Abstract
Verruconis gallopava is an uncommon cause of phaeohyphomycosis. We describe an unusual case of disseminated V. gallopava infection in a renal transplant recipient involving the endocardium but without endocarditis, associated with fungaemia and infection in the skin, oral cavity, brain and lung. The isolate was first detected from blood cultures which is rare. Surgical resection of cardiac fungal mass was not possible. The patient died despite resolution of fungaemia and combination antifungal therapy.
Keywords: Verruconis gallopava, Endocarditis, Dermatiaceous moulds, Antifungal therapy
1. Introduction
Dematiaceous moulds are increasingly recognised as human pathogens typically in immunocompromised hosts, but also occur in others including trauma patients [1], [2]. Verruconis (previously Ochroconis) gallopava is a thermotolerant environmental dematiaceous fungus is an emergent pathogen [2], [3]. Most descriptions of V. gallopava infection have been in the form of case reports [4], [6], although two small case series in solid organ transplant recipients are reported with mixed patient outcomes [3], [6]. Other patients at high risk of infection include those with haematological malignancies [2], [4].
Infection is likely acquired by inhalation of environmental fungal spores, with the lung the commonest site of infection [1], [7]. However, clinical manifestations are protean given the potential for dissemination to other organs [1], [3], [6]. The optimal antifungal treatment regimen and duration of therapy is uncertain and reliant on expert opinion [8].
Herein, we describe a case of fatal V. gallopava infection in an immunosuppressed renal transplant recipient, diagnosed after isolation of the fungus in blood culture. The case is notable for its substantive involvement of the cardiac ventricular wall and dissemination to the brain, lungs and skin. It is also instructive for its first manifestation as apparently indolent disease of the oral cavity, and underscores the importance of source control by surgical resection of fungal mass lesions for patient survival.
2. Case
A 67-year old Egyptian born lady was admitted to our hospital (day 0) with an 18-d history of increasing confusion and difficulty walking. Approximately 70 days after multiple tooth extraction and restoration procedures at another facility (day −18), she developed right nasolabial swelling overlying a known periodontal apical cyst from a recently restored tooth at position 1–3. She was afebrile. Neurological examination was normal except for minimal disorientation. An pre-existing ejection systolic murmur in the cardiac apical region consistent with aortic sclerosis was present. No pathogens were isolated from blood cultures.
An orthopantomogram showed lucency of the apex of the root of the tooth at position 1–3, suspicious for infection. This abnormality was likewise noted on a cerebral computed tomography (CT) scan which also showed a hypodensity in the left frontal lobe, representative of an old cerebral infarct. Intravenous ceftriaxone and metronidazole were administered for 3 days for a presumed dental abscess followed by oral amoxicillin-clavulanate for 14 days (ending at day −3) with apparent clinical response. Follow up surgical review determined that the tooth was not infected but she was re-hospitalised 3 days later (day 0) for ongoing confusion.
The patient's medical history was significant for deceased donor renal transplantion 18 months prior to this illness for end stage kidney disease from hypertensive nephrosclerosis. Her medications at presentation included azathioprine 150 mg daily, tacrolimus 1 mg bd, prednisolone 10 mg daily and trimethoprim-sulfamethoxazole prophylaxis against Pneumocystis jiroveci infection.
On admission (day 0), her temperature was 37.5 °C. She was confused to time, however detailed questioning from her family revealed since the dental the dental procedures she was now unable to perform routine simple household chores. She displayed increased difficulty in walking and was requiring a full arm support frame. Neurological examination including fundoscopy by an ophthalmologist revealed no focal abnormalities. Laboratory investigations revealed a serum creatinine of 240 μmol/L (baseline 180 μmol/L), serum albumin of 25 mg/L, white cell count of 10.4×109/L and CRP of 121 mg/L.
