Abstract
Background
The aim of this study was to assess the long-term clinical tolerance and cardiac safety during trastuzumab treatment for patients diagnosed as having breast cancer with human epidermal growth factor receptor 2 (HER2) overexpression.
Material/Methods
A total 105 female cases diagnosed as having breast cancer with high expression of Her2, were treated with trastuzumab (T). All of them underwent electrocardiography monitoring in the process of T treatment. Left ventricular ejection fractions (LVEFs) were estimated using echocardiography before the T treatment and every 3 months. General clinical data and above parameters were collected and reviewed as analysis.
Results
The mean value of LVEFs with baseline was higher than those at other time points. All LVEFs were more than 50% during the course of trastuzumab treatment. The decline scope ≥15% of LVEFs ranged from 2 months to 16 months, and the ratios were counted for 3.1% at 2 months, 4.3% at 6 months, 3.8% at 10 months, and 5.4% at 16 months. Furthermore, a larger decrease of LVEF during the course occurred mainly in the patients with cumulative dose of A >300 mg/m2, without CPD and 16-month duration of T treatment. There was a strong correlation between cumulative dose of A, cyto/cardio-protection drugs (CPD), duration of T, and the change of LVEF (P=0.82, P=0.744, and P=0.717, respectively), which indicated that 3 factors may be associated with the change in LVEF (P<0.05).
Conclusions
The LVEF in patients with trastuzumab treatment was significantly decreased, which may be seen as a favorable benefit-risk ratio for patients undergoing long-term trastuzumab treatment.
MeSH Keywords: Receptor, Epidermal Growth Factor; Triple Negative Breast Neoplasms; Ventricular Dysfunction, Left
Background
Treatment with trastuzumab, a humanized monoclonal antibody binding to the extracellular domain of human epidermal growth factor receptor 2 (HER2) to target the HER2 pathway, significantly improves outcomes for women with HER2-positive breast cancer [1–4]. Either concurrent or sequential with systemic chemotherapy, trastuzumab can improve both disease-free survival (DFS) and overall survival (OS) in patients with HER2-positive breast cancer; however, cardiotoxicity remains an important clinical issue especially for concurrent anthracyclines regimen. Trastuzumab-related cardiotoxicity manifests mainly as a decrease in left ventricular ejection fraction (LVEF) and abnormality of cardiac function [5], but the overall incidence of cardiac toxicity is variable in various treatment centers. It has been reported to occur in up to 7% of patients when trastuzumab was used as a single agent, while cardiotoxicity occurred in up to 27% of patients receiving trastuzumab concurrently with anthracycline therapy and up to 13% of patients receiving paclitaxel with trastuzumab in one of the first clinical trials [6–8]. Due to the variation in reported incidence among different centers, the situation in China is unclear.
Given the short-term cardiac safety surveillance reported in previous studies, long-term tolerance in trastuzumab treatment has rarely been reported, and the factors associated with increased risk of cardiac events (CE) are not fully known. We wondered what factors influence CE during the whole complicated process of treatment.
In the current study, we evaluated the long-term cardiac safety and incidence of cardiotoxicity in patients treated with trastuzumab, and further analyzed the probable factors associated changes in LVEF.
Material and Methods
Participants
A total of 105 women diagnosed as having breast cancer with overexpression of Her-2 underwent trastuzumab treatment and were enrolled into the study from 2010 to 2016. All subjects met the following criteria: (1) Her2 was over-expressed. The standard is 3+ using IHC or fluorescence in situ hybridization (FISH) ratio >2.0; (2) ECOG PS ≤2; (3) no concomitant congenital heart disease or myocardial infarction; (4) baseline LVEF>50%; and (5) good compliance. All patients’ clinical characteristics are listed in Table 1. Among of these factors, cardiovascular disease risk factors were analyzed according to the CDC/ACSM guidelines, including hypertension, high BMI, dyslipidemia, and metabolic syndrome.
Table 1.
