Abstract
Purpose:
Deep venous thrombosis (DVT) and pulmonary embolism (PE) are considered as similar disease entities representing different clinical manifestations. The objectives of this study were: 1) to determine the prevalence and outcome of DVT in patients with PE; 2) to identify additional risk factors for PE-related unfavorable outcome and 30-day all-cause mortality; and 3) to establish the clinical importance of screening for concomitant DVT.
Materials and Methods:
From January 2013 to December 2015, a total of 141 patients with confirmed PE were evaluated. The prevalence and outcome of DVT in patients with PE was determined. Furthermore, the potential risk factors for PE-related unfavorable outcome and 30-day all-cause mortality were also analyzed.
Results:
The prevalence of concomitant DVT was 45.4%. PE-related unfavorable outcome was observed in 21.9% of all concomitant DVT, with all-cause mortality of 21.9%. There was no significant relationship between the presence of concomitant DVT and the development of PE-related unfavorable outcome or all-cause mortality. Our results indicated that heart rate >100/min and peripheral oxygen saturation <90% were independent predictors for PE-related unfavorable outcome. Regarding all-cause mortality, active malignancy and hypotension or shock were significant risk factors.
Conclusion:
Our findings demonstrate that approximately half of patients with PE possess DVT. However, this study failed to establish any clinical significance of concomitant DVT for PE-related unfavorable outcome and all-cause mortality. Tachycardia and hypoxemia were identified as significant predictors for PE-related unfavorable outcome along with active malignancy and hypotension or shock as significant risk factors of all-cause mortality.
Keywords: Venous thrombosis, Pulmonary embolism, Prevalence, Outcome, Risk factors
INTRODUCTION
Deep venous thrombosis (DVT) and pulmonary embolism (PE) are considered as two different clinical manifestations of a single disease. Approximately 90% of symptomatic PEs are reported to originate from thrombus located in the venous system of the lower extremity [1–3]. DVT and PE also share the same risk factors including age, immobilization, active malignancy, and history of surgery or trauma [4]. In recent years, the concept of venous thromboembolism (VTE) has been introduced as a result of both DVT and PE sharing similar etiology. VTE is known as a potential life-threatening condition with >200,000 new cases annually reported in the United States [5]. Mortality rates of VTE during the first 3 months in patients with PE are in the range of 1.4% to 17.4% despite advanced therapeutic options [6–9]. PE-related complications including pulmonary artery hypertension (HTN) and right heart failure are known to be the most common causes of early mortality and medical comorbidity that cause late death after PE [10]. VTE treatment seeks to prevent the development of PE-related complications and the occurrence of postthrombotic syndrome (PTS) from DVT as well as the recurrence of VTE.
Several studies have shown that a number of patients with symptomatic DVT possess silent PE whereas asymptomatic DVT might be present in many patients with PE [11]. However, the exact prevalence of DVT in patients with PE and the clinical significance of concomitant DVT are vastly unknown [12–14]. In addition, little information is available on the differences in outcome between PE patients with DVT and those without DVT [4].
Therefore, the objectives of this study were: 1) to determine the prevalence and outcome of DVT in patients with PE; 2) to identify potential risk factors for PE-related unfavorable outcome and 30-day all-cause mortality; and 3) to establish the clinical importance of screening for concomitant DVT to prevent the development of PTS and determine the effect of concomitant DVT on the outcome of patients with PE.
MATERIALS AND METHODS
From January 2013 to December 2015, a total of 177 patients with confirmed PE were enrolled in this study. All PEs were diagnosed by computed tomography pulmonary angiography (CTPA). All CTPAs were interpreted by two board-certified radiologists specializing in chest imaging. Among the 177 patients with PE, 141 patients (79.7%) were assessed for the presence of DVT in the lower extremities using duplex ultrasonography or lower extremity CT venography and were evaluated retrospectively in this study.
Based on the findings of duplex ultrasonography or CT venography, DVT was divided into proximal DVT and distal DVT (Fig. 1). Proximal DVT was defined as thrombus involving the common and external iliac veins, common femoral vein, femoral vein, and popliteal vein with or without calf vein thrombosis. Distal DVT was diagnosed if the thrombosis involved calf veins solely [4]. PE was confirmed if a filling defect outlined by contrast media or complete occlusion was seen on CTPA. Each confirmed PE on CTPA was further evaluated for the localization of the following vessels: main pulmonary arteries, lobar pulmonary arteries, and segmental or subsegmental branches (Fig. 2).
