Abstract
Telomeres are the capping ends of chromosomes that protect the loss of genetic material and prevent chromosomal instability. In human tissue-specific stem/progenitor cells, telomere length is maintained by the telomerase complex, which consists of a reverse transcriptase catalytic subunit (TERT) and an RNA template (TERC). Very short telomeres and loss-of-function mutations in the TERT and TERC have been reported in acute myeloid leukemia, but the role of telomeres in acute promyelocytic leukemia (APL) has not been well established. We report the results for a large cohort of 187 PML/RARα positive APL patients. No germline mutations in TERT or TERC genes were identified. Codon 279 and 1062 TERT polymorphisms were present at a frequency similar to that of the general population. Telomere length measured in blood or marrow mononuclear cells at diagnosis was significantly lower in the APL patients than in healthy volunteers, and shorter telomeres at diagnosis were significantly associated with high-risk disease. For patients who achieved complete remission, the median increase in telomere length from diagnosis to remission (delta TL) was 2.0 kilobase (kb) and we found delta TL to be the most powerful predictor for overall survival when compared with well-established risk factors for poor outcome in APL.
Keywords: telomere, length, survival, acute, promyelocytic, leukemia
Introduction
Telomeres are composed of tandem hexameric TTAGGG nucleotide repeats that protect the telomeres from the loss of genetic material due to end-replication deficiency of the DNA polymerase, as well as conferring stability to the chromosome ends. Telomeres are maintained by the telomerase enzyme complex, made up of a reverse transcriptase catalytic subunit (TERT) and an RNA template (TERC)[1]. TERT enzymatically adds TTAGGG nucleotide repeats to the 3′ end of the telomere leading strand by using TERC as a template. Present in very specific cells, such as embryonic and adult stem cells, telomerase elongates the linear telomeric sequence by the addition of new hexameric repeats, thus slowing the attrition rate of telomeric repeats and preventing replicative cellular aging. Loss-of-function mutations in the telomerase complex genes lead to telomere shortening in humans, and have been linked to malignant transformation and alteration of normal bone marrow hematopoiesis[2]. Telomerase activity is also often increased in neoplastic cells and is important for continuous cancer cell proliferation in human malignancies[3]. Though very short telomeres and germline mutations in TERT and TERC have been associated with acute myeloid leukemia (AML)[4], the association between telomere length (TL) or telomerase complex integrity and outcomes in patients with acute promyelocytic leukemia (APL) has not been thoroughly analyzed.
The majority of patients with APL are characterized by a balanced reciprocal translocation between the long arms of chromosomes 15 and 17, giving rise to the novel PML/RARα fusion gene. Currently, APL is effectively treated with the cell differentiation inducer, all-trans retinoic acid (ATRA) and with arsenic trioxide (ATO)[5]. In vitro studies have demonstrated that the success of ATRA/ATO treatment may be at least in part explained by the synergistic effect in triggering down regulation of telomerase enzyme, leading to TL shortening and subsequent cell death[6]. The association between TL and telomerase activity in patients with APL has been addressed sporadically, one report recognizing that shortened TL and elevated telomerase activity in APL patients correlate with inferior overall survival[7].
Mutations in the human TERT and TERC genes have been described in dyskeratosis congenita and other bone marrow failure syndromes[8–10], placing these patients at a high risk of secondary malignant transformations[11]. The link between mutations in the TERT and TERC genes in association with APL is anecdotal, with only a few APL patients reported with TERT gene polymorphisms among a larger group of AML patients[4].
This report describes the largest analysis to date of TL and germline mutations in TERT and TERC in mononuclear cells (MNC) from the blood or bone marrow of APL patients at all stages of disease including time of diagnosis, complete remission (CR), and at relapse. We measured TL and delta TL, the difference between TL in MNCs at diagnosis and in remission, in comparison with clinical and laboratory parameters, including disease risk status defined by presenting white blood cell (WBC) count, early mortality, achievement of CR, disease-free survival (DFS) and overall survival (OS).
Materials/Subjects and Methods
APL cohort
One hundred and eighty seven previously untreated APL patients enrolled in the intergroup trials E2491, C9710, S0521, and S0535 with available DNA from blood or bone marrow MNCs from the ECOG-ACRIN Leukemia Tissue Bank were included in this study. E2491 was a phase III randomized study of ATRA versus cytosine arabinoside and daunorubicin as induction therapy in both high and low risk patients[12]; C9710 was a phase III randomized study of concurrent ATRA and chemotherapy with or without ATO as initial consolidation therapy followed by maintenance therapy with intermittent ATRA versus intermittent ATRA plus mercaptopurine and methotrexate[5]; S0521 was a Phase III randomized trial of maintenance versus observation for low and intermediate risk APL; and S0535 was a phase II study of ATRA, ATO and gemtuzumab ozogamicin in high-risk APL (both S0521 and S0535 are ongoing, but not recruiting participants). The diagnosis of APL was established based on the presence of PML/RARα by qualitative polymerase chain reaction (PCR). Disease risk groups were defined as low risk (WBC ≤ 10 ×109/L) and high risk (WBC > 10 ×109/L)[5]. All studies were carried out in accordance with local institution-approved regulations, and informed written consent was obtained from each patient. Telomere length in the APL samples was compared with the samples from the healthy, age matched controls.
