Abstract
Neurotoxic viral protein TAT may contribute to deficits in dopaminergic and cognitive function in individuals infected with human immunodeficiency virus. Transgenic mice with brain-specific doxycycline-induced TAT expression (TAT+, TAT- control) show impaired cognition. However, previously reported TAT-induced deficits in reversal learning may be compromised by initial learning deficits. We investigated the effects of TAT expression on memory retention/recall and reversal learning, and neurotransmitter function. We also investigated if TAT-induced effects can be reversed by improving dopamine function with selegiline, a monoamine oxidase inhibitor. Mice were tested in the Barnes maze and TAT expression was induced after the task acquisition. Selegiline treatment continued throughout behavioral testing. Dopamine, serotonin and glutamate tissue levels in the prefrontal/orbitofrontal cortex, hippocampus and caudate putamen were measured using high performance liquid chromatography. Neither TAT expression nor selegiline altered memory retention. On day 2 of reversal learning testing, TAT+ mice made fewer errors and used more efficient search strategies than TAT- mice. TAT expression decreased dopamine turnover in the caudate putamen, increased serotonin turnover in the hippocampus and tended to increase the conversion of glutamate to glutamine in all regions. Selegiline decreased dopamine and serotonin metabolism in all regions and increased glutamate levels in the caudate putamen. In the absence of impaired learning, TAT expression does not impair spatial memory retention/recall, and actually facilitates reversal learning. Selegiline-induced increases in dopamine metabolism did not affect cognitive function. These findings suggest that TAT-induced alterations in glutamate signaling, but not alterations in monoamine metabolism, may underlie the facilitation of reversal learning.
Keywords: l-Deprenyl, Barnes maze, Dopamine, Glutamate, Prefrontal cortex, AIDS
1. Introduction
Mild neuropsychological impairments associated with Human Immunodeficiency Virus (HIV) infection are relatively common, occurring in approximately 50% of people with AIDS [1] receiving combination antiretroviral therapy [2]. HIV-related brain dysfunction is associated with frontal-subcortical mediated patterns of cognitive deficits, characterized by impairments in working memory, processing speed, executive function, learning and motor skills [1,3,4]. This collection of symptoms suggest the brain regions most commonly damaged in HAD are the basal ganglia, hippocampus, and cerebral cortex [5]. Due to the persistence of cognitive deficits in HIV patients, identifying neurobiological mechanisms and subsequently, therapeutics for HIV-related cognitive deficits is a growing area of interest in the field of HIV research [3].
HIV-induced neurodegeneration involves, in part, HIV viral products including the non-structural protein TAT which plays a central role in the pathogenesis of the HIV infection (for review, [6]) and may contribute to cognitive deficits in treated patients. For example, the TAT protein has been found in the post-mortem brain tissue of patients with HIV [7,8]. Transgenic mice that express the viral TAT protein under the glial fibrillary acidic protein (GFAP) promoter provide a useful in vivo model to study the impact of TAT protein in cognitive function. TAT-induced mice show neuropathology similar to that observed in HIV-infected humans including apoptosis, astrocytosis, neurodegeneration of the cortex, degeneration of dendrites, inflammation and premature death [9]. TAT protein also induces dysfunction of dopaminergic neurotransmission in corticolimbic brain circuits [10–13] that are involved in memory and executive function [14–16]. TAT expression in mice leads to impaired learning, memory and cognitive flexibility (reversal learning) [17,18]. However, an important caveat to these studies is that TAT expression was induced prior to the task acquisition. Therefore, deficits in learning may compromise the subsequent testing of memory retrieval and reversal learning. Thus, it is important to design experiments that can discretely assess memory and reversal learning independent of concomitant impairments in learning after TAT expression.
HIV infection has been shown to preferentially target the basal ganglia, leading to decreased caudate/basal ganglia volume [19,20]. Both caudate atrophy and decreased dopamine levels have been associated with impaired cognitive performance in HIV-infected humans [20–22]. TAT infusions into the striatum resulted in decreased levels of potassium-evoked dopamine release 24 and 48 h later [10] and TAT has been shown, in vitro, to induce rapid and reversible effects on dopamine uptake and storage [12,13]. Dopamine function in the prefrontal cortex (PFC) and caudate putamen (CPu) of rodents has been associated with memory retrieval and reversal learning [23,24]. Furthermore, interactions with dopamine systems via other brain regions such as the orbitofrontal cortex (ORB) [25] and hippocampus [26] are also important for both memory and adaptive responses. Selegiline, a monoamine oxidase (MAO) inhibitor, decreases dopamine and serotonin (5-HT) metabolism [27], in addition to having antioxidant and neuroprotective functions [28]. Selegiline treatment improved age-related memory deficits in rodents [29] as well as memory deficits induced by a variety of insults in rodent models [30–32]. Moreover, selegiline treatment in monkeys with SIV has improved dopamine-related function in the brain [33]. Based on these findings, we hypothesized that selegiline may be promising treatment for TAT-induced memory impairments by improving brain dopamine function with possible downstream effects on glutamate and γ-aminobutyric acid (GABA) systems.
The goal of the present study was to determine the impact of TAT expression and chronic selegiline treatment on memory retention, reversal learning and neurotransmitter function. To discretely assess memory and reversal learning without confounding alterations in learning, mice were trained to learn the location of the escape tunnel in the Barnes maze test prior to TAT expression or selegiline treatment. Subsequently, the effects of TAT expression, with and without selegiline, on memory retention/recall and reversal learning were assessed. Dopamine, 5-HT, glutamate and GABA function in regions associated with memory and reversal learning including the PFC, ORB, CPu and hippocampus was determined using high performance liquid chromatography (HPLC).
2. Materials and methods
2.1. Animals
A total of 60 male mice all containing the GFAP-null alleles but only half containing the TAT protein transgene were used in this study. Inducible TAT transgenic mouse colonies with a C57BL/6J background were obtained by generation of two separate transgenic lines Teton-GFAP mice and TRE-Tat86 mice, and then cross-breeding of these two lines of transgenic mice as previously described in Ref. [9]. The mice were housed in groups of 2–4 in a humidity- and temperature-controlled animal facility on a 12 h/12 h reverse light/dark cycle (lights off at 7:00 AM) with ad libitum access to food and water. Behavioral testing was conducted during the dark phase of the light/dark cycle. All of the experiments were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and National Research Council's Guide for the Care and Use of Laboratory Animals and approved by the University of California San Diego Institutional Animal Care and Use Committee.
