We write this commentary as 2 animal lovers who run a nonhuman primate (NHP) laboratory. Steve Helms Tillery is a principal investigator (PI), and Rachele McAndrew is the Laboratory Coordinator for all of the primate labs at Arizona State University. Rachele was always an animal lover and grew up with many different pets ranging from mice to dogs. Even as a child, she dreamed of working with animals in some form. She entered college in pre-veterinary studies. Though plans changed, she did not give up on a career with animals. Instead, she ended up with a different focus, working with research animals. Steve grew up in a different environment. He did not have animals in his home, but he always loved being around animals of any kind. He was fascinated by their behaviors and their perception of the world, and enjoyed the casual bond that he was able to develop with domestic animals from rodents and dogs to horses and cows.
And yet we both grew up to be active participants in animal research. To the outsider it may appear as if we turned our backs on early leanings toward animals: Rachele in particular gave up on her life goal of helping animals as a veterinarian. Indeed, we are both often asked how we can do what we do if we love animals. Steve feels no need to apologize for what he does: his work is part of an enterprise which contributes dramatic changes to health care and human health, and he understands that healthy primates provide the best data. Rachele amplifies that by pointing out that her love of animals is a crucial part of her job: it is important in this field to care for and provide routine veterinary care for the research animals. At times, laboratory animals receive even more care and attention than the average pet.
However, it is clear to us that the general public does not understand the whole story regarding animal research. They may hear or read about the newest scientific breakthroughs with the help of research with animals, but in many cases the public perception of animal research is colored by the claims of animal activists who frequently foment an environment of controversy regarding animal research. The result is often negative. Public attacks on scientists can result in bad press for the researcher. This can lead to public antipathy toward animal scientists, who are often represented in popular media as unlikeable and untrustworthy.1 It is of little surprise that the average researcher does not feel comfortable discussing the day to day routine of their laboratory. Therefore we think it is important that the public gain another perspective regarding animal research, one directly from people like ourselves who carry out research. This editorial is one component of a larger effort to reach out to the scientific and broader communities about research with NHPs. Our goals include making positive changes in the way nonhuman primates are handled in research and raising a discussion regarding end-of-study decisions for these intelligent and social animals.
We agree with major advocacy groups that it is important to clearly and publicly define why we work with animals in research. There are a number of online resources providing this information. The National Association for Biomedical Research, the Foundation for Biomedical Research, and Americans for Medical Progress, to name a few, have information on their websites that delve into this topic. The advances in science and medicine that are dependent upon animal research have been substantial.2 Before any new medication is released in the market it must first be tested for efficacy and potential risks. We would not have medicines such as antibiotics, drugs to combat cancer, HIV/AIDS, and Alzheimer, to name a few, without thorough testing and research with lab animals including side effects, efficacy, and proper dosing. Animals are vital in the development of vaccines, learning about infectious diseases, behavior, cognition, development, genetics, and the brain. Fundamentally, any visit to a doctor is informed by animal research. This article, however, will mainly focus on monkeys in biomedical research, as those are the animals with whom we work on a daily basis. In particular, we will describe the laboratory lives of 2 of our animals. The first is Tiberius, a 5 y old rhesus macaque and the second is River, a 3 y old rhesus macaque.
Before we get into details on those 2 animals, we think it important to note that this field is highly regulated by multiple agencies. Monkeys are considered a USDA regulated species, so researchers must follow the detailed statutes in the Animal Welfare Act (AWA). This act governs the use of all research primates from the time of their birth. Most animal research in the US is regulated by the Public Health Service (PHS), which requires that anyone conducting animal research follow the guidelines set in the Guide for the Care and Use of Laboratory Animals (The Guide). The Office of Laboratory Animal Welfare (OLAW) enforces these guidelines at the federal level. Each institution is required to have an Institutional Animal Care and Use Committee (IACUC), which is the governing body to which the researcher must apply to in order to begin a study. The application requires that the study be scientifically sound, uses the appropriate animal model, and follows the regulations set forth by the AWA, The Guide, and other pertinent regulatory agencies. The IACUC must comprise at least one of each of the following: a scientist, a veterinarian, a member of the community with no relationship to the institution, and a non-scientist. Finally, animal research programs can apply for accreditation to the Assessment and Accreditation of Laboratory Animal Care (AAALAC) International, which requires the institution to not only follow, but exceed, the rules and regulations. Additional agencies can be involved depending on how research is funded. For example, with Department of Defense (DoD) funded projects, the Animal Care Use Review Office (ACURO) must also review and approve the IACUC approved protocol. Laboratories, breeding facilities and suppliers all fall under at least one regulatory agency.