TERT/TERC sequencing and telomere length assay
DNA was isolated from Ficoll-Hypaque separated MNCs using the Qiagen DNA isolation kit.Mean telomere length was measured by a validated, CLIA-certified quantitative PCR (qPCR) method, using the Rotor-Gene SYBR Green Kit (Qiagen)[13]. Relative telomere length was calculated by generating a ratio between total telomere DNA (T) and DNA from amplification of a single copy gene (S), thereby providing a relative T/S ratio normalized to the reference sample. Age-adjusted telomere length was calculated using 299 healthy volunteers, ages 0 to 99, for whom telomere length was measured from peripheral blood leukocytes. All healthy volunteers were enrolled in clinical research trials at National Heart, Lung, and Blood Institute. For ease of reading, we refer to mean telomere content as telomere length (TL) in the current paper. TERT and TERC sequencing was performed as previously described[4].
DNA was isolated from Ficoll-Hypaque separated MNCs using the Qiagen DNA isolation kit. Mean telomere content by qPCR and Sanger sequencing of TERC and TERT were performed as previously described[4,5]. For ease of reading, we refer to mean telomere content as telomere length (TL) in the current paper.
Statistical Analysis
Patient demographic factors and laboratory parameters were compared among groups using Fisher's exact test and the Kruskal-Wallis test when appropriate. Spearman correlation was calculated between numeric variables. OS was measured from study entry to death from any cause with follow-up censored at the date of last contact. DFS was defined as the time from hematologic CR to relapse or death from any cause, whichever occurred earlier. Patients without documented relapse or death reported were censored at the time of last contact. Given the heterogeneity of the study and treatment schedules, the analysis performed here was stratified by the combination of study and treatment. To examine the association between TL length and clinical outcomes, odds ratio (OR) and its corresponding 95% confidence interval (CI) were calculated using stratified logistic models. OS and DFS were estimated using the Kaplan-Meier method and compared using stratified log-rank tests. Hazard ratios were computed and tested using stratified univariate and multivariable Cox proportional hazards (PH) models, with ECOG performance status (0 vs. 1 vs. 2-3), WBC risk status (low vs. high), age, platelet counts, and hemoglobin counts serving as covariates in the multivariable model. Cox PH models with forward selection (with entry criterion set at 0.20) were used to find the most potent predictor of OS and DFS. All reported p values are two-sided. A level of 5% was considered statistically significant.
Results
Patient characteristics
Patient pretreatment characteristics by study are presented in Table 1. Median follow up for survivors among the 187 patients was 3.9 years (range: 0-14.5 years), from the time of study enrollment. No statistically significant difference was observed in age, sex, ECOG performance status (PS), platelet or hemoglobin values at baseline between the four studies; however, WBC count was statistically different among the four cohorts -whether characterized by the numerical values on the continuous scale (median of 3.4, 2.1, 2.0 and 21.9 ×109/L for C9710, E2491, S0521 and S0535 respectively, p<0.001) or by risk status. Early death (defined as death occurring ≤ 30 days from entering the study), CR, and relapse status by study are also summarized in Table 1. Although there was no statistically significant difference in early mortality rate across the four studies (p=0.65), a significant difference was noted with respect to CR (p=0.03) and relapse status (p<0.0001).
Table 1. Pretreatment Characteristics for Patients with Telomere Data (N=190).
