Abstract
Curcumin, a curcuminoid from Curcuma longa, presents antioxidant and anti-inflammatory actions and, among pathological changes of cerebral ischemic injury, inflammation is an important one. The objectives were to study the neuroprotective action of curcumin, in a model of global ischemia. Male Wistar rats (sham-operated, ischemic untreated and ischemic treated with curcumin, 25 or 50 mg/kg, p.o.) were anesthesized and their carotid arteries occluded, for 30 min. The SO group had the same procedure, except for carotid occlusion. In the 1st protocol, animals were treated 1 h before ischemia and 24 h later; and in the 2nd protocol, treatments began 1 h before ischemia, continuing for 7 days. Twenty four hours after the last administration, animals were euthanized and measurements for striatal monoamines were performed, at the 1st and 7th days after ischemia, as well as histological and immunohistochemical assays in hippocampi. We showed in both protocols, depletions of DA and its metabolites (DOPAC and HVA), in the ischemic group, but these effects were reversed by curcumin. Additionally, a decrease seen in 5-HT contents, 1 day after ischemia, was also reversed by curcumin. This reversion was not seen 7 days later. On the other hand, a decrease observed in NE levels, at the 7th day, was totally reversed by curcumin. Furthermore, curcumin treatments increased neuronal viability and attenuated the immunoreactivity for COX-2 and TNF-alpha, in the hippocampus in both protocols. We showed that curcumin exerts neuroprotective actions, in a model of brain ischemia that are probably related to its anti-inflammatory activity.
Keywords: Curcumin, Brain ischemia, Neuroprotection, Neuroinflammation, Cytokines
Graphical abstract
1. Introduction
Curcumin is the major chemical component of turmeric, produced from the rhizome of Curcuma longa, a traditional plant belonging to the Zingiberaceae family and used in Ayurvedic medicine for over 6,000 years. Curcumin is a polyphenol that possesses anti-inflammatory, antioxidant, antidiabetic, anticarcinogenic properties, among others.
Brain ischemia is a condition that occurs when there is not enough blood flow to the brain for meeting metabolic demands. This leads to limited oxygen supply or cerebral hypoxia and often to the death of brain tissues, cerebral infarction, or ischemic stroke. Stroke is currently the second most common cause of death and major cause of disability worldwide. Because of the aging population, the burden will greatly increase during the next 20 years.1 However, stroke recently declined in the USA from the third to the fourth leading cause of death.2
Cerebral ischemia results from severe reductions in cerebral blood flow (CBF) after cardiac arrest, the occlusion of cerebral and extracerebral vessels supplying nervous tissues, or periods of prolonged systemic hypotension. Severe and/or prolonged reduction in CBF leads to deprivations of oxygen and glucose, as well as to the building up of potentially toxic substances. Because nerve cells do not store alternative energy sources, these hemodynamic reductions can result in the reduction of metabolites, as ATP, leading to metabolic stress, energy failure, ionic perturbations and ischemic injury.3
Cells that undergo severe ischemia may die within minutes of the insult or display a delayed vulnerability. Ischemic insults can be focal or global, as well as permanent or transient ones, leading to reperfusion in post-ischemia areas. Depending on how early reperfusion is initiated, metabolic and ionic homeostases can return and cell survival maintained.4
Both necrotic and apoptotic cell death mechanisms have been implicated in the pathogenesis of brain ischemia injury.5, 6, 7, 8 The brain is vulnerable to oxidative stress, due to its high rate of oxidative metabolic activity.9 Oxidative stress, leading to calcium accumulation, mitochondrial dysfunction and the production of reactive oxygen radicals, is an important mechanism of cell death, following brain ischemia.10, 11
Inflammation is a host defense mechanism initiated by injury, through which blood leukocytes and soluble factors, as cytokines, chemokines, complement and lipid by-products attempt to restore tissue homeostasis.12 Inflammation plays an important role in the pathogenesis of ischemic brain injury. Experimental and clinical studies have shown that the brain responds to ischemic injury with an acute and prolonged inflammatory process, characterized by rapid activation of resident cells, as microglia, production of inflammatory mediators and infiltration of inflammatory cells into the brain ischemic tissue.13
Considering that curcumin presents anti-inflammatory and antioxidative properties, as shown by us14 and others,15, 16 and the importance of inflammation and oxidative stress in brain injury, the objectives of the present work were to evaluate the neuroprotective effects of curcumin on neurochemical (striatal DA and DOPAC) determinations and on histological (fluoro-jade staining) and immuno-histochemical (COX-2 and TNF-alpha) assays in the hippocampus, in the model of global ischemia in rats.
2. Material and methods
2.1. Drugs
Commercial curcumin was purchased from Sigma-Aldrich (MO, USA) and presented ≥94% of curcuminoid content and ≥80% of curcumin. Ketamine and xylazine were from Konig Laboratory (Santana de Parnaíba, São Paulo, Brazil). Antibodies for immunohistochemistry assays were from Santa Cruz Biotechnology (Dallas, TX, USA) or Merck-Millipore (Darmstadt, Germany). All other reagents were of analytical grade.
2.2. Animals and experimental protocols
Male Wistar rats from the Animal House of the Faculty of Medicine Estácio of Juazeiro do Norte, Brazil, were maintained under standard conditions and at a controlled temperature (23 ± 1 °C), with a 12 h dark/12 h light cycle, and food and water ad libitum. The animals (4–10 per group) were divided into four groups: controls treated with distilled water (SO and ischemic untreated with curcumin) or treated orally with curcumin (from Sigma-Aldrich, USA), at the doses of 25 or 50 mg/kg. Two protocols were used. In the 1st one, the animals were subjected to ischemia and treated 1 h before ischemia and 24 h later, and they were euthanized, 1 h after the drug second administration. In the 2nd protocol, the animals were subjected to ischemia, but daily treatments began 1 h before ischemia and continued, at the next day, daily for 7 days. For the experimental procedure, the rats were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (20 mg/kg), then submitted or not (SO groups) to transient global brain ischemia by the occlusion of the left common carotid artery, for 30 min, followed by reperfusion. The sham-operated groups (SO) were submitted to the entire procedure, except for the artery occlusion. Twenty four hours after the last drug administration, the animals were euthanized for dissection of striata and hippocampi. Neurochemical alterations (DA and DOPAC determinations in striata) were assessed, at the 1st and 7th days after ischemia. Besides, immunohistochemical assays in hippocampi were also performed, at those same periods. The study had the approval of the Animal Experimentation Committee of the Federal University of Ceará and the experiments were carried out in accordance with the current law and the NIH Guide for the Care and Use of Laboratory Animals, 2011.