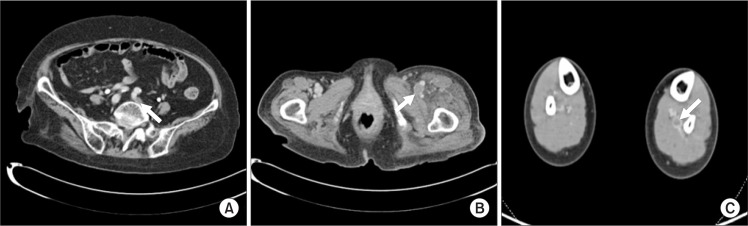
Fig. 1.
Computer tomography (CT) angiographies in patients with concomitant deep venous thrombosis (DVT). (A, B) CT angiographic findings indicate proximal DVT, involving left common iliac vein and left femoral vein. (C) CT angiographies show distal location of DVT, involving left posterior tibial vein.
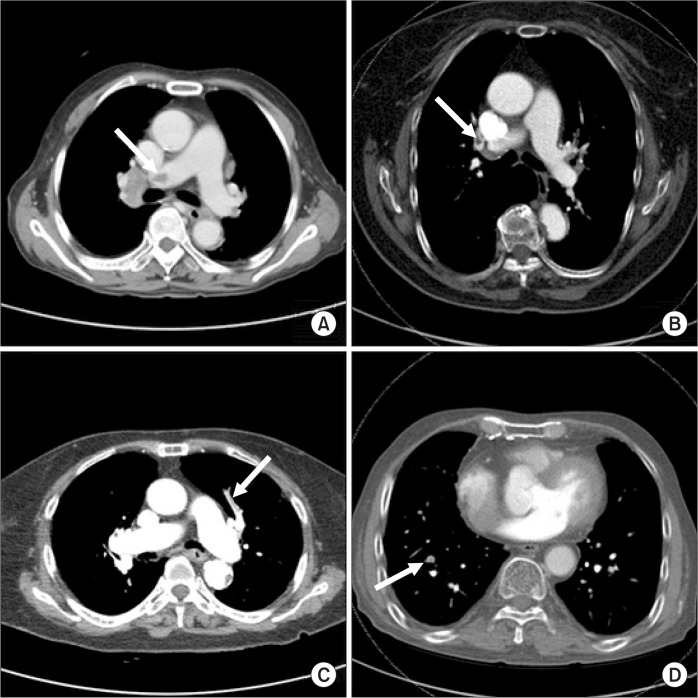
Fig. 2.
Computer tomography (CT) angiographies in patients with pulmonary embolism. (A–D) CT angiographic findings indicate embolic lesion of right main pulmonary artery, right lobar artery, left segmental artery, and right subsegmental artery, respectively.
Anticoagulation therapy was performed for all patients with PE. Patients received unfractionated or low molecular weight heparin followed by oral vitamin K antagonist or a new oral anticoagulant (including rivaroxaban) for at least 6 months. An international normalized ratio of 2–3 was considered as sufficient therapeutic range. An inferior vena cava filter was inserted only in patients with contraindication for anticoagulation therapy. Anticoagulation was initiated only after the contraindication had been resolved.
Initially, patients with confirmed PE were classified into non-DVT and concomitant DVT groups. Their demographic features and risk factors for VTE were investigated, including age, gender, previous history of VTE, immobilization ≥3 days, history of surgery or trauma ≤4 weeks, active malignancy and/or chemotherapy, HTN, diabetes mellitus, coronary artery disease, cerebrovascular accident, dementia, underlying pulmonary disease, and smoking status (Table 1). In addition, the following PE-related risk factors were collected: PE-related symptoms (chest pain, dyspnea, cough, and so on), systolic blood pressure (SBP) <100 mmHg, respiratory rate (RR) >30/min, heart rate (HR) >100/min, and peripheral oxygen saturation (SpO2) <90%.
Table 1.