Characteristics of study population and LVEF level at different time points (n=94).
| Characteristics | N | Characteristics | Mean ±SD |
|---|---|---|---|
| PS | Age | 46.73±8.91 | |
| 0 | 44 | Cumulative dose of A (mg/m2) | 228.12±174.4 |
| 1 | 34 | Interval between A and T (M) | 4.47±1.45 |
| 2 | 16 | Interval between R and T (M) | 2.44±3.47 |
| Stage | Duration of T (M) | 15.73±13.18 | |
| I | 10 | LVEFbaseline | 72.13±4.93 |
| II | 26 | LVEF3 | 69.93±6.36 |
| III | 40 | LVEF6 | 69.12±5.32 |
| IV | 18 | LVEF9 | 69.82±6.12 |
| Heart disease | LVEF12 | 69.46±5.54 | |
| Yes | 0 | LVEF15 | 68.49±6.39 |
| No | 94 | LVEF18 | 70.12±6.17 |
| A | LVEF21 | 69.25±6.67 | |
| With | 84 | LVEF24 | 69.39±6.41 |
| Without | 10 | LVEF27 | 69.55±4.84 |
| CPD | LVEF30 | 69.40±4.77 | |
| With | 57 | LVEF33 | 69.64±5.44 |
| Without | 37 | LVEF36 | 70.19±5.03 |
| Radiation | LVEF39 | 69.89±5.59 | |
| Left | 57 | LVEF42 | 70.69±6.32 |
| Right/without | 37 | LVEF45 | 71.49±7.01 |
| ECG | LVEF48 | 70.36±4.79 | |
| N | 63 | LVEF51 | 69.23±4.45 |
| AN | 31 | LVEF54 | 73.19±3.49 |
| CVD risk | LVEF57 | 70.05±4.18 | |
| With | 20 | LVEF60 | 71.40±5.46 |
| Without | 74 | ||
| Symptom | |||
| Yes | 88 | ||
| No | 6 |
PS – performance score; A – anthracycline; CPD – cyto/cardio-protection drugs; ECG – electrocardiography; N – normal; AN – abnormal; CVD – cardiovascular disease; T – trastuzumab; LVEF – left ventricular ejection fraction.
The Medical Ethics Committee of Laiwu Hospital Affiliated to Taishan Medical College approved this study. Written informed consent conforming to the tenets of the Declaration of Helsinki was obtained from each participant prior to the study.
Therapy protocols
According to the trastuzumab manufacturer’s instruction, all subjects were administered trastuzumab (initial 8 mg/Kg followed by 6 mg/Kg every 3 weeks, Myl 1401O, Mylan) during a 90-minute period. The regimen including chemotherapy or radiotherapy may be concurrent with or followed by trastuzumab. It was recommended but not mandated that patients received cyto/cardio-protection drugs during the course of trastuzumab treatment (i.e., Shenmai injection, Amifostine, and Levocarnitine (Qilu Pharmaceutical Co., Ltd.).
Detection of ECG and evaluation of cardiac function and treatment
All subjects received an ECG examination before and 1 month later after trastuzumab treatment and were examined for heart-related symptoms such as chest distress, dyspnea, and palpitation. If the subjects were symptomatic, an ECG examination was given every month. Echocardiography was given to all subjects before and every 3 months during trastuzumab treatment to acquire the value of LVEF. Changes in LVEF at all time points were determined relative to the LVEF measured at baseline level and defined as LVEFratio.
| (1) |
If LVEFratio was ≥16% or LVEF was <50%, trastuzumab treatment was halted temporarily more than 4 weeks, and echocardiography was performed every 4 weeks. Trastuzumab was continued if LVEF restored to normal level or absolute decline <15% in 4–8 weeks. If the duration was over 8 weeks, trastuzumab was halted permanently. To evaluate the correlation between the main clinical factors and the change in LVEF, the maximal shift of LVEF in each case during the course of trastuzumab treatment was calculated and defined as LVEFmax.
| (2) |
Data on patient demographic and baseline characteristics, cardiovascular disease risk factors, radiotherapy or no radiotherapy, interval between anthracycline (A) and trastuzumab (T), duration of trastuzumab treatment, LVEF level at every point, and ECG results were obtained from existing data.
Statistical analysis
All statistical analyses were carried out using SPSS 18.0 software. Descriptive statistics were produced for continuous variables. Results are presented as mean ±SD for continuous variables. The chi-square test was used to determine the significance of rate variables, while the significance of continuous variables was assessed by the analysis of variance (ANOVA). Spearman correlation coefficient was used to analyze the correlation between main clinical factors and change of LVEF. P-values less than 0.05 were considered statistically significant.