3. Neurochemical assays
3.1. Concentrations of striatal monoamine (NE, DA, DOPAC, 5-HT and HVA) by HPLC
The striata from all groups, at different post-ischemia times, were used for the preparation of 10% homogenates in 0.1 M perchloric acid. This mixture was sonicated for 30 s, centrifuged at 4 °C for 15 min, at 15,000 rpm. The supernatants were filtered (0.2 μm, Millipore) and 20 μL injected into the HPLC column (Shim-Pack CLC-ODS, 25 cm) for electrochemical detection (Shimadzu, model LCD-6A, Japan), with a 0.6 mL/min flux. The mobile phase was prepared in 0.163 M citric acid, pH 3.0, containing 0.02 mM EDTA and 0.69 mM sodium octanosulfonic acid, 4% acetonitrile (v/v) and 1.7% tetrahydrofuran (v/v). Monoamine concentrations were determined by comparison to standards and the values expressed as ng/mg tissue.
3.2. Histological study for neuronal viability (fluoro-jade staining)
Fluoro-jade is an anionic fluorescein derivative, useful for the histological staining of neurons undergoing degeneration. After paraffin removal (by immersion in xylol), sections (5 μm) from hippocampi were mounted on slides surrounded by gelatin. The tissue was rehydrated by immersion in ethanol for 3 min, followed by immersions in 70 and 50% ethanol solutions and distilled water. The slices were placed into a 0.06% potassium permanganate solution, for 15 min, washed in distilled water and transferred to a fluoro-jade solution where they stayed for 30 min (with gentle stirring). After staining, the slices were washed in distilled water (3 times, 2 min each time). The excess of water was discarded and the dry slices mounted in Fluoromount® media and examined with a fluorescence microscope. The data were quantified with the Image J software (National Institute of Health, USA).
3.3. Immunohistochemical assays for COX-2 and TNF-alpha in rat hippocampi
Sections were fixed in 10% buffered formol, for 24 h, followed by immersion in a 70% alcohol solution. They were embedded into paraffin wax, for slices processing on appropriate glass slides. These were placed into the oven at 58 °C, for 10 min, followed by deparaffinization in xylol, rehydration in alcohol at decreasing concentrations, washing in distilled water and PBS (0.1 M sodium phosphate buffer, pH 7.2) for 10 min. The endogenous peroxidase was blocked with a 3% hydrogen peroxide solution, followed by incubation with appropriate primary anti-antibodies, diluted according to the manufacturers' instructions (Santa Cruz or Millipore, USA), for 2 h, at room temperature in a moist chamber. The glass slides were then washed with PBS (3 times, 5 min each) and incubated with the biotinylated secondary antibody, for 1 h, at room temperature in the moist chamber. Then, they were washed again in PBS and incubated with streptavidin-peroxidase, for 30 min, at room temperature, again in a moist chamber. After another wash in PBS, they were incubated in 0.1% DAB solution (in 3% hydrogen peroxide). Finally, the glass slides were washed in distilled water, dehydrated in alcohol (at increasing concentrations), diaphanized in xylol and mounted on Entelan® for optic microscopy examination. The data were quantified with the Image J software (National Institute of Health, USA).
