Abstract
Background
Tendinopathy is an overuse tendon injury that occurs in loaded tendons and results in pain and functional impairment. Although many treatments for painful tendons are described, the scientific evidence for most of the conservative and surgical treatments is not always conclusive.
Objectives
This study was designed to evaluate the efficacy of 3 different interventions in patients with Achilles tendinopathy. The interventions include the combination of 2 physical therapy programs (eccentric training [EC] or passive stretching [PS]) with a supplement containing mucopolisaccharides. The efficacy of the interventions was evaluated depending on the stage of the disease.
Methods
Fifty-nine patients were randomly assigned to 1 of 3 treatment groups, and classified according to the disease stage: reactive versus degenerative tendinopathy. Treatment groups were EC; EC + a dietary supplement containing mucopolisaccharides, type I collagen, and vitamin C (MCVC); and a passive stretching program + MCVC. Patients were evaluated at baseline, 6 weeks, and 12 weeks with the Victorian Institute of Sports Assessment-Achilles questionnaire for function, a visual analog scale for pain, and ultrasound characterization for the evolution of tendon structure.
Results
A significant improvement in Victorian Institute of Sports Assessment-Achilles questionnaire score, pain at rest, and pain during activity were detected in all 3 treatment groups at 6 and 12 weeks’ follow-up when compared with baseline. In patients with reactive tendinopathy, the reduction in pain at rest was greater in the groups who took the supplemental MCVC than in the EC alone group (P < 0.05).
Conclusions
MCVC seems to be therapeutically useful for management of tendinopathies, providing some additional benefit to physical therapy. This is especially evident in early stages of the disease, when the tendon does not present severe matrix and vascular changes.
ClinicalTrials.gov identifier
Key words: eccentric training, food supplement, passive stretching, tendinopathy
Highlights
-
•
Efficacy and safety on Achilles’ tendinopathy of a food supplement (MCVC) is tested
-
•
All patients showed pain relief and improved functionality after the treatments
-
•
Greater pain reduction was obtained in the groups treated with MCVC
-
•
MCVC adds benefit to physical therapy, especially in early stages of the disease
-
•
Passive stretching+MCVC showed similar results to Eccentric training
Background
Tendinopathy is an overuse tendon injury that occurs in loaded tendons and results in pain and functional impairment, a major problem in sports and occupational medicine. Characteristic changes in tendon structure include loss of type I collagen, increase in proteoglycan content, and neovascularization.1 This has been termed failed healing response and results in the tendon being less capable of sustaining repeated tensile load.2 Recently, a new pathology model has been accepted to explain the clinical course of tendinopathy. The Cook and Purdam model3 states there is a continuum of tendon pathology that, for easier clinical use, has been divided into 2 clear groups: reactive tendinopathy/early tendon disrepair and degenerative tendinopathy/late tendon disrepair. Because the same intervention to all presentations of tendinopathy is unlikely to show efficacy, applying this model might be useful to rationally place specific treatments through the evolution of the pathology.
Although many treatments are described for painful tendons, the scientific evidence for most of the conservative and surgical treatments is not always conclusive. Eccentric exercise has evidential basis obtained from randomized controlled trials and is currently included in most treatment algorithms.4, 5 It involves active lengthening of the muscle–tendon unit and short-term improvements in tendon pain6, 7, 8 and normalization of tendon structure9 have been demonstrated. Nevertheless, why eccentric exercise reduces pain in tendinopathy and what is the correct intensity, speed, load, and frequency remains unclear.2, 10 Other therapeutic options that have shown some positive outcomes for tendinopathies’ treatment include shockwaves, Sclerosing Polidocanol Injections, topical nitroglycerin therapy, and cooling therapies. Costicosteoid injections have also shown very good outcomes in the short term but should be avoided due to the cortisone’s negative influence on the tendon structure.11
A composition containing mucopolysaccharides, type I collagen, and vitamin C (MCVC) demonstrated efficacy both in in vitro models12 and human studies.13, 14, 15 MCVC induces changes in tenocytes metabolism in vitro, thereby helping to preserve the structure of the extracellular matrix. Results indicate that this formulation promotes the endogenous synthesis of collagen type I, avoiding the accumulation of collagen type III and aggrecan,11 and therefore may interfere with the degeneration of tendon tissue. These findings suggest that MCVC may improve tendinopathy symptomatology, reducing discomfort and improving tendon function. Indeed, in 3 studies in humans, 2 observational and 1 controlled, the consumption of the supplement might be associated with clinical and structural improvements in the recovery of tendinopathies.13, 14, 15
The goal of this trial was to determine whether daily consumption of a supplement containing MCVC, added to an eccentric exercise program, provided some additional benefit in improving symptoms of chronic noninsertional tendinopathy in patients with a gradually evolving painful condition in the midportion of the Achilles tendon for at least 3 months. In parallel, the specific effect of MCVC when combined with 2 different physical therapies was compared: eccentric training (EC) or passive stretching (PS). Finally, whether there is a different response pattern to the aforementioned treatments depending on the pathology stage of the patient (reactive tendinopathy vs degenerative tendinopathy) was assessed.