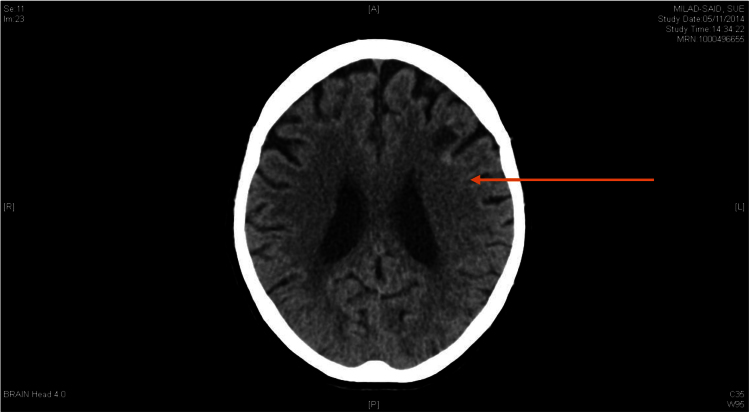
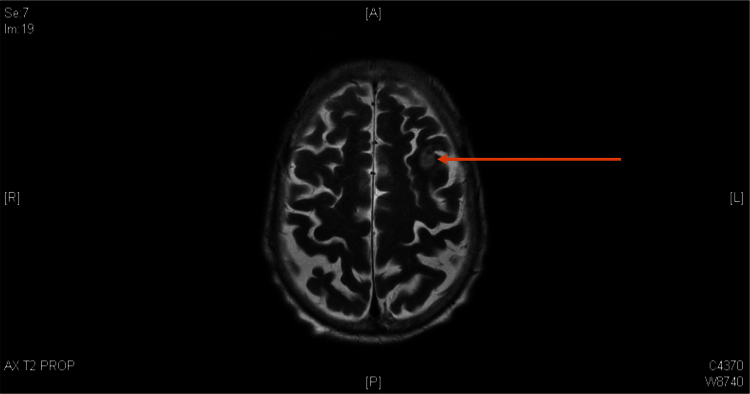
Contrast-enhanced cerebral CT scan showed that the previous frontal lobe lesion had reduced in size however new foci of high density lining the cortex of the left frontal lobe were noted (Fig. 1). On day +2, due to ongoing confusion, a lumbar puncture was performed. Cerebrospinal fluid analysis included glucose 4.2 mmol/L, protein 0.46 g/L, white blood cells 0×10^6/L; no organisms were seen on Gram stain or cultured and the cryptococcal antigen test was negative. Magnetic resonance imaging (MRI) of the brain showed a hyperintense T2 signal with restricted diffusion in the left frontal lobe, consistent with recent infarction associated with hemosiderin staining (Fig. 2).
Fig. 1.
: CT Brain Day 0 with arrow indicating hypodense frontal lesion (resolving) and rim of high density lining the cortex of the more superficial part of the left frontal lobe.
Fig. 2.
: MRI Brain Day +2 T2 weighted image with arrow indicating hyperintense T2 signal with corresponding restricted diffusion in the left frontal lobe likely an area of recent infarction with area of hemosiderin staining.
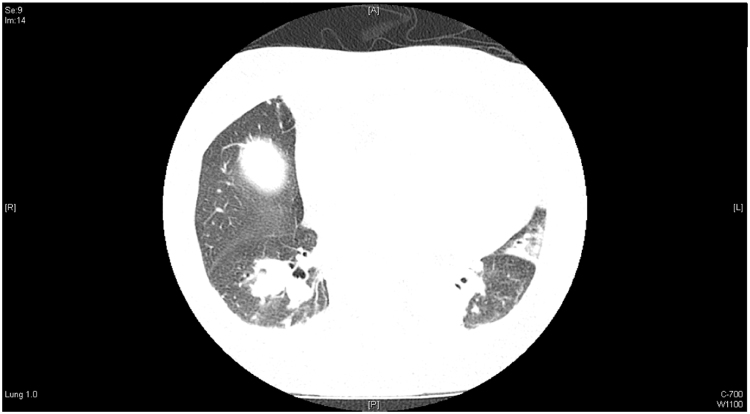
Blood cultures collected on admission flagged positive on day +4 and Gram stain revealed long septate hyphae. At this time, the patient became increasingly drowsy (Glasgow coma scale 10), with generalised hyperreflexia, and multiple small haemorrhagic vesicles on her thighs. Liposomal amphotericin B (L-AMB) 5 mg/kg every second day, voriconazole (200 mg twice daily) and terbinafine 250 mg daily were commenced. A chest CT scan showed a 3.0 cm diameter lesion with cavitation in the right lower lobe not present 8 months prior (Fig. 3).