Comparison of demographic features between non-DVT and concomitant DVT in patients with PE
| Demographic features | All patients (n=141) | Non-DVT (n=77, 54.6%) | Concomitant DVT (n=64, 45.4%) | P-valuea |
|---|---|---|---|---|
| Age (y) | 69.90±15.13 | 71.05±14.02 | 68.52±16.36 | 0.616 |
| <50 | 16 (11.3) | 7 (9.1) | 9 (14.1) | |
| 51–69 | 38 (27.0) | 21 (27.3) | 17 (26.5) | 0.647 |
| ≥70 | 87 (61.7) | 49 (63.6) | 38 (59.4) | |
| Male | 65 (46.1) | 37 (48.1) | 28 (43.8) | 0.610 |
| PE-related risk factors | ||||
| PE-related symptoms | 87 (61.7) | 51 (66.2) | 36 (56.3) | 0.225 |
| HR >100/min | 51 (36.2) | 26 (33.8) | 25 (39.1) | 0.515 |
| Respiration rate >30/min | 9 (6.4) | 4 (5.2) | 5 (7.8) | 0.732 |
| SpO2 <90% | 41 (29.1) | 21 (27.3) | 20 (31.3) | 0.605 |
| Risk factor for VTE | ||||
| History of VTE | 15 (10.6) | 6 (7.8) | 9 (14.1) | 0.229 |
| Immobilization ≥3 days | 61 (43.3) | 29 (37.7) | 32 (50.0) | 0.141 |
| History of surgery or trauma ≤4 weeks | 21 (14.9) | 11 (14.3) | 10 (15.6) | 0.824 |
| Active malignancy and/or chemotherapy | 39 (27.7) | 20 (26.0) | 19 (29.7) | 0.624 |
| Comorbidities | ||||
| HTN | 80 (56.7) | 42 (54.5) | 38 (59.4) | 0.564 |
| DM | 44 (31.2) | 29 (37.7) | 15 (23.4) | 0.070 |
| CAD | 10 (7.1) | 7 (9.1) | 3 (4.7) | 0.348 |
| Chronic kidney disease | 5 (3.5) | 3 (3.9) | 2 (3.1) | 1.000 |
| Congestive heart failure | 12 (8.5) | 8 (10.4) | 4 (6.3) | 0.547 |
| CVA | 30 (21.3) | 16 (20.8) | 14 (21.9) | 0.874 |
| Dementia | 10 (7.1) | 3 (3.9) | 7 (10.9) | 0.185 |
| Underlying pulmonary disease | 52 (36.9) | 28 (36.4) | 24 (37.5) | 0.889 |
| Smoking | 11 (7.8) | 7 (9.1) | 4 (6.3) | 0.754 |
| Location of PE | ||||
| Main & lobar arteries | 86 (61.0) | 42 (54.5) | 44 (68.8) | 0.085 |
| Segmental & subsegmental arteries | 55 (39.0) | 35 (63.6) | 20 (36.4) | |
| Anticoagulation treatment | 122 (86.5) | 67 (87.0) | 55 (85.9) | 0.852 |
| PE-related unfavorable outcome | 25 (17.7) | 11 (14.3) | 14 (21.9) | 0.240 |
| 30-day all-cause mortality | 28 (19.9) | 14 (18.2) | 14 (21.9) | 0.584 |
Values are presented as mean±standard deviation or number (%).
DVT, deep venous thrombosis; PE, pulmonary embolism; HR, heart rate; SpO2, peripheral oxygen saturation; VTE, venous thromboembolism; HTN, hypertension; DM, diabetes mellitus; CAD, coronary artery disease; CVA, cerebrovascular accident.
Chi-square test or Fisher’s exact test.
PE-related clinical composite outcomes and all-cause mortality were obtained through medical chart review within one month post PE diagnosis. PE-related clinical composite outcomes were divided into PE-related favorable outcome and PE-related unfavorable outcome. PE-related unfavorable outcome was defined when at least one of the following criteria was met: (1) need for cardiopulmonary resuscitation, (2) hypotension (SBP <100 mmHg) or shock, (3) need for invasive or non-invasive mechanical ventilation, (4) need for inotropic agents to maintain adequate organ perfusion and SBP ≥100 mmHg, and (5) PE-related death.
In this study, we first determined the prevalence and outcome of DVT in patients with PE and compared the demographic features between the non-DVT group and the concomitant DVT group using chi-square test or Fisher’s exact test. The presence of concomitant DVT and additional risk factors as independent predictors for PE-related unfavorable outcome were then analyzed. Furthermore, 30-day all-cause mortality was reviewed using univariate analysis and multiple logistic regression analysis. Multivariate analysis was used to estimate the odds ratio (OR) for statistically significant correlation between risk factors and PE-related unfavorable outcome or early mortality. Statistical significance was indicated when a P-value was less than <0.05 for all analyses carried out in this investigation. All statistical analyses were performed using PASW Statistics ver. 18.0 software (IBM Co., Armonk, NY USA).