Patients and Methods
Experimental Approach to the Problem
This was a nutrition-based, randomized, controlled, multicenter trial to assess the efficacy and safety of a supplement containing MCVC combined with either an EC or PS program, and compared with EC alone. Patients were randomly assigned to 1 of 3 treatment groups: EC, combination of EC and MCVC, and combination of PS and MCVC. Patients were assigned a randomization number taken from a computer-generated randomization list following the chronological order by which they were included in the study, independent of the stage of their tendinopathy.
It was conducted in men and nonpregnant women, aged 18 to 70 years, with painful noninsertional Achilles tendinopathy of the midportion for at least 3 months. Diagnosis was based on clinical examination showing a painful thickening of the Achilles tendon located 2 to 6 cm above the tendon insertion, and confirmed by ultrasonography; specifically, local thickening of the tendon, irregular tendon structure with hypoechoic areas, and irregular fiber orientation. Clinical suspicions of insertional tendinopathy, tendon rupture (Thomson test and palpable gap), neural disorder, systemic disease, like gout, spondylarthropaty, rheumatoid arthritis and sarcoidosis, or pregnancy were considered exclusion criteria. Patients which already received a previous treatment with eccentric training or PRP for the studied injury were also excluded.
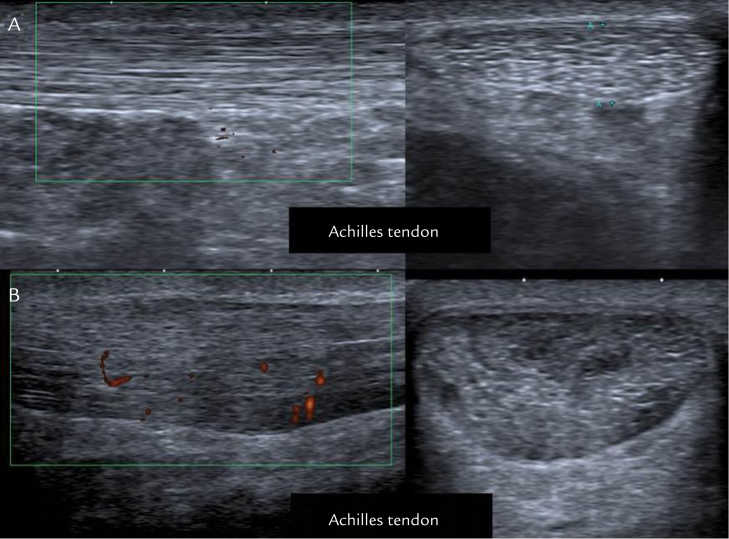
The stage of the tendinopathy was classified into 2 subgroups according to the Cook and Purdam pathology model: patients with reactive tendinopathy and patients with degenerative tendinopathy. Based on ultrasonographic assessment, reactive tendinopathy is characterized by swollen tendon and diffuse hypoechogenicity occurring between intact collagen structures (Figure 1A), whereas degenerative tendinopathy shows increased matrix disorganization represented by hypoechoic regions with few reflections from collagen fascicles and clear neovascularization usually visible on Doppler ultrasound (Figure 1B). Stage of tendinopathy was assessed using imaging criteria. To avoid variations between centers, a single specialist blinded to the study group of the participants evaluated the imaging across all study sites.
Figure 1.
Echographic imaging. (A) Reactive tendinopathies. (B) Degenerative tendinopathies. Reactive tendinopathies do not present disorganized matrix but swollen and diffuse hypoechogenicity. Degenerative tendinopathies present matrix disorganization, hypoechoic regions, and clear neovascularization (red marks).