Fig. 3.
: CT chest demonstrating right lower lobe 30 mm nodule.
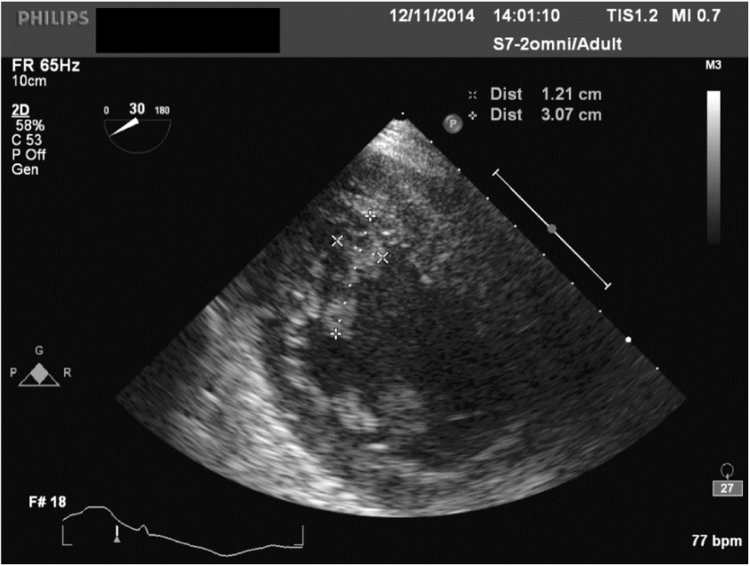
Transthoracic echocardiogram on day +6 demonstrated a 3.0 cm diameter mobile mass adherent to the left ventricular wall, confirmed as a 3.5×1.1 cm ‘filamentous’ lesion by transoesophageal echocardiogram (TOE) (Fig. 4). No vegetations were observed on the cardiac valves, and myocardial contractility was normal. A diagnosis of fungal endocardial disease, with dissemination to the skin and lungs was made. Despite antifungal therapy, hypotension and renal failure developed on day +8, prompting substitution of L-AMB with anidulafungin 100 mg daily.
Fig. 4.
: Echocardiogram with crosses showing dimensions of left ventricular wall mass.
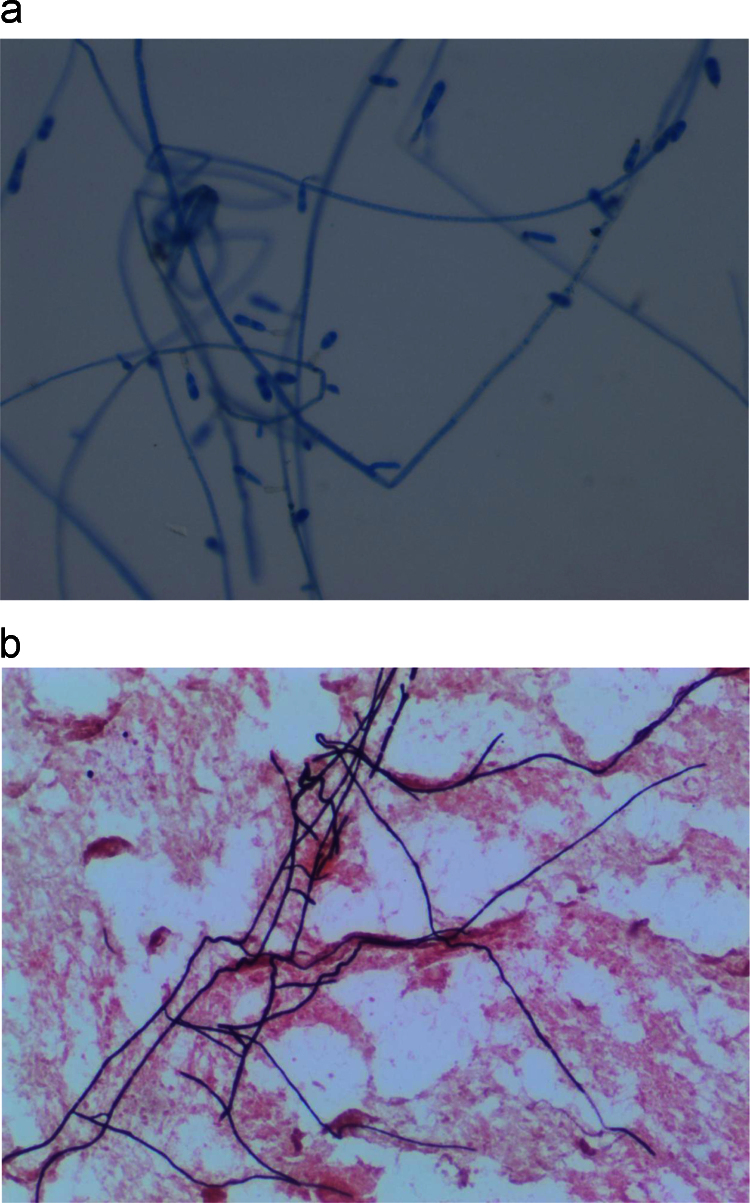
The blood culture grew a fungus which manifest as brownish black colonies with a dark reddish brown diffusible pigment on Sabouraud's Dextrose Agar. Microscopic examination showed cylindrical conidiophores bearing two-celled smooth walled conidia at the tip (Fig. 5). The isolate was identified as V. gallopava on day +9. V. gallopava was also isolated from skin biopsy and dental tissue specimens collected on days +9 and +12, respectively. The morphological identification was confirmed by DNA sequence analysis of the internal transcribed spacer (ITS) region of the ribosomal DNA (rDNA) gene using published primers and standard sequencing methodologies [9], resulting in a 648 base pair amplified fragment. The sequence of the isolate had 100% sequence similarity to those of archived V. gallopava sequences in the GenBank database (Accession numbers: AB272161; AB125284; AB125283; AB125282; AB125280; AB125281; HQ667551; HQ667559; HQ667555). Antifungal susceptibility testing was performed using the Sensititre® YeastOne® YO10 (TREK Diagnostic Systems, Cleveland, OH); drug minimum inhibitory concentrations (MICs) or minimum effective concentrations (MECs) are listed in Table 1. With the exception of fluconazole, the MIC of azole antifungal agents were low (≤0.25 mg/L).
