ABSTRACT
Purpose: Thermotolerance is an acquired state of increased cytoprotection achieved following single or repeated exposures to heat stress, in part characterized by changes in the intracellular 72 kda heat shock protein (HSP72; HSPA1A). Females have demonstrated reduced exercise induced HSP72 in comparison to males. This study examined sex differences in heat shock protein 72 messenger ribonucleic acid (Hsp72 mRNA) transcription during heat acclimation (HA) to identify whether sex differences were a result of differential gene transcription. Methods: Ten participants (5M, 5F) performed 10, 90 min controlled hyperthermia [rectal temperature (Tre) ≥ 38.5°C] HA sessions over 12 d. Leukocyte Hsp72 mRNA was measured pre and post D1, D5, and D10, via Reverse transcription polymerase chain reaction (RT-QPCR). Results: HA was evidenced by a reduction in resting Tre (−0.4 ± 0.5°C) and resting heart rate [(HR); −13 ± 7 beats.min−1] following HA (p ≤ 0.05). During HA no difference (p > 0.05) was observed in ΔTre between males (D1 = 1.5 ± 0.2°C; D5 = 1.6 ± 0.4°C; D10 = 1.8 ± 0.3°C) and females (D1 = 1.5 ± 0.5°C; D5 = 1.4 ± 0.2°C; D10 = 1.8 ± 0.3°C). This was also true of mean Tre demonstrating equality of thermal stimuli for mRNA transcription and HA. There were no differences (p > 0.05) in Hsp72 mRNA expression between HA sessions or between males (D1 = +1.8 ± 1.5-fold; D5 = +2.0 ± 1.0 fold; D10 = +1.1 ± 0.4-fold) and females (D1 = +2.6 ± 1.8-fold; D5 = +1.8 ± 1.4-fold; D10 = +0.9 ± 1.9-fold). Conclusions: This experiment demonstrates that there is no difference in Hsp72 mRNA increases during HA between sexes when controlled hyperthermia HA is utilised. Gender specific differences in exercise-induced HSP72 reported elsewhere likely result from post-transcriptional events.
KEYWORDS: controlled hyperthermia, females, heat shock protein, males, thermotolerance
Introduction
Repeated exposure to stressful thermal environments initiates heat adaptation in humans.1 Heat adaptation incorporates the interrelated acclimation and thermotolerance.2,3 A heat acclimated phenotype describes enhanced heat loss effector responses and hypervolemia which mitigate physiological, perceptual, and functional detriments to heat exposure.1,4 Thermotolerance or acquired cellular thermotolerance is the nomenclature used to describe cellular adaptations caused by a single, or repeated severe, but non-lethal heat exposure [e.g. heat acclimation (HA)].5
HA repeatedly initiates the heat shock response (HSR), typically increasing various basal heat shock proteins (HSP), including HSP72 (HSP72). In response to 10 d HA, baseline intracellular HSP72 has been shown to increase by 18%6 while heat shock protein 72 messenger ribonucleic acid (Hsp72 mRNA) demonstrates a pattern whereby transcription occurs within each HA session (+195%) before returning to baseline 24 hr later.7,8 These transient HA mediated cellular adaptations to iHSP72 can confer cytoprotection to subsequent thermal6 and non-thermal8 stressors in vitro6 and in vivo.9 Eloquent in-vitro data demonstrates that cytoprotection to stress (thermal or otherwise) is abolished when HSP72 is knocked out10-12 or blocked.13 HA mediated in-vivo cytoprotection is dependent upon sufficient Hsp72 mRNA transcription14 and subsequent HSP72 protein translation.15
Controlled hyperthermia HA results in a greater Hsp72 mRNA compared with matched training in cool conditions as a result of greater endogenous stimuli for transcription.8 Due to the consistent endogenous thermal stimulus there is an equality of Hsp72 mRNA transcription during 10 d controlled hyperthermia HA.7 Thus, controlled hyperthermia is a preferred HA method compared with traditional exogenously prescribed HA since it induces robust Hsp72 mRNA responses, ensuring sufficient and consistent increases in endogenous stimuli throughout an in vivo chronic intervention, particularly when comparing independent groups.