4. Statistical analyses
For statistical analyses, One-way ANOVA, followed by the Newman–Keuls as the post hoc test for multiple comparisons were used. Whenever needed, the paired or unpaired Student's t-test was used for comparisons between two means. Differences were considered significant at p < 0.05.
5. Results
5.1. Determination of monoamine contents in striatal tissue from ischemic rats, after 1 and 7 post-ischemia days
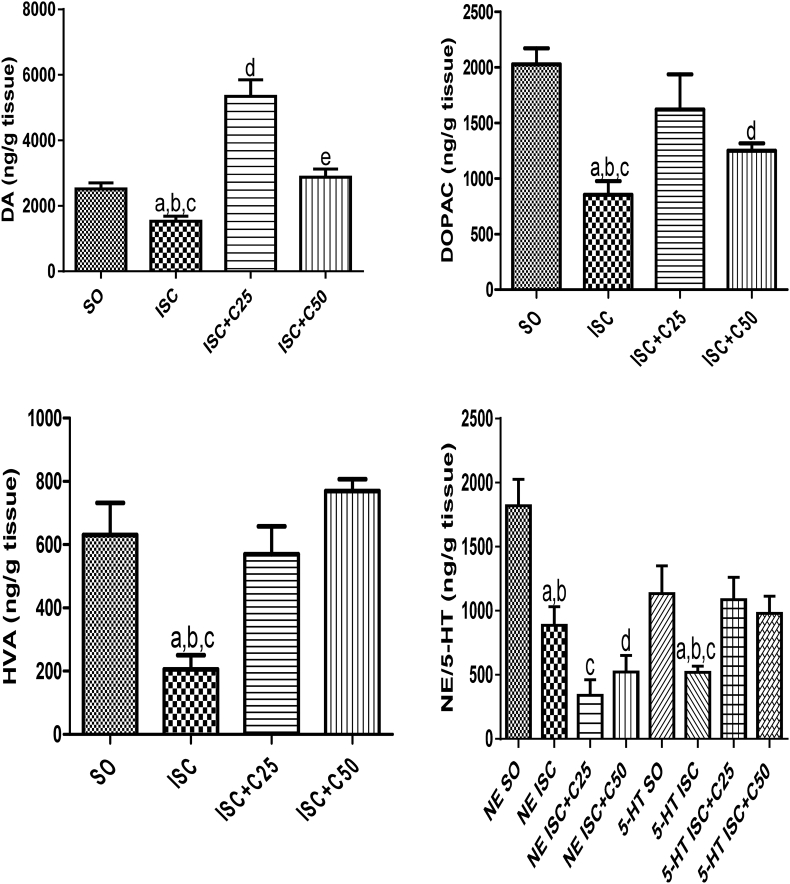
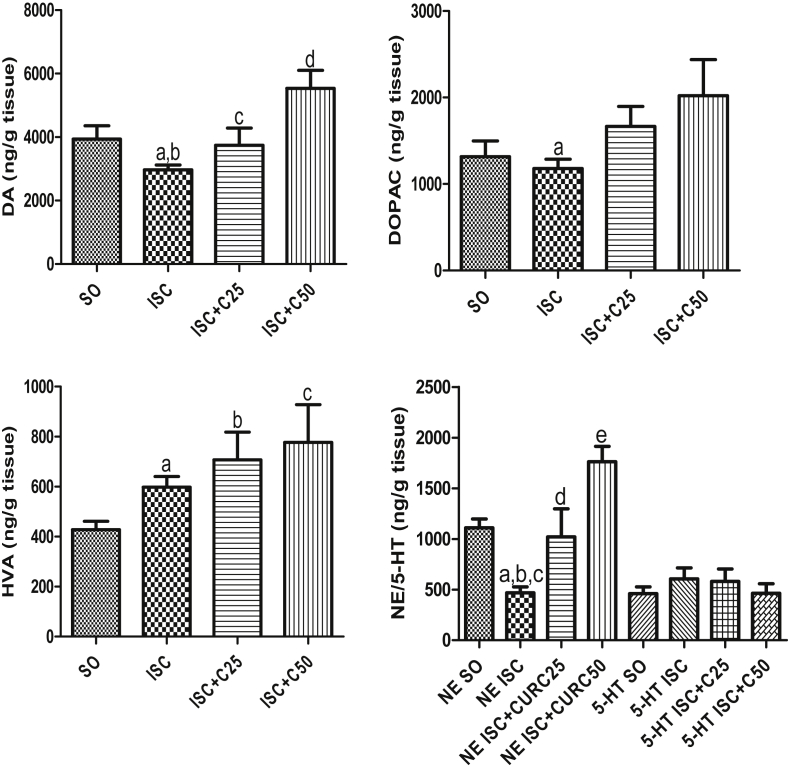
Our results demonstrated that all the monoamine striatal concentrations decreased (39 to 67%) in the untreated ischemic group, as related to the SO group, at 1 post-ischemia day (Fig. 1A, B, C and D). Except in the case of NE contents, where the decreases in the untreated ischemic group were maintained, these alterations were totally (e.g. DA, HVA and 5-HT) or partially (DOPAC) reversed in the curcumin (25 and 50 mg/kg) treated ischemic groups. As far as DA is concerned, its levels in the curcumin treated ischemic groups were even higher than those in the SO group, mainly after the lower curcumin dose (2.1-fold increase). The pattern of changes, at the 7th post-ischemia day, was different as related to that observed in the 1st post-ischemia day. Thus, while significant decreases were seen in NE (58%) and DA (25%) contents in the untreated ischemic group, no significant changes were observed in the levels of other monoamines, as related to the SO group. These changes were totally reversed in the ischemic group after curcumin treatments. Interestingly, in this protocol (7th post-ischemia day), 5-HT contents were similar in all groups tested (Fig. 2A, B, C, D).
Fig. 1.
Curcumin treatments reverse DA and its metabolites, DOPAC and HVA, as well as NE and 5-HT alterations, as related to the curcumin-untreated ischemic group, at the 1st day after ischemia. DA: a. vs. SO, q = 3.220; b. vs. ISC + C25, q = 12.49; c. vs. ISC + C50, q = 3.857; d. vs. SO, q = 9.269; e. vs. ISC + C25, q = 7.097. DOPAC: a. vs. SO, q = 7.487; b. vs ISC + C25, q = 4.269; c. vs. ISC + C50, t = 2.858, df = 8; d. vs. SO, q = 4.957. HVA: a. vs. SO, q = 6.256; b. vs. ISC + C25, q = 4.167; c. vs. ISC + C50, q = 6.971. NE: a. vs. SO, q = 5.629; b. vs. ISC + C25, t = 2.800, df = 7; c. vs. SO, q = 8.425; d. vs. SO, q = 7.383. 5-HT: a. vs. SO, q = 4.649; b. vs. ISC + C25, q = 4.101; c. vs. ISC + C50, t = 3.976, df = 12 (One-way ANOVA followed by Newman–Keuls as the post hoc test and two-tailed unpaired t-test).
Fig. 2.
Curcumin treatments partly reversed DA and its metabolites, DOPAC and HVA, as well as NE, but not 5-HT contents, as related to the curcumin-untreated ischemic group, at the 7th day after ischemia. DA: a. vs. SO, t = 2.306, df = 11; b. vs. ISC + C50, q = 6.551; c. vs. ISC + C50, q = 3.994; d. vs. SO, q = 3.949. DOPAC: a. vs. ISC + C50, t = 2.225, df = 12. HVA: a. vs. SO, t = 2.986, df = 14; b. vs. SO, t = 3.043, df = 9; c. vs. SO, t = 2.445, df = 11. NE: a. vs. SO, q = 5.124; b. vs. ISC + C25, t = 2.384, df = 8; c. vs. ISC + C50, q = 10.85; d. vs. ISC + C25, q = 5.565; e. vs. SO, q = 5.222 (One-way ANOVA followed by Newman–Keuls as the post hoc test and two-tailed unpaired t test).