| C9710 | E2491 | S0521 | S0535 | p1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | N | % | N | % | N | % | N | % | |
| Age (median, range) | 44.5 (18, 75) | 42.5 (16, 76) | 44 (22, 59) | 46 (19, 63) | 0.77 | ||||
| WBC (×109/L) (median, range)2 | 3.4 (0.4, 117.4) | 2.1 (0.3, 550.0) | 2.0 (0.4, 12.4) | 21.9 (10.4, 91.7) | <0.0001 | ||||
| Platelets (×109/L) (median, range)3 | 28.0 (4.0, 232.0) | 24.5 (2.0, 144.0) | 20.0 (7.0, 65.0) | 15.5 (12.0, 72.0) | 0.18 | ||||
| Hemoglobin (g/dL) (median, range)4 | 9.5 (6.1, 29.6) | 9.7 (2.1, 13.8) | 8.8 (7.3, 14.8) | 9.2 (5.9, 12.3) | 0.92 | ||||
| Sex | 0.53 | ||||||||
| Male | 52 | 58 | 39 | 53 | - | - | - | - | |
| Female | 38 | 42 | 35 | 47 | - | - | - | - | |
| ECOG Performance Status | 0.93 | ||||||||
| 0 | 35 | 41 | 30 | 41 | 4 | 31 | 4 | 31 | |
| 1 | 38 | 45 | 34 | 46 | 8 | 61 | 7 | 54 | |
| 2 | 6 | 7 | 7 | 9 | 1 | 8 | 2 | 15 | |
| 3 | 6 | 7 | 2 | 4 | 0 | 0 | 0 | 0 | |
| Unknown | 5 | - | 0 | - | 0 | - | 0 | - | |
| Risk Group by WBC2, 5 | <0.0001 | ||||||||
| Low | 64 | 74 | 58 | 78 | 12 | 92 | 0 | 0 | |
| High | 22 | 26 | 16 | 22 | 1 | 8 | 13 | 100 | |
| Early Death6 | 0.65 | ||||||||
| No | 84 | 93 | 67 | 91 | 13 | 100 | 13 | 100 | |
| Yes | 6 | 7 | 7 | 9 | 0 | 0 | 0 | 0 | |
| Complete Remission | 0.03 | ||||||||
| No | 10 | 11 | 17 | 23 | 0 | 0 | 0 | 0 | |
| Yes | 80 | 89 | 57 | 77 | 13 | 100 | 13 | 100 | |
| Relapse | <0.0001 | ||||||||
| No | 70 | 88 | 30 | 53 | 13 | 100 | 13 | 100 | |
| Yes | 10 | 12 | 27 | 47 | 0 | 0 | 0 | 0 | |
Fisher's exact test or the Kruskal-Wallis test when appropriate.
Four patients in C9710 without WBC data.
Four patients in C9710 without platelet data; 1 patient in S0535 without platelet data.
Seven patients in C9710 without hemoglobin data.
Low risk group, WBC ≤ 10 ×109/L and high risk group > 10 ×109/L.
Defined as death occurring ≤ 30 days from entering the study.
Telomere length at diagnosis, remission, and relapse of APL
Quantitative PCR measurements of TL in kilobase (kb) pairs was performed at various disease time points across all four studies in patients who had samples available for extraction of DNA, and the results are presented in Table 2. Of 187 patients analyzed at diagnosis, 143 had DNA available in hematologic and molecular CR (PML/RARα negative), and 18 at relapse. At diagnosis, DNA was extracted from blood or bone marrow MNCs that contained a median of 75% (range, 13-99%) of leukemic promyelocytes. No statistically significant association between TL at presentation and percentage of leukemic promyelocytes at presentation was observed (p=0.12, n=172 across all four studies).
Table 2. Telomere Length (kb) (across Studies) at Various Time Point.
| Time Point | N | Median | 1st Quartile | 3rd Quartile | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Diagnosis | 187 | 5.1 | 4.6 | 5.6 | 3.9 | 7.7 |
| Remission | 143 | 7.2 | 6.7 | 8.1 | 4.6 | 13.2 |
| Delta TL1 | 140 | 2.0 | 1.3 | 2.7 | 0.1 | 7.1 |
| Relapse | 18 | 6.5 | 5.5 | 8.4 | 4.5 | 10.3 |
Delta TL refers to change in telomere length, defined as telomere length at remission minus telomere length at diagnosis.
TL at diagnosis was significantly different among all four studies (p=0.002; Table 3). Similar results were observed for TL in remission (p=0.002). Specifically, patients in S0535 had the lowest TL distribution at diagnosis (median 4.8, range 4.2 - 7.2 kb), while those in S0521 had the highest TL distribution (median 5.7, range 4.6 - 6.4 kb), echoing the risk status of APL patients in these studies. Delta TL (change in TL at time of diagnosis of APL to time of CR) was not significantly different among the four studies (p=0.32). Telomere length at relapse was only available for 5 patients from C9710 and 13 patients from E2491, and it demonstrated no statistically significant difference in TL between these two studies (p=0.08). TL at diagnosis was significantly shorter in the APL samples compared with the samples from healthy controls (median 5.1 (range 3.9 – 7.7) vs. 7.8 (range 5.3 – 14.2) kb, p<0.0001; Figure 1A). For patients in CR, the median delta TL was 2.0 kb (range 0.1 - 7.1 kb; signed rank test against 0, p<0.0001, Figure 1B); however, TL at remission in these patients was still shorter than that in age-matched healthy control subjects [median 7.2 (range 4.6 – 13.2) vs. 7.8 kb, p<0.0001]. We observed statistically significant increase in TL from the time points of diagnosis to relapse (median 5.1 vs. 6.5 kb, p=0.005, n=16). However, no significant decrease in TL was found between CR and relapse (median 7.2 vs. 6.5 kb, respectively, p=0.10). Noted that this finding was based on 14 patients only.