Once a protocol has been approved by all pertinent groups and agencies, the PIs may begin his or her research. This first requires the necessary species to be purchased. Animals can be bought from a commercial supplier, another lab, or can be acquired from a breeding colony maintained at the research institution. Upon arrival at the institution, they will most likely be placed in quarantine, away from any current research animals for a short period of time. During the quarantine time, the incoming animals are tested for tuberculosis (Tb) and various parasites so as not to infect the animals that are currently housed in the institution. After this period is lifted, the new animals are introduced to the current colony, and the research may begin. Since incoming animals need to be housed separately from the colony upon arrival, we try to order more than one at a time so no monkey is ever housed alone. Tiberius and River were purchased from another laboratory, and were research naïve. They were housed in quarantine for a little over a month; they were sedated twice, 2 weeks apart for Tb and fecal tests. Once they tested negative the second time, they were introduced to the rest of the colony.
Monkeys are housed in a variety of ways, depending on the type of research conducted and the available space of the institution. They can be housed in large groups, smaller groups, paired with one other partner, or singly housed. Housing an NHP alone requires special permission and oversight from the institution's Attending Veterinarian and the IACUC. Social interaction is highly important for most species of NHPs. However, some species of monkeys, particularly rhesus macaque and African green monkey adult males are highly aggressive with each other. This can make forming pairs difficult if a colony only consists of adult males. The AWA allows for exemptions to be made in special cases, such as a lack of compatible partners, aggression, disease, or surgical and study related reasons. In these particular cases, the need for a quality environmental enrichment program that includes visual access to other conspecifics is crucial. Even so, the Attending Veterinarian and the IACUC must review all singly housed animals annually to ensure that it is still a necessary option. The Guide establishes requirements for an environmental enrichment program including social housing, as well as other parameters such as room temperature, humidity, noise control, veterinary care, and minimum cage sizes for research animals. The minimum cage size for NHPs are set according to weight and includes horizontal and vertical dimensions per animal. Caging can range from large outdoor or indoor pens, smaller pens, to indoor caging systems. These systems are normally very adjustable, and can be connected to each other to turn a smaller single cage into a large system of connected cages (Fig. 1). The monkeys in our facility are housed in the indoor caging systems described above. Each monkey gets at least 2 cages. If they are pair housed, they will get a total of four. Tiberius is currently singly housed. He was paired with another of our monkeys, but those 2 animals did not get along. He was then paired with another, but was placed on a surgically-related pairing restriction after his cortical implant procedure while the implant healed. Tiberius is now off the restriction, but there is a current lack of potential partners. On the other hand, River is paired housed with another young male of about the same age.
Figure 1.

Caging system: Two of our NHPs (River peeking around the corner, Severus behind the Primahedron) in a play cage. Their individual apartment cages are attached to the right of the play cage. When this photograph was shot, the animals had access to a total of 3 apartment cages along the bottom as well as the larger play cage.
Animal Care staff provide enrichment items daily, with the goal of providing the monkey with stimuli and occupying their time with things to do while they are not on study. Enrichment plans should be well thought-out and aim to stimulate all 5 senses.3,4 Care should be taken to mimic naturalistic behaviors as much as possible. Monkeys spend a majority of their time in the wild foraging for food. In captivity it is very easy for them to get food, as they merely need to go to their food bin. It is vital they be provided the opportunity to search or work for their food. Food puzzles and foraging devices work well for this purpose. Variety is also important, as monkeys can become bored if their options continually remain the same, therefore, items offered should be rotated often.3 Our program rotates at least one item daily in order to keep some novelty. The Guide does not specify how often items are rotated, but requires the IACUC and Attending Veterinarian review the institution's enrichment program on a regular basis to assess its effectiveness. A popular saying in this field is “the best enrichment is another monkey,” so social housing, as stated earlier, is a very important piece of a quality enrichment program. If social housing is not available, mirrors are a good way of introducing another monkey. Because Tiberius is singly housed, he has access to mirrors so he may use them to look at himself or other monkeys. A mirror in a cage also allows the monkey to use it as a tool to get a better view of the room or what's around the corner. Placing a mirror on the wall of the room can help monkeys see each other. Tiberius also receives protected access to other monkeys via mesh doors placed in between his cage and another monkey's cage. This way he has the ability to interact with another monkey without worry of injury. Positive reinforcement training can also be considered a form of enrichment. Training provides a sense of control for the monkey. Their actions directly produce certain results; if they perform the task correctly, they receive a reinforcer. A variety of behaviors can be trained, from routine veterinary procedures to specific research behavioral tasks.