Morton and colleagues (2009) reported a sex specific HSP adaptation in human skeletal muscle following 6 weeks of continuous and interval training. Specifically, HSP70 increased by 38 ± 41% and 23 ± 36% following continuous and interval training respectively in males (n = 5); however females (n = 5) had no changes (3 ± 37% and 4 ± 14% increase respectively), despite similar training status, training prescription and training adaptations (V̇O2 max).16 Differential sex responses reported by Morton et al. (2009) may be attributed to cytoprotective effects of estrogen. Elevated estrogen has been shown to afford cellular protection,17 accordingly increased estrogen in females versus males may provide a mechanism for inhibited changes in HSP72 expression.18 Estrogen binds to the estrogen receptor, which is a member of the steroid family of nuclear receptors and is the estrogen response element in target genes, leading to the transcriptional regulation of many genes.19 Gillum et al. (2013) reported higher intracellular HSP72 concentrations following a single bout of exercise in the heat in males compared with females (in both the follicular and luteal phase of the menstrual cycle), despite similar baseline values and identical endogenous stimuli for Hsp72 mRNA transcription.20 Differential sex responses were also suggested to be a result of estrogen providing cellular protection and thus, decreasing the necessity for translation of HSP72 in females. Although, stress-mediated sex specific differences in the HSP72 have been seen,16,20 they have not been examined at an mRNA level across the course of controlled hyperthermia HA.
Determination of Hsp72 mRNA transcription in females would facilitate identification of whether the inhibited HSP72 response resulted from absent gene signaling, or mitigated protein translation, potentially due to elevated estrogen.16,20 Absence of data in female populations could be problematic for practitioners who may adopt HA protocols that are informed by mechanistic cellular adaptations from male only cohorts.7,21 This may reduce the magnitude to which females are protected against heat injury.14
The aim of the current study was to determine whether the Hsp72 mRNA response during controlled hyperthermia HA, differed between males and females. It was hypothesized that the Hsp72 mRNA response would be attenuated in females compared to males across the course of controlled hyperthermia HA.
Materials and methods
Participants
Based on a priori power analyses using previous experimental data with identical methods,7,8 4 participants in each group would result in 95% probability of detecting a difference in Hsp72 mRNA across the course of controlled hyperthermia HA. In line with power analysis, and previous work in the area,16 5 males and 5 females (Table 1) provided written informed consent to participate in the current study. All procedures were performed in accordance with the ethical standards of the institute and with the 1964 Declaration of Helsinki, as revised in 2013. Experimental trials were performed between 07:00 and 10:00 h to control for the time of day effects.7,22 Confounding variables of smoking, caffeine, glutamine, alcohol, generic supplementation, and prior thermal, hypoxic, and hyperbaric exposures were all controlled in line with previous work in the field.23 To control for hormonal fluctuations associated with the menstrual cycle, female participants began testing during the early follicular phase (3 d after the onset of menstruation) of their self-reported menstrual cycle; where estrogen (∼30 pg.ml−1) and progesterone (∼1 ng.ml−1) value are expected to be stable.
Table 1.
Participant characteristics. Mean ± SD.
| V̇O2 peak |
||||||
|---|---|---|---|---|---|---|
| Age (years) | Height (cm) | BM (kg) | (L.min−1) | (mL.kg−1.min−1) | ||
| Males (N = 5) | 24 ± 7 | 175 ± 3 | 70.1 ± 5.1 | 2.64 ± 0.34 | 45.7 ± 4.4 | |
| Females (N = 5) | 20 ± 1 | 163 ± 9 | 57.1 ± 4.9 | 3.22 ± 0.50 | 46.2 ± 4.1 | |
Notes: BM, body mass; V̇O2 peak, peak oxygen uptake.
Preliminary testing
Two hr prior to arrival participants consumed 3–5 mL.kg−1 of water. On arrival to the laboratory for all experimental sessions, participants voided their bladder to provide a mid-flow urine sample. When two out of the following 3 criteria were achieved, adequate hydration to perform the trial was assumed based upon an osmolality value of ≤ 700 mOsm.kg−1, a urine specific gravity value of ≤ 1.020 or body mass within 1% of daily average.24 These experimental controls were not violated for any participant for any of the preliminary or experimental procedures. Height was measured using a fixed stadiometer recorded to 1 cm (Detecto Physicians Scales; Cranlea & Co., Birmingham, UK), and nude body mass (BM) recorded to 0.01 kg from digital scales (ADAM GFK 150, USA). A graded exercise test was performed in temperate laboratory conditions [20°C, 40% relative humidity (RH)] to determine V̇O2 peak using a cycle ergometer (Monark e724, Vansbro, Sweden). The cycling intensity was set to 80 W and resistance was applied to the flywheel to elicit a 16 to 24 W.min−1 increase (selected depending on the BM of the participant). Expired air was measured using online gas analysis (Metalyzer Sport, Cortex, Germany). Peak V̇O2 was considered the highest V̇O2 obtained in any 30 s period.