Table 3. Median and Range of Telomere Length (kb) by Study and Time Point.
| C9710 | E2491 | S0521 | S0535 | P1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Time Point | N | Length | N | Length | N | Length | N | Length | |
| Diagnosis | 90 | 5.0 (3.9, 6.9) | 71 | 5.3 (4.1, 7.7) | 13 | 5.7 (4.6, 6.4) | 13 | 4.8 (4.2, 7.2) | 0.002 |
| Remission | 72 | 6.9 (4.6, 10.7) | 45 | 7.8 (5.1, 13.2) | 13 | 7.4 (6.4, 9.2) | 13 | 7.1 (5.4, 9.9) | 0.002 |
| Delta TL2 | 72 | 1.9 (0.1, 4.5) | 42 | 2.3 (0.1, 7.1) | 13 | 1.9 (0.6, 3.5) | 13 | 2.3 (0.6, 4.8) | 0.32 |
| Relapse | 5 | 6.0 (4.5, 6.9) | 13 | 6.9 (4.6, 10.3) | 0 | - | 0 | - | 0.08 |
Kruskal-Wallis Test
Delta TL refers to change in telomere length, defined as telomere length at remission minus telomere length at diagnosis.
Figure 1. Telomere length at Diagnosis and at Remission.
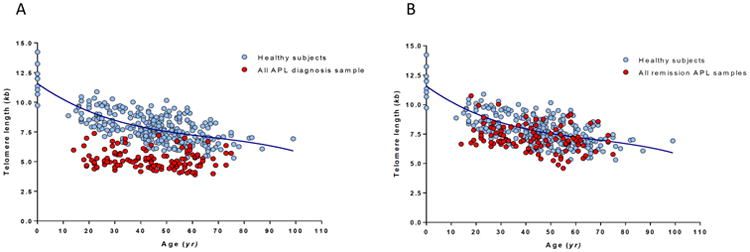
Telomere length at diagnosis (A) and at remission (B), both displayed as telomere length in (kb) over the age (years) of APL patients, as compared to a healthy, age-matched subjects.
Longer telomeres at diagnosis correlate with low-risk disease and better clinical outcomes
Telomere length at diagnosis was significantly associated with disease risk status (OR=4.32, 95% CI=(2.02, 9.22), p=0.0002). Our results indicate that a one-kb higher TL at diagnosis translated into 332% increase in the odds of having low risk APL. The association between TL at diagnosis and APL risk status once again supported the finding of differences in TL at diagnosis among the 4 cohorts with different risk levels. Among the 187 patients with TL data at diagnosis, 160 (85.6%) patients achieved CR and 13 (7.0%) patients died early across all studies. Neither CR (OR=1.10, 95% CI=(0.60, 2.00), p=0.76) nor early death (OR=0.62, 95% CI=(0.25, 1.53), p=0.30) were significantly associated with TL at diagnosis.
Preliminary analysis of impact of telomere length at diagnosis and delta TL on OS and DFS using the Akaike information criterion (AIC) values from stratified univariate Cox PH regression models showed that using the third quartile (Q3) as the cutoff point to dichotomize TL fits the model best (i.e., decreased the AIC value most from the null model), compared to using the first quartile (Q1), the median (Q2), or TL on the continuous scale (data not shown; no significant difference in AIC values with respect to various cutoff points for TL at remission). Consequently, hazard ratio (HR) (TL: long/short or delta TL: big/small) and its 95% CI with respect to OS and DFS were calculated using Q3 as the TL cutoff point for each time point. Table 4 summarizes the hazard ratio and its corresponding 95 % CI for OS and DFS by disease time point, using univariate and multivariable Cox PH models. Both OS (log-rank p=0.023; Figure 2A) and DFS (log-rank p=0.019; Figure 2B) were statistically superior in patients who presented with longer telomeres. However, this effect on OS and DFS disappeared after controlling for risk status (low vs. high), ECOG PS (0 vs. 1 vs. 2-3), age, platelet count, and hemoglobin value (Table 4). A Cox PH model with forward selection on all these baseline predictors showed that risk status was selected into the model first to predict either OS (χ2=6.55, p=0.0002) or DFS (χ2=6.55, p=0.01), followed by hemoglobin, then TL at diagnosis.
