferroptosis. However, Arts increased Nrf2 expression, which contributed to ferroptosis resistance. Thus, suppression of Nrf2 enhances ferroptosis and causes the death of resistant HNC cells.
Artesunate has been successfully repurposed as an anticancer drug for various cancer types [10], [11], [12], [13]. The role of artesunate as an effective anticancer drug is under active examination in phase I–II clinical trials [43], [44]. The selective killing of cancer cells but not normal cells may be the major advantage of artesunate, which is already known to be generally safe and well tolerated, even in the fetus during first-trimester pregnancies [45]. However, Nrf2 inhibitory therapy combined with artesunate will be cautiously examined in the preclinical setting because Nrf2 has a protective effect against oxidative damage in normal tissue [46], [47]. Our study will lead to a better molecular and therapeutic understanding of ferroptosis and artesunate, which may lead to the elimination of resistant cancer cells, e.g., cisplatin-resistant HNC cells.
5. Conclusion
Our study showed that artesunate selectively killed HNC cells by inducing a novel form of iron-dependent, lipid ROS-accumulated ferroptosis. However, ferroptotic cell death by artesunate may be suboptimal in some cisplatin-resistant HNCs because of activation of the Nrf2–ARE antioxidant signaling pathway. Inhibition of the Nrf2–ARE pathway increased artesunate sensitivity and reversed the ferroptosis resistance in HNC cells. Additional in vivo and clinical investigations should be conducted to explore this promising artesunate-based combination cancer therapy for patients with resistant cancer types.
Author contributions
Jong-Lyel Roh and Eun Hye Kim conceived and designed the experiments. Jong-Lyel Roh, Eun Hye Kim, Hyejin Jang, and Daiha Shin performed the experiments. Jong-Lyel Roh and Eun Hye Kim analyzed the data. Jong-Lyel Roh and Eun Hye Kim contributed reagents/materials/analysis tools; Jong-Lyel Roh and Eun Hye Kim wrote the draft, and checked and revised. All authors approved to submit this version to this publication.
Fundings
This study was supported by a Grant (no. 2015R1A2A1A15054540) from the Basic Science Research Program through the National Research Foundation of Korea (NRF), Ministry of Science, ICT, and Future Planning and a Grant (no. HI15C2920) from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), Ministry of Health & Welfare, Seoul, Republic of Korea (J.L. Roh).
Conflict of interest statement
The authors declare no conflicts of interest.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012, CA. Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Haddad R.I., Shin D.M. Recent advances in head and neck cancer. N. Engl. J. Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 3.Kong M., Hong S.E. Tumor regression patterns based on follow-up duration in patients with head and neck squamous cell carcinoma treated with radiotherapy or chemoradiotherapy. Clin. Exp. Otorhinolaryngol. 2015;8:416–421. doi: 10.3342/ceo.2015.8.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon S.H., Choi J.Y., Lee H.J., Son Y.I., Baek C.H., Ahn Y.C., Ahn M.J., Park K., Kim B.T. Prognostic value of volume-based positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma treated with concurrent chemoradiotherapy. Clin. Exp. Otorhinolaryngol. 2015;8:142–148. doi: 10.3342/ceo.2015.8.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrelli F., Coinu A., Riboldi V., Borgonovo K., Ghilardi M., Cabiddu M., Lonati V., Sarti E., Barni S. Concomitant platinum-based chemotherapy or cetuximab with radiotherapy for locally advanced head and neck cancer: a systematic review and meta-analysis of published studies. Oral. Oncol. 2014;50:1041–1048. doi: 10.1016/j.oraloncology.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L., Senovilla L., Vitale I., Michels J., Martins I., Kepp O., Castedo M., Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Conrad M., Angeli J.P., Vandenabeele P., Stockwell B.R. Regulated necrosis: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2016;15:348–366. doi: 10.1038/nrd.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dondorp A., Nosten F., Stepniewska K., Day N., White N. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 10.Vandewynckel Y.P., Laukens D., Geerts A., Vanhove C., Descamps B., Colle I., Devisscher L., Bogaerts E., Paridaens A., Verhelst X., Van Steenkiste C., Libbrecht L., Lambrecht B.N., Janssens S., Van Vlierberghe H. Therapeutic effects of artesunate in hepatocellular carcinoma: repurposing an ancient antimalarial agent. Eur. J. Gastroenterol. Hepatol. 2014;26:861–870. doi: 10.1097/MEG.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 11.Jeong da E., Song H.J., Lim S., Lee S.J., Lim J.E., Nam D.H., Joo K.M., Jeong B.C., Jeon S.S., Choi H.Y., Lee H.W. Repurposing the anti-malarial drug artesunate as a novel therapeutic agent for metastatic renal cell carcinoma due to its attenuation of tumor growth, metastasis, and angiogenesis. Oncotarget. 2015;6:33046–33064. doi: 10.18632/oncotarget.5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kast R.E., Boockvar J.A., Bruning A., Cappello F., Chang W.W., Cvek B., Dou Q.P., Duenas-Gonzalez A., Efferth T., Focosi D., Ghaffari S.H., Karpel-Massler G., Ketola K., Khoshnevisan A., Keizman D., Magne N., Marosi C., McDonald K., Munoz M., Paranjpe A., Pourgholami M.H., Sardi I., Sella A., Srivenugopal K.S., Tuccori M., Wang W., Wirtz C.R., Halatsch M.E. A conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma care. Oncotarget. 2013;4:502–530. doi: 10.18632/oncotarget.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augustin Y., Krishna S., Kumar D., Pantziarka P. The wisdom of crowds and the repurposing of artesunate as an anticancer drug. Ecancermedicalscience. 2015;9:50. doi: 10.3332/ecancer.2015.ed50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efferth T., Benakis A., Romero M.R., Tomicic M., Rauh R., Steinbach D., Hafer R., Stamminger T., Oesch F., Kaina B., Marschall M. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic. Biol. Med. 2004;37:998–1009. doi: 10.1016/j.freeradbiomed.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Ji Y., Chen Q., Jiao X., Hou L., Zhu X., Zhang Z. Enhancement of cytotoxicity of artemisinin toward cancer cells by transferrin-mediated carbon nanotubes nanoparticles. J. Drug Target. 2015;23:552–567. doi: 10.3109/1061186X.2015.1016437. [DOI] [PubMed] [Google Scholar]
- 16.Efferth T., Giaisi M., Merling A., Krammer P.H., Li-Weber M. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS One. 2007;2:e693. doi: 10.1371/journal.pone.0000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamacher-Brady A., Stein H.A., Turschner S., Toegel I., Mora R., Jennewein N., Efferth T., Eils R., Brady N.R. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J. Biol. Chem. 2011;286:6587–6601. doi: 10.1074/jbc.M110.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P.C., Lam E., Roos W.P., Zdzienicka M.Z., Kaina B., Efferth T. Artesunate derived from traditional Chinese medicine induces DNA damage and repair. Cancer Res. 2008;68:4347–4351. doi: 10.1158/0008-5472.CAN-07-2970. [DOI] [PubMed] [Google Scholar]
- 19.Berdelle N., Nikolova T., Quiros S., Efferth T., Kaina B. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol. Cancer Ther. 2011;10:2224–2233. doi: 10.1158/1535-7163.MCT-11-0534. [DOI] [PubMed] [Google Scholar]
- 20.Wang B., Hou D., Liu Q., Wu T., Guo H., Zhang X., Zou Y., Liu Z., Liu J., Wei J., Gong Y., Shao C. Artesunate sensitizes ovarian cancer cells to cisplatin by downregulating RAD51. Cancer Biol. Ther. 2015;16:1548–1556. doi: 10.1080/15384047.2015.1071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papanikolaou X., Johnson S., Garg T., Tian E., Tytarenko R., Zhang Q., Stein C., Barlogie B., Epstein J., Heuck C. Artesunate overcomes drug resistance in multiple myeloma by inducing mitochondrial stress and non-caspase apoptosis. Oncotarget. 2014;5:4118–4128. doi: 10.18632/oncotarget.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eling N., Reuter L., Hazin J., Hamacher-Brady A., Brady N.R. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2:517–532. doi: 10.18632/oncoscience.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J., Zhu W., Tang Y., Cao H., Zhou Y., Ji R., Zhou X., Lu Z., Yang H., Zhang S., Cao J. Artemisinin derivative artesunate induces radiosensitivity in cervical cancer cells in vitro and in vivo. Radiat. Oncol. 2014;9:84. doi: 10.1186/1748-717X-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beccafico S., Morozzi G., Marchetti M.C., Riccardi C., Sidoni A., Donato R., Sorci G. Artesunate induces ROS- and p38 MAPK-mediated apoptosis and counteracts tumor growth in vivo in embryonal rhabdomyosarcoma cells. Carcinogenesis. 2015;36:1071–1083. doi: 10.1093/carcin/bgv098. [DOI] [PubMed] [Google Scholar]