RESULTS
1. Prevalence, outcome, and demographic features
In this study, the overall prevalence rate of DVT in patients with PE was 45.4% (64 patients). Among the total of 141 PE patients, 55 (39.0%) had proximal DVT. PE-related unfavorable outcomes were observed in 25 (17.7%) patients, with 30-day all-cause mortality found in 28 (19.9%) of the 141 PE patients.
Demographic characteristics between the non-DVT group and the concomitant DVT group of patients with PE did not show any statistically significant differences (Table 1). The presence of concomitant DVT or proximal DVT demonstrated slightly more proximally localized PE (68.8% in concomitant DVT, 65.5% in proximal DVT) (Table 1). However, such difference was not statistically significant according to the presence or the location of DVT (P=0.085, P=0.385, respectively).
2. Concomitant DVT and unfavorable outcome
Of the 141 PE patients, 25 (17.7%) patients had PE-related unfavorable outcome. Of the 64 concomitant DVT patients, 14 (21.9%) had PE-related unfavorable outcome. In addition, in 102 PE patients without active malignancy and/or chemotherapy, 18 (17.6%) showed unfavorable outcome. Potential risk factors including the presence of concomitant DVT were tested by univariate analysis. Results are shown in Table 2. HR >100/min, RR >30/min, and SpO2 <90% were found to be statistically significant risk factors for PE-related unfavorable outcome (P=0.001, P=0.009, and P=0.001, respectively). However, there was no statistically significant relationship between the presence of concomitant DVT and the development of PE-related unfavorable outcome (P=0.240). There was no significant relationship between the location of PE and unfavorable outcome (P=0.734). Moreover, the presence of pulmonary symptoms at the diagnosis of PE did not significantly affect the development of PE-related unfavorable outcome (P=0.847). Several potential risk factors identified in univariate analysis were subjected to multivariate analysis. Results are also shown in Table 2. HR >100/min (OR, 5.19; 95% confidence interval [CI], 1.521–17.713; P=0.009) and SpO2 <90% (OR, 3.51; 95% CI, 1.072–11.513; P=0.038) were found to be statistically significant independent predictors for PE-related unfavorable outcome.
Table 2.
Prevalence and risk factors for PE-related unfavorable outcome in patients with pulmonary embolism
| Risk factor | Unfavorable outcome (n=25, 17.7%) | Univariatea | Multivariateb | |
|---|---|---|---|---|
|
|
|
|||
| P-value | P-value | 95% CI | ||
| Age (y) | 71.0±12.5 | 0.304 | 0.338 | 0.267–4.058 |
| <50 | 1 (4.0) | |||
| 51–69 | 9 (36.0) | |||
| ≥70 | 15 (60.0) | |||
| Male | 11 (44.0) | 0.816 | 0.352 | 0.513–6.524 |
| Location of PE | 0.734 | 0.993 | 0.303–3.265 | |
| Main & lobar arteries | 16 (64.0) | |||
| Segmental & subsegmental arteries | 9 (36.0) | |||
| PE-related symptoms | 15 (60.0) | 0.847 | 0.586 | 0.198–2.499 |
| DVT | 14 (56.0) | 0.240 | 0.318 | 0.594–4.961 |
| Proximal | 10 (40.0) | |||
| Immobilization ≥3 days | 13 (52.0) | 0.331 | 0.860 | 0.261–3.069 |
| History of surgery or trauma ≤4 weeks | 5 (20.0) | 0.429 | 0.827 | 0.256–5.489 |
| Active malignancy and/or chemotherapy | 7 (28.0) | 0.967 | 0.995 | 0.295–3.410 |
| Comorbidities | ||||
| HTN | 14 (56.0) | 0.935 | 0.429 | 0.181–2.067 |
| DM | 8 (32.0) | 0.925 | 0.915 | 0.307–3.739 |
| CAD | 2 (8.0) | 1.000 | 0.795 | 0.147–12.200 |
| Congestive heart failure | 3 (12.0) | 0.446 | 0.987 | 0.155–6.667 |
| Cerebrovascular disease | 6 (24.0) | 0.714 | 0.647 | 0.161–3.113 |
| Underlying pulmonary disease | 10 (40.0) | 0.721 | 0.597 | 0.211–2.443 |
| Smoking | 2 (8.0) | 1.000 | 0.623 | 0.239–10.947 |
| HR >100/min | 17 (68.0) | 0.001 | 0.009 | 1.521–17.713 |
| RR >30/min | 5 (20.0) | 0.009 | 0.176 | 0.544–28.150 |
| SpO2 <90% | 14 (56.0) | 0.001 | 0.038 | 1.072–11.513 |
| Anticoagulation treatment | 19 (76.0) | 0.089 | 0.169 | 0.077–1.569 |
Values are presented as mean±standard deviation or number (%).