Patients
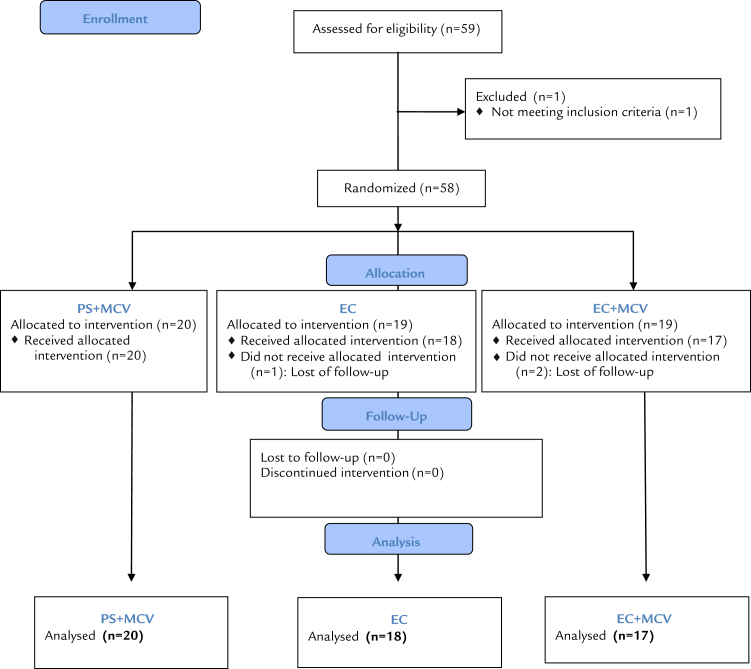
Fifty-nine potentially eligible patients were recruited between September 2012 and June 2013 in 5 Spanish sports medicine centers. In each clinical center, a team composed of an orthopedist and a physiotherapist was in charge of the study. A Consolidated Standards of Reporting Trials diagram of participant flow through the trial is shown in Figure 2. A total of 58 participants fulfilled the inclusion criteria and consented to take part in the study. The intention to treat (ITT) population was defined by all the participants who took at least 1 unit of the trial product. The subjects did not receive any financial incentive to participate in the study but received the assigned treatment free of charge.
Figure 2.
Consolidated Standards of Reporting Trials flow diagram.
The study was approved by the official Clinical Research Ethical Committees of Consell Català de l’Esport, Barcelona, Spain. The protocols followed the principles found in the Helsinki Declaration. This trial was registered with ClinicalTrials.gov (No. NCT01691716). All participants provided written informed consent before enrollment into the trial.
Procedures
Patients allocated in the EC training group were instructed to perform a training protocol according to Alfredson et al.16 To guarantee the consistency of the procedures among different research centers, the protocol was agreed upon by all the researchers in prestudy meetings, and its correct execution was confirmed by a physiotherapist every week. Two types of eccentric exercises were used. The gastrocnemius muscle was eccentrically loaded both with the knee straight and, to maximize the activation of the soleus muscle, also with the knee bent. Each of the 2 exercises included 15 repetitions done in 3 sets. This protocol was repeated twice per day, 7 days a week, for 12 weeks. Compliance with the exercise protocol was recorded weekly by the physiotherapist. The minimum compliance to allow inclusion in the final analyses was 70%.
Patients in the EC + MCVC group consumed 3 capsules per day containing 435 mg mucopolysaccharides, 75 mg collagen type I, and 60 mg vitamin C (TendoActive; Bioiberica SA, Palafolls, Spain) and followed the EC protocol. Patients in the PS + MCVC group consumed the dietary supplement and were instructed to perform a personalized protocol of passive stretching that was supervised weekly by a physiotherapist. PS routines were included in the program to guarantee the same weekly monitoring in all the study groups. A standard protocol was applied, including 2 exercises to stretch the gastrocnemius muscle and 2 for the soleus muscle. Each exercise was performed for 30 seconds. Patients performed the stretching exercises consecutively, switching between the muscle groups without rest.
Primary Outcome
Patients were evaluated at baseline, 6 weeks, and 12 weeks after the beginning of the treatment protocol with the original English version of the validated Victorian Institute of Sports Assessment-Achilles (VISA-A) questionnaire. The VISA-A questionnaire scores range from 0 to 100, with higher scores corresponding with less pain and increased activity.
Secondary Outcome
Every week, pain was evaluated at rest and during activity using a visual analog scale (VAS). The evolution of the affected tendons was monitored ultrasonographically on every visit and bilateral thickness was recorded. Bilateral thickness was measured in the transversal section of the more thickened cross point at a level of 2 to 6 cm above the tendon insertion. The safety profile of the given therapeutic approaches was also assessed during the study.