Results
Clinical characteristics
A total of 105 patients were enrolled from 2010 through 2016 and had a full set of clinical data. Median age was 46 years (range, 26–64 years). There were 43 cases with PS scores of 0, 35 with scores of 1, and 27 with scores of 2. Over half of the patients (68%) had stage II/III disease. There were 61 patients who underwent adjuvant trastuzumab treatment, and 44 underwent salvage trastuzumab treatment for advanced-stage disease or recurrence. About 30 patients had cardiovascular-related risk factors. There were 67 patients who underwent left chest irradiation. Ninety-four (89.4%) patients also received anthracycline-based adjuvant chemotherapy. The mean duration of trastuzumab treatment was 15.73 months (range 5–60 m). The mean interval between anthracycline and trastuzumab was 4.47 months. Sixty-seven patients accepted cyto/cardio-protection drug treatment. Of these patients, 16 felt cardio-related symptoms but all continued with the treatment. Thirty-one patients had abnormal ECG results; the most common electrocardiography abnormalities were sinus tachycardia, sinus bradycardia, and ST-T segment change. The interval between initial trastuzumab treatment and electrocardiography abnormalities was 5.47±3.66 months.
Monitoring of LVEF level
All the patients underwent echocardiography from baseline to 6 months; mean baseline LVEF was 72.12% (60.0–82.43%), mean LVEF at 2 months was 69.93% (53.7–82.8%), and mean LVEF at 6 months was 69.12% (58.86–79. 7%). Of all the patients, 85 underwent echocardiography until 12 months, 69 at 24 months, 65 at 24 months, 44 at 36 months, 35 at 48 months, and 27 at 60 months. Change in LVEF was calculated according to the equation. As shown in Figure 1, the descending scopes of LVEF were expanded from 2 months to 16 months, and then contracted after 16 months. The lowest point of LVEF was at 16 months, and the ratios were counted for 3.1% at 2 months, 4.3% at 6 months, 3.8% at 10 months, and 5.4% at 16 months.
Figure 1.
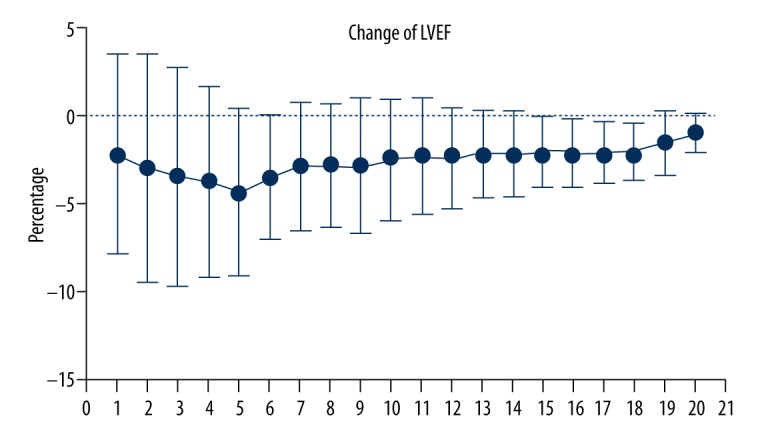
Changes in LVEF level from 2 months to 60 months were determined relative to the LVEF measured at baseline level. The points from 1 to 20 at X axis were represented changes of LVEF from 3 to 60 months, respectively. From 2 months to 16 months, descending scopes of LVEF expanded gradually and a distinct trough was seen at 16 months. Data are displayed as mean ±SD.