2.2. Experimental design
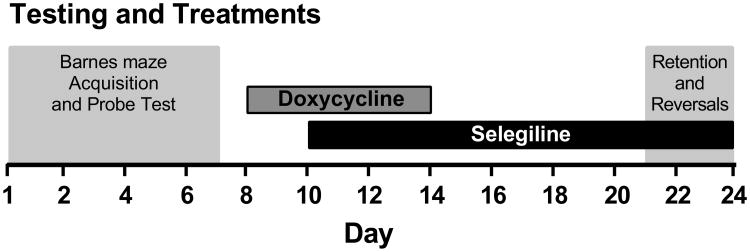
A graphical representation of the experimental design is presented in Fig. 1. TAT- (n = 30) and TAT+ (n = 30) mice were trained to learn the spatial location of the escape tunnel during the acquisition trials followed by the probe test in the Barnes maze. Subsequently, TAT+ and TAT- mice were divided into two equally performing groups based on latency, strategy and reference errors during the final three days of acquisition trials. Then all mice were treated with a doxycycline hyclate regimen (100 mg/kg, Sigma, St. Louis, MO, USA) consisting of intraperitoneal injections once a day for 7 days at 08:00 and starting two days after the probe test. This doxycycline regimen is based on the previously demonstrated efficacy of TAT induction at this dose of doxycycline [9,17]. Selegiline hydrochloride (Sel+; Sigma), 2 mg/kg subcutaneously once per day at 17:00 [29], or saline (Sel-) treatment began two days after the first doxycycline administration and continued throughout behavioral testing. The final number of mice included in each test group were as follows: TAT-/Sel- n = 16, TAT+/Sel- n = 16, TAT-/Sel+ n = 13 and TAT+/Sel+ n = 15. Effects of TAT and selegiline on memory retention were assessed 15-days after the completion of acquisition trials and followed immediately with reversal trials. Brain samples were taken the day following the final reversal trials.
Fig. 1.
A graphical representation of the testing and treatment timeline.
2.3. Barnes maze test
The Barnes maze testing was conducted similar to that described previously [34,35]. The maze consisted of a white, acrylic, circular disc (90 cm diameter) that was elevated 90 cm above the floor, with 20 equally spaced holes (San Diego Instruments, San Diego, CA) with a black acrylic escape box (20 × 5 × 6 cm) placed under one of the holes. The maze was surrounded by four spatial cues at the height of the maze. Illumination in the center of the maze was approximately 900 l×. The maze was rotated 90° each day to avoid the use of local cues on the maze by the mice.
2.3.1. Acquisition trials
Each mouse underwent 20 acquisition trials over 5 days, tested four times a day with an intertrial interval of 10–15 min. Immediately prior to the first trial, all of the mice were individually placed into the escape tunnel for 1 min to avoid any neophobic responses. During testing, the mice were placed into a starting cylinder (10 cm diameter) in the center of the maze for 30 s. The cylinder was then removed, and the mouse was allowed to explore the arena to find the escape tunnel. The trial ended when the mouse entered the escape tunnel (i.e., when all four paws left the maze). When the mouse entered the escape tunnel, the entry was blocked, and the mouse was left in the tunnel for 1 min. If the mouse did not find or enter the escape tunnel within 3 min, then it was manually placed into the escape tunnel.
2.3.2. Probe trial
The 3-min probe trial was conducted on day 6 and was identical to the acquisition trials, with the exception that the escape tunnel was removed.
2.3.3. Memory retention
Two weeks after the probe test, the mice were tested for memory retention over four trials identical to the acquisition trials.
2.3.4. Reversal learning
For two days after the memory retention trials mice were tested for reversal learning. Each day consisted of four trials identical to the acquisition trials, but the location of the escape tunnel for each mouse was shifted 180°.
2.3.5. Behavioral measures
All behaviors were scored from video files by an experimenter who was blind to the experimental conditions. The measures assessed were the latency to find the target hole, number of reference errors, number of working memory errors, and number of perseverative errors. Reference errors were defined as any incorrect hole inspection. Working memory errors were defined as searching the same hole twice within a trial when the revisit occurred after the inspection of other holes. Perseverative errors were defined as repeated searches of the same hole without searching another hole in between. Search strategy was also assessed in the acquisition, retention, and reversal trials. The search strategy was defined as one of three categories: spatial, serial, and random/mixed [34,35]. A spatial strategy was defined as finding the target hole directly or after inspecting one of the adjacent holes first (≤one reference error). Random/mixed (<74%) and serial (≤75%) strategy scores were defined based on the percentage of reference errors that were made in a serial fashion. For an error to be defined as serial, this error had to be part of a minimum of three consecutive errors made in either direction around the maze without skipping a hole or changing direction. The percentages of each strategy used during the 4 daily trials were calculated. In the probe trial, the time spent by each mouse in the quadrant of the maze that contained the target hole was calculated.
2.4. High performance liquid chromatography and analysis
Catecholamines and amino acids from brain tissue were measured by HPLC with electrochemical detection for catecholamines and fluorescence detection for amino acids [36,37]. Brain tissues were homogenized in 0.1 M perchloric acid with 50 ng/mL deoxyepinephrine (catecholamine internal standard) using probe sonication (Vibra-Cell, Sonics & Materials, CT, USA) and centrifuged at 13,000 rpm for 5 min. The supernatant was filtered by a 4 mm 0.22 μM nylon syringe filter (MicroSolv Technology Corporation, NJ, USA). For catecholamines, 15 μl of sample was injected into the HPLC system, which consisted of an autosampler (Dionex UltiMate 3000, Thermo Scientific, CA, USA), an isocratic HPLC pump (Model 584, ESA Laboratories, MA, USA), a Sunfire C18 column, (4.6 mm × 100 mm, 3 μm; Waters Corporation, MA, USA) and a Coulochem III (ESA Laboratories) electrochemical detector. The mobile phase consisted of a 12% acetonitrile/50 mM citric acid and 25 mM potassium dihydrogen phosphate buffer containing 1 mM EDTA and 1.4 mM octane sulfonic acid adjusted to pH 4.3 with phosphoric acid. Flow rate was 0.5 ml/min. An analytical cell (Model 5014B, ESA Laboratories) with the first and second electrodes maintained at −150 and +300 mV, respectively, was used for detection. Amino acids were analyzed using pre-column derivatization at 4°C and fluorescence detection. The derivatisation protocol was conducted by the autosampler as follows: 10 μl of 1 nM/μL homoserine (amino acid internal standard) was mixed with 10 μl of sample; then 20 μl of borate buffer (0.4 M at pH 10) was added and mixed; then 5 μl of OPA reagent (100 mg ophthalaldehyde in 1 ml methanol with 9 ml borate buffer and 50 μl mercaptoethanol) was added and mixed; then after a 30 s wait, 50 μl of mobile phase was added and mixed; 5 μl of the final solution was injected into the HPLC system. The system consisted of an isocratic pump and autosampler (Dionex UltiMate 3000, Thermo Scientific), and fluorescence detector (Model 2475, Waters Corporation) equipped with a Phenomenex Gemini C18 column (4.6 mm μ× 150 mm, 3 um; Phenomenex, CA, USA). The mobile phase consisted of 0.05 M sodium acetate, tetrahydrofuran and acetonitrile (74:1:25, v/v) adjusted to pH 4.0 using 100% acetic acid. Flow rate was 1 ml/min and the fluorescence detector was set to an excitation wavelength of 337 nm and an emission wavelength of 454 nm. All data was stored and processed with Dionex Chromeleon software (version 7.2, Thermo Scientific). Data was quantified by calculating peak-area ratios of each compound compared to the relevant internal standard and expressed as pg/mg (catecholamines) or ng/mg (amino acids) of tissue.