Statistical Analysis
The statistical analysis followed the statistical plan according to the principles specified in the guidelines of the International Council for Harmonization of Statistical Principles for Clinical Trials and of the Committee for Proprietary Medicinal Products with the Efficacy Working Party Points to Consider on Multiplicity Issues in Clinical Trials. All statistical tests were applied with an α = 0.05 2-sided significance level. No interim analyses of efficacy were planned for this study and, therefore, no multiplicity adjustments were needed. The primary efficacy analysis was conducted to evaluate the absolute change from baseline to last visit of the VISA-A score, using an ANCOVA model with the baseline value as a covariate and a baseline observation carried forward approach on ITT. All secondary efficacy variables were assessed similarly as primary variables using an ANCOVA model with the baseline value as a covariate.
Treatment effect was also evaluated in 2 subgroups designed according to the Cook and Purdam pathology model: patients with reactive tendinopathy and patients with degenerative tendinopathy.
Results
Three participants were excluded from the randomized population for not having attended the inclusion visit (1 in the PS + MCVC group and 2 in the EC +MCVC group). Fifty-five participants (94.83% of those randomized) entered the ITT population; 23 had reactive tendinopathy and 32 degenerative tendinopathy. A CONSORT diagram of participant flow through the trial is shown in Figure 2. The average (SD) age was 38.9 (6.6) years for the EC group, 43.5 (14.5) years for the EC + MCVC group, and 40.2 (10.6) years for the PS + MCVC group. The participants were mostly men: 78%, 82%, and 80% in the EC, EC + MCVC, and PS + MCVC groups, respectively. There were 5, 9, and 9 patients with reactive tendinopathy and 15, 8, and 9 patients with degenerative tendinopathy in the EC, EC + MCVC, and PS + MCVC groups, respectively. There were no significant differences at baseline in any of the clinical outputs of the study. No participants were excluded from the ITT population due to protocol violation.
When analyzing the obtained data regardless of the patient’s stage of disease, a significant improvement in VISA-A score was detected in all 3 treatment groups at 6 and 12 weeks’ follow-up, when compared with baseline. There was no between-group effect for the primary outcome of VISA-A questionnaire between baseline and the end of the study (P > 0.1). In all the studied groups, VAS-rated pain was reduced both at rest and during activity after the 12-week treatment. Differences in pain reduction among treatments were found between the PS + MCVC and the EC groups, being significant at rest: for PS + MCV (–3.7 [0.8] cm) and EC (–2.7 [1.3] cm) (P < 0.05), and tending toward significance during activity: PS + MCV (–4.4 [1.1] cm) and EC (–3.5 [1.4] cm) (P = 0.074). Although no significant variation was found in the tendon bilateral thickness among treatments (P > 0.1), reduction from baseline was only detected in the PS + MCVC treatment group (–0.63 [0.73] mm) (P < 0.05).
In patients with reactive tendinopathy, the improvement of the VISA-A score at 12 weeks’ follow-up was 56% for the PS + MCV group, 44% for the EC +MCV group, and 30% for the EC group (Table I) (P = 0.154). Although no statistically significant results were found, a tendency toward significance was found in the EC and EC + MCVC groups (P = 0.069). In patients with degenerative tendinopathy, improvement in VISA-A score had the same magnitude among treatments (Table II). Similarly, pain at rest improved in all 3 groups of treatment in all randomized patients. In patients with reactive tendinopathy, the pain reduction at 12 weeks’ follow-up was greater for the group PS + MCVC (–3.815 [0.8] cm) compared with the EC group (–2.80 [1.0] cm) (P < 0.05). For the EC + MCVC group the pain reduction cannot be compared in absolute values because the mean pain value at rest was not balanced at baseline (Table I). In patients with degenerative tendinopathy, the magnitude of the pain reduction was similar among the study groups (Table II). The evaluation of pain during activity followed the same pattern of response as pain at rest, although differences among treatment groups failed to reach statistical significance, either in reactive or in degenerative tendinopathy.
Table I.