- 25.Greenshields A.L., Shepherd T.G., Hoskin D.W. Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol. Carcinog. 2016 doi: 10.1002/mc.22474. [DOI] [PubMed] [Google Scholar]
- 26.Yang W.S., Stockwell B.R. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., Kang R., Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roh J.L., Kim E.H., Jang H.J., Park J.Y., Shin D. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett. 2016;381:96–103. doi: 10.1016/j.canlet.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura M., Nakatani K., Uzawa K., Ono K., Uesugi H., Ogawara K., Shiiba M., Bukawa H., Yokoe H., Wada T., Fujita S., Tanzawa H. Establishment and characterization of a cisplatin-resistant oral squamous cell carcinoma cell line, H-1R. Oncol. Rep. 2005;14:1281–1286. [PubMed] [Google Scholar]
- 31.Chou T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization . second edition. World Health Organization; Geneva, Switzerland: 2010. (Gulideline for the Treatment of Malaria). [Google Scholar]
- 33.The Nobel Prize in Physiology or Medicine . The Nobel Assembly at Karolinska Institutet; Solna, Sweden: 2015. Nobel Foundation; p. 2015. [Google Scholar]
- 34.Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011;17:1217–1220. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- 35.Winzeler E.A., Manary M.J. Drug resistance genomics of the antimalarial drug artemisinin. Genome Biol. 2014;15:544. doi: 10.1186/s13059-014-0544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou J., Wang D., Zhang R., Wang H. Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin. Cancer Res. 2008;14:5519–5530. doi: 10.1158/1078-0432.CCR-08-0197. [DOI] [PubMed] [Google Scholar]
- 37.Manz D.H., Blanchette N.L., Paul B.T., Torti F.M., Torti S.V. Iron and cancer: recent insights. Ann. N. Y Acad. Sci. 2016;1368:149–161. doi: 10.1111/nyas.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 39.Sporn M.B., Liby K.T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 41.Wang X.J., Sun Z., Villeneuve N.F., Zhang S., Zhao F., Li Y., Chen W., Yi X., Zheng W., Wondrak G.T., Wong P.K., Zhang D.D. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi A., Kang M.I., Watai Y., Tong K.I., Shibata T., Uchida K., Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ericsson T., Blank A., von Hagens C., Ashton M., Abelo A. Population pharmacokinetics of artesunate and dihydroartemisinin during long-term oral administration of artesunate to patients with metastatic breast cancer. Eur. J. Clin. Pharm. 2014;70:1453–1463. doi: 10.1007/s00228-014-1754-2. [DOI] [PubMed] [Google Scholar]
- 44.Krishna S., Ganapathi S., Ster I.C., Saeed M.E., Cowan M., Finlayson C., Kovacsevics H., Jansen H., Kremsner P.G., Efferth T., Kumar D., Randomised A. Double Blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EBioMedicine. 2015;2:82–90. doi: 10.1016/j.ebiom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark R.L. Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester. Reprod. Toxicol. 2009;28:285–296. doi: 10.1016/j.reprotox.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Ho W.E., Cheng C., Peh H.Y., Xu F., Tannenbaum S.R., Ong C.N., Wong W.S. Anti-malarial drug artesunate ameliorates oxidative lung damage in experimental allergic asthma. Free Radic. Biol. Med. 2012;53:498–507. doi: 10.1016/j.freeradbiomed.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 47.Cao T.H., Jin S.G., Fei D.S., Kang K., Jiang L., Lian Z.Y., Pan S.H., Zhao M.R., Zhao M.Y. Artesunate protects against sepsis-induced lung injury via heme Oxygenase-1 modulation. Inflammation. 2016;39:651–662. doi: 10.1007/s10753-015-0290-2. [DOI] [PubMed] [Google Scholar]