2.5. Statistical analyses
All of the analyses were performed with IBM SPSS Statistics 20 (Armonk, NY, USA). All neurochemical and behavioral data were analyzed using analysis of variance (ANOVA), with TAT and Selegiline as the between-subject factors. For Barnes maze acquisition data repeated-measures ANOVAs were used with Day and Trial as the repeated measures. For Barnes maze retention and reversal learning data repeated-measures ANOVAs were used with Trial as the repeated measure. When appropriate, post hoc comparisons were performed using Least Significant Difference (LSD) test. Results are expressed as mean ± standard error of the mean (SEM). Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Barnes maze acquisition
All mice learnt to find the escape tunnel during the acquisition phase. The final 3 days of acquisition were used to balance groups for subsequent selegiline treatment. There were no differences between experimental groups in the relevant outcome measures including reference errors, working memory errors, perseverative errors and search strategies during the final three days of acquisition trials (Supplementary Fig. 1). There were also no differences between experimental groups in the time spent in the target quadrant during the probe trial (Supplementary Fig. 2). In all cases there were no significant main effects of TAT or Selegiline, and no significant interactions of TAT × Selegiline. The lack of any observable deficits in performance prior to TAT induction support other studies demonstrating that transgene leakage in this model is small or negligible [38].
3.2. Memory retention
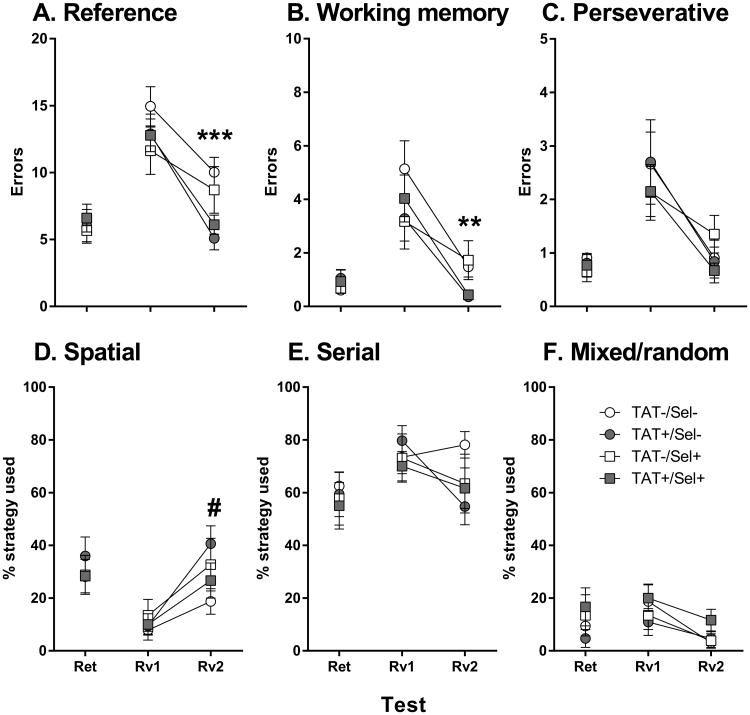
There was no effect of TAT or Selegiline on behavioral measures of memory retention (Fig. 2). All mice maintained memory of the escape tunnel location as demonstrated by no significant differences between the retention trials and the last day of acquisition trials in any measure.
Fig. 2.
Average reference errors (A), working memory errors (B), perseverative errors (C) and the percentage of spatial (D), serial (E) and mixed/random (F) strategy use during days of retention (Ret) and reversal learning (Rv) testing in the Barnes maze test. **p < 0.01, ***p < 0.001; significant main effect of TAT. #p < 0.053; near significant interaction of TAT × Selegiline.
3.3. Reversal learning
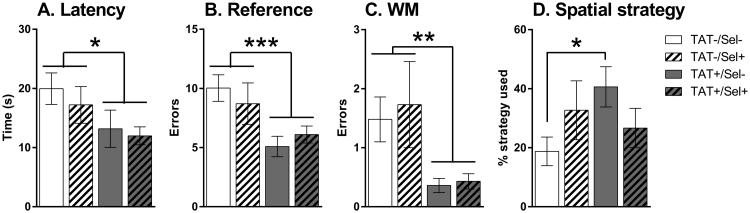
Neither Gene nor Selegiline led to any significant effects on latency, errors or strategy use on the first day of reversal learning (Fig. 2). All mice improved performance across the four trials as demonstrated by significant main effects of Trial on latency (F3,168 = 32.2, p < 0.001), reference errors (F3,168 = 38.2, p < 0.001), working memory errors (F3,168 = 34.4, p < 0.001) and perseverative errors (F3,168 = 19.7, p < 0.001). On the second day of reversal learning there were significant main effects of TAT on latency (F1,56 = 4.9, p < 0.05), reference errors (F1,56 = 11.2, p < 0.001) and working memory errors (F1,56 = 9.9, p < 0.01). To find the escape tunnel, TAT+ mice took less time (Fig. 3A), and made less reference (Fig. 3B) and working memory (Fig. 3C) errors compared with control mice. There was also a near significant interaction of TAT × Selegiline on the spatial strategy use for the second day of reversal learning (F1,56 = 3.9, p = 0.053) with TAT+/Sel- mice using a spatial strategy on a greater percentage of trials compared with TAT-/Sel- mice (Fig. 3D; p < 0.05).
Fig. 3.
Average latency (A), reference errors (B), working memory (WM) errors (C) and the percentage of spatial strategy used (D) on the second day of reversal learning trials in the Barnes maze test. TAT+ mice (gray bars) took less time, and made fewer reference and working memory errors compared with TAT- control mice (white bars). TAT+ mice treated with saline (grey, non-hatched bar) utilised a spatial strategy on a greater percentage of trials compared with TAT- control mice treated with saline (white, non-hatched bar; D). Selegiline treatment tended to increase spatial strategy use in TAT- control mice and decrease spatial strategy use in TAT+ mice. *p < 0.05, **p < 0.01, ***p < 0.001.
3.4. Neurochemistry
3.4.1. Caudate putamen
In the CPu (Table 1), chronic selegiline treatment significantly increased levels of dopamine (F1,54 = 32.7, p < 0.001) and 3-methoxytyramine (3-MT; F1,54 = 70.6, p < 0.001), but decreased levels of dihydroxyphenylacetic acid (DOPAC; F1,54 = 147.3, p < 0.001) and homovanillic acid (HVA; F1,54 = 12.2, p < 0.001). Decreased dopamine turnover in response to selegiline treatment was demonstrated by significant decreases in the ratio of HVA/dopamine (F1,54 = 102.7, p < 0.001), DOPAC/dopamine (F1,54 = 180.9, p < 0.001) and DOPAC/HVA (F1,54 = 106.4, p < 0.001). Significant increases in both 5-HT (F1,54 = 5.7, p < 0.05) and glutamate (F1,54 = 5.4, p < 0.05) were also observed.
Table 1.