PE, pulmonary embolism; CI, confidence interval; DVT, deep venous thrombosis; HTN, hypertension; DM, diabetes mellitus; CAD, coronary artery disease; HR, heart rate; RR, respiratory rate; SpO2, peripheral oxygen saturation.
Chi-square test or Fisher’s exact test,
logistic regression model.
3. Concomitant DVT and all-cause mortality
Overall, 28 of all PE patients (19.9%) and 14 (21.9%) of 64 patients with concomitant DVT died within 30 days after being diagnosed with PE. There was no statistically significant difference in 30-day all-cause mortality (18.2% vs. 21.9%, P=0.584) between patients with DVT and those without DVT. Among 39 PE patients with active malignancy and/or chemotherapy, 30-day all-cause mortality occurred in 18 patients (46.2%). However, in 102 PE patients without active malignancy and/or chemotherapy, only 10 (9.8%) patients showed early mortality. In univariate analysis, patients with active malignancy and/or chemotherapy (P=0.001) and hypotension or shock (P=0.007) were significant risk factors for 30-day all-cause mortality after PE diagnosis (Table 3). Multivariate analyses also showed that active malignancy and/or chemotherapy (OR, 28.87; 95% CI, 6.564–126.950; P=0.001) and hypotension or shock (OR, 11.40; 95% CI, 1.581–82.201; P=0.016) were statistically significant risk factors for 30-day all-cause mortality (Table 3). Interestingly, both univariate and multivariate analysis undertaken in this study indicated that PE patients with active malignancy and/or chemotherapy had significantly poorer prognosis compared to those without active malignancy and/or chemotherapy (30-day mortality: 46.2% vs. 9.8%, P=0.001). However, despite the fact that patients who required inotropic agents showed higher mortality rate compared to those who did not require such agents (36.4%, 4 of 11 patients vs. 18.5%, 24 of 130 patients), there was no statistical significance between the two by univariate and multivariate analyses (P=0.229, P=0.607, respectively).
Table 3.
Prevalence and risk factors for 30-day all-cause mortality in patients with PE
| Risk factor | 30-day all-cause mortality (n=28, 19.9%) | Univariatea | Multivariateb | |
|---|---|---|---|---|
|
|
|
|||
| P-value | P-value | 95% CI | ||
| Age (y) | 69.2±12.6 | 0.638 | 0.728 | 0.182–9.966 |
| <50 | 2 (7.1) | |||
| 51–69 | 9 (32.1) | |||
| ≥70 | 17 (60.7) | |||
| Male | 14 (50.0) | 0.644 | 0.832 | 0.357–3.598 |
| Location of PE | 0.183 | 0.172 | 0.698–7.422 | |
| Main & lobar arteries | 14 (50.0) | |||
| Segmental & subsegmental arteries | 14 (50.0) | |||
| DVT | 14 (50.0) | 0.584 | 0.873 | 0.351–3.425 |
| Immobilization ≥3 days | 12 (42.9) | 0.961 | 0.467 | 0.444–5.866 |
| Active malignancy and/or chemotherapy | 18 (64.3) | 0.001 | 0.001 | 5.047–61.320 |
| Comorbidities | ||||
| HTN | 14 (50.0) | 0.421 | 0.408 | 0.175–2.033 |
| DM | 9 (32.1) | 0.905 | 0.747 | 0.229–2.879 |
| CAD | 2 (7.1) | 1.000 | 0.823 | 0.070–8.298 |
| Congestive heart failure | 3 (10.7) | 0.705 | 0.654 | 0.223–10.925 |
| Cerebrovascular disease | 5 (17.9) | 0.621 | 0.362 | 0.103–2.287 |
| Dementia | 1 (3.6) | 0.687 | 0.363 | 0.013–4.863 |
| Smoking | 2 (7.1) | 1.000 | 0.740 | 0.214–8.730 |
| HR >100/min | 11 (39.3) | 0.702 | 0.788 | 0.338–4.188 |
| SpO2 <90 mmHg | 7 (25.0) | 0.596 | 0.127 | 0.066–1.403 |
| Anticoagulation treatment | 22 (78.6) | 0.169 | 0.214 | 0.080–1.762 |
| Hypotension or shock | 9 (32.1) | 0.007 | 0.005 | 2.117–69.706 |
| Need for inotropics | 4 (14.3) | 0.229 | 0.651 | 0.210–12.164 |
Values are presented as mean±standard deviation or number (%).