Clinical outcomes of the different treatment groups in patients with reactive tendinopathy.
| Outcome measure | Mean (SD) |
Change over time |
Treatment comparison |
||
|---|---|---|---|---|---|
| EC | PS + MCVC | EC + MCVC | P value | ||
| n=5 | n=5 | n=9 | |||
| VISA-A (%) | |||||
| Basal | 66 (2) | 54 (17) | 63 (10) | ||
| 6 wk | 66 (11) | 69 (30) | 79 (15) | ||
| 12 wk | 86 (11) | 84 (17) | 91 (13) | 0.045 | 0.154 |
| Pain at rest (cm) | |||||
| Basal | 4.2 (2.0) | 4.1 (2.3) | 2.2 (1.7) | ||
| 6 wk | 3.0 (1.4) | 2.0 (2.0) | 2.6 (3.2) | ||
| 12 wk | 1.4 (1.9) | 0.4 (0.5) | 0.4 (0.7) | 0.020 | < 0.001 |
| Pain during activity (cm) | |||||
| Basal | 6.8 (0.8) | 4.3 (2.8) | 5.1 (3.0) | ||
| 6 wk | 5.0 (1.4) | 2.0 (2.3) | 2.9 (2.1) | ||
| 12 wk | 2.4 (1.1) | 1.0 (0.9) | 1.6 (2.6) | < 0.001 | 0.148 |
EC = eccentric training; MCVC = food supplement containing mucopolysaccharides, collagen, and vitamin C; PS = passive stretching; VISA-A = Victorian Institute of Sports Assessment-Achilles.
Table II.
Clinical outcomes of the different treatment groups in patients with degenerative tendinopathy.
| Outcome measure | Mean (SD) |
Change over time |
Treatment comparison |
||
|---|---|---|---|---|---|
| EC | PS + MCVC | EC + MCVC | P value | ||
| n=15 | n=9 | n=8 | |||
| VISA-A (%) | |||||
| Basal | 49 (18) | 52 (10) | 49 (18) | ||
| 6 wk | 63 (24) | 72 (19) | 61 (26) | ||
| 12 wk | 76 (19) | 83 (28) | 82 (18) | < 0.001 | 0.763 |
| Pain at rest (cm) | |||||
| Basal | 4.1 (2.1) | 4.1 (1.4) | 3.9 (2.5) | ||
| 6 wk | 2.8 (2.0) | 3.1 (3.0) | 3.2 (2.6) | ||
| 12 wk | 1.7 (2.0) | 0.7 (1.0) | 1.2 (1.5) | 0.003 | 0.822 |
| Pain during activity (cm) | |||||
| Basal | 5.1 (2.9) | 6.0 (2.1) | 6.1 (3.0) | ||
| 6 wk | 3.5 (2.4) | 4.1 (3.2) | 2.6 (1.7) | ||
| 12 wk | 2.1 (1.8) | 0.7 (0.9) | 1.2 (1.0) | < 0.001 | 0.572 |
EC = eccentric training; MCVC = food supplement containing mucopolysaccharides, collagen, and vitamin C; PS = passive stretching; VISA-A = Victorian Institute of Sports Assessment-Achilles.
The bilateral thickness of the affected tendon remained constant during the study period in patients in the EC and EC + MCVC groups from the ITT population. In contrast, a significant reduction (–0.63 [0.3] mm; P < 0.05) was detected in patients in the PS + MCVC group. In patients with degenerative tendinopathy, a significant reduction was registered in the PS + MCVC group (P < 0.05), whereas no differences were detected in patients with reactive tendinopathy (Table III).
Table III.
Mean (SD) thickness of the affected tendon (in mm) in patients with reactive tendinopathy and patients with degenerative tendinopathy.
| Disease state | EC | PS + MCVC | EC + MCVC |
|---|---|---|---|
| Reactive | n = 5 | n = 9 | n = 9 |
| Basal | 5.10 (1.68) | 5.09 (0.83) | 5.06 (0.81) |
| 6 wk | 4.74 (1.29) | 5.33 (1.35) | 4.75 (0.68) |
| 12 wk | 4.72 (1.31) | 5.13 (0.81) | 5.19 (1.36) |
| Degenerative | n = 15 | n = 9 | n = 8 |
| Basal | 5.92 (1.62) | 5.83 (1.09) | 5.72 (1.25) |
| 6 wk | 5.57 (2.38) | 5.47 (0.80) | 6.35 (1.43) |
| 12 wk | 6.01 (1.77) | 4.59 (1.81)* | 5.59 (1.76) |
EC = eccentric training; MCVC = food supplement containing mucopolysaccharides, collagen, and vitamin C; PS = passive stretching.
*P < 0.05 compared with EC.