Neurotransmitter levels in the caudate putamen.
| TAT-/Sel- | TAT+/Sel- | TAT-/Sel+ | TAT+/Sel+ | SME | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Sel | TAT | |
| Dopamine system | ||||||||||
| DA | 12644.2 | 471.1 | 13213.7 | 508.9 | 16027.2 | 458.1 | 15171.4 | 417.5 | *** | |
| DOPAC | 1564.3 | 80.7 | 1355.3 | 75.3 | 777.6 | 28.1 | 698.2 | 19.4 | *** | * |
| 3-MT | 608.28 | 25.29 | 597.96 | 24.78 | 838.10 | 26.13 | 779.65 | 21.58 | *** | |
| HVA | 1013.0 | 37.9 | 1006.4 | 49.0 | 881.6 | 24.3 | 864.3 | 37.4 | *** | |
| DOPAC/DA | 0.1253 | 0.0076 | 0.1032 | 0.0053 | 0.0487 | 0.0017 | 0.0465 | 0.0019 | *** | * |
| HVA/DA | 0.0805 | 0.0021 | 0.0762 | 0.0024 | 0.0553 | 0.0016 | 0.0572 | 0.0024 | ||
| DOPAC/HVA | 1.5644 | 0.0898 | 1.3578 | 0.0605 | 0.8841 | 0.0286 | 0.8194 | 0.0254 | *** | * |
| Serotonin system | ||||||||||
| 5-HT | 387.32 | 13.80 | 379.24 | 14.69 | 404.90 | 21.21 | 435.36 | 11.93 | * | |
| 5-HIAA | 233.57 | 11.15 | 236.72 | 14.17 | 223.84 | 10.44 | 249.17 | 11.58 | ||
| 5-HIAA/5-HT | 0.6124 | 0.0355 | 0.6339 | 0.0436 | 0.5616 | 0.0242 | 0.5743 | 0.0239 | ||
| Amino acids | ||||||||||
| GLU | 1404.5 | 46.5 | 1397.3 | 53.4 | 1487.0 | 41.2 | 1522.4 | 34.4 | * | |
| GABA | 180.84 | 10.40 | 177.50 | 8.06 | 183.44 | 5.07 | 188.58 | 9.08 | ||
| Glutamine | 1317.1 | 86.9 | 1397.5 | 100.0 | 1280.6 | 59.5 | 1533.5 | 116.5 | ||
| GLU/GABA | 7.95 | 0.31 | 7.93 | 0.19 | 8.19 | 0.35 | 8.33 | 0.43 | ||
| Gln/GLU | 0.9312 | 0.0427 | 0.9989 | 0.0582 | 0.8689 | 0.0477 | 0.9941 | 0.0561 | ||
Sel, Selegiline; SME, significant main effects; SEM, standard error of the mean; DA, dopamine; DOPAC, dihydroxyphenylacetic acid; 3-MT, 3-methoxytyramine; HVA, homovanillic acid; 5-HT, serotonin; 5-HIAA, 5-hydroxy-indoleacetic acid; GLU, glutamate; GABA, γ-aminobutyric acid; Gln, glutamine.
p < 0.05,
p < 0.001.
TAT expression decreased levels of DOPAC (F1,54 = 5.9, p<0.05) and decreased the ratios of DOPAC/dopamine (F1,54 = 6.0, p < 0.05) and DOPAC/HVA (F1,54 = 5.3, p < 0.05). A significant interaction of TAT × Selegiline was observed for the DOPAC/dopamine ratio (F1,54 = 4.0, p < 0.05) with TAT+/Sel- mice having a significantly lower ratio when compared with TAT-/Sel- mice (p < 0.01). In addition, there were trends for increased levels of glutamine (F1,54 = 3.1, p = 0.086) and an increased glutamine/glutamate ratio (F1,54 = 3.4, p = 0.069) after TAT expression.
3.4.2. Hippocampus
In the hippocampus (Table 2), chronic selegiline treatment significantly increased levels of dopamine (F1,56 = 21.5, p < 0.001) and 3-MT (F1,56 = 13.3, p < 0.001) but decreased levels of DOPAC (F1,56 = 63.3, p< 0.001) and HVA (F1,56 = 9.5, p<0.01). Decreased dopamine turnover in response to selegiline treatment was also demonstrated by significant decreases in the ratio of HVA/dopamine (F1,56 = 58.3, p < 0.001), DOPAC/dopamine (F1,56 = 104.4, p < 0.001) and DOPAC/HVA (F1,56 = 67.6, p < 0.001). Selegiline treatment also significantly impacted the 5-HT system with increased levels of 5-HT (F1,56 = 31.2, p < 0.001), decreased levels of 5-hydroxy-indoleacetic acid (5-HIAA; F1,56 = 16.3, p < 0.001) and a decreased ratio of 5-HIAA/5-HT (F1,56 = 30.6, p < 0.001) observed.
Table 2.
Neurotransmitter levels in the hippocampus.
| TAT-/Sel- | TAT+/Sel- | TAT-/Sel+ | TAT+/Sel+ | SME | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Sel | TAT | |
| Dopamine system | ||||||||||
| DA | 13.31 | 0.88 | 13.91 | 1.10 | 21.55 | 2.01 | 22.06 | 2.67 | *** | |
| DOPAC | 17.20 | 0.70 | 17.41 | 0.78 | 11.41 | 0.49 | 11.61 | 0.82 | *** | |
| 3-MT | 0.71 | 0.07 | 0.71 | 0.05 | 1.82 | 0.17 | 2.09 | 0.66 | *** | |
| HVA | 22.72 | 0.52 | 23.63 | 1.00 | 19.29 | 0.59 | 20.53 | 1.65 | ** | |
| DOPAC/DA | 1.3707 | 0.0962 | 1.3197 | 0.0768 | 0.5661 | 0.0399 | 0.5982 | 0.0605 | *** | |
| HVA/DA | 1.8074 | 0.1100 | 1.8034 | 0.1123 | 0.9644 | 0.0692 | 1.0519 | 0.1073 | *** | |
| DOPAC/HVA | 0.7553 | 0.0219 | 0.7379 | 0.0182 | 0.5919 | 0.0205 | 0.5727 | 0.0187 | *** | |
| Serotonin system | ||||||||||
| 5-HT | 702.67 | 19.71 | 714.07 | 20.70 | 828.35 | 29.12 | 842.96 | 22.37 | *** | |
| 5-HIAA | 410.20 | 10.85 | 424.11 | 17.63 | 335.72 | 9.67 | 380.45 | 17.05 | *** | * |
| 5-HIAA/5-HT | 0.5947 | 0.0283 | 0.6085 | 0.0397 | 0.4109 | 0.0182 | 0.4574 | 0.0259 | *** | |
| Amino acids | ||||||||||
| GLU | 1465.8 | 45.9 | 1434.7 | 19.0 | 1431.3 | 30.0 | 1490.9 | 28.2 | ||
| GABA | 219.31 | 5.86 | 208.58 | 3.74 | 206.30 | 6.19 | 219.96 | 4.77 | ||
| Glutamine | 1148.6 | 90.6 | 1208.5 | 84.8 | 1086.5 | 51.0 | 1335.3 | 101.8 | ||
| GLU/GABA | 6.70 | 0.15 | 6.90 | 0.11 | 6.99 | 0.18 | 6.80 | 0.14 | ||
| Gln/GLU | 0.7744 | 0.0378 | 0.8377 | 0.0518 | 0.7638 | 0.0419 | 0.8964 | 0.0670 | ||
Sel, Selegiline; SME, significant main effects; SEM, standard error of the mean; DA, dopamine; DOPAC, dihydroxyphenylacetic acid; 3-MT, 3-methoxytyramine; HVA, homovanillic acid; 5-HT, serotonin; 5-HIAA, 5-hydroxy-indoleacetic acid; GLU, glutamate; GABA, γ-aminobutyric acid; Gln, glutamine.
p < 0.05,
p < 0.01,
p < 0.001.