PE, pulmonary embolism; CI, confidence interval; DVT, deep venous thrombosis; HTN, hypertension; DM, diabetes mellitus; CAD, coronary artery disease; HR, heart rate; SpO2, peripheral oxygen saturation.
Chi-square test or Fisher’s exact test,
logistic regression model.
DISCUSSION
In recent years, there has been an understanding that DVT and PE are similar disease entities presenting different clinical manifestations. However, few studies have investigated the overall prevalence of DVT in patients with PE. Several previous reports have shown a relatively high prevalence of concomitant DVT, ranging from 10% to 93% in various studies with differing methodologies [15–21]. Recently, Yamaki et al. [4] have reported that 58.1% of patients with PE have concomitant DVT with proximal DVT being found in 30% of these patients. Girard et al. [22] have stated that 60% of patients with CTPA-proven symptomatic PE possess concomitant DVT. In this study, we found that the prevalence of concomitant DVT among PE patients was 45.5% (64 of all 141 PE patients), while proximal DVT was shown in 39% (55) of all PE patients. Our results suggest that screening for DVT in patients with PE is necessary to prevent the development of PTS because approximately half of all patients with PE might have concomitant DVT.
Unfortunately, different studies have revealed contradiction and conflict in data concerning the clinical significance of concomitant DVT in the outcome of patients with PE. The International Cooperative Pulmonary Embolism Registry (ICOPER) study has reported that there is no association between concomitant DVT among PE patients and all-cause mortality [6]. In contrast, Wicki et al. [23] have demonstrated that patients with concomitant DVT have higher risk of death compared to patients without DVT. Furthermore, a recent meta-analysis has indicated that the presence of DVT in patients with acute PE is significantly associated with an increased risk of 30-day mortality [24]. In order to evaluate the clinical significance of DVT in patients with PE, we investigated PE-related clinical composite outcomes and 30-day all-cause mortality in this study and compared PE-related outcome and early mortality between non-DVT group and concomitant DVT group. Our results showed that 17.7% of all PE patients had PE-related unfavorable outcome, while 21.9% of concomitant DVT patients had unfavorable outcome. There was no statistically significant relationship between concomitant DVT and unfavorable outcome in patients with PE (P=0.240). In addition, 30-day all-cause mortality occurred in 19.9% of all PE patients and 21.9% of all concomitant DVT patients. However, the presence of concomitant DVT did not result in statistically significant difference in 30-day all-cause mortality for patients with PE (P=0.584). In summary, this study demonstrated that the presence of concomitant DVT did not affect PE-related unfavorable outcome or early mortality in patients with PE.
Furthermore, additional clinical risk factors for PE-related clinical outcome and all-cause mortality in patients with PE were determined in this study. Our multivariate analyses showed that HR >100/min (P=0.009) and SpO2 <90% (P=0.038) were significant risk factors for PE-related unfavorable outcome. Souza et al. have also reported that SpO2 <90% is one of the most accurate predictors of unfavorable outcome [25]. However, the present study revealed that there was no statistically significant relationship between pre-exiting comorbidities, including cardiovascular and respiratory system, and PE-related unfavorable outcome. Our results indicated that tachycardia and hypoxemia at the time of diagnosis of PE were potential risk factors for PE-related unfavorable outcome. In addition, our univariate and multivariate analyses showed that patients with active malignancy and/or chemotherapy (P=0.001) and hypotension or shock (P=0.016) were significant risk factors for 30-day all-cause mortality in patients with PE. Consequently, this study showed that the presence of active malignancy and cardiovascular compromise at the point of PE diagnosis might indicate poor prognosis for patients with PE.