Table 4. Hazard Ratio of OS and DFS Using Stratified Univariate and Multivariable Cox Proportional Hazards Regression Models.
| Efficacy | Parameter | # of Events/Total N | Univariate | Wald p | # of Events/Total N | Multivariable1 | Wald p |
|---|---|---|---|---|---|---|---|
| HR (long/short or big/small) & 95% CI | HR (long/short or big/small) & 95% CI | ||||||
| OS2 | TL at diagnosis (by Q3) | 49/186 | 0.44 (0.21, 0.90) | 0.026 | 48/177 | 0.65 (0.29, 1.45) | 0.29 |
| TL at remission (by Q3) | 24/143 | 1.05 (0.44, 2.53) | 0.91 | 23/137 | 1.36 (0.50, 3.69) | 0.54 | |
| Delta TL(by Q3)3 | 21/140 | 3.60 (1.44, 9.01) | 0.006 | 20/134 | 3.51 (1.20, 10.31) | 0.02 | |
| DFS | TL at diagnosis (by Q3) | 37/159 | 0.36 (0.15, 0.87) | 0.023 | 35/151 | 0.43 (0.16, 1.14) | 0.09 |
| TL at remission (by Q3) | 30/143 | 0.79 (0.35, 1.77) | 0.56 | 29/137 | 0.82 (0.34, 2.00) | 0.67 | |
| Delta TL(by Q3)3 | 27/140 | 1.69 (0.76, 3.79) | 0.20 | 26/134 | 1.30 (0.51, 3.29) | 0.58 |
Adjusted for ECOG performance status (0 vs. 1 vs. 2-3), WBC risk group (low vs. high), age, platelet counts, and hemoglobin counts.
One patient in C9710 is missing survival indicator.
Delta refers to change in telomere length, defined as telomere length at remission minus telomere length at diagnosis.
Figure 2. Overall-Survival and Disease-Free Survival by Telomere Length at Diagnosis.
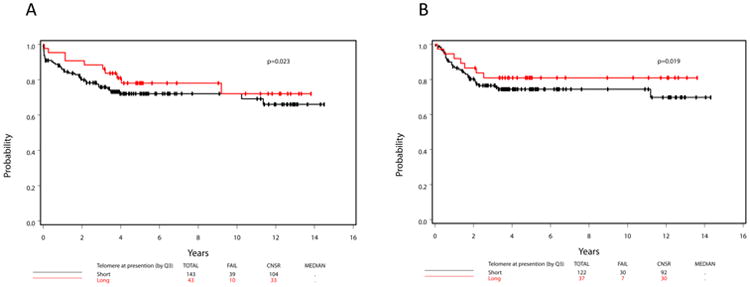
Overall survival (OS) (A) and disease-free survival (DFS) (B) curves by short or long telomere lengths (TL) at diagnosis (with the third quartile as the cutoff point).
Telomere length in remission does not correlate with clinical outcomes
Among the 143 patients with available TL in CR, 29 (20.3%) patients relapsed. We found no statistically significant association between TL at remission and the likelihood of relapse (OR=0.96, 95% CI=(0.64, 1.42), p=0.82). Similarly, delta TL did not significantly associate with risk of relapse (OR=1.06, 95% CI=(0.70, 1.59), p=0.79). TL at remission had no statistically significant effect on either OS (HR (long/short)=1.05, 95% CI=(0.44, 2.53), p=0.91) or DFS (HR (long/short)=0.79, 95% CI=(0.35, 1.77), p=0.56).
We subsequently examined the change in TL as it occurred during the disease course. For patients who achieved CR, the change in telomere length from diagnosis of APL to CR was defined as delta TL, with a positive delta TL indicating an increase in TL at remission. There was a negative correlation between TL at remission and TL at diagnosis (Spearman ρ=-0.27, p=0.0012), suggesting that patients with shorter TL at diagnosis experience bigger delta TL, and patients with longer starting TL tended to achieve smaller delta TL.
Telomere length recovery (delta TL) is the strongest predictor of OS
Next, we analyzed the association of delta TL with clinical outcomes [change in TL, small vs. big, dichotomized by Q3 (at 2.7 kb)]. Patients with small delta TL had significantly longer OS than patients with a big delta TL (HR (big/small)=3.60, 95% CI=(1.44, 9.01), p=0.006). Results from multivariable Cox regression indicated that delta TL remained a statistically significant predictor for OS (HR=3.51, 95% CI=(1.20, 10.31), p=0.02) after adjusting for known risk factors (WBC risk status, ECOG PS, age, platelet count, and hemoglobin value) at baseline. This effect of delta TL on OS was even more pronounced in patients who achieved a CR (χ2=9.61, p=0.002) compared to TL at diagnosis, age, ECOG PS, platelet count, or hemoglobin value (χ2=1.31, 6.29, 0.54, 2.13, 4.58, and 0.52, respectively). A Cox model with forward selection on these predictors showed that delta TL was entered into the model first, followed by platelet counts, then hemoglobin. DFS was not statistically different between patients with small and big delta TL (log-rank p=0.20; Figure 3B)
Figure 3. Overall-Survival and Disease-Free Survival by Telomere Length Change.