TAT expression lead to increased turnover of 5-HT as demonstrated by increased levels of the metabolite 5-HIAA (F1,56 = 4.0, p < 0.05). In addition, there were trends towards increased levels of glutamine (F1,56 = 3.2, p = 0.081) and an increased glutamine/glutamate ratio (F1,54 = 3.6, p = 0.062) after TAT expression. A significant interaction of TAT × Selegiline was also observed for GABA levels (F1,56 = 5.6, p < 0.05) with trends toward the TAT-/Sel+ mice having lower GABA levels compared with both TAT-/Sel- mice (p < 0.086) andTAT+/Sel+ mice (p < 0.076).
3.4.3. Prefrontal cortex
In the PFC (Table 3), chronic selegiline treatment significantly increased levels of dopamine (F1,56 = 6.3, p < 0.05) and 3-MT (F1,56 = 9.0, p < 0.01) but decreased levels of DOPAC (F1,56 = 7.2, p < 0.05). Decreased dopamine turnover in response to selegiline treatment was also demonstrated by significant decreases in the ratio of HVA/dopamine (F1,56 = 12.0, p < 0.001) and DOPAC/dopamine (F1,56 = 24.0, p < 0.001). Selegiline treatment also significant impacted the 5-HT system with significantly increased levels of 5-HT (F1,56 = 10.6, p < 0.01) and a trend for a decreased ratio of 5-HIAA/5-HT (F1,56 = 30.6, p = 0.057) observed.
Table 3.
Neurotransmitter levels in the prefrontal cortex.
| TAT-/Sel- | TAT+/Sel- | TAT-/Sel+ | TAT+/Sel+ | SME | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Sel | TAT | |
| Dopamine system | ||||||||||
| DA | 114.67 | 5.51 | 117.04 | 7.39 | 142.23 | 10.60 | 146.42 | 18.23 | * | |
| DOPAC | 74.07 | 3.71 | 68.39 | 2.64 | 59.16 | 2.80 | 65.59 | 3.74 | ** | |
| 3-MT | 22.21 | 1.09 | 23.20 | 1.13 | 26.32 | 1.22 | 27.64 | 2.03 | ** | |
| HVA | 65.48 | 2.88 | 68.11 | 3.40 | 64.71 | 5.08 | 67.37 | 5.59 | ||
| DOPAC/DA | 0.6568 | 0.0325 | 0.6103 | 0.0338 | 0.4294 | 0.0204 | 0.4994 | 0.0437 | *** | |
| HVA/DA | 0.5857 | 0.0311 | 0.6042 | 0.0343 | 0.4609 | 0.0249 | 0.4928 | 0.0412 | *** | |
| DOPAC/HVA | 1.1434 | 0.0534 | 1.0287 | 0.0505 | 0.9539 | 0.0545 | 1.0786 | 0.1172 | ||
| Serotonin system | ||||||||||
| 5-HT | 516.53 | 22.76 | 554.75 | 20.47 | 604.34 | 23.08 | 608.98 | 20.76 | ** | |
| 5-HIAA | 151.60 | 8.59 | 175.19 | 10.18 | 160.82 | 9.26 | 172.50 | 14.21 | ||
| 5-HIAA/5-HT | 0.2999 | 0.0179 | 0.3175 | 0.0175 | 0.2666 | 0.0116 | 0.2822 | 0.0205 | ||
| Amino acids | ||||||||||
| GLU | 1702.7 | 71.0 | 1775.8 | 55.6 | 1774.2 | 32.0 | 1765.8 | 73.4 | ||
| GABA | 162.81 | 6.00 | 169.81 | 6.93 | 176.33 | 6.59 | 182.31 | 9.13 | ||
| Glutamine | 1101.1 | 88.9 | 1219.0 | 91.1 | 1121.9 | 73.1 | 1401.5 | 158.5 | ||
| GLU/GABA | 10.47 | 0.31 | 10.58 | 0.30 | 10.21 | 0.38 | 9.82 | 0.39 | ||
| Gln/GLU | 0.6451 | 0.0378 | 0.6823 | 0.0390 | 0.6300 | 0.0345 | 0.7787 | 0.0688 | ||
Sel, Selegiline; SME, significant main effects; SEM, standard error of the mean; DA, dopamine; DOPAC, dihydroxyphenylacetic acid; 3-MT, 3-methoxytyramine; HVA, homovanillic acid; 5-HT, serotonin; 5-HIAA, 5-hydroxy-indoleacetic acid; GLU, glutamate; GABA, γ-aminobutyric acid; Gln, glutamine.
p < 0.05,
p < 0.01,
p < 0.001.
TAT expression did not alter dopamine or 5-HT systems but trends for increased levels of glutamine (F1,56 = 3.3, p = 0.075) and an increased glutamine/glutamate ratio (F1,56 = 3.8, p = 0.056) were observed.
3.4.4. Orbitofrontal cortex
In the ORB (Table 4), chronic selegiline treatment tended to increase levels of dopamine (F1,56 = 3.5, p = 0.065), but significantly increased levels of 3-MT (F1,56 = 22.1, p < 0.001) and decreased levels of DOPAC (F1,56 = 8.9, p < 0.01). Furthermore, decreased dopamine turnover in response to selegiline treatment was also demonstrated by significant decreases in the ratio of HVA/dopamine (F1,56 = 5.9, p < 0.05) and DOPAC/dopamine (F1,56 = 12.3, p < 0.001). Selegiline treatment also significant impacted the 5-HT system with significantly increased levels of 5-HT (F1,56 = 12.1, p < 0.001) and a decreased ratio of 5-HIAA/5-HT (F1,56 = 5.7, p < 0.05) observed.
Table 4.