Discussion
In this study, 3 different interventions have been evaluated: EC alone, the combination of EC with a dietary supplement containing MCVC, and an alternative physical therapy such as PS combined with the dietary supplement in patients with Achilles tendinopathy. The main goal was to determine the additional benefit of MCVC to a physical therapy program.
After 90 days, all 3 treatment groups experienced significant improvements from baseline in VISA-A, VAS-rated pain at rest, and VAS-rated pain during activity. These improvements were not only statistically, but also clinically, significant because the minimum clinically important difference is 9 mm for VAS-rated pain and 6.5 points in the VISA-A scale.17, 18 The clinical improvements registered as a result of the EC training agree with those previously described16, 19 and were expected because EC is currently the gold standard for Achilles’ tendinopathy treatment. The addition of MCVC to EC significantly improved pain, but not function-related outcomes. When patients were divided according to severity of tendinopathy, a statistically significant clinical benefit from MCVC in patients with reactive but not degenerative tendinopathy, as determined by VAS-rated pain, was clearly observed. Although functionality, assessed with the VISA-A test, did not reach a statistically significant improvement it showed a tendency toward significance, although statistical significance was not reached due to low patient number. However, it reached a clinically significant improvement because it increased more than 6.5 points in both MCVC groups compared with controls. According to the model described by Cook and Purdam,3 in reactive tendinopathy a homogeneous, noninflammatory cell response to load that leads to metaplastic change in cells and cell proliferation is found. Theoretically, this cellular deregulation could be counterbalanced by the effects of MCVC on tenocyte metabolism, as described by Shakibaei et al.12 In degenerative tendinopathy, areas of cell death due to apoptosis, trauma, or tenocyte exhaustion in tendons have been reported.20 In this stage of the pathology the efficacy of MCVC could be limited by the reduced viability of the tenocytes.
In this study, a protocol of PS supervised by a physiotherapist and adjusted personally and supplemented with MCVC was shown to improve pain reduction and function recovery as good as a standard EC in both reactive and degenerative tendinopathy. Although the efficacy of the eccentric gastrocnemius muscle training has been widely described,21 less data about the efficacy of a protocol of PS are available. It has been recently published that isometric exercises could induce pain reduction in patellar tendinopathy.22 Because PS implies a certain degree of isometric contractions, the potential analgesic effect of this intervention could partially explain the clinical findings for this group. To elucidate the contribution of the PS protocol and the MCVC supplement to the clinical improvement, an additional group of patients following the PS program but without the MCVC supplement would have been required. From our study the placebo effect cannot be assessed because the groups not receiving the MCVC supplement did not receive an inert capsule. Another weakness from the study is the lack of blinding of the therapists treating the patients. Although the lack of blinding could potentially interfere with the results, we think that this should not have had a major impact on the patients’ evaluation and reported outcomes as greater improvements were expected from the EC+MCVC treatment compared to the PS+MCVC group and the obtained data did not match the expectations.
Regarding the structural changes during the study period, it has been previously described that a program of eccentric exercise is valid in the symptomatic nonoperative management of tendinopathy, probably by promoting collagen fiber cross-linkage formation within the tendon and, thereby, facilitating tendon remodeling.23 Previous studies report increased Type I collagen synthesis and reduction of fluid within the tendon after eccentric training offering a possible explanation for the mechanism of tendon healing.24, 25 Nevertheless, actual histologic adaptations after a program of EC or PS remain unclear. In this trial, the stretching program improved tendon structure when compared with baseline, as evidenced by a reduction in tendon thickness in patients with degenerative tendinopathy. During the study period no structural improvement was registered in the groups following the EC. This might be caused by the harshness of EC, which applies some degree of aggression to the tendon structure, and could maintain longer a temporary reactive disorganization of the tendon tissue.
Eccentric training is not the only kind of exercises that have been used for treating tendinopathies, other exercises like concentric ones have been tested for tendinopathy management. Some studies have compared the effectiveness of eccentric vs. concentric training but conflicting results have been obtained; some studies found that eccentric training was statistically superior to concentric training and others did not find any statistical difference between the treatments.8, 26, 27 Some protocols combine eccentric and concentric training in the same sessions, like the Silbernagel-combined program8 or the Stanish and Curwin28 programs. Besides concentric exercises, one study found that isometric muscle contractions reduce pain in patellar tendinopathy. Further research on this field is required as the effectiveness on the Achilles tendon cannot be assessed.22 Due to the existing uncertainty in the programs equivalence, the routine that has shown higher effectivity was chosen.