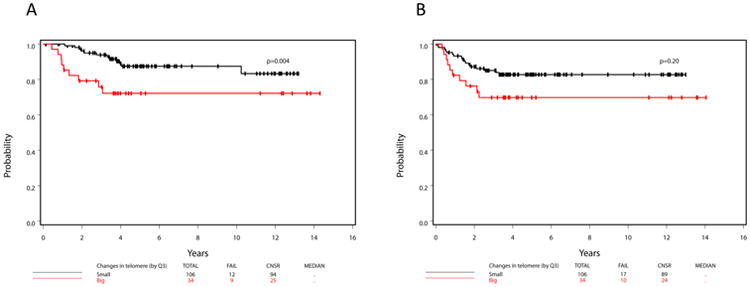
Overall survival (A) and disease-free survival (DFS) (B) curves by small or big telomere length changes (delta TL) (with the third quartile as the cutoff point).
TERT and TERC Mutational Status in APL
Sanger sequencing for TERT and TERC genes of diagnosis samples in the first 90 patients of this cohort failed to identify mutations, apart from the exon 2 codon 279 (GCC/ACC - Ala/Thr) and exon 15 codon 1062 (GCC/ACC - Ala/Thr) polymorphisms in the TERT, which were identified at a frequency of 4.4% and 2.2% respectively. These rates are ultimately similar to those found in 528 previously reported healthy volunteers (1.9% and 1.3%, p=0.13 and p=0.63, respectively by Fisher exact T-test)[14] (Table 1S). For the observed polymorphisms in our patients, sequencing of the remission samples showed the heterozygous presence of the same single nucleotide variants, consistent with a germline inheritance.
Discussion
The treatment of APL is among the most unique in the myeloid leukemias, and represents a major triumph in the field of hematologic malignancies. The exquisite sensitivity of PML/RARα containing leukemic promyelocytes to ATRA and ATO result in a cure rates of 85% to 90%, provided that potentially lethal hemorrhagic complications at presentation or during induction are avoided[15]. To date, the most important prognostic factor in APL besides age, and the sole factor influencing treatment decisions, has been the WBC count at presentation. Other biologic features, such as PML/RARα isoform[16], expression of the neural cell adhesion factor CD56[17], additional cytogenetic abnormalities[18] or mutations in the FLT3 gene[19] have been considered and found to have prognostic impact in some but not all studies. Considering survivorship issues in APL patients as the final frontier in the management of this disease, identification of stronger predictors of the long-term outcome may prove invaluable, potentially directing post-remission therapy.
In the largest study of TL in APL patients analyzed to date, we investigated TL in 187 subjects enrolled across four intergroup trials. As seen in AML[4] and various solid tumors[20], DNA from untreated APL patients consistently had very short TL when compared with healthy individuals. We assumed that the low-density MNCs collected after the Ficoll gradient separation at diagnosis were all APL blasts. In fact, this cell fraction is frequently heterogeneous, particularly in APL cases with low WBC count at presentation, in which the percentage of APL cells is typically much less that 100%. Nevertheless, in our study, we did not find an association between TL and the percentage of leukemic promyelocytes, even though the DNA, that was extracted from the entire MNC fraction of blood or bone marrow samples contained a median of 75% leukemic promyelocytes (range 13-99%). As an adjustment to this variance, separating the APL promyelocytes with flow cytometric sorting and analyzing a pure APL blast population could in future trials provide a more refined measurement of TL.
At diagnosis, TL was a statistically significant predictor of both DFS and OS. However, very short telomeres were associated with higher WBC count, suggesting that increased proliferative rate of the leukemic cells led to enhanced telomere attrition.
The only existing study of telomere biology in APL is a retrospective analysis of 32 newly diagnosed and 8 relapsed APL patients from a single institution.7 In this study, telomerase activity was significantly higher in relapsed patients versus newly diagnosed, and a higher degree of TL recovery (corresponding to the delta TL in our study) was a predictor for longer OS. In our cohort, delta TL was the most powerful predictor of OS, more powerful than WBC risk status which currently stands as the only clinical parameter that segregates patients into low and high risk subgroups, ultimately informing treatment[5]. This biological association, now found in two independent retrospective APL cohorts, may appear paradoxical, as recovery to normal hematopoiesis with longer telomeres in circulating leukocytes should not be associated with poorer outcomes. As TL in remission is likely representative of the TL of normal hematopoietic cells, delta TL is a measure of the degree of telomere attrition occurring from the time of acquisition of t(15:17) in a normal myeloid stem/progenitor to the overt presentation of APL. Thus, large delta TL, better than WBC count at diagnosis of APL, identifies a group of patients with very active disease, and inferior prognosis. In our analysis, we have accounted for known clinical risk factors for poor OS in APL but we cannot rule out that other indicators of inferior outcome play a role in this patient population. Molecular markers such as FLT3-ITD[19], CD95 promoter polymorphism varients[21] or minimal residual disease by reverse transcription PCR[22] have been proposed as relevant predictors of outcome, and are likely responsible for refractory/relapsed disease through various, postulated or proven, mechanisms. Telomere dynamics in recovery from APL should be investigated as an additional factor in identifying the high-risk disease regardless of the available molecular mechanisms.