Neurotransmitter levels in the orbitofrontal cortex.
| TAT-/Sel- | TAT+/Sel- | TAT-/Sel+ | TAT+/Sel+ | SME | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Sel | TAT | |
| Dopamine system | ||||||||||
| DA | 39.74 | 1.89 | 41.23 | 2.29 | 44.44 | 3.39 | 45.87 | 2.41 | ||
| DOPAC | 36.98 | 1.26 | 38.92 | 2.73 | 33.26 | 1.30 | 31.64 | 1.38 | ** | |
| 3-MT | 2.41 | 0.26 | 2.44 | 0.28 | 4.25 | 0.53 | 4.15 | 0.43 | *** | |
| HVA | 52.20 | 3.65 | 63.27 | 6.56 | 51.89 | 5.44 | 54.40 | 5.29 | ||
| DOPAC/DA | 0.9587 | 0.0519 | 0.9957 | 0.0953 | 0.7804 | 0.0460 | 0.7140 | 0.0451 | *** | |
| HVA/DA | 1.3422 | 0.0963 | 1.6125 | 0.1941 | 1.1625 | 0.0784 | 1.1741 | 0.0772 | * | |
| DOPAC/HVA | 0.7438 | 0.0433 | 0.6431 | 0.0281 | 0.7066 | 0.0613 | 0.6511 | 0.0646 | ||
| Serotonin system | ||||||||||
| 5-HT | 509.95 | 16.04 | 553.55 | 18.42 | 584.30 | 19.68 | 606.35 | 18.76 | *** | |
| 5-HIAA | 136.83 | 8.15 | 146.35 | 6.90 | 129.03 | 8.89 | 146.61 | 9.41 | ||
| 5-HIAA/5-HT | 0.2729 | 0.0193 | 0.2677 | 0.0142 | 0.2219 | 0.0144 | 0.2423 | 0.0144 | * | |
| Amino acids | ||||||||||
| GLU | 1685.4 | 44.0 | 1657.1 | 43.0 | 1746.1 | 60.5 | 1718.2 | 65.3 | ||
| GABA | 153.64 | 5.48 | 151.66 | 4.04 | 156.07 | 6.35 | 156.65 | 5.70 | ||
| Glutamine | 1052.1 | 71.2 | 1126.2 | 93.1 | 1066.4 | 89.5 | 1264.6 | 134.3 | ||
| GLU/GABA | 11.06 | 0.26 | 10.96 | 0.22 | 11.23 | 0.16 | 10.99 | 0.21 | ||
| Gln/GLU | 0.6282 | 0.0431 | 0.6754 | 0.0482 | 0.6064 | 0.0377 | 0.7186 | 0.0551 | ||
Sel, Selegiline; SME, significant main effects; SEM, standard error of the mean; DA, dopamine; DOPAC, dihydroxyphenylacetic acid; 3-MT, 3-methoxytyramine; HVA, homovanillic acid; 5-HT, serotonin; 5-HIAA, 5-hydroxy-indoleacetic acid; GLU, glutamate; GABA, γ-aminobutyric acid; Gln, glutamine.
p < 0.05,
p < 0.01,
p < 0.001.
TAT expression did not alter dopamine or 5-HT systems but a trend for an increased glutamine/glutamate ratio (F1,54 = 2.8, p = 0.097) was observed.
4. Discussion
The main findings of this study are that TAT expression in mice, when induced after task acquisition, did not affect memory retention/recall but facilitated reversal learning on the second day of testing. Even though prior work has demonstrated reversal learning deficits in TAT-expressing mice [17], TAT expression was induced before the acquisition of the behavioral tasks and led to impaired learning during the task acquisition [17]. Thus, it would appear that previously observed deficits in memory and reversal learning may be a consequence of impairments in the initial learning rather than reversal learning deficits per se. Further, although chronic selegiline treatment significantly altered dopamine and 5-HT systems throughout the brain, it did not affect memory retention and had subtle effects on reversal learning. These results suggest that chronic selegiline treatment does not significantly impact spatial reversal learning in the absence of learning impairments.
4.1. Effects of TAT protein on memory retention/recall
In contrast to our hypothesis, prior TAT expression did not impair memory retention/recall but, in fact facilitated reversal learning. Given that TAT expression is likely to have substantially decreased or even ceased by the time of memory retention/recall testing [38], it is still likely that deficits may be observed during periods of active TAT expression. However, the present results suggest that there are no persisting effects of TAT expression on these outcome measures. TAT-induced deficits in spatial learning and memory have been observed in mice using both the Barnes maze [17] and the Morris water maze [18], but in both studies TAT-expression was induced prior to or throughout testing. Thus, it would appear that spatial memory retention/recall is not negatively affected by prior TAT expression in mice. Memory impairments have been observed in rats given intra-hippocampal TAT protein infusion after task acquisition in the Morris water maze [39] suggesting that a greater level of TAT protein may be required to induce memory impairments. Alternatively, the training used prior to TAT protein infusion featured significantly less training trials compared with the current study. A strong bias toward the target quadrant in the probe trial provides clear evidence that mice in the current protocol were using spatial cues to determine the escape tunnel position. Moreover, the low number of reference errors made on day 5 of acquisition (approximately 2.5) was less than would be made by using a serial strategy without any spatial cues (e.g., approximately 9.5 if randomly choosing a starting hole and turning left or right 50% of the time). Thus, the effects of TAT exposure on memory recall may depend on the strength of the memory, i.e. weaker memories in less trained mice are more susceptible to TAT-induced impairments.
4.2. Effects of TAT protein on reversal learning
TAT expression facilitated reversal learning on day 2 but not day 1 suggesting that prior TAT exposure may affect reversal learning in a phase-dependent manner. For example, reversal learning features an initial phase whereby errors are mainly due to the previously learnt association; while a late phase is driven by the learning of the new association [40]. Paradoxically, lesions of the ventromedial PFC in mice facilitated late-stage reversal learning in an operant visual reversal learning task [40]. HIV disease has been shown to decrease cortical gray matter, which has been associated with neurocognitive impairments [41], decrease PFC activity during neurocognitive tasks [42] and induce frontocortical astrocytosis [43]. Similarly, TAT expression in mice resulted in cortical astrocytosis and degeneration of neuronal dendrites [9] suggesting that PFC neuropathology after TAT expression may underlie the observed reversal learning facilitation. Such impairments in PFC function may facilitate reversal learning by disinhibition of subcortical areas involved in habit formation [40]. The inability to maintain an appropriate balance between goal-directed behaviors and habitual responding is key to multiple neuropsychiatric disorders. Particularly in obsessive-compulsive disorder where repetitive actions interfere significantly with daily functioning [44]. Given the lack of observed TAT-induced changes in cortical dopamine and 5-HT neurochemistry, it is unlikely that impairments in cortical dopamine or 5-HT function underlie TAT-induced facilitation of reversal learning. However, receptor expression and signaling may be altered. Further studies are required to determine the full extent of TAT exposure on cortical neurotransmission.
One mediator of TAT-induced behavioral alterations may be altered glutamate function. We observed global trends toward TAT-induced increases in glutamine levels and the ratio of glutamine/glutamate. Excess glutamate levels result in excitotoxicity which has been suggested as a neuropathological mechanism associated with HIV infection [45]. In a healthy brain, glutamate levels are tightly regulated via astrocytic conversion to a less toxic alternative, glutamine. Thus, TAT-induced increases in glutamine levels may be a compensatory response to reduce elevated glutamate release. Combined increases in glutamate and glutamine levels have been observed in the cortex of patients with acute HIV infection [46]. Furthermore, it has recently been shown that increases in cerebrospinal fluid glutamine levels were associated with improvements in the cognitive status of HIV-infected subjects [47] supporting the premise that this may be a compensatory factor. Given that acute TAT has been shown to decrease glutamine and increase glutamate levels in cultured neuroblastoma cells [48], it may be that the observed opposite trends for increased glutamine levels in TAT+ mice are in response to the long-term effects of prior TAT protein expression. TAT protein expression in this mouse model persists for less than 14 days after doxycycline treatment [38]. The brain samples in the current study were collected 11-days after the final doxycycline injection making it likely that the TAT protein was no longer present.