The NHP's daily activity varies depending on the type of study conducted. Our lab's primary focus lies in neuro-controlled prosthetics. These state of the art devices can be controlled from a variety of biological signals, but do not generate sensation: it is like using an arm that has no feeling. A primary focus in the field is to use the same devices and neural interfaces to generate sensation from the prosthetic devices, which will make them much more usable for a broad population of users. Therefore, we record neural activity while the monkey performs different activities. Behavioral tasks we use rely on the monkey to respond to a certain stimulus in specific ways. For example; reaching out and grabbing an object or a virtual object in a specific way (Fig. 2), using the monkey's neural activity to control a curser on a screen, discriminating whether 2 presented stimuli are the same or different from each other, or simply responding whether or not they detect a stimulus.
Figure 2.
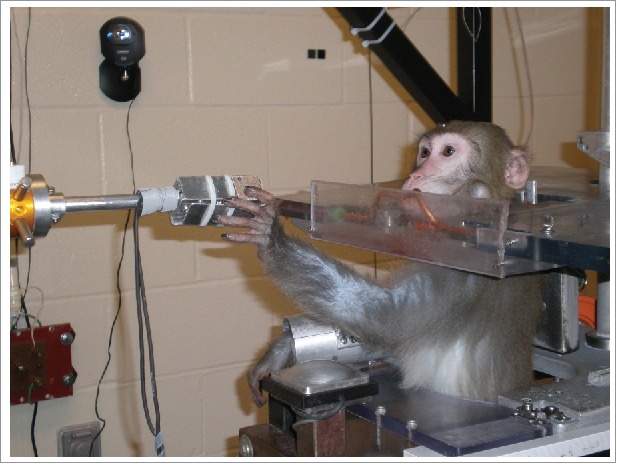
Monkey performing a task: A previous lab animal (Kringle) is shown interacting with an object that is being presented by a robotic arm. His task is to grasp the object once it has been positioned by the robot. If he grasps the object properly, he receives a reinforcer, a drop of juice through the copper tubing.
Training these complex tasks can take time. We follow a shaping plan for each experiment. These plans are separated into small steps, which are chained together until the desired behavior is obtained. For example, Tiberius and River focus on different aspects of the same experiment. This task requires the monkey to respond after they feel a small vibration on either their hand or arm. The first aim is to help them become used to and comfortable with the sensation. This is achieved by introducing the vibration, followed by a bridge and a treat. The bridge, usually a click in our laboratory, serves as an indication that the reinforcer is coming. After they are comfortable with the sensation, we move on to introducing a button that they will press upon feeling the vibration. The button would be brought to their attention by placing a food item on it, so they would interact with it. The vibration would then be presented; at this time we would wait for them to push the button on their own. When they do, a larger than normal reinforcer is given. During this time, we use the bridge as indication that the button is important, if their hand approaches the button, the bridge is signaled, followed by the reinforcer. This helps indicate to the monkey that he is doing something correct. Small steps like these are used to eventually lead up to the full task. Once the full task is learned, the experiment can begin. Tiberius is a part of the first phase of the study; this part does not require him to participate in a task. For both monkeys, we first record from the cortical arrays to map the hand and forearm. Once that is complete, arrays will be placed in the ulnar, median and radial nerves. The remainder of the experiment for Tiberius will be conducted while he is under anesthesia. On the other hand, River is a part of the chronic phase of the study. After we implant the cortical and peripheral nerve arrays, he will then recover, and perform a task while he is awake and alert. While Tiberius was not scheduled to participate in this phase of the study, he still received this training as a form of occupational enrichment so he was occupied while in the testing room.