In patients with AML, germline mutations in human TERT have been described, with the most common mutation 3 times more prevalent than in healthy controls[4]. In our analysis of APL patients, TERT mutations in APL exon 2 codon 279 (Ala/Thr) and polymorphisms in exon 15 codon 1062 (Ala/Thr) were identified at a frequency similar to that in normal healthy volunteers[14], consistent with t(15:17) being sufficient to drive the leukemic process in APL[16]. Moreover, APL was not reported in a cohort of patients with telomere disease[23], making it unlikely that TERT mutations, or short telomeres, are associated with the acquisition of t(15:17).
Recently reported phase III trials from Europe that utilized the ATRA/ATO combinations upfront across all APL risk-subgroups did not explore the role of telomere dynamics in disease stratification and/or low rates of relapse[24,25]. Our findings of TL, and delta TL in particular, as powerful predictors of OS in APL warrant prospective confirmation. Given that most of the deaths in our analysis (all E2491 cases and a majority of C9710 cases) occurred in patients that did not receive primary ATO, this analysis needs to be applied in particular to updated and ongoing protocols that use upfront ATO. If corroborated, TL measurement may be useful as a simple and inexpensive parameter in disease risk stratification and as a meaningful predictor of OS in patients with APL.
Acknowledgments
This study was conducted by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180820, CA180794, CA189859, CA180791, CA180816, CA180819, CA180821, CA180790, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Appendix A.
APLs and Controls Sequencing for human TERT and TERC.
Sanger sequencing for TERT and TERC genes failed to identify mutations, apart from the exon 2 codon 279 (GCC/ACC - Ala/Thr) and exon 15 codon 1062 (GCC/ACC -Ala/Thr) polymorphisms in the TERT, which were previously identified.
| SNP | Cohort | SNP present | SNP absent | SNP Percentage | Fisher exact Test |
|---|---|---|---|---|---|
| TERT, Exon 2, codon 279 GCC/ACC (Ala/Thr) | Control | 10 | 518 | 1.9 | P=0.13 |
| APL | 4 | 86 | 4.4 | ||
| TERT, Exon 15, codon 1062 GCC/ACC (Ala/Thr) | Control | 7 | 521 | 1.3 | P=0.63 |
| APL | 2 | 88 | 2.2 |
Footnotes
Disclosure of Conflicts of Interest: Authors report no conflicts of interest.
Authorship Contributions: M.B. and M.S.T. designed the study, performed data analysis, wrote the manuscript; B.D. and N.S.Y. performed the TL measurements and TERT/TERC mutation analysis, analyzed the results, co-wrote the manuscript; C.C. performed the TL measurements and TERT/TERC mutation analysis; J.L. performed all statistical analysis and statistical interpretation of the data and co-wrote the manuscript; E.P. isolated patient sample DNA, analyzed the data and co-wrote the manuscript; P.H.W. and J.R. isolated patient sample DNA; E.S., R.E.G., J.M.R., F.R.A., B.L.P., R.A.L., S.E.C., J.L., M.R.L. and S.L. provided patient samples.