Correlation between main clinical factors and change in LVEF
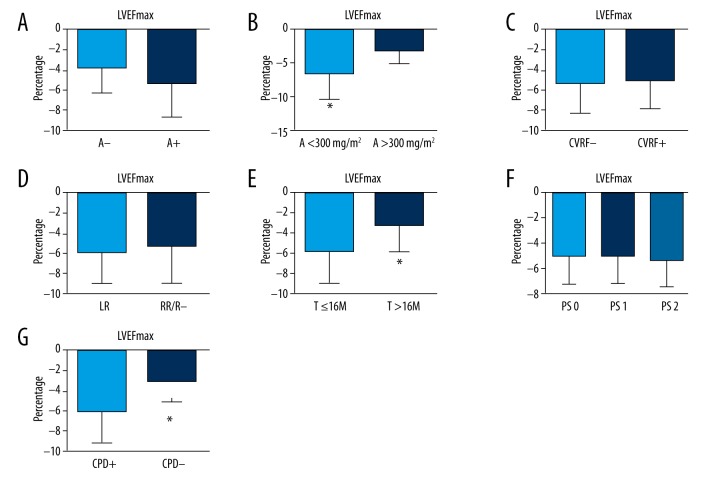
To observe the correlation between main clinical factors and change in LVEF, the maximal shift of LVEF in each patient was calculated and defined as LVEFmax. LVEFmax was divided into groups according to clinical factors: PS scoring, anthracycline (A) (+/−), chest radiation, cardiovascular risk factors (+/−), drugs of cyto/cardio-protection (+/−), and duration of trastuzumab (T) (Figure 2). We found that cumulative dose of A, cyto/cardio-protection drugs (CPD), and duration of T may be associated with the change in LVEF (P<0.05). A larger decrease in LVEF during the course occurred mainly in the patients with cumulative dose of A >300 mg/m2, without CPD and duration of 16-month T treatment. A Spearman correlation coefficient was calculated to analyze the correlation between above factors and the maximal change in LVEF (LVEFmax). Among of these factors, the factors of cyto/cardio-protection drugs and cumulative dose of anthracycline had strong correlations with LVEFmax to different extents (ρ=0.82, ρ=0.744, and ρ=0.717), indicating that the utility of cyto/cardio-protection drugs helped preserve cardiac function during trastuzumab treatment.
Figure 2.
Several main clinical factors were analyzed to identify the correlation with change in LVEF, and the maximal shift of LVEF was calculated and defined as LVEFmax. The histograms were obstructed and the LVEFmax was expressed as mean ±SD. (A) LVEFmax was categorized as the 2 groups in which the patients underwent Anthracycline-containing chemotherapy or not. The LVEFmax was lower in the group without Anthracycline regimen (A−) than in the group with anthracycline regimen (A+), but the difference was not significant (P=0.068). (B) LVEFmax was divided into 2 groups according to cumulative dose of Anthracycline. LVEFmax was lower in the group with A <300 mg/m2 than in the group with A >300 mg/m2, and the difference was significant (* P<0.05). (C) It did not show a statistically significant difference between the group with cardio-vascular diseases risk factors (CVRF) and the group without CVRF (−5.76±3.42 vs. −5.35±3.28). (D) The LVEFmax was −5.83±3.51 in the group that underwent left chest radiation (LR), and −5.49±3.71 in the group that underwent right chest radiation/no radiation (RR/R−). (E) The LVEFmax in the group of T ≤16M was higher than that in the group of T >16M (T – trastuzumab), and the difference was significant (* P<0.05), which indicates the decline of LVEF appears in the first 16 months of trastuzumab treatment. (F) The LVEFmax was divided into 3 groups according to PS scoring. It showed no significant difference between the 3 groups, though there was a trend toward increasing LVEFmax with PS scoring increase. (G) The LVEFmax in the group using cyto/cardio-protection drugs (CPD) was clearly lower than that in the group using CPD (* P<0.05).
Discussions
Trastuzumab, as an efficacious treatment for breast cancer with overexpression of HER2, was approved by the United States Food and Drug Administration in September 1999. Its cardiotoxicity has been a focus of clinical oncologists since then. It has been reported that the incidence of cardiac events is influenced by individual differences, and many factors that are still not understood affect the variance in cardiac function. In the present study, the results demonstrated low rates of symptomatic cardiac events after trastuzumab treatment with/without anthracycline, and the decline scope of LVEF ranged from 18.56% to 17.95% with large variance, and the interval of decline was ranged from 2 months to 16 months, and the trough happened at 16 months. Over time we observed cases of LVEF decline ≥16%, which reveals that the late onset of cardiac dysfunction with trastuzumab is rare and long-term use of trastuzumab is relatively safe. The phenomenon was fairly consistent with other pivotal adjuvant trastuzumab trials [9,10].