In many cases, including ours, one or more surgical procedures may be necessary before beginning data collection. It is important to note that according to the AWA, only one major surgical procedure per animal is allowed. A major surgical procedure is defined by the AWA as “any surgical intervention that penetrates and exposes a body cavity or any procedure which produces permanent impairment of physical or physiological functions.” The Attending Veterinarian, the IACUC and the USDA must approve any deviation from that set amount. A thorough scientific justification is required. The AWA also requires that aseptic technique be followed, including the use of sterile instruments and gowning, as well as pre and post-operative care in addition to proper pain management. We implant either electrocorticography (ECoG) grids or microelectrode arrays to record neural activity. Tiberius received both, an ECoG grid on his left cortical hemisphere and microelectrode arrays on his right hemisphere. In order to access the arrays, the external connectors are stored in an external chamber that is affixed to the monkey's skull. This keeps the connectors protected and clean, otherwise they would be easily reachable by the monkey to tug on and break off. In this experiment we are working to understand how the brain responds to electrical microstimulation in a peripheral nerve: the type of stimulation that will likely be used to provide feedback from neuroprosthetic hands. Unfortunately, we saw only limited activity from the grid, which is why we then implanted the microelectrode arrays. To map the area we presented a stimulus on particular parts of his hand and arm and recorded the neural data. Data collection continued until the arm/hand areas of the cortex had been carefully mapped. We have yet to implant River. We are completing the experiment first with Tiberius in order to learn what procedures will work best so we can optimize the experiment.
Once all data have been collected and the study is completed, the PI is left with a decision of what to do with the monkey. They can continue on to another study in our lab, be sold to another lab, or be euthanized for tissue histology. However, in our case, they rarely can move on to another experiment or another lab due to the regulation that only one major surgical procedure is allowed per animal. Though, if the same implants are needed for another study, we can move that monkey to a different project. It is important to note, however, that generally microelectrode arrays do have a life expectancy of a year or two. Due to this, moving a monkey to another project isn't always feasible. At times histology is necessary because we need to demonstrate that we were recording from the correct area of cortex or, in the case of Tiberius, assess biocompatibility of the implants and confirm their positioning. In his case, the ECoG grid worked for only a short time, thus we need to examine the tissue for clues regarding the quick failure of the device. Was it in the wrong location? Did it have an adverse effect on the tissue? Thus, Tiberius will be euthanized at the end of this study in order for us to investigate the cause of the problems.
There is an additional option that is not as yet widely used in research: to retire the monkey to an animal sanctuary (Fig. 3). There are some substantial challenges in retiring a research animal to a sanctuary. First, the cost can be quite prohibitive. Sanctuaries are non-profits that operate from donations. Caring for a monkey for the remainder of its life can be costly, and the sanctuaries need to ensure they will have enough money to continue to care for the animals in their possession. It is common for sanctuaries to require a monetary donation to accompany the monkey. Labs conduct their research from funds received from granting agencies. Most of these funds are allocated directly to the progress of science, not to cover the cost of the animal once the study is completed. However, we believe, labs should start including retirement costs in their budget proposals. Unfortunately, if budget cuts are requested, these types of costs would most likely be the first to be cut. If that is the case, labs can start small and keep retirement costs for one or 2 animals in the budget. If that is not possible, then it will be up to the lab to pay for retirement, which can be difficult for them to afford this cost. Because of this, euthanasia ends up being the cheaper option. This is an unfortunate ending.
Figure 3.

Monkeys at Born Free, an NHP sanctuary in Texas. Images used with permission from Born Free USA. Photographer: Kirk Parker.
We believe euthanasia should not be the default outcome for nonhuman primates who have completed their terms as research animals. These animals make a great contribution to the advancement of science. Don't we, as researchers, owe our animals a different life after they have completed their contributions to science? Other research animals have the ability to be adopted after the end of their studies, as they are considered common pets. Research dogs, cats, rats, rabbits, guinea pigs, and even mice can end a study and be adopted into a home with a loving family. Unfortunately, this cannot be the case for research monkeys. Monkeys are wild animals, can be very dangerous, and do not thrive in a home environment as a pet. They can, however, thrive in the atmosphere of a sanctuary.
Sanctuaries strive to mimic a monkey's natural habitat. In contrast to the lab setting, sanctuaries have more outdoor space and can spend a majority of their funds on housing, enrichment, and the care of their animals. They have more opportunities to find a suitable partner or group for their monkeys, so singly housed monkeys are uncommon. The animals at these sanctuaries are able to spend time outside, climbing trees, feeling the sun and foraging in a more natural way (Fig. 3). Indoor space is also usually available in the case of extreme weather situations.
However, there are challenges other than financial, that may be seen as unfavorable to the research community.5 First, unlike research, sanctuaries are not as well-regulated. This can appear to be negative as there is a lack of oversight into the care of these animals. It is up to the lab to be proactive and look into the sanctuary before they send animals there. Sanctuaries can be accredited by the American Sanctuary Association (ASA), the Global Federation of Animal Sanctuaries (GFAS) and the North American Primate Sanctuary Alliance (NAPSA), where they must follow a set of standards. They can also volunteer to be USDA licensed. Most sanctuaries will allow the PIs to visit before settling on one. Many labs are also concerned about negative publicity from activists. A sanctuary may post negative comments on their website or social media about where the animal came from and display animal research in a negative light. For example, a particular sanctuary has a dedicated page on their website that highlights stories from individual monkeys they have taken in. In a few stories they highlight the horrific condition they arrived in, the words they use are meant to create a feeling of empathy and anger for how the monkeys were treated. They continue to speculate how they were treated in the lab and what may have happened to other monkeys in that lab's care. Clearly, the animal research community does not want to solicit comments such as these. It may be easier to avoid it all together and choose to not retire their animals. It is very important for labs to collect information and thoroughly research any sanctuary they are interested in. There are many that are research friendly, and will respect the privacy of the labs to either not mention where the monkeys came from or portray them in a negative light. Confidentiality agreements between the sanctuary and the lab can easily be signed to ease this concern. Generally, labs and sanctuaries have the same goal: to provide a life for animals after research. Another challenge is available space. There are not enough sanctuaries to be able to accept all research monkeys that can be retired. This is a larger issue that cannot be easily solved.
The Research Animal Retirement Foundation was created to help with these challenges. The aim of the foundation is to provide the necessary financial resources for NHP retirement initiatives, as well as advocacy for a paradigm shift among the research community at large in perspectives on end-of-career options for these animals. The aim of this foundation is to bridge the gap between the science community and the general public, promoting positive attitudes toward research that upholds our ethical responsibility to provide animals with a life after the lab. The hope is that with more labs favorable to and supportive of the retirement of research monkeys, more money will be donated to sanctuaries, allowing for creation of more available space. It is vital that the scientific, sanctuary, and general communities work collectively to change old ways of thinking about animal research, and provide the respectful treatment the animals deserve at the end of the study.5
It is unfortunate that Tiberius will not be able to retire to a sanctuary. However, we will learn much from his contribution. On the other hand, what we learned from Tiberius will directly help River, who we hope will be our lab's first monkey to be retired. We are working to make this the common outcome for our end-of-study NHPs.
References
- [1].Meredith J. Animal Research in the Movies. Understanding Animal Research. http://www.understandinganimalresearch.org.uk/news/staff-blog/animal-research-in-the-movies/ [Google Scholar]
- [2].Bontrop RE. Non-human primates: essential partners in biomedical research. Immunological Reviews 2001; 183:5-9; PMID:11782243; http://dx.doi.org/ 10.1034/j.1600-065x.2001.1830101.x [DOI] [PubMed] [Google Scholar]
- [3].Kuczaj S, Lacinak T, Fad O, Trone M, Solangi M, Ramos J. Keeping Environmental Enrichment Enriching. Int J Comparative Psychol 2002; 15:127-37. [Google Scholar]
- [4].Tarou LR, Bashaw MJ. Maximizing the effectiveness of environmental enrichment: Suggestions from the experimental analysis of behavior. App Anim Behav Sci 2007; 102:189-204; http://dx.doi.org/ 10.1016/j.applanim.2006.05.026 [DOI] [Google Scholar]
- [5].Kerwin A. Overcoming the barriers to the retirement of old and new world monkeys from research facilities. J Appl Anim Welf Sci 2006; 9:337-47; PMID:17209758; http://dx.doi.org/ 10.1207/s15327604jaws0904_9 [DOI] [PubMed] [Google Scholar]