4.3. Effects of selegiline on reversal learning
Although selegiline tended to increase spatial strategy use in TAT- mice and decrease its use in TAT+ mice during reversal learning, overall selegiline treatment had little effect on memory retention/recall and reversal learning. The impact of selegiline treatment on neurochemistry was consistent with its primary mode of action, i.e. inhibition of MAO function [27]. MAO is required for the conversion of dopamine and 5-HT to DOPAC and 5-HIAA, respectively. Increased levels of dopamine and 5-HT, reduced levels of DOPAC and 5-HIAA or both were observed in all the brain regions assessed. Interestingly, selegiline treatment also increased glutamate levels specifically in the dopamine rich CPu but did not alter GABA levels in any of the brain regions assessed. Previous work has shown stimulant-induced dopamine release to be increased after MAO inhibition [49]. However, our findings suggest that under the current experimental conditions, any potential increases in dopamine function by selegiline do not impact spatial memory retention or reversal learning. Furthermore, given that selegiline did not alter TAT-induced changes in reversal learning, we conclude that it is unlikely that altered dopamine function was a causative factor in this behavioral outcome.
4.4. Conclusions
Our results suggest that prior TAT protein expression in the brain of mice leads to the facilitation of late-phase reversal learning. This behavioral pattern is consistent with results observed after PFC damage [40] suggesting that TAT-induced cortical pathology may mediate this behavioral alteration. Furthermore, TAT-induced facilitation of reversal learning is unlikely to be attributed to dopamine or 5-HT function because selegiline treatment did not normalize TAT-induced effects or alter behavior in mice not exposed to TAT in this task. In conclusion, this work suggests that, in the absence of impaired learning, TAT protein does not impair spatial memory retention/recall but facilitates late phase reversal learning in a manner consistent with PFC damage. Furthermore, TAT-induced alterations in glutamate signaling, but not alterations in dopamine and 5-HT metabolism, may underlie TAT-induced changes in reversal learning.
Supplementary Material
highlights.
TAT protein expression in the brain facilitates reversal learning in mice.
TAT expression tended to increase the conversion of glutamate to glutamine.
MAO inhibitor selegiline decreased the metabolism of dopamine and serotonin.
Chronic selegiline treatment increased glutamate levels in the caudate putamen.
Chronic selegiline treatment does not alter spatial memory or reversal learning.
Acknowledgments
This work was supported by the Translational Methamphetamine AIDS Research Center funded by the National Institute on Drug Abuse (P50DA026306), a TMARC developmental grant to JPK, and the Interdisciplinary Research Fellowship in NeuroAIDS (IRFN, R25MH081482) to JPK. AM has received contract research support from Forest Laboratories and Astra-Zeneca and honoraria/consulting fees from AbbVie during the past 2 years.
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD), the Sanford-Burnham Medical Research Institute (SBMRI), and the University of California, Irvine (UCI). The TMARC is composed of: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Scott L Letendre, M.D., and Cristian L. Achim, M.D., Ph.D.; Center Manager – Mariana Cherner, Ph.D.; Associate Center Manager – Erin E. Morgan, Ph.D.; Assistant Center Manager – Aaron M. Carr, B.A.; Behavioral Assessment and Medical (BAM) Core–Neuromedical and Laboratory Unit (NLU): Scott L. Letendre, M.D. (Core Co-Director/NLU Chief), Ronald J. Ellis, M.D., Ph.D.; BAM Core–Neuropsychiatric Unit (NPU): Robert K. Heaton, Ph.D. (Core Co-Director/NPU Chief), J. Hampton Atkinson, M.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D., Matthew Dawson (NPU Manager); Neuroimaging (NI) Core: Gregory G. Brown, Ph.D. (Core Director), Thomas T. Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John R. Hesselink, M.D., Mary Jane Meloy, Ph.D., Craig E.L. Stark, Ph.D.; Neuroscience and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Marcus Kaul, Ph.D., Virawudh Soontornniyomkij, M.D.; Pilot and Developmental (PAD) Core: Mariana Cherner, Ph.D. (Core Director), Stuart A. Lipton, M.D., Ph.D.; Pilot Study Leaders: Jennifer E. Iudicello, Ph.D., Assawin Gongvatana, Ph.D., Rachel D. Schrier, Ph.D., Virawudh Soontornniyomkij, M.D., Marta Massanella, Ph.D.; Administrative Coordinating Core (ACC)–Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Chief), Clint Cushman, B.A. (Unit Manager); ACC–Statistics Unit: Florin Vaida, Ph.D. (Unit Chief), Ian S. Abramson, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC–Participant Unit: J. Hampton Atkinson, M.D. (Unit Chief), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark A. Geyer, Ph.D., Jared W. Young, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Susan F. Tapert, Ph.D., Assawin Gongvatana, Ph.D.; Project 3: Erin E. Morgan, Ph.D. (Project Director), Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director), Athina Markou, Ph.D., James Kesby, Ph.D.; Project 5: Marcus Kaul, Ph.D. (Project Director).
Abbreviations
- 3-MT
3-methoxytyramine
- 5-HIAA
5-hydroxy-indoleacetic acid
- 5-HT
serotonin
- AIDS
acquired immunodeficiency syndrome
- ANOVA
analysis of variance
- CPu
caudate putamen
- DA
dopamine
- DOPAC
dihydroxyphenylacetic acid
- GABA
γ-aminobutyric acid
- GFAP
glial fibrillary acidic protein
- Gln
glutamine
- GLU
glutamate
- HAD
HIV associated dementia
- HIV
human immunodeficiency virus
- HPLC
high performance liquid chromatography
- HVA
homovanillic acid
- LSD
least significant difference
- MAO
monoamine oxidase
- ORB
orbitofrontal cortex
- PFC
prefrontal cortex
- Ret
retention
- Rv
reversal learning
- Sel
selegiline
- SEM
standard error of the mean
- SIV
simian immunodeficiency virus
- SME
significant main effects
- WM
working memory
Footnotes
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbr.2016.05.034.
JPK, AM and SS have no competing financial interests in relation to the work described.