5.2. Fluoro-jade staining in rat hippocampi
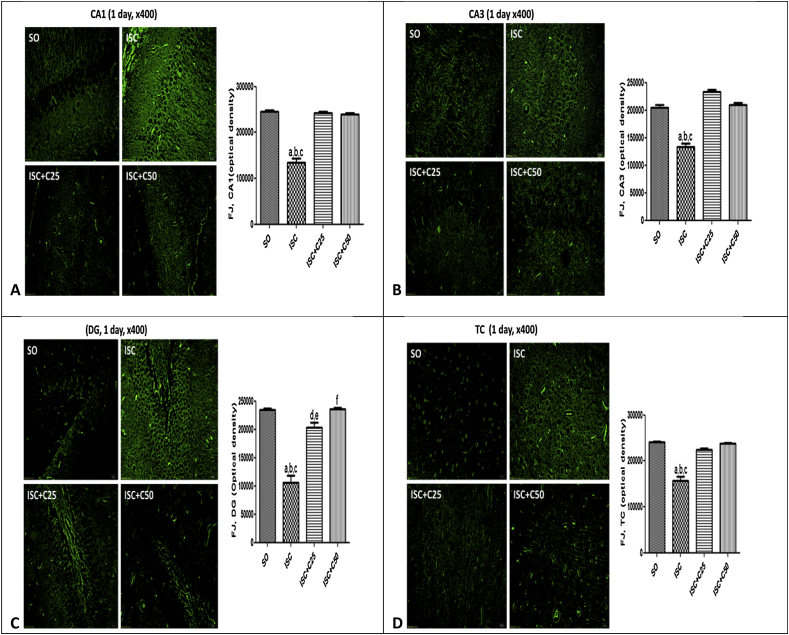
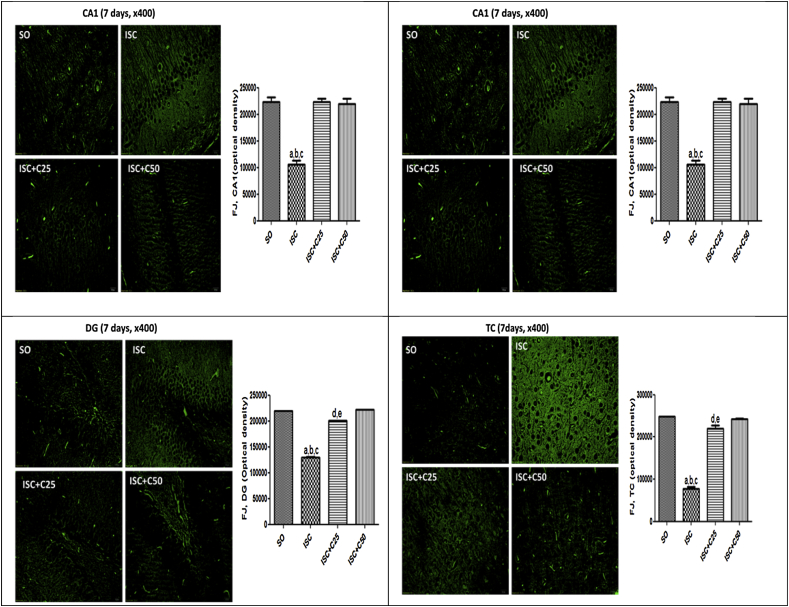
Fig. 3 are photomicrographs of sham operated (SO), untreated ischemic groups and ischemic groups after curcumin treatments (25 and 50 mg/kg), at 1 day after ischemia. The data show lower neuron viabilities in the CA1, CA3, DG and temporal cortex (TC) of the untreated ischemic group, as related to those of the SO group. However, changes were observed mainly in the CA1 and DG areas which showed 45 and 55% reductions in neuron viability, respectively, as related to the SO group. The ischemic groups after treatments with curcumin presented a profile similar to those of the SO group. Fig. 4 presents the same areas in the 2nd protocol (7th day after ischemia). Lower neuron viabilities were seen in the untreated ischemic group, mainly at CA1 and TC areas (52 and 69% reductions, respectively, as related to the SO group) and these changes were completely reversed after curcumin treatments.
Fig. 3.
Representative photomicrographs (×400 magnification) of fluoro-jade staining, in the CA1, CA3, DG and TC areas, at 1 day after ischemia, showing lower neuron viability in the untreated ischemic group, as related to the SO group. Alterations were mainly seen at the CA1 area. The curcumin treatments significantly improved neuronal viability in all areas, and the optical density values were brought towards those of the SO group. The data were quantified by the Image J software. CA1: a. vs. SO, q = 24.19; b. vs. ISC + C25, q = 23.29; c. vs. ISC + C50, q = 22.70. CA3: a. vs. SO, q = 14.36; b. vs. ISC + C25, q = 18.93; c. vs. ISC + C50, q = 14.40. DG: a. vs. SO, q = 15.64; b. vs. ISC + C25, q = 11.89; c. vs. ISC + C50, q = 15.80. TC: a. vs. SO, q = 17.41; b. vs. ISC + C25, q = 13.92; c. vs. ISC + C50, q = 16.83 (One-way ANOVA and Newman–Keuls as the post hoc test).
Fig. 4.
Representative photomicrographs (×400 magnification) of fluoro-jade staining, in the CA1 (A), CA3 (B), DG (C) and TC (D) areas, at 7 days after ischemia, showing lower neuron viability in the untreated ischemic group, as related to the SO group. Alterations were observed mainly at the CA1 and TC areas. The curcumin treatments significantly improved neuronal viability in all areas, and the optical density values were brought towards those of the SO group. The data were quantified by the Image J software. A-CA1: a. vs. SO, q = 14.47; b. vs. ISC + C25, q = 14.37; c. vs. ISC + C50, q = 14.02. B-CA3: a. vs. SO, q = 7.511; b. vs. ISC + C25, q = 14.44; c. vs. ISC + C50, q = 9.092; d. vs. SO, q = 6.9.34; e. vs. ISC + C25, q = 5.353. C-DG: a. vs. SO, q = 103.3; b. vs. ISC + C25, q = 81.56; c. vs. ISC + C50, q = 105.8; d. vs. SO, q = 21.78; e. vs. ISC + C50, q = 24.20. D-TC: a. vs. SO, q = 35.08; b. vs. ISC + C25, q = 29.43; c. vs. ISC + C50, q = 33.78; d. vs. SO, q = 6.103; e. vs. ISC + C50, q = 4.695 (One-way ANOVA and Newman–Keuls as the post hoc test).