Experimental design
Two, 5 d consecutive blocks (10 d total) of controlled hyperthermia HA were completed separated by 48 hrs. Immediately prior to each HA session participants inserted a rectal thermometer (Henley, Reading, UK) 10 cm past the anal sphincter and affixed a heart rate (HR) monitor (Polar Electro Oy, Kempele, Finland). After a 20 min seated stabilization period, resting measures were recorded and participants entered the environmental chamber (TISS, Hampshire, UK). The daily sessions consisted of a 90 min exposure to 40°C, 40% RH. Exercise intensity was set at 65% V̇O2 peak from the outset and adjusted with work: rest intervals to maintain a rectal temperature (Tre) ∼38.5°C.25,26 Tre and HR were recorded at 5 min intervals. Fluid intake was restricted during the 90 min HA session.
Blood sampling, RNA extraction, and one-step reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Venous blood samples were taken immediately before and immediately after exercise heat exposure on D1, D5, and D10 of controlled hyperthermia HA. All blood samples were drawn from the antecubital vein into 6 mL EDTA Vacuette tubes (Grenier BIO-One, Stonehouse, UK). 1 mL of venous blood was pipetted into 10 mL of 1 in 10 red blood cell lysis solution (10X red blood Cell Lysis Solution; Miltenyi Biotech, Bisley, UK). Samples were incubated for 15 min at room temperature then isolated via centrifugation at 5°C and 400 g for 5 min and washed twice in 2 mL phosphate-buffered saline at 400 g for 5 min to isolate all leukocytes. RNA was then extracted via the previously validated acid guanidium thiocyanate–phenol–chloroform extraction method.27 Quantity was determined at an optical density of 260 nm while quality was determined via the 260/280 and 260/230 ratios using a nanodrop spectrophotometer (NanoDrop 2000c; Thermo Scientific, Waltham, MA, USA).
Hsp72-relative mRNA expression (Hsp72 mRNA) was quantified using Reverse transcription polymerase chain reaction (RT-QPCR). Primers (Table 2) were designed using primer design software (Primer Quest and Oligoanalyzer; Integrated DNA Technologies, Coralville, IA, USA). Twenty μL reactions containing 10 μL SYBR-Green RT-PCR Mastermix (Quantifast SYBRgreen Kit; Qiagen, Manchester, UK), 0.15 μL forward primer, 0.15 μL reverse primer, 0.2 μL reverse transcription mix (Quantifast RT Mix; Qiagen) and 9.5 μL sample (70 ng RNA/μL) were prepared in separate tubes. Each PCR reaction (Rotorgene Q; Qiagen) was then performed as follows: 10 min, 50°C (reverse transcription), 5 min 95°C (transcriptase inactivation and initial denaturation); followed by: 10 s, 95°C (denaturation), 30 s, 60°C (annealing and extension) for 40 cycles. Fluorescence was measured following each cycle as a result of the incorporation of SYBR green dye into the amplified PCR product. Melt curves (50 to 95°C; ramp protocol 5-s stages) were analyzed for each reaction to ensure only the single gene of interest was amplified. The relative quantification of mRNA expression for each sample was assessed by determining the ratio between the cycle threshold (CT) value of the target mRNA and the CT values for β2-microglobulin. Fold change in relative mRNA expression was calculated using the 2-ΔΔCT method.28
Table 2.
Hsp72 mRNA primer sequences.
| Gene | NCBI Accession # | Primer | Sequence (5′→3′) | Amplitude length |
|---|---|---|---|---|
| B2- Microglobulin (β2-M) | NM_004048 | Forward Reverse | CCGTGTGAACCATGTGACT TGCGGCATCTTCAAACCT | 91 |
| Hsp72 | NM_005345 | Forward Reverse | CGCAACGTGCTCATCTTTGA TCGCTTGTTCTGGCTGATGT | 198 |
Notes: NCBI National Center for Biotechnology Information.