References
- 1.Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, et al. The HNRC 500: neuropsychology of HIV infection at different disease stages. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 2.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Charter H. Grp HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesby JP, Heaton RK, Young JW, Umlauf A, Woods SP, Letendre SL, Markou A, Grant I, Semenova S. Methamphetamine exposure combined with HIV-1 disease orgp120 expression: comparison of learning and executive functions in humans and mice. Neuropsychopharmacology. 2015;40:1899–1909. doi: 10.1038/npp.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Wesselingh SL, Purcell DFJ. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1:463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- 6.Li WX, Li GH, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res. 2009;16:205–220. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- 7.Hudson L, Liu JK, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- 8.Parmentier HK, Vanwichen DF, Meyling F, Goudsmit J, Schuurman HJ. Epitopes of human-immunodeficiency-virus regulatory proteins Tat, Nef, and Rev are expressed in normal human tissue. Am J Pathol. 1992;141:1209–1216. [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BO, Liu Y, Ruan YW, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. In vivo microdialysis in awake, freely moving rats demonstrates HIV-1 Tat-induced alterations in dopamine transmission. Synapse. 2009;63:181–185. doi: 10.1002/syn.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theodore S, Cass WA, Dwoskin LP, Maragos WF. HIV-1 protein Tat inhibits vesicular monoamine transporter-2 activity in rat striatum. Synapse. 2012;66:755–757. doi: 10.1002/syn.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Midde NM, Gomez AM, Zhu J. HIV-1 Tat protein decreases dopamine transporter cell Surface expression and vesicular monoamine transporter-2 function in rat striatal synaptosomes. J Neuroimmune Pharmacol. 2012;7:629–639. doi: 10.1007/s11481-012-9369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in H-3 dopamine uptake: dissociation of H-3 dopamine uptake and H-3 2 beta-carbomethoxy-3-beta-(4fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castane A, Theobald DEH, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behav Brain Res. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cognit. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 16.Leon WC, Bruno MA, Allard S, Nader K, Cuello AC. Engagement of the PFC in consolidation and recall of recent spatial memory. Learn Mem. 2010;17:297–305. doi: 10.1101/lm.1804410. [DOI] [PubMed] [Google Scholar]
- 17.Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su JM, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 tat transgenic mice. Biol Psychiatry. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD. Reduced basal ganglia volume in HIV-1 associated dementia: results from quantitative neuroimaging. Neurology. 1993;43:2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- 20.Kieburtz K, Ketonen L, Cox C, Grossman H, Holloway R, Booth H, Hickey C, Feigin A, Caine ED. Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Arch Neurol. 1996;53:155–158. doi: 10.1001/archneur.1996.00550020059016. [DOI] [PubMed] [Google Scholar]
- 21.Hestad K, McArthur JH, Dalpan GJ, Selnes OA, Nancesproson TE, Aylward E, Mathews VP, McArthur JC. Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test-performance. Acta Neurol Scand. 1993;88:112–118. doi: 10.1111/j.1600-0404.1993.tb04201.x. [DOI] [PubMed] [Google Scholar]
- 22.Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- 23.Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, but not serotonin regulates reversal learning in the marmoset caudate nucleus. J Neurosci. 2011;31:4290–4297. doi: 10.1523/JNEUROSCI.5066-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D-1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- 25.Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Laux G, Volz HP, Moller HJ. Newer and older monoamine-oxidase inhibitors—a comparative profile. CNS Drugs. 1995;3:145–158. [Google Scholar]
- 28.Bainbridge JL, Page RL, Ruscin JM. Elucidating the mechanism of action and potential interactions of MAO-B inhibitors. Neurol Clin. 2008;26:S85–+. doi: 10.1016/j.ncl.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 29.de Lima MN, Laranja DC, Caldana F, Bromberg E, Roesler R, Schroder N. Reversal of age-related deficits in object recognition memory in rats with l-deprenyl. Exp Gerontol. 2005;40:506–511. doi: 10.1016/j.exger.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 30.de Lima MNM, Laranja DC, Caldana F, Grazziotin MM, Garcia VA, Dal-Pizzol F, Bromberg E, Schroder N. Selegiline protects against recognition memory impairment induced by neonatal iron treatment. Exp Neurol. 2005;196:177–183. doi: 10.1016/j.expneurol.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Maia FD, Pitombeira BSS, Araujo DT, Cunha GMA, Viana GSB. l-deprenyl prevents lipid peroxidation and memory deficits produced by cerebral ischemia in rats. Cell Mol Neurobiol. 2004;24:87–100. doi: 10.1023/B:CEMN.0000012727.59502.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsunekawa H, Noda Y, Mouri A, Yoneda F, Nabeshima T. Synergistic effects of selegiline and donepezil on cognitive impairment induced by amyloid beta (25-35) Behav Brain Res. 2008;190:224–232. doi: 10.1016/j.bbr.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Scheller C, Sopper S, Jenuwein M, Neuen-Jacob E, Tatschner T, Grunblatt E, ter Meulen V, Riederer P, Koutsilieri E. Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. J Neurochem. 2005;95:377–387. doi: 10.1111/j.1471-4159.2005.03373.x. [DOI] [PubMed] [Google Scholar]
- 34.Kesby JP, Markou A, Semenova S. TMARC Cognitive deficits associated with combined HIVgp120 expression and chronic methamphetamine exposure in mice. Eur Neuropsychopharmacol. 2015;25:141–150. doi: 10.1016/j.euroneuro.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesby JP, Kim JJ, Scadeng M, Woods G, Kado DM, Olefsky JM, Jeste DV, Achim CL, Semenova S. Spatial cognition in adult and aged mice exposed to high-fat diet. PLoS One. 2015;10:e0140034. doi: 10.1371/journal.pone.0140034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groves NJ, Kesby JP, Eyles DW, McGrath JJ, Mackay-Sim A, Burne THJ. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav Brain Res. 2013;241:120–131. doi: 10.1016/j.bbr.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Kesby JP, Cui X, Ko P, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain. Neurosci Lett. 2009;461:155–158. doi: 10.1016/j.neulet.2009.05.070. [DOI] [PubMed] [Google Scholar]
- 38.Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression ofthe HIV-1 regulatory protein. Tat, Psychopharmacology (Berl) 2014;231:2349–2360. doi: 10.1007/s00213-013-3385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harricharan R, Thaver V, Russell VA, Daniels WM. Tat-induced histopathological alterations mediate hippocampus-associated behavioural impairments in rats. Behav Brain Funct: BBF. 2015;11:3. doi: 10.1186/s12993-014-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci. 2011;14:1507–1509. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TL, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- 42.Melrose RJ, Tinaz S, Castelo JMB, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res. 2008;188:337–347. doi: 10.1016/j.bbr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 43.Weis S, Haug H, Budka H. Astroglial changes in the cerebral-cortex of AIDS brains: a morphometric and immunohistochemical investigation. Neuropathol Appl Neurobiol. 1993;19:329–335. doi: 10.1111/j.1365-2990.1993.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 44.Gillan CM, Robbins TW. Goal-directed learning and obsessive-compulsive disorder. Philos Trans R Soc Lond B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr HIV Res. 2012;10:392–406. doi: 10.2174/157016212802138832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sailasuta N, Ross W, Ananworanich J, Chalermchai T, DeGruttola V, Lerdlum S, Pothisri M, Busovaca E, Ratto-Kim S, Jagodzinski L, Spudich S, Michael N, Kim JH, Valcour V, Teams RSP. Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickens AM, Anthony DC, Deutsch R, Mielke MM, Claridge TD, Grant I, Franklin D, Rosario D, Marcotte T, Letendre S, McArthur JC, Haughey NJ. Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS. 2015;29:559–569. doi: 10.1097/QAD.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samikkannu T, Atluri VS, Arias AY, Rao KV, Mulet CT, Jayant RD, Nair MP. HIV-1 subtypes B and C Tat differentially impact synaptic plasticity expression and implicates HIV-associated neurocognitive disorders. Curr HIV Res. 2014;12:397–405. doi: 10.2174/1570162x13666150121104720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mishra N, Sasmal D. Modulations of brain amines and dopaminergic behavior by a novel, reversible and selective MAO-B inhibitor. Brain Res. 2012;1470:45–51. doi: 10.1016/j.brainres.2012.06.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.