References
- 1.Blackburn EH. Switching and signaling at the telomere. Cell. 2001 Sep 21;106:661–73. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 2.Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008 May 1;111:4446–55. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 4.Calado RT, Regal JA, Hills M, Yewdell WT, Dalmazzo LF, Zago MA, Lansdorp PM, Hogge D, Chanock SJ, Estey EH, Falcão RP, Young NS. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A. 2009 Jan 27;106:1187–92. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM, Rowe JM, Coutre S, Feusner JH, Gregory J, Couban S, Appelbaum FR, Tallman MS, Larson RA. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116:3751–3757. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarkanyi I, Dudognon C, Hillion J, Pendino F, Lanotte M, Aradi J, Ségal-Bendirdjian E. Retinoid/arsenic combination therapy of promyelocytic leukemia: induction of telomerase-dependent cell death. Leukemia. 2005 Oct;19:1806–11. doi: 10.1038/sj.leu.2403923. [DOI] [PubMed] [Google Scholar]
- 7.Ghaffari SH, Shayan-Asl N, Jamialahmadi AH, Alimoghaddam K, Ghavamzadeh A. Telomerase activity and telomere length in patients with acute promyelocytic leukemia: indicative of proliferative activity, disease progression, and overall survival. Ann Oncol. 2008 Nov;19:1927–34. doi: 10.1093/annonc/mdn394. [DOI] [PubMed] [Google Scholar]
- 8.Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998 May;19:32–8. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 9.Vulliamy TJ, Walne A, Baskaradas A, Mason PJ, Marrone A, Dokal I. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol Dis. Jan;34:257–63. doi: 10.1016/j.bcmd.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001 Sep 27;413:432–5. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 11.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009 Jun 25;113:6549–57. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Willman C, Bloomfield CD, Rowe JM, Wiernik PH. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 13.Winkler T, Hong SG, Decker JE, Morgan MJ, Wu C, Hughes WM, 5th, Yang Y, Wangsa D, Padilla-Nash HM, Ried T, Young NS, Dunbar CE, Calado RT. Defective telomere elongation and hematopoiesis from telomerase-mutant aplastic anemia iPSCs. J Clin Invest. 2013 May;123:1952–63. doi: 10.1172/JCI67146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005 May 7;352:1413–24. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, Altman JK, Douer D, Rowe JM, Tallman MS. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118:1248–1254. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baljevic M, Park JH, Stein E, Douer D, Altman JK, Tallman MS. Curing all patients with acute promyelocytic leukemia: are we there yet? Hematol Oncol Clin North Am. 2011 Dec;25:1215–33, viii. doi: 10.1016/j.hoc.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray CK, Estey E, Paietta E, Howard RS, Edenfield WJ, Pierce S, Mann KP, Bolan C, Byrd JC. CD56 expression in acute promyelocytic leukemia: a possible indicator of poor treatment outcome? J Clin Oncol. 1999 Jan;17:293–7. doi: 10.1200/JCO.1999.17.1.293. [DOI] [PubMed] [Google Scholar]
- 18.Hiorns LR, Swansbury GJ, Mehta J, Min T, Dainton MG, Treleaven J, Powles RL, Catovsky D. Additional chromosome abnormalities confer worse prognosis in acute promyelocytic leukaemia. Br J Haematol. 1997 Feb;96:314–21. doi: 10.1046/j.1365-2141.1997.d01-2037.x. [DOI] [PubMed] [Google Scholar]
- 19.Au WY, Fung A, Chim CS, Lie AK, Liang R, Ma ES, Chan CH, Wong KF, Kwong YL. FLT-3 aberrations in acute promyelocytic leukaemia: clinicopathological associations and prognostic impact. Br J Haematol. 2004 May;125:463–9. doi: 10.1111/j.1365-2141.2004.04935.x. [DOI] [PubMed] [Google Scholar]
- 20.Heaphy CM, Meeker AK. The potential utility of telomere-related markers for cancer diagnosis. J Cell Mol Med. 2011 Jun;15:1227–38. doi: 10.1111/j.1582-4934.2011.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunter NJ, Scott K, Hills R, Grimwade D, Taylor S, Worrillow LJ, Fordham SE, Forster VJ, Jackson G, Bomken S, Jones G, Allan JM. A functional variant in the core promoter of the CD95 cell death receptor gene predicts prognosis in acute promyelocytic leukemia. Blood. 2012 Jan 5;119:196–205. doi: 10.1182/blood-2011-04-349803. [DOI] [PubMed] [Google Scholar]
- 22.Chendamarai E, Balasubramanian P, George B, Viswabandya A, Abraham A, Ahmed R, Alex AA, Ganesan S, Lakshmi KM, Sitaram U, Nair SC, Chandy M, Janet NB, Srivastava VM, Srivastava A, Mathews V. Role of minimal residual disease monitoring in acute promyelocytic leukemia treated with arsenic trioxide in frontline therapy. Blood. 2012 Apr 12;119:3413–9. doi: 10.1182/blood-2011-11-393264. [DOI] [PubMed] [Google Scholar]
- 23.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009 Dec 10;361:2353–65. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo-Coco F, 1, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona E, Specchia G, Sica S, Divona M, Levis A, Fiedler W, Cerqui E, Breccia M, Fioritoni G, Salih HR, Cazzola M, Melillo L, Carella AM, Brandts CH, Morra E, von Lilienfeld-Toal M, Hertenstein B, Wattad M, Lübbert M, Hänel M, Schmitz N, Link H, Kropp MG, Rambaldi A, La Nasa G, Luppi M, Ciceri F, Finizio O, Venditti A, Fabbiano F, Döhner K, Sauer M, Ganser A, Amadori S, Mandelli F, Döhner H, Ehninger G, Schlenk RF, Platzbecker U. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013 Jul 11;369:111–21. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 25.Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, Morgan YG, Lok J, Grech A, Jones G, Khwaja A, Friis L, McMullin MF, Hunter A, Clark RE, Grimwade D. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015 Oct;16:1295–305. doi: 10.1016/S1470-2045(15)00193-X. [DOI] [PubMed] [Google Scholar]