To better understanding of factors affecting change in LVEF, various probable clinical factors were entered into the analysis. Among of these general clinical factors, cyto/cardio-protection drugs, cumulative dose of A, and duration of T were important factors related with the change in LVEF. The results showed that the cardiotoxic effect of trastuzumab may be alleviated in cases with preventive CPD. It has been reported that these patients benefit from the cyto/cardio-protection drugs used during chemotherapy treatment [11]. A popular herbal preparation in China is widely used for maintaining atherosclerotic coronary and myocardial function [9,10,12,13]. However, few studies have investigated the efficacy of Shenmai injection in trastuzumab treatment. It has been reported that Amifostine, a cyto-protection drug, is feasible to include in protocols including trastuzumab in the aggressive radiochemotherapy when supported with Amifostine [11,14,15]. Levocarnitine, a naturally occurring essential co-factor of fatty acid metabolism, plays protective roles in patients with ischemic heart disease, which are related to the attenuation of oxidative stress injury [16], but its role in the trastuzumab treatment is still unknown. In the present study, the decline scope was significant lower in the group receiving the drugs discussed above than in the group not receiving these drugs. All of these 3 kinds of cyto/cardio-protection drugs seem to play a positive role during trastuzumab treatment to some extent. A study stratifying these 3 kinds of drugs would be useful. Patients who underwent preferential anthracycline treatment with a cumulative dose >300 mg/m2 suffered from decreased LVEF. Retrospective analyses from clinical trials in adults suggest that the incidence of cardiac function damage due to doxorubicin was 1.7% at a cumulative dose of 300 mg/m2 [17], which is similar to results in our study. In a previous analysis of pooled data from 6 trials on metastatic breast cancer, 11.6% of cases treated with trastuzumab after prior anthracyclines experienced a decline of LVEF of ≥15 points to a level<50% [10], and the ratio was higher than that in our study, which may be partially attributed to different disease stage of patients and the doses of anthracycline.
Previous studies have reported that patients with multiple risk factors, such as hypertension, obesity, dyslipidemia, and metabolic syndrome, have further worsening cardiovascular reserve and increasing the likelihood of subsequent cardiotoxicity [9,12,13,18]. However, we found that cardiovascular disease risk did not obviously affect LVEF during trastuzumab treatment. It has traditionally been widely accepted that cardiovascular response is one of the main complications in breast cancer patients undergoing radiotherapy; therefore, radiotherapy, especially left chest radiotherapy, theoretically may aggravate heart injury from trastuzumab; however, the results did not demonstrate a synergistic effect, and there was no significant difference in the change in LVEF between patients receiving left/right radiation and those receiving no radiation.
Conclusions
In summary, decreased LVEF might occur in the first year in patients with trastuzumab treatment, but progressive decrease in LVEF seldom appeared with the prolonged of treatment interval. There was good patient tolerance of trastuzumab used after anthracycline or concurrent/sequential with radiotherapy. These patients may benefit from use of cyto/cardio-protection drugs.
Acknowledgment
This work was supported by grants from Laiwu Hospital Affiliated to Taishan Medical College. Ma Ying, Ph.D., who works at Tsinghua University, helped us with data analysis and manuscript revision.