This study has a number of potential limitations. Its major limitation is its retrospective study design for investigating the prevalence and clinical significance of DVT in patients with PE. Although this study used consistent diagnostic methods and treatment strategies for PE patients, the assessment of concomitant DVT was determined with two different diagnostic modalities (duplex ultrasonography and CT venography). In this study, a relatively lower rate of prevalence of DVT in patients with PE might be due to the use of two different methods for the diagnosis of concomitant DVT, although it has been reported that CT venography and duplex ultrasonography have similar accuracies in detecting DVT [26]. Another limitation of this study was the small number of patients with PE evaluated. This might have resulted in a high rate of PE-related unfavorable outcome and 30-day all-cause mortality in patients with PE. The lack of sample patients might have also resulted in the failure to establish clinical significances of concomitant DVT for the development of PE-related unfavorable outcome and early mortality. In addition, this study did not investigate biomarkers, such as D-dimer or serum lactate level, as additional independent risk factors for PE-related unfavorable outcome or 30-day all-cause mortality. Consequently, only clinical risk factors as potential predictors for PE-related unfavorable outcome and early mortality could be subjected to univariate and multivariate analyses.
CONCLUSION
In conclusion, our results revealed that approximately half of patients with PE possessed concomitant DVT. Therefore, our present study supports that the screening test for concomitant DVT is needed by all patients with confirmed PE.
Our results also demonstrated that 17.7% (25 of 141) of all PE patients and 21.9% (14 of 64) of patients with concomitant DVT had PE-related unfavorable outcome. Moreover, 30-day all-cause mortality rate was 19.9% in patients with PE and 21.9% in patients with concomitant DVT. However, this study failed to establish any clinical significance of concomitant DVT in the development of PE-related unfavorable outcome or early mortality. Based on our analysis for additional clinical risk factors, tachycardia and peripheral hypoxemia were identified as significant predictors for PE-related unfavorable outcome. In addition, active malignancy and/or chemotherapy and hypotension or shock were found to be significant risk factors for 30-day all-cause mortality in PE patients.
REFERENCES
- 1.Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I22–I30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 2.Hyers TM. Venous thromboembolism. Am J Respir Crit Care Med. 1999;159:1–14. doi: 10.1164/ajrccm.159.1.9803109. [DOI] [PubMed] [Google Scholar]
- 3.Perrier A, Bounameaux H. Cost-effective diagnosis of deep vein thrombosis and pulmonary embolism. Thromb Haemost. 2001;86:475–487. [PubMed] [Google Scholar]
- 4.Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Presence of lower limb deep vein thrombosis and prognosis in patients with symptomatic pulmonary embolism: preliminary report. Eur J Vasc Endovasc Surg. 2009;37:225–231. doi: 10.1016/j.ejvs.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 6.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/S0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 7.Douketis JD, Kearon C, Bates S, Duku EK, Ginsberg JS. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA. 1998;279:458–462. doi: 10.1001/jama.279.6.458. [DOI] [PubMed] [Google Scholar]
- 8.Büller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, et al. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003;349:1695–1702. doi: 10.1056/NEJMoa035451. [DOI] [PubMed] [Google Scholar]
- 9.Low-molecular-weight heparin in the treatment of patients with venous thromboembolism. The Columbus Investigators N Engl J Med. 1997;337:657–662. doi: 10.1056/NEJM199709043371001. [DOI] [PubMed] [Google Scholar]
- 10.Conget F, Otero R, Jiménez D, Martí D, Escobar C, Rodríguez C, et al. Short-term clinical outcome after acute symptomatic pulmonary embolism. Thromb Haemost. 2008;100:937–942. [PubMed] [Google Scholar]