According to these findings, a dietary supplement containing MCVC seems to present a high safety profile and to be useful for managing tendinopathies, providing some additional benefit to EC, especially in early stages of the disease. In the reactive phase, tendons present a short-term adaptation to overload with thickening and increased stiffness, but without severe matrix and vascular changes. A reactive tendon has the potential to revert to normal if the overload is sufficiently reduced or if there is enough time between loading sessions.3 In this type of patient, supplementation with MCVC may be an efficient therapy when combined with EC or PS. Conversely, in degenerative pathology the tendon presents areas of cell death, vessel-filled matrix, matrix breakdown products, and little collagen, and therefore the reversibility of pathologic changes is limited.3 Although a nutrition-based approach is less likely to offer a clinical benefit at this stage, certain clinical improvements were observed in the PS-MCVC group during our study. However, further studies with larger sample sizes are required to confirm the efficacy of MCVC for the dietary management of tendinopathy.
Conclusions
The data obtained from this study confirm the therapeutic potential of EC for the management of tendinopathy, both at reactive and degenerative stages. The novelty of this study is that it shows the effects of a new therapeutic approach consisting of personalized stretching routines and dietary supplementation with MCVC. In patients with reactive tendinopathy, supplementation with MCVC further decreased pain levels compared to EC alone, so it could be included in the therapeutic arsenal for tendinopathy. In patients with degenerative tendinopathy, supplementation with MCVC could not improve the outcome of EC. Despite the lack of blinding and placebo treatment, the obtained results suggested that for patients with difficulties in performing EC due to advanced age, illness, or extreme tendon injury, a PS program supplemented with MCVC could be an alternative treatment to be taken into account.
Conflicts of Interest
Financial support for this work was provided by Bioiberica, SA, Palafolls, Spain. E. Costa and D. Martínez-Puig are employed by Bioibérica SA. Bioiberica SA is the trademark owner, manufacturer, and distributor of TendoActive, the supplement used in this study. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Acknowledgments
The authors thank Pablo Ramirez for helpful comments and fruitful discussions of this work.
R. Balius and D. Martínez-Puig conceived and designed the experiments and contributed to drafting the manuscript. E. Costa contributed to the preparation of the manuscript. G. Álvarez, F. Baró, F. Jiménez, and C. Pedret performed the clinical trial. All authors read and approved the final manuscript.
References
- 1.Xu Y., Murrell G.A.C. The Basic Science of Tendinopathy. Clin. Orthop. 2008;466:1528–1538. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rees J.D., Maffulli N., Cook J. Management of tendinopathy. Am. J. Sports Med. 2009;37:1855–1867. doi: 10.1177/0363546508324283. [DOI] [PubMed] [Google Scholar]
- 3.Cook J.L., Purdam C.R. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br. J. Sports Med. 2009;43:409–416. doi: 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 4.Alfredson H., Cook J. A treatment algorithm for managing Achilles tendinopathy: new treatment options. Br. J. Sports Med. 2007;41:211–216. doi: 10.1136/bjsm.2007.035543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinking M. Tendinopathy in athletes. Phys. Ther. Sport. 2012;13:3–10. doi: 10.1016/j.ptsp.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Mafi N., Lorentzon R., Alfredson H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA. 2001;9:42–47. doi: 10.1007/s001670000148. [DOI] [PubMed] [Google Scholar]
- 7.Roos E.M., Engström M., Lagerquist A., Söderberg B. Clinical improvement after 6 weeks of eccentric exercise in patients with mid-portion Achilles tendinopathy - a randomized trial with 1-year follow-up. Scand. J. Med. Sci. Sports. 2004;14:286–295. doi: 10.1111/j.1600-0838.2004.378.x. [DOI] [PubMed] [Google Scholar]
- 8.Silbernagel K.G., Thomeé R., Thomeé P., Karlsson J. Eccentric overload training for patients with chronic Achilles tendon pain--a randomised controlled study with reliability testing of the evaluation methods. Scand. J. Med. Sci. Sports. 2001;11:197–206. doi: 10.1034/j.1600-0838.2001.110402.x. [DOI] [PubMed] [Google Scholar]