Footnotes
Source of support: Departmental sources
References
- 1.Bashari MH, Fan F, Vallet S, et al. Mcl-1 confers protection of Her2-positive breast cancer cells to hypoxia: therapeutic implications. Breast Cancer Res. 2016;18(1):26. doi: 10.1186/s13058-016-0686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Datta J, Fracol M, McMillan MT, et al. Association of depressed anti-HER2 T-helper type 1 response with recurrence in patients with completely treated HER2-positive breast cancer: Role for immune monitoring. JAMA Oncol. 2016;2(2):242–46. doi: 10.1001/jamaoncol.2015.5482. [DOI] [PubMed] [Google Scholar]
- 3.Murthy P, Kidwell KM, Schott AF, et al. Clinical predictors of long-term survival in HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2016;155(3):589–95. doi: 10.1007/s10549-016-3705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arpino G, Michelotti A, Truini M, et al. Demographic, tumor and clinical features of clinical trials versus clinical practice patients with HER2-positive early breast cancer: Results of a prospective study. J Cancer Res Clin Oncol. 2016;142(3):669–78. doi: 10.1007/s00432-015-2033-z. [DOI] [PubMed] [Google Scholar]
- 5.Xue J, Jiang Z, Qi F, et al. Risk of trastuzumab-related cardiotoxicity in early breast cancer patients: A prospective observational study. J Breast Cancer. 2014;17(4):363–69. doi: 10.4048/jbc.2014.17.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang I, Bell R, Feng FY, et al. Trastuzumab retreatment after relapse on adjuvant trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer: Final results of the Retreatment after HErceptin Adjuvant trial. Clin Oncol (R Coll Radiol) 2014;26(2):81–89. doi: 10.1016/j.clon.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. Outcome of patients with HER2-positive breast cancer treated with or without adjuvant trastuzumab in the Finland Capecitabine Trial (FinXX) Acta Oncol. 2014;53(2):186–94. doi: 10.3109/0284186X.2013.820840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieras V, Bachelot T. The success story of trastuzumab emtansine, a targeted therapy in HER2-positive breast cancer. Target Oncol. 2014;9(2):111–22. doi: 10.1007/s11523-013-0287-4. [DOI] [PubMed] [Google Scholar]
- 9.G DIC, Marengo G, Albanese NN, et al. Proteomic profiling of Trastuzumab (Herceptin(R))-sensitive and -resistant SKBR-3 breast cancer cells. Anticancer Res. 2013;33(2):489–503. [PubMed] [Google Scholar]
- 10.Metzger-Filho O, de Azambuja E, Bradbury I, et al. Analysis of regional timelines to set up a global phase III clinical trial in breast cancer: The adjuvant lapatinib and/or trastuzumab treatment optimization experience. Oncologist. 2013;18(2):134–40. doi: 10.1634/theoncologist.2012-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki Y, Ogiya R, Oshitanai R, et al. Feasibility and pharmacokinetics of combined therapy with S-1 and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic or recurrent breast cancer. Int J Clin Oncol. 2014;19(2):274–79. doi: 10.1007/s10147-013-0547-4. [DOI] [PubMed] [Google Scholar]
- 12.Wynne C, Harvey V, Schwabe C, et al. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53(2):192–201. doi: 10.1177/0091270012436560. [DOI] [PubMed] [Google Scholar]
- 13.Shigekawa T, Takeuchi H, Misumi M, et al. [Successful treatment of trastuzumab-resistant HER2-positive breast cancer with extensive liver metastases using the combination of trastuzumab and capecitabine – a case report]. Gan To Kagaku Ryoho. 2013;40(2):225–27. [in Japanese] [PubMed] [Google Scholar]
- 14.Moreno-Aspitia A, Dueck AC, Ghanem-Canete I, et al. RC0639: phase II study of paclitaxel, trastuzumab, and lapatinib as adjuvant therapy for early stage HER2-positive breast cancer. Breast Cancer Res Treat. 2013;138(2):427–35. doi: 10.1007/s10549-013-2469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi N, Niikura N, Yamauchi H, et al. Adding hormonal therapy to chemotherapy and trastuzumab improves prognosis in patients with hormone receptor-positive and human epidermal growth factor receptor 2-positive primary breast cancer. Breast Cancer Res Treat. 2013;137(2):523–31. doi: 10.1007/s10549-012-2336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Castillo B, Oliveras-Ferraros C, Vazquez-Martin A, et al. Basal/HER2 breast carcinomas: integrating molecular taxonomy with cancer stem cell dynamics to predict primary resistance to trastuzumab (Herceptin) Cell Cycle. 2013;12(2):225–45. doi: 10.4161/cc.23274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrelli F, Barni S. A pooled analysis of 2618 patients treated with trastuzumab beyond progression for advanced breast cancer. Clin Breast Cancer. 2013;13(2):81–87. doi: 10.1016/j.clbc.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Gullo G, Bettio D, Zuradelli M, et al. Level of HER2/neu amplification in primary tumours and metastases in HER2-positive breast cancer and survival after trastuzumab therapy. Breast. 2013;22(2):190–93. doi: 10.1016/j.breast.2013.01.005. [DOI] [PubMed] [Google Scholar]