- 11.Monreal M, Barba R, Tolosa C, Tiberio G, Todolí J, Samperiz AL, RIETE Investigators Deep vein thrombosis and pulmonary embolism: the same disease? Pathophysiol Haemost Thromb. 2006;35:133–135. doi: 10.1159/000093555. [DOI] [PubMed] [Google Scholar]
- 12.Giachino A. Relationship between deep-vein thrombosis in the calf and fatal pulmonary embolism. Can J Surg. 1988;31:129–130. [PubMed] [Google Scholar]
- 13.Philbrick JT, Becker DM. Calf deep venous thrombosis. A wolf in sheep’s clothing? Arch Intern Med. 1988;148:2131–2138. doi: 10.1001/archinte.1988.00380100029007. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb RH, Widjaja J, Mehra S, Robinette WB. Clinically important pulmonary emboli: does calf vein US alter outcomes? Radiology. 1999;211:25–29. doi: 10.1148/radiology.211.1.r99ap0125. [DOI] [PubMed] [Google Scholar]
- 15.Turkstra F, Kuijer PM, van Beek EJ, Brandjes DP, ten Cate JW, Büller HR. Diagnostic utility of ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med. 1997;126:775–781. doi: 10.7326/0003-4819-126-10-199705150-00005. [DOI] [PubMed] [Google Scholar]
- 16.van Rossum AB, van Houwelingen HC, Kieft GJ, Pattynama PM. Prevalence of deep vein thrombosis in suspected and proven pulmonary embolism: a meta-analysis. Br J Radiol. 1998;71:1260–1265. doi: 10.1259/bjr.71.852.10318998. [DOI] [PubMed] [Google Scholar]
- 17.Girard P, Musset D, Parent F, Maitre S, Phlippoteau C, Simonneau G. High prevalence of detectable deep venous thrombosis in patients with acute pulmonary embolism. Chest. 1999;116:903–908. doi: 10.1378/chest.116.4.903. [DOI] [PubMed] [Google Scholar]
- 18.Cham MD, Yankelevitz DF, Shaham D, Shah AA, Sherman L, Lewis A, et al. The Pulmonary Angiography-Indirect CT Venography Cooperative Group Deep venous thrombosis: detection by using indirect CT venography. Radiology. 2000;216:744–751. doi: 10.1148/radiology.216.3.r00se44744. [DOI] [PubMed] [Google Scholar]
- 19.Loud PA, Katz DS, Bruce DA, Klippenstein DL, Grossman ZD. Deep venous thrombosis with suspected pulmonary embolism: detection with combined CT venography and pulmonary angiography. Radiology. 2001;219:498–502. doi: 10.1148/radiology.219.2.r01ma26498. [DOI] [PubMed] [Google Scholar]
- 20.Coche EE, Hamoir XL, Hammer FD, Hainaut P, Goffette PP. Using dual-detector helical CT angiography to detect deep venous thrombosis in patients with suspicion of pulmonary embolism: diagnostic value and additional findings. AJR Am J Roentgenol. 2001;176:1035–1039. doi: 10.2214/ajr.176.4.1761035. [DOI] [PubMed] [Google Scholar]
- 21.Elias A, Colombier D, Victor G, Elias M, Arnaud C, Juchet H, et al. Diagnostic performance of complete lower limb venous ultrasound in patients with clinically suspected acute pulmonary embolism. Thromb Haemost. 2004;91:187–195. doi: 10.1160/TH03-05-0278. [DOI] [PubMed] [Google Scholar]
- 22.Girard P, Sanchez O, Leroyer C, Musset D, Meyer G, Stern JB, et al. Evaulation du Scanner Spiralé dans l’Embolie Pulmonaire Study Group Deep venous thrombosis in patients with acute pulmonary embolism: prevalence, risk factors, and clinical significance. Chest. 2005;128:1593–1600. doi: 10.1378/chest.128.3.1593. [DOI] [PubMed] [Google Scholar]
- 23.Wicki J, Perrier A, Perneger T V, Bounameaux H, Junod AF. Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thromb Haemost. 2000;84:548–552. [PubMed] [Google Scholar]
- 24.Becattini C, Cohen AT, Agnelli G, Howard L, Castejón B, Trujillo-Santos J, et al. Risk stratification of patients with acute symptomatic pulmonary embolism based on presence or absence of lower extremity DVT: systematic review and meta-analysis. Chest. 2016;149:192–200. doi: 10.1378/chest.15-0808. [DOI] [PubMed] [Google Scholar]
- 25.Souza CS, Resende FS, Rodrigues MP. Severe hypoxaemia can predict unfavourable clinical outcomes in individuals with pulmonary embolism aged over 40 years. Singapore Med J. 2014;55:483–487. doi: 10.11622/smedj.2014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson JC, Brown MD, McCullough N, Smith S. CT lower extremity venography in suspected pulmonary embolism in the ED. Emerg Radiol. 2006;12:160–163. doi: 10.1007/s10140-005-0459-3. [DOI] [PubMed] [Google Scholar]