- 9.Ohberg L. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up * Commentary. Br. J. Sports Med. 2004;38:8–11. doi: 10.1136/bjsm.2001.000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habets B., van Cingel R.E.H. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: A systematic review on different protocols: A systematic review on different protocols. Scand. J. Med. Sci. Sports. 2015;25:3–15. doi: 10.1111/sms.12208. [DOI] [PubMed] [Google Scholar]
- 11.Knobloch K., Hüfner T. Konservative Behandlung der Achillestendinopathie. Unfallchirurg. 2010;113:705–711. doi: 10.1007/s00113-010-1808-6. [DOI] [PubMed] [Google Scholar]
- 12.Shakibaei M., Buhrmann C., Mobasheri A. Anti-inflammatory and anti-catabolic effects of TENDOACTIVE® on human tenocytes in vitro. Histol. Histopathol. 2011;26:1173–1185. doi: 10.14670/HH-26.1173. [DOI] [PubMed] [Google Scholar]
- 13.Arquer A. The efficacy and safety of oral mucopolysaccharide, type I collagen and vitamin C treatment in tendinopathy patients. Apunts Med. Esport. 2014;49:31–36. [Google Scholar]
- 14.Nadal F., Bové T., Sanchís D., Martinez-Puig D. Effectiveness of treatment of tendinitis and plantar fascitis by TendoactiveTM. Osteoarthritis Cartilage. 2009;17:S253. [Google Scholar]
- 15.Hai Binh B., Ramirez P., Martinez-Puig D. A Randomized, Placebo-Controlled Study to Evaluate Efficacy and Safety of A Dietary Supplement Containing Mucopolysaccharides, Collagen Type I and Vitamin C for Management of Different Tendinopathies. Ann. Rheum. Dis. 2009;17 299–299. 299-299. [Google Scholar]
- 16.Alfredson H., Pietilä T., Jonsson P., Lorentzon R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am. J. Sports Med. 1998;26:360–366. doi: 10.1177/03635465980260030301. [DOI] [PubMed] [Google Scholar]
- 17.McCormack J., Underwood F., Slaven E., Cappaert T. The minimum clinically important difference on the VISA-A and LEFS for patients with insertional Achilles tendinopathy. Int. J. Sports Phys. Ther. 2015;10:639. [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly A.-M. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Acad. Emerg. Med. 1998;5:1086–1090. doi: 10.1111/j.1553-2712.1998.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 19.Herrington L., McCulloch R. The role of eccentric training in the management of Achilles tendinopathy: A pilot study. Phys. Ther. Sport. 2007;8:191–196. [Google Scholar]
- 20.Lian Ø. Excessive apoptosis in patellar tendinopathy in athletes. Am. J. Sports Med. 2007;35:605–611. doi: 10.1177/0363546506295702. [DOI] [PubMed] [Google Scholar]
- 21.Alfredson H. Clinical commentary of the evolution of the treatment for chronic painful mid-portion Achilles tendinopathy. Braz. J. Phys. Ther. 2015;19:429–432. doi: 10.1590/bjpt-rbf.2014.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rio E. Isometric exercise induces analgesia and reduces inhibition in patellar tendinopathy. Br. J. Sports Med. 2015;49:1277–1283. doi: 10.1136/bjsports-2014-094386. [DOI] [PubMed] [Google Scholar]
- 23.Rompe J.D., Furia J., Maffulli N. Eccentric Loading Versus Eccentric Loading Plus Shock-Wave Treatment for Midportion Achilles Tendinopathy: A Randomized Controlled Trial. Am. J. Sports Med. 2009;37:463–470. doi: 10.1177/0363546508326983. [DOI] [PubMed] [Google Scholar]
- 24.Langberg H. Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis. Scand. J. Med. Sci. Sports. 2007;17:61–66. doi: 10.1111/j.1600-0838.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 25.Lorenz D., Reiman M. The role and implementation of eccentric training in athletic rehabilitation: tendinopathy, hamstring strains, and acl reconstruction. Int. J. Sports Phys. Ther. 2011;6:27–44. [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsson P., Alfredson H. Superior results with eccentric compared to concentric quadriceps training in patients with jumper’s knee: a prospective randomized study. Br. J. Sports Med. 2005;39:847–850. doi: 10.1136/bjsm.2005.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Silvestrini J.A. Chronic lateral epicondylitis: comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J. Hand Ther. Off. J. Am. Soc. Hand Ther. 2005;18:411–419. doi: 10.1197/j.jht.2005.07.007. quiz 420. [DOI] [PubMed] [Google Scholar]
- 28.Stanish W.D., Rubinovich R.M., Curwin S. Eccentric exercise in chronic tendinitis. Clin. Orthop. 1986:65–68. [PubMed] [Google Scholar]