Statistical analysis
All data were first checked for normality using the Shapiro-Wilk method and corrected for sphericity using the Greenhouse Geisser method. A two way mixed design analysis of variance (ANOVA) was performed to determine differences between the physiological and performance characteristics between D1, D5, and D10 in males and females. A three-way mixed design ANOVA was performed to identify differences between the Hsp72 mRNA, pre and post, on D1, D5, and D10 of controlled hyperthermia HA between males and females. When a main effect or interaction effect was found, results were followed up using a Bonferroni corrected post hoc comparison. Effect sizes [partial eta squared (np2)] were calculated to analyze the magnitude of trends associated with controlled hyperthermia HA. All data were analyzed using a standard statistical package (SPSS version 20.0, IBM, Armonk, New York, USA) and reported as mean ± SD. Statistical significance was accepted at the level of p ≤ 0.05.
Results
Evidence of heat acclimation
Figure 1 presents the resting Tre and resting HR data for D1, D5, and D10 of controlled hyperthermia HA. There was a main effect of day on Tre rest (F (2, 16) = 11.219, p ≤ 0.001, np2 = 0.584). No differences were observed from D1 to D5 (p = 0.563). However, Tre rest reduced from D1 to D10 (p = 0.027) and from D5 to D10 (p = 0.003). There was no interaction effect between day and sex on Tre rest (F (2, 16) = 3.287, p = 0.064, np2 = 0.291), however this was approaching significance with a moderate effect. The mean reduction in Tre rest from D1 to D5 was −0.3 ± 0.2°C in males, whereas in females there were no changes (+0.1 ± 0.2°C). The mean reduction in Tre rest from D5 to D10 was greater in females (−0.4 ± 0.2°C) compared with males (−0.1 ± 0.1°C).
There was a main effect of day on HR rest (F (2, 16) = 15.227, p ≤ 0.001, np2 = 0.656). HR rest reduced from D1 to D5 (p = 0.040), from D1 to D10 (p = 0.008), and from D5 to D10 (p = 0.008). There was no interaction effect between day and sex on HR rest (F (2, 16) = 0.383, p = 0.688, np2 = 0.046).
Hsp 72 mRNA responses to heat acclimation between sexes
Figure 2 presents the means ± SD for Hsp72 mRNA, pre and post on D1, D5, and D10 of controlled hyperthermia HA between males and females. Figure 2 also presents individual participants percentage change relative to D1. There was a main effect of time on Hsp72 mRNA response (F (1,8) = 32.998, p ≤ 0.001, np2 = 0.805). Hsp 72 mRNA increased pre to post on D1 (1.7 ± 0.8-fold, 3.9 ± 1.8-fold; p = 0.003), D5 (1.6 ± 0.8-fold, 3.5 ± 1.7-fold; p ≤ 0.001), and D10 (2.0 ± 0.7-fold, 3.0 ± 1.4-fold; p = 0.050). There was no interaction effect between time and sex (F (2, 16) = 1.027, p = 0.381, np2 = 0.114). The increase in Hsp72 mRNA from pre to post was similar between males (D1 = 1.8 ± 1.5-fold; D5 = 2.0 ± 1.0 fold; D10 = 1.1 ± 0.4-fold) and females (D1 = 2.6 ± 1.8-fold; D5 = 1.8 ± 1.4-fold; D10 = 0.9 ± 1.9-fold).
Figure 1.
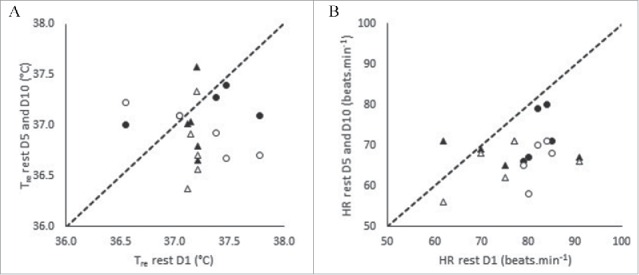
Resting rectal temperature (Tre) (A) and resting heart rate (HR) (B) on D1, D5 and D10 of heat acclimation. Dotted line represents line of equality. N = 10 (5M, 5F). Note: Males (Δ) Females (O) STHA (D1 to D5) (closed markers) LTHA (D1 to D10) (open markers).
Figure 2.
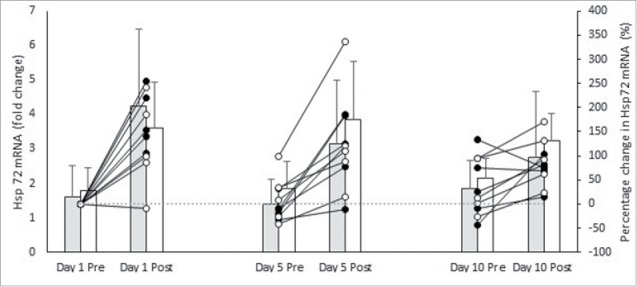
Hsp72 mRNA pre and post session on D1, D5, and D10 of heat acclimation in females (grey bars) and males (white bars). Individual percentage change relative to D1 pre are presented as lines for females (closed circles) and males (open circles). Mean±SD. N = 10 (5M, 5F). Notes: *Denotes significant pre to post difference within session in all participants (p ⩽ 0.05).