Fig. 5.
a. Verruconis gallopava stained with lactophenol cotton blue demonstrating cylindrical conidiophores bearing two-celled smooth walled conidia at the tip. Image at 400x magnification. b. Gram's stain from blood culture showing long septate hyphae at 400x magnification.
Table 1.
Minimum inhibitory concentration values for the Verruconis gallopava isolate.
| MIC (mg/L) | |
|---|---|
| Amphotericin B | <0.12 |
| Anidulafungin | 0.06 |
| Micafungin | 0.03 |
| Caspofungin | 0.25 |
| Flucytosine | 2 |
| Posaconazole | 0.06 |
| Voriconazole | 0.25 |
| Itraconazole | 0.06 |
| Fluconazole | 128 |
Of note, histopathological examination of alveolar tissue demonstrated areas of a radicular cyst lined by histiocytes, multinucleated giant cells and proliferating blood vessels. Darkly pigmented hyphal structures characteristic of dematiaceous fungi were seen after Grocott methenamine silver (GMS) staining within the histiocyte collections (not shown). Sequential blood cultures collected on day +8 also isolated V. gallopava, however blood cultures were negative on day +12 and thereafter.
Antifungal therapy was rationalised to combination voriconazole and anidulafungin. However, a cerebral CT on day +13 showed progressive meningeal thickening and increased size of the frontal lobe lesion. Repeat echocardiography on day +27 showed persistence of the cardiac mass. Options for radical oro-facial and cardiothoracic surgery were not considered to be feasible. The patient died on day +55. Post mortem was not performed.
3. Discussion
V. gallopava is a melanised thermotolerant saprophytic mould found in various environments including soil, decaying vegetation, chicken litter, thermal springs and effluent of thermal nuclear reactors [7]. Previously, Dactylaria gallopava and then O. gallopava, modern molecular methods have now placed this fungus into the genus Verruconis because of its thermotolerance, separating it from the thermolabile genus Ochroconis [7], [10].
Our patient is notable as a rare example of cardiac (endocardial) infection caused by V. gallopava which led to histopathologically-confirmed disseminated disease. Additionally, the case demonstrates that early clinical manifestations may present insidiously; in our patient, we postulate that infection likely originated from the oral cavity and later gave rise to fungaemia with subsequent dissemination including to the heart, brain, skin and lungs. Typically however, infection from V. gallopava is reported to result from inhalation of fungal conidia or direct inoculation where melanin may act as a virulence factor [11]. The present case is unusual with dematiaceous fungus being isolated from blood cultures, and it is likely that the patient was fungaemic prior to endocardial infection. This underscores the importance of performing blood cultures in immunocompromised patients where definitive diagnoses have yet been established even in the absence of fever, and taking steps to ensure that dematiaceous fungi are not dismissed as laboratory contaminants without consideration of their potential clinical relevance.
V. gallopava infection manifesting as subcutaneous abscesses was first described in 1986 by Fukushiro [4] in a patient with acute myeloblastic leukaemia and is an uncommon cause of phaeohypomycosis. Infection has been described in canine, feline and avian hosts, where the organism has caused cerebral abscesses and encephalitis [7], [12]. In humans, it predominately affects immunocompromised hosts, as was the case herein, with solid organ transplant (SOT), haematologic malignancy and stem cell transplants recipients at greatest risk [6], [13]. Cases have also been documented in patients with advanced HIV and chronic granulomatous disease and in immunocompetent individuals [12], [14], [15].
There are two predominant clinical patterns of presentation in SOT recipients; (i) cutaneous or subcutaneous involvement and (ii) systemic infections predominately involving the lungs, brain, liver and spleen [3], [6]. Our patient had disseminated disease affecting at least four non-contiguous body sites, likely originating from localised oral infection and then producing fungaemia, complicated by substantive endovascular infection. Singh et al. reviewed 34 SOT patients with systemic dematiaceous mould infections [5]. V. gallopava was the fungal agent in only three, all of whom had cerebral lesions and one also had pulmonary involvement. Another study of 12 cases of V. gallopava infections in SOT patients, found isolated pulmonary involvement in five, six involving the brain alone, and one patient with had pulmonary and skin spread [6]. Single case reports describe endophthalmitis, peritoneal, and kidney infections [13], [16] caused by V. gallopava consistent with the broad spectrum of disease caused by dematiaceous fungi in general including bursitis and joint infections, and sub-cutaneous infection in association with peritoneal dialysis catheter insertion sites [1], [5], [15].
Because Verucconis fungi are ubiquitous in the environment, their isolation from clinical specimens must be interpreted in the appropriate clinical context. As a group, dematiaceous fungi are common laboratory contaminants with as few as 10% of isolates having clinical significance [17]. Apart from performing blood cultures (as above), tissue sampling of affected sites for culture and histopathological examination is usually required for diagnosis of Verruconis infections. Verruconis species grow relatively easily on mycological media with distinctive macro- and micro-scopic characteristics as observed for our patient's isolates [1], [18]. However, DNA sequence analysis of the rDNA ITS may be required for species identification [18], [19]. Evidence for invasive infection should always be sought where possible by examining tissue for characteristic pigmented hyphae using fungal stains. Diagnosis is often supported by radiological abnormalities as was the case herein. There are no data on the utility of serum 1,3-beta D glucan testing for Verruconis infections [1].
Optimal antifungal treatment and its duration for invasive Verruconis disease have not been established. We initially chose combination antifungals, incorporating L-AMB, voriconazole and terbinafine as empiric treatment, and then selected voriconazole and anidulafungin once the organism had been definitively identified and MIC results known. However, the benefits (if at all) of combination antifungal therapy are uncertain. In the study by Singh et al. [5], all three patients received treatment with amphotericin B, one patient with additional flucytosine, and one patient with flucytosine and itraconazole; both patients receiving combination therapy survived whilst the other died. Outcomes appear to be dependent on the site of infection. Marked differences in outcomes of cutaneous/subcutaneous versus systemic infection are reported with mortality rates of 7% and 57–100%, respectively [3], [6], [20]. Furthermore, in SOT recipients, the mortality of central nervous system (CNS) disease was 100% (6/6 patients) compared with 33% (2/6) in those without CNS disease [6]. The high mortality associated with disseminated disease was highlighted in our case.
Antifungal susceptibility testing was performed on our patient's isolates yet MIC data to guide selection of therapy are uncertain. As for other dematiaceous fungi, interpretive MIC breakpoints have not been established. Studies show that terbinafine, posaconazole, and voriconazole have the greatest in vitro activity [8]. Eighteen V. gallopava isolates tested by Seyedmousavi et al. [10] showed the following MIC ranges; amphotericin B 0.125–4.0 mg/L, andidulofungin 0.016–0.25 mg/L, caspofungin 0.25–1.0 mg/L, fluconazole 4 – >64 mg/L, flucytosine 0.125–>16 mg/L, itraconazole <0.016 – >16 mg/L, posaconazole <0.016–0.25 mg/L and voriconazole 0.125–8 mg/L (19), similar to the MICs obtained for our patient.
Surgical debridement or resection of affected sites in Verruconis infections appears necessary for optimal outcomes. A review of cerebral phaeohyphomycoses by Revankar et al. [20] found improved survival associated with complete surgical debridement (mortality rates of 62% (23 of 37 patients)) compared to limited resection or aspiration (mortality rates 86% (19 of 22 patients) and overall mortality of 73% (66 of 91 patients) [20]. In another study, aggressive debulking was associated with increased survival in cerebral infection caused by another dematiaceous fungus, Cladophialophora bantiana [21]. In our patient, while it was clear that surgical resection was needed for survival, because of the nature and site of infection, combined by her poor surgical state, this was not possible.
Summary
This case describes the first case of cardiac infection caused by V. gallopava. The patient presented with subtle clinical manifestations resulting in late diagnosis with disseminated disease not amenable to surgery, resulting in an unfavourable clinical outcome. A high index of suspicion for invasive fungal disease and early diagnostic sampling should be considered in immunocompromised patients where the diagnosis is uncertain.
Conflict of interest
There are none.
Acknowledgements
No additional acknowledgements.
Footnotes
Ethical Form: Please note that this journal requires full disclosure of all sources of funding and potential conflicts of interest. The journal also requires a declaration that the author(s) have obtained written and signed consent to publish the case report from the patient or legal guardian(s).
The statements on funding, conflict of interest and consent need to be submitted via our Ethical Form that can be downloaded from the submission site www.ees.elsevier.com/mmcr. Please note that your manuscript will not be considered for publication until the signed Ethical Form has been received.
References
- 1.Revankar S.G., Sutton D.A. Melanized fungi in human disease. Clin. Microbiol. Rev. 2010;23(4):884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slavin M., van Hal S., Sorrell T.C. Invasive infections due to filamentous other than Aspergillus: epidemiology and determinants of mortality. Clin. Microbiol. Infect. 2015;21(5):490e1–490e10. doi: 10.1016/j.cmi.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi Z.A., Kwak E.J., Nguyen M.H., Silveira F.P. Ochroconis gallopava: a dermatiaceous mould causing infections in transplant recipients. Clin. Transpl. 2012;26(1):E17-2. doi: 10.1111/j.1399-0012.2011.01528.x. [DOI] [PubMed] [Google Scholar]
- 4.Fukushiro R., Udagawa S., Kawashima Y., Kawamura Y. Subcutaneous abscesses caused by Ochroconis galloparvum. J. Med Vet. Mycol. 1986;14(3):175–182. [PubMed] [Google Scholar]
- 5.Singh N., Chang F.Y., Gayowski T. Infections due to Dermatiaceous fungi in organ transplant recipients: case report and review. Clin. Infect. Dis. 1997;24:369–374. doi: 10.1093/clinids/24.3.369. [DOI] [PubMed] [Google Scholar]
- 6.Shoham S., Pic-Aluas L., Taylor J. Transplant-associated Ochroconis gallopava infections. Transpl. Infect. Dis. 2008;10(6):442–448. doi: 10.1111/j.1399-3062.2008.00327.x. [DOI] [PubMed] [Google Scholar]
- 7.Giraldo A., Sutton D.A., Samerpitak K. Occurrence of ochroconis and verruconis species in clinical specimens from the United States. J. Clin. Microbiol. 2014;52(12):4189–4201. doi: 10.1128/JCM.02027-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhary A., Meiss J.F., Guarro J. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Cinical Microbiol. Infect. 2014;20(3):47–75. doi: 10.1111/1469-0691.12515. [DOI] [PubMed] [Google Scholar]
- 9.White T., Burns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M., Gelfand D., Sninsky J., White T., editors. PCR Protocols: a Guide to Methods and Applications. Academic Press; New York: 1990. pp. 315–322. [Google Scholar]
- 10.Seyedmousavi S., Samerpitak K., Rijs A.J. Antifungal susceptibility patterns of opportunistic fungi in the genera Verruconis and Ochroconis. Antimicrob. Agents Chemother. 2014;58(6):3285–3292. doi: 10.1128/AAC.00002-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson E. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 2000;13(4):708–717. doi: 10.1128/cmr.13.4.708-717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollingsworth J.W., Shofer S., Zaas A. Successful treatment of Ochroconis gallopavum infection in an immunocompetent host. Infection. 2007;Vols. 35(5):367–369. doi: 10.1007/s15010-007-6054-7. [DOI] [PubMed] [Google Scholar]
- 13.Cardeau-Desangles I., Fabre A., Cointault O. Disseminated Ochroconis gallopava infection in a heart transplant patient. Transpl. Infect. Dis. 2013;15(3):115–118. doi: 10.1111/tid.12084. [DOI] [PubMed] [Google Scholar]
- 14.Boggild A.K., Poutanen S.M., Mohan S., Ostrowski M.A. Disseminated phaeohyphomycosis due to Ochroconis gallopavum in the setting of advanced HIV infection. Med. Mycol. 2006;44(8):777–782. doi: 10.1080/13693780600900098. [DOI] [PubMed] [Google Scholar]
- 15.Meriden Z., Marr K.A., Lederman H.M. Ochroconis gallopava infection in a patient with chronic granulomatous disease: case report and review of the literature. Med. Mycol. 2012;50:883–889. doi: 10.3109/13693786.2012.681075. [DOI] [PubMed] [Google Scholar]
- 16.Bowyer J., Johnson E., Horn E., Gregson R. Ochroconis gallopava endopthalmitis in fludarabine treated chronic lymphocytic leukaemia. Br. J. Opthalmol. 2000;84(1):117. doi: 10.1136/bjo.84.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Ami R., Lewis R.E., Raad I.I., Kontoyiannis D.P. Phaeohyphomycosis in a tertiary care cancer centre. Clin. Infect. Dis. 2009;48:1033–1041. doi: 10.1086/597400. [DOI] [PubMed] [Google Scholar]
- 18.D. Ellis Mycology Online The University of Adelaide. 〈http://www.mycology.adelaide.edu.au/Descriptions.html〉. [Online] The University of Adelaide. [Cited: 15 72016.] 〈http://www.mycology.adelaide.edu.au/Descriptions.html〉
- 19.Seyedmousavi S., Netea M.G., Mouton J.W. Black yeasts and their filamentous. Clin. Microbiol. Rev. 2014;27(3):527–542. doi: 10.1128/CMR.00093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revankar S.G., Suttin D.A., Rinaldi M.G. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin. Infect. Dis. 2004;38(2):206–216. doi: 10.1086/380635. [DOI] [PubMed] [Google Scholar]
- 21.Garg N., Devi I.B., Vajramani G.V. Central nervous system cladosporiosis: an account of 10 culture proven cases. Neurol. India. 2007;55:282–288. doi: 10.4103/0028-3886.35690. [DOI] [PubMed] [Google Scholar]