5.3. Immunohistochemistry assays for COX-2 and TNF-alpha in rat hippocampi
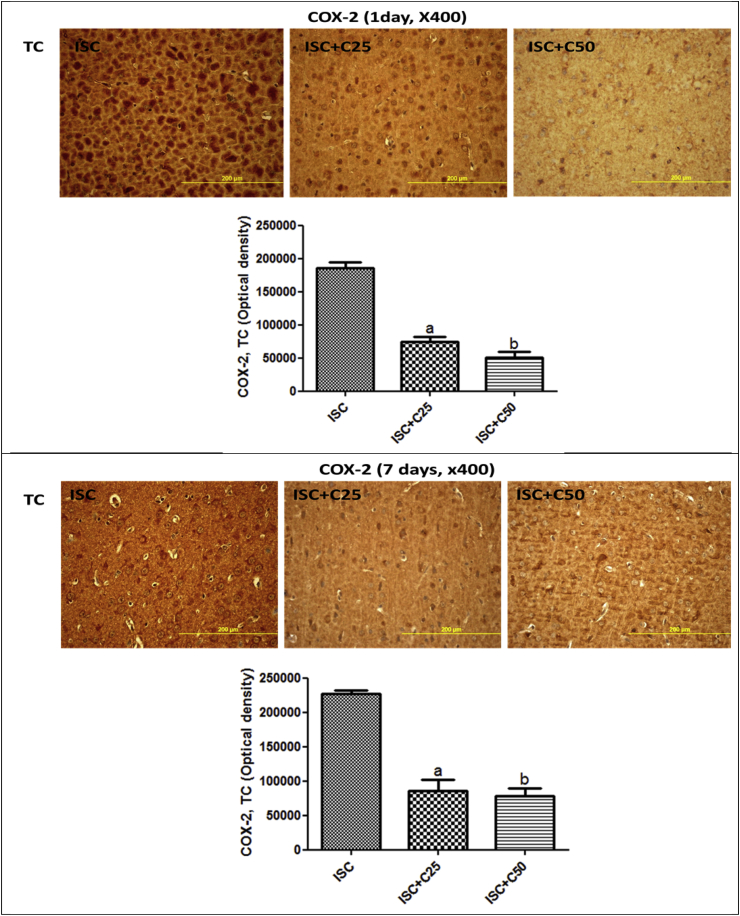
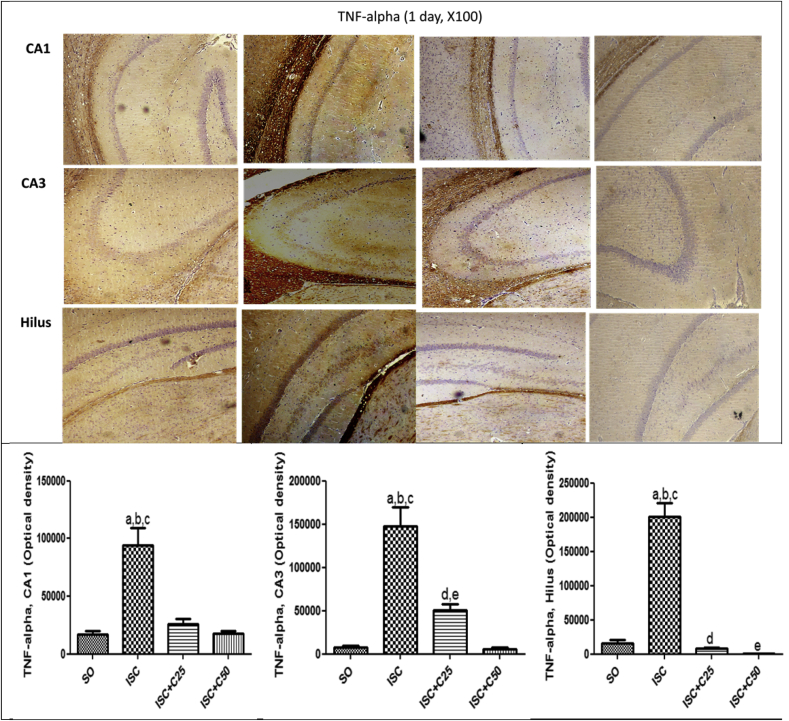
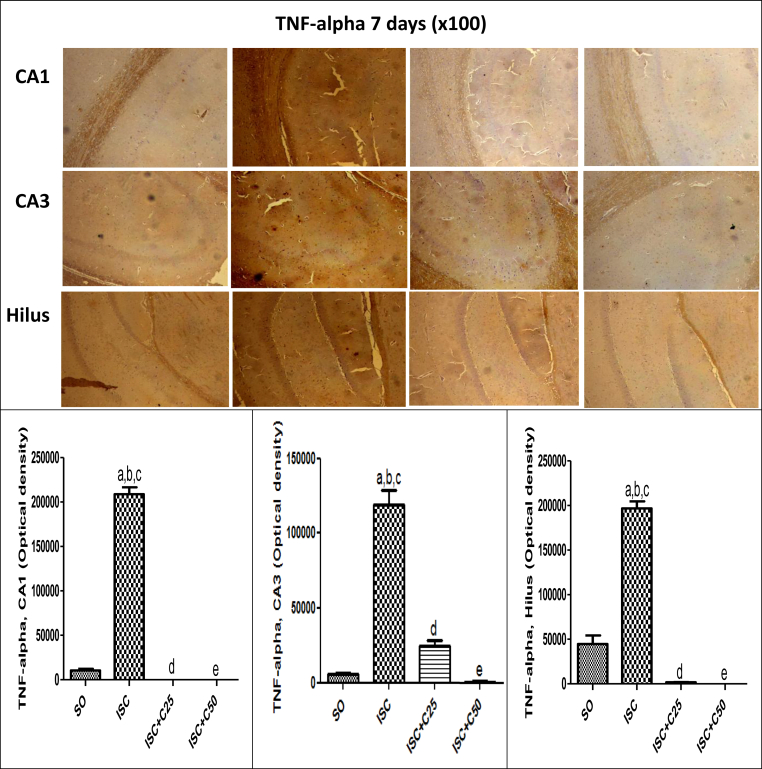
Fig. 5 shows immunohistochemistry assays for COX-2, an inducible inflammatory enzyme. A higher immunostaining was observed in the TC area in the untreated ischemic groups, at 1 and 7 days after ischemia. This effect was drastically decreased in the ischemic group after curcumin treatments (25 and 50 mg/kg). A much higher immunostaining for TNF-alpha, a pro-inflammatory cytokine, was also demonstrated in CA1 (5.5-fold), CA3 (19-fold) and hilus (12.7-fold) in the untreated ischemic group, as related to the SO groups. These values were drastically attenuated in the ischemic group after curcumin treatments (25 and 50 mg/kg), at the 1st day after ischemia (Fig. 6). At the 7th post-ischemia day, the untreated ischemic group showed a 20-fold increase in TNF-alpha immunoreactivity, in both CA1 and CA3 areas, and only a 4.4-fold increase in the hylus, as related to the SO group. Similarly, immunostainings for TNF-alpha were highly attenuated after curcumin treatments, emphasizing the involvement of this cytokine in the neuroprotective effect of curcumin (Fig. 7).
Fig. 5.
Representative photomicrographs showing less immunostaining for COX-2 in the temporal cortex (TC) area, in the ischemic group after curcumin treatments, as related to curcumin-untreated ischemic animals, at the 1st and 7th post-ischemia days (x400). The data were quantified by the Image J software and the results expressed as optical densities. TC, 1day: a. vs. ISC + C25, q = 13.27; b. vs. ISC + C50, q = 16.01. TC, 7 days: a. vs. ISC + C25, q = 13.74; b. vs. ISC + C50, q = 14. 57 (One-way ANOVA and Newman–Keuls as the post hoc test).
Fig. 6.
Representative photomicrographs (×100 magnification) showing a high immunoreactivity for TNF-alpha in CA1, CA3 and hilus areas, in the untreated ischemic group, as related to the SO group, at 1 day after ischemia. The effects were more intense in the CA3 area which showed a 19-fold increase in TNF-alpha immunoreactivity. The curcumin treatment completely reversed these effects, which were quantified by the Image J software. CA1: a. vs. SO, q = 9.887; b. vs. ISC + C25, q = 8.775; c. vs. ISC + C50, q = 9.804. CA3: a. vs. SO, q = 12.23; b. vs. ISC + C25, q = 8.501; c. vs. ISC + C50, q = 12.44; d. vs. SO, q = 3.727; e. vs. ISC + C50, q = 3.942. Hilus: a. vs. SO, q = 18.64; b. vs. ISC + C25, q = 19.36; c. vs. ISC + C50, q = 20.16 (One-way ANOVA and Newman–Keuls as the post hoc test).
Fig. 7.
Representative photomicrographs (×100 magnification) showing a high immunoreactivity for TNF-alpha in CA1, CA3 and hilus areas, in the untreated ischemic group, as related to the SO group, at 7 days after ischemia. The effects were more intense in CA1 and CA3 areas which showed a 20-fold increase in TNF-alpha immunoreactivity. The curcumin treatment completely reversed these effects which were quantified by the Image J software. CA1: a. vs. SO, q = 50.77; b. vs. ISC + C25, q = 53.44; c. vs. ISC + C50, q = 53.44. CA3: a. vs. SO, q = 22.45; b. vs. ISC + C25, q = 18.72; c. vs. ISC + C50, q = 23.52; d. vs. SO, q = 3.729; e. vs. ISC + C50, q = 4.799. Hilus: a. vs. SO, q = 24.43; b. vs. ISC + C25, q = 31.30; c. vs. ISC + C50, q = 31.59; d. vs. SO, q = 6.867; e. vs, SO, q = 7.162 (One-way ANOVA and Newman–Keuls as the post hoc test).
6. Discussion
Curcuma longa is probably one of the most studied medicinal plants and presents curcumin as its main bioactive compound. Curcumin shows a great number of biological effects, ranging from anti-inflammatory to anticancer properties, among several others.17, 18 More recently, several studies have shown the neuroprotective effects of curcumin in animal models of brain ischemia.15, 19, 20, 21, 22, 23 In addition, it is largely accepted that inflammation plays an important role in brain ischemia.13 Lastly, curcumin can cross the blood brain barrier and has therapeutic potential in different disorders, including neurological diseases.24
Evidences from in vitro and in vivo experimental as well as clinical studies indicate that the brain responds to ischemic injury with an acute and prolonged inflammatory process, characterized by rapid activation of resident cells, production of proinflammatory mediators and infiltration of inflammatory cells into the ischemic brain tissue.13, 25, 26, 27 The brain is particularly vulnerable to ischemia and the interruption of blood flow to the brain, even for a short period of time, as 5 min, triggers the death of neurons in several brain regions.28 The severe and prolonged reduction of cerebral blood flow leads to deprivations in oxygen and glucose, as well as to the increase of potentially toxic substances.4
In the present study, we showed that brain ischemia significantly decreases not only striatal DA contents, but also its metabolites (DOPAC and HVA). Although this effect was observed mainly at the 1st day after ischemia, it was still seen 7 days later. Earlier studies demonstrated that, during brain ischemia, the blood flow decreased up to 95%, but recovered to control levels during reperfusion.29 These authors showed that dopamine increased markedly in the cerebral cortex and striatum, during recirculation, while its metabolites DOPAC and HVA were not much affected. This massive increase in DA was observed in the hippocampus as well.30 Others,31, 32 showed that the marked increase in extracellular levels of DA in the striatum persisted throughout ischemia and was rapidly cleared up during reperfusion, mainly via reuptake.
Recently,33 the extracellular concentration of DA was shown to increase abruptly, 3 min after the ischemic insult, reaching a maximum after 20–40 min and decreasing subsequently. For 120 min, DOPAC and HVA concentrations also decreased significantly. These data indicated that a large increase of extracellular DA concentration, in cerebral ischemia, probably results from energy failure of cell membranes and might play a role in neuronal damage associated to cerebral ischemia. In our study, all monoamine measurements were performed several hours or several days after ischemia and the initial increase in these monoamine contents was replaced by a significant decrease instead, particularly because, in our case, we used a transient brain ischemia model. This result was similar to other ones,34 showing that, after different time intervals, ranging from 24 up to 96 h, DA and its metabolites in the striatum decreased markedly, as related to control values.
In the present study, most of these alterations in monoamine contents were reversed in the ischemic group after curcumin treatments. We also showed significant decreases in NE, at the 1st and 7th days after ischemia that were reversed only after a 7-day treatment of the ischemic group. While no changes were observed in 5-HT levels, 7 days after ischemia, a decrease had occurred at the 1st post-ischemia day and this effect was completely reversed by curcumin treatments. Thus, we showed a different profile in NE and 5-HT contents in the striatum, after ischemia and curcumin treatments. Curcumin was shown35, 36, 37 to increase DA levels and to cause no change in NE, when combined to antidepressant drugs, and this effect could at least partly be involved with the curcumin antidepressant effect. This antidepressant effect of curcumin seems to involve not only 5-HT but also dopamine systems.38
Although, in the present study, besides much lower neuron viability, we also showed a high immunoreactivity in almost all hippocampal areas of the untreated ischemic group that was in great part blocked in the ischemic group after curcumin treatments. These results were observed for COX-2 and TNF-alpha. Inflammatory processes are very important in the pathophysiology of stroke where a key initial event is the activation of microglia.39 Furthermore, in vitro studies demonstrate that curcumin reduces the production of ROS and inflammatory mediators from activated microglia.40 Microglial cells are important effectors of the neuronal innate immune system, and in vitro studies show that curcumin attenuates microglial migration, triggering a cell phenotype with anti-inflammatory and neuroprotective properties.41 These data agree with ours, suggesting of a curcumin neuroprotective action.
The anti-inflammatory effect of curcumin is likely mediated through its ability to inhibit not only COX-2, but also LOX and ions, important enzymes that mediate inflammatory processes. Indeed, at cellular and molecular levels, curcumin has been shown to regulate signaling pathways involving COX and LOX.42, 43 In the present study, we showed that the curcumin treatment of ischemic animals decreases COX-2 immunostaining in hippocampal areas, as related to the curcumin-untreated ischemic group, what points out to its anti-inflammatory property.
Earlier studies in rats44 showed that focal cerebral ischemia results in elevated TNF-alpha mRNA, in ischemic neurons. TNF-alpha is a pleiotrophic polypeptide, known to play a significant role in brain immune and inflammatory activities.45 It is produced in the brain in response to various pathological processes, including ischemia.
Others46 showed that exogenous TNF-alpha exacerbates focal ischemic injury, thus the blocking of TNF-alpha is neuroprotective. This suggests that inhibiting TNF-alpha may represent a novel pharmacological strategy to treat brain ischemia and related processes. More recently, curcumin was found to inhibit the expression of TNF-alpha-induced IL-1beta and IL-6, in HaCaT cells.47 These authors concluded that curcumin exerts anti-inflammatory and growth inhibitory effects in TNF-alpha-treated HaCaT cells, through inhibition of NF-kappa B and MAPK pathways.
Curcumin was shown to block the activation of NF-kappa B by TNF-alpha, in human endothelial cells.48 More recently,49 evidences from a review study indicate that curcumin blocks TNF-alpha production in vitro and in vivo models, as well as in humans, and is active against all diseases for which TNF-alpha blockers are currently being used. Clinical studies indicate that inflammatory processes are associated with the early stage of ischemic stroke, and TNF-alpha and IL-6 are higher in patients with a bad prognostic.50
Despite concerns about its poor oral biodisponibility, curcumin has been shown to present at least ten neuroprotective actions.25 Thus, we feel that curcumin and/or its more stable metabolite, tetrahydrocurcumin (TC), are responsible for the observed effects shown by us. A previous study51 clearly demonstrated the antioxidant, anti-inflammatory and anti-amyloidogenic effects of dietary curcumin and TC, chronically or acutely administered on LPS-injected mice. These authors showed that, despite higher drug plasma levels after TC as compared to curcumin, the resulting brain levels of parent compounds were similar and correlated with the reduction in LPS-stimulated iNOS and other endogenous compounds. Curcumin and TC also reduced chronic inflammation and IL-1beta. In addition, TC was detected in mice brain and plasma. Surprisingly, curcumin but not TC prevented Aβ aggregation in models of neuroinflammation and Alzheimer's disease. Furthermore, all these data support our findings and indicate that curcumin and/or TC are responsible for the neuroprotective properties.
7. Conclusion
We showed that curcumin reverses most of the neurochemical and immunohistochemical alterations of the ischemic group. Considering the involvement of inflammation in brain ischemic episodes and the anti-inflammatory property of curcumin, as well as its antioxidant activity observed even at lower doses, as shown by us14 and others,42, 52, 53, 54 our data emphasize the potential benefit of curcumin in the prevention or treatment of stroke.
Conflict of interest
The authors declare no conflict of interest.
Authors' contribution
GFTA and ES-N carried out the brain ischemia experiments; GMPC and MEPN helped with the statistical analyses; KRTN, GMA and GACB performed all histological and immunohistochemistry assays; and GSBV participated in the design and coordination of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the financial support from the Ceará State Foundation for the Development of Technology and Science (FUNCAP), the technical assistance from Ms. Janice Lopes and Ms. Auryclennedy Araújo and to Prof. M.O.L. Viana for the orthographic revision of the manuscript.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Glaura Fernandes Teixeira de Alcântara, Email: gfta_fernandes@hotmail.com.
Eudes Simões-Neto, Email: eudesimoes@gmail.com.
Giovany Michely Pinto da Cruz, Email: giovanycruz@hotmail.com.
Maria Elizabeth Pereira Nobre, Email: bethpn@bol.com.br.
Kelly Rose Tavares Neves, Email: kelly.rose@hotmail.com.
Geanne Matos de Andrade, Email: gmatos@ufc.br.
Gerly Anne de Castro Brito, Email: gerlybrito@gmail.com.
Glauce Socorro de Barros Viana, Email: gbviana@live.com.
References
- 1.Donnan G.A., Fisher M., Macleod M., Davis S.M. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Towfighi A., Saver J.L. Stroke declines from third to fourth leading cause of death in the United States: historical perspectives and challenges ahead. Stroke. 2011;42:2351–2355. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- 3.Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 4.Bramlett H.M., Dietrich W.D. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- 5.Graham S.H., Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Liu P.K., Hamilton W.J., Hsu C.Y. Apoptosis: DNA damage and repair in stroke. In: Miller L.P., editor. vol. 11. John Wiley & Sons Inc; 1999. pp. 299–320. (Stroke Therapy Basic, Preclinical, and Clinical Directions). [Google Scholar]
- 7.Snider B.J., Gottron F.J., Choi D.W. Apoptosis and necrosis in cerebrovascular disease. Ann N Y Acad Sci. 1999;893:243–253. doi: 10.1111/j.1749-6632.1999.tb07829.x. [DOI] [PubMed] [Google Scholar]
- 8.Martin L.J., Sieber F.E., Traystman R.J. Apoptosis and necrosis occur in separate neuronal populations in hippocampus and cerebellum after ischemia and are associated with differential alternations in metabotropic glutamate receptor signaling pathways. J Cereb Blood Flow Metab. 2000;20:153–167. doi: 10.1097/00004647-200001000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Maier C.M., Chan P.H. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist. 2002;8:323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- 10.Globus M.Y., Alonso O., Dietrich W.D., Busto R., Ginsberg M.D. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- 11.Chan P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Bethea J.R., Dietrich W.D. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol. 2002;15:355–360. doi: 10.1097/00019052-200206000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira J.A., Carvalho E.T., Araújo A.C., Lopes M.J.P., Cruz G.M.P., Viana G.S.B. XXIII Simposium of Brazilian Medicinal Plantas, Goiânia, GO, September 2014. 2014. Evaluation of the analgesic and anti-inflammatory properties of curcumin at low doses. [Google Scholar]
- 15.Liu L., Zhang P., Li Y., Yu G. Curcumin protects brain from oxidative stress through inducing expression of UCP2 in chronic cerebral hypoperfusion aging-rats. Mol Neurodegener. 2012;7(suppl 1):S10. [Google Scholar]
- 16.Zhu H.T., Blan C., Yuan J.C. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-kB signaling pathway in experimental traumatic brain injury. J Neuroinflammation. 2014;11:59. doi: 10.1186/1742-2094-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattopadhyay I., Biswas K., Bandyopadhyay, Banerjee R.K. Turmeric and curcumin: biological actions and medicinal application. Curr Sci. 2004;87:44–53. [Google Scholar]
- 18.Sharma R.A., Gescher A.J., Steward W.P. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Yang C., Zhang X., Fan H., Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Yu G., Liu L., Zhang P., Li Y. Protective effect of curcumin on chronic cerebral ischemia by altering expression of alpha-synuclein in 2VO model. Mol Neurodegener. 2012 [Google Scholar]
- 21.Liu Z.-J., Liu W., Liu L., Xiao C., Wang Y., Jiao J.-S. Curcumin protects neuron against cerebral ischemia-induced inflammation through improving PPAR-gamma function. Evid Based Complement Altern Med. 2013;2013:10. doi: 10.1155/2013/470975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J., Li Q., Wang X. (2013). Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PloS One. 2013;8(3):e59843. doi: 10.1371/journal.pone.0059843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu X.K., Yang W.Z., Chen J.P. Curcumin inhibits TLR2/4-NF-kB signaling pathway and attenuates brain damage in permanent focal cerebral ischemia in rats. Inflammation. 2014;37:1544–1551. doi: 10.1007/s10753-014-9881-6. [DOI] [PubMed] [Google Scholar]
- 24.Mukunda M., Mythri S.B., Harish R.B. 16th International Congress of Parkinson's Disease and Movement Disorders, Dublin, Ireland. 2012. Neuroprotection by the dietary polyphenol curcumin: therapeutic implications for Parkinson's disease. [Google Scholar]
- 25.Cole G.M., Teter B., Frautschy S.A. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakhan S.E., Kirchgessner A., Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shichita T., Sakaguchi R., Suzuki M., Yoshimura A. Post-ischemic inflammation in the brain. Front Immunol. 2012;3 doi: 10.3389/fimmu.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S., Suh S., Kim S. Protective effects of the green tea polyphenol (-)-epigallocatechin gallate against hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci Lett. 2000;287:191–194. doi: 10.1016/s0304-3940(00)01159-9. [DOI] [PubMed] [Google Scholar]
- 29.Harik S.I., Yoshida S., Busto R., Ginsberg M.D. Monoamine neurotransmitters in difuse reversible forebrain ischemia and early recirculation: increased dopaminergic activity. Neurology. 1986;36:971–976. doi: 10.1212/wnl.36.7.971. [DOI] [PubMed] [Google Scholar]
- 30.Vamyakidès A. Cerebral ischemia: massive increase of the dopamine release or stagnation? Ann Pharm Fr. 1992;50:277–289. [PubMed] [Google Scholar]
- 31.Phebus L.A., Clemens J.A. Effects of transient, global, cerebral ischemia on striatal extracellular dopamine, serotonin and their metabolites. Life Sci. 1989;44:1335–1342. doi: 10.1016/0024-3205(89)90390-1. [DOI] [PubMed] [Google Scholar]
- 32.Obrenovitch T.P., Sarna G.S., Matsumoto T., Symon L. Extracellular striatal dopamine and its metabolites during transient cerebral ischaemia. J Neurochem. 1990;54:1526–1532. doi: 10.1111/j.1471-4159.1990.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 33.Kawano T., Tsutsumi K., Miyake H., Mori K. Striatal dopamine in acute cerebral ischemia of stroke-resistant rats. Stroke. 1988;19:1540–1543. doi: 10.1161/01.str.19.12.1540. [DOI] [PubMed] [Google Scholar]
- 34.Frölich I., Dirr A., Riederer P., Hoyer S. Effects of long-term recovery from transient cerebral ischemia in rat brain: tissue levels of acetylcholine, monoamines, and their metabolites. Neurochem Res. 1993;18:1239–1244. doi: 10.1007/BF00975041. [DOI] [PubMed] [Google Scholar]
- 35.Kulkarni S.K., Bhutani M.K., Bishoi M. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology. 2008;201:435–442. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- 36.Kulkarni S.K., Dhir A., Akula K.K. Potentials of curcumin as an antidepressant. Sci World J. 2009;9:1233–1241. doi: 10.1100/tsw.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buthani M.K., Bishnoi M., Kulkarni S.K. Antidepressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical chnges. Pharmacol Biochem Behav. 2009;92:39–43. doi: 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Lopresti A.L., Hood S.D., Drummond P.D. Multiple antidepressant modes of action of curcumin: a review of its anti-inflammatory, monoaminergic, antioxidant, immunomodulatin and neuroprotective effects. J Psychopharmacol. 2012;26:1512–1524. doi: 10.1177/0269881112458732. [DOI] [PubMed] [Google Scholar]
- 39.Patel A.R., Ritzel R., McCullough L.D., Liu F. Microglia and ischemic stroke: a double-edge sword. Int J Physiol Pathophysiol Pharmacol. 2013;5:73–90. [PMC free article] [PubMed] [Google Scholar]
- 40.Guo L., Xing Y., Pan R. Curcumin protects microglia and primary rat cortical neurons against HIV-1 gp120-mediated inflammation and apoptosis. Plos One. 2013;8(8):e70565. doi: 10.1371/journal.pone.0070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlstetter M., Lippe E., Walczak Y. Curcumin is a potent modulator of microglial gene expression and migration. J Neuroinflammation. 2011;2011(8):12. doi: 10.1186/1742-2094-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menon V.P., Sudheer A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 43.Rao C.V. Regulation of COX and LOX by curcumin. Adv Exp Med Biol. 2007;595:213–226. doi: 10.1007/978-0-387-46401-5_9. [DOI] [PubMed] [Google Scholar]
- 44.Liu T., Clark R.K., McDonnell P.C. Tumor necrosis factor-alpha expression inmischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 45.Feuerstein G.Z., Liu T., Barone F.C. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev. 1994;6:341–360. [PubMed] [Google Scholar]
- 46.Barone F.C., Arvin B., White R.F. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 47.Cho J.W., Lee K.S., Kim C.W. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med. 2007;19:469–474. [PubMed] [Google Scholar]
- 48.Kim Y.S., Ahn Y., Hong M.H. Curcumin attenuates inflammatory responses of TNF-alpha stimulated human endothelial cells. J Cardiovasc Pharmacol. 2007;50:41–49. doi: 10.1097/FJC.0b013e31805559b9. [DOI] [PubMed] [Google Scholar]
- 49.Aggarwal B.B., Gupta S.C., Sung B. Curcumin: an orally bioavailable blocker of TNF-alpha and other pro-inflammatory biomarkers. Brit J Pharmacol. 2013;169:1672–1692. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Domaç F., Somay G., Missrlt H., Erenoglu N.Y. Tumor necrosis factor alpha serum levels and inflammatory response in acute stroke. Neurosciences. 2007;12:25–30. [PubMed] [Google Scholar]
- 51.Begum A.N., Jones M.R., Lim G.P. Curcumim structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. Pharmacol Exp Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sikora E., Scapagnini G., Barbagallo M. Curcumin, inflammation, ageing and age-related diseases. Immun Ageing. 2010;7:1. doi: 10.1186/1742-4933-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basnet P., Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koeberle A., Muñoz E., Appendino G.B. SAR studies on curcumin's pro-inflammatory targets: discovery of prenylated pyrazolocurcuminoids as potent and selective novel inhibitors of 5-lipoxygenase. J Med Chem. 2014;57:5638–5648. doi: 10.1021/jm500308c. [DOI] [PubMed] [Google Scholar]