There was no main effect of day on Hsp72 mRNA (F (1, 8) = 0.052, p = 0.826, np2 = 0.006). There was no interaction effect between day and sex on Hsp72 mRNA (F (2, 16) = 1.027, p = 0.381, np2 = 0.114). There was no interaction effect between time, day and sex on Hsp72 mRNA (F (2, 16) = 0.479, p = 0.628, np2 = 0.057).
Comparable heat acclimation sessions
Table 3 presents the mean ± SD for performance and physiological variables during D1, D5, and D10 of controlled hyperthermia HA for males and females. There was no main effect of day (F (2, 16) = 3.042, p = 0.076, np2 = 0.275) or interaction effect between day and sex (F (2, 16) = 0.234, p = 0.794, np2 = 0.028) on ΔTre. There was no main effect of day (F (2, 16) = 2.536, p = 0.143, np2 = 0.241) or interaction effect between day and sex (F (2, 16) = 1.880, p = 0.185, np2 = 0.190) on mean Tre. There was no main effect for day (F (2, 16) = 0.488, p = 0.623, np2 = 0.057) or interaction effect between day and sex (F (2, 16) = 0.242, p = 0.788, np2 = 0.029) on mean HR.
Table 3.
Physiological and performance measures on D1, D5, and D10 of controlled hyperthermia heat acclimation. Mean ± SD.
| D1 |
D5 |
D10 |
|||||
|---|---|---|---|---|---|---|---|
| Males | Female | Males | Females | Males | Females | ||
| Tre change (°C) | 1.5 ± 0.2 | 1.5 ± 0.5 | 1.6 ± 0.4 | 1.4 ± 0.2 | 1.8 ± 0.3 | 1.8 ± 0.3 | |
| Mean Tre (°C) | 38.2 ± 0.2 | 38.3 ± 0.2 | 38.1 ± 0.18 | 38.1 ± 0.1 | 38.2 ± 0.1 | 38.3 ± 0.9 | |
| Mean HR (beats.min−1) | 146 ± 14 | 152 ± 9 | 145 ± 8 | 146 ± 10 | 143 ± 12 | 148 ± 9 | |
| Intensity (% V̇O2 peak) | 38 ± 5 | 35 ± 8 | 50 ± 6 | 44 ± 7 | 52 ± 11 | 53 ± 14 | |
Notes: HR, heart rate; Tre, rectal temperature; V̇O2 peak, peak oxygen uptake.
There was a main effect of day on relative exercise intensity (F (2, 16) = 21.593, p ≤ 0.001, np2 = 0.730). Relative exercise intensity was higher on D5 (p ≤ 0.001) and D10 (p ≤ 0.001) compared to D1. There were no differences between D5 and D10 (p = 0.221). There was no interaction effect between day and sex on relative exercise intensity (F (2, 16) = 0.1.034, p = 0.378, np2 = 0.114).
Discussion
This is the first study to compare Hsp72 mRNA expression in males and females across the course of controlled hyperthermia HA. In contrast to our hypothesis, this experiment demonstrates that the Hsp72 mRNA response is similar between males and females on D1, D5, and D10 of a controlled hyperthermia HA. This suggests that sex differences in HSP following acute20 and chronic16 in vivo exercise bouts are due to post transcriptional events. Controlled hyperthermia HA resulted in typical phenotypic adaptations, evidenced by reduction in resting Tre and HR across the course of controlled hyperthermia HA. Males and females demonstrated equal physiological responses (∆Tre, mean Tre and HR) to each HA session where Hsp72 mRNA was measured. Accordingly, equality of these endogenous stimuli, both between groups, and throughout HA elicited equal increases in Hsp72 mRNA transcription. Comparable transcription of Hsp72 mRNA between males and females, suggests endogenous stimuli which induce the HSR are the most important criteria for increasing Hsp72 mRNA,7 with no sex dependent inhibition or amplification in transcription.
In the current study, HA produced a significant increase in Hsp72 mRNA providing further evidence that the controlled hyperthermia HA method presents a sufficient endogenous stress to surpass the minimum requirement to elicit