The transient receptor potential melastatin 8 (TRPM8) plays a crucial part in cold detection by the somatosensory system. In heterologous expression systems, TRPM8 activity steeply increases upon cooling and in the presence of substances that are known to produce a cooling sensation, including menthol, and the ‘super-cooling agent’ icilin.1 TRPM8-deficient mice exhibited a striking deficit in avoiding cool temperatures (18–30°C). Moreover, whereas mild cooling can evoke analgesia in wild-type mice, cooling-induced analgesia was absent in TRPM8-deficient mice.2,3 Importantly, increased functional expression of TRPM8 contributes to pathological cold hypersensitivity and cold allodynia in various animal models of neuropathic and inflammatory pain.3 In recent years, important advances have been made in our knowledge about the biophysical properties of TRPM8.1 However, the knowledge about the trafficking mechanism that determine the abundance of TRPM8 at the plasma membrane is very sparse.4 Nevertheless, modulation of the number of active cold sensitive TRPM8 channels at the plasma membrane represents an important regulatory mechanism under normal and pathophysiological conditions. In this article we discuss our recent findings published in the article ’VAMP7 regulates constitutive membrane incorporation of the cold-activated channel’ in which we have uncovered a cellular pathway that controls functional plasma membrane incorporation of TRPM8, and thus regulates thermo-sensitivity in vivo.5
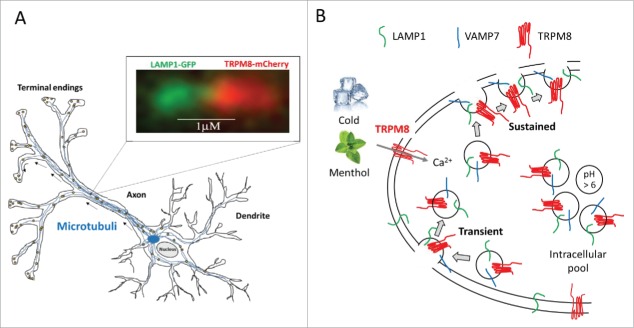
By the use of Total internal reflection fluorescence (TIRF) microscopy, in which only a thin layer of illumination above the interface is created and only fluorophores within this thin layer (∼100–300 nm) in the sample are excited, we revealed that fluorescently tagged TRPM8 channels are located in a population of highly dynamic vesicular and tubular structures. By treatment of TRPM8-mCherry expressing cells with microtubule- or actin-depolymerizing agents and additional TIRF Recovery after Photobleaching (TIRF-FRAP) experiments, we were able to show that TRPM8-positive structures use microtubules as principal track for rapid near-membrane intracellular movement. Further characterization of the mobile TRPM8-positive structures was done by co-expression of TRPM8-mCherry along with known markers of various cellular compartments tagged with GFP, and quantified by dual-color TIRFM to simultaneously monitor the movement of TRPM8-mCherry along with GFP-tagged marker proteins. These results showed strong dynamic co-localization of TRPM8 and the Lysosomal associated membrane protein 1 (LAMP1), which was also observed in neurites of TGN co-expressing TRPM8-mCherry and LAMP1-GFP (Fig. 1A). Although LAMP1 is typically associated with endo-lysosomal structures, additional TIR-FRAP experiments indicated that TRPM8- and LAMP1-positive mobile vesicles transport TRPM8 from the cell center toward the plasma membrane via microtubules. The pool of mobile TRPM8-positive vesicles is a stable compartment rather than a lysosomal structure targeted for degradation. This was further supported by the fact that the large majority of TRPM8– or LAMP1-positive structures observed in the near-membrane zone were not stained by lysotracker red or pHrodo red dextran, fluorescent dyes that selectively stain acidic lysosomal compartments, indicating that the luminal pH of these structures is higher than that of classical lysosomes (pH < 6).
Figure 1.
TRPM8 trafficking in neuronal cells and model of membrane incorporation. (A) Schematic overview of a neuronal cell overexpressing TRPM8-mCherry and LAMP1-GFP. TRPM8- and LAMP1-positive mobile vesicles transport TRPM8 from the cell toward the plasma membrane via microtubules. Insert shows dual-color TIRF images at consecutive time interval showing the movement of TRPM8-mCherry (red) along with the LAMP1-GFP (green), illustrating the dynamic co-localization of LAMP1 and TRPM8. (B) Cartoon illustrating the dynamic interaction of TRPM8 vesicles with the plasma membrane. TRPM8 is expressed in non-acidic (pH > 6), LAMP1 and VAMP7 positive vesicles and may undergo 2 different types of fusion with the plasma membrane. A transient fusion, where the vesicles fuse transiently with the plasma membrane and a sustained fusion, where the vesicle fuse with the plasma membrane for a longer period.
Further co-localization studies showed association between TRPM8 and the vesicular SNARE protein VAMP7. VAMP7 is present in LAMP1-positive structures and mediates their fusion with the plasma membrane. TIR-FRAP experiments showed dynamic co-localization of VAMP7-GFP and TRPM8-mCherry in mobile structures that repopulated the evanescent field following photobleaching. To further investigate whether the TRPM8 – VAMP7 structures are exclusively intracellular vesicles or also include vesicles that fuse with the plasma membrane, we have expressed TRPM8-mCherry together with VAMP7 tagged with the ecliptic pHluorin (VAMP7-pHluorin). TIRF imaging revealed a strong VAMP7-pHluorin signal in TRPM8-positive mobile structures, confirming the non-acidic character of these structures. Superfusion with a solution containing Na-acetate, which causes acidification of intracellular compartments, resulted in a rapid quenching of 50% of the VAMP7-pHluorin fluorescence, whereas the associated TRPM8-mCherry remained stable. Subsequent acidification of the extracellular solution to pH 5.5, in the continuous presence of acetate resulted in total quenching of the VAMP7-pHluorin signal. These results indicate that TRPM8 co-localizes with VAMP7, both in non-acidic intracellular vesicles as well as in membrane regions that are accessible to the extracellular medium. Next, we analyzed distinct types of TRPM8-positive structures repopulating the evanescent field following photobleaching by TIR-FRAP and identified 3 distinct structures. A first type included structures in which VAMP7-pHluorin was rapidly quenched by acetate, while TRPM8-mCherry fluorescence was sustained. This was interpret as intracellular vesicles. A second type included structures where both TRPM8-mCherry and VAMP7-pHluorin remained stable in response to the acetate treatment, and was interpret as structures that are in contact with the extracellular space. A last type of structures was observed as incoming punctate structures that initially appeared in the red (mCherry) channel, and suddenly also acquired green (pHluorin) fluorescence and are indicative of exocytosis of TRPM8- and VAMP7-positive vesicles. These exocytotic events were observed as either transient or more sustained (Fig. 1B). Overall, these data provide for the first time direct evidence for the constitutive fusion of TRPM8- and VAMP7-positive vesicles with the plasma membrane.
Fura-2-based calcium imaging and whole cell patch-clamp experiments in TRPM8 expressing HEK293 cells along with either wild type VAMP7 or the N-terminus of VAMP7, known as a specific inhibitor of VAMP7 function, indicated that VAMP7 regulates the number of active TRPM8 channels in the plasma membrane. A similar result was observed in TGN from WT and VAMP7−/− mice with respect to their responsiveness to a cold stimulus. In the subset of capsaicin-insensitive VAMP7−/− neurons, we observed a significant reduction in the both the number of cells that showed a detectable cold response as well as in the amplitude of the cold-induced calcium increase. These results indicate that VAMP7 deficiency leads to reduced TRPM8 activity in non-nociceptive sensory neurons. Finally, we provide evidence that the absence of VAMP7 leads to a specific deficit in the avoidance of innocuously cold temperatures in the thermal gradient assay, and to a strong deficit in icilin-induced cold hypersensitivity in VAMP7−/− mice.
Altogether, these results suggest that fusion of VAMP7- and TRPM8-containing vesicles represents a mechanism for the constitutive transport of TRPM8 to the plasma membrane. Notably, the VAMP7-dependent transport is not unique for TRPM8 but may be utilized by a subset of TRP channels, including the heat-sensitive TRPM3. These findings suggest that targeting VAMP7 function may represent an interesting alternative to classical channel antagonists for treatment of patients with cold allodynia.
References
- [1].Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci 2014; 15:573-89; PMID:25053448; http://dx.doi.org/ 10.1038/nrn3784 [DOI] [PubMed] [Google Scholar]
- [2].Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007; 448:204-8; PMID:17538622; http://dx.doi.org/ 10.1038/nature05910 [DOI] [PubMed] [Google Scholar]
- [3].Colburn RW, Lubin ML, Stone DJ Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007; 54:379-86; PMID:17481392; http://dx.doi.org/ 10.1016/j.neuron.2007.04.017 [DOI] [PubMed] [Google Scholar]
- [4].Toro CA, Eger S, Veliz L, Sotelo-Hitschfeld P, Cabezas D, Castro MA, Zimmermann K, Brauchi S. Agonist-dependent modulation of cell surface expression of the cold receptor TRPM8. J Neurosci 2015; 35:571-82; PMID:25589752; http://dx.doi.org/ 10.1523/JNEUROSCI.3820-13.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ghosh D, Pinto S, Danglot L, Vandewauw I, Segal A, Van Ranst N, Benoit M, Janssens A, Vennekens R, Vanden Berghe P, et al. VAMP7 regulates constitutive membrane incorporation of the cold-activated channel TRPM8. Nat Commun 2016; 7:10489; PMID:26843440; http://dx.doi.org/ 10.1038/ncomms10489 [DOI] [PMC free article] [PubMed] [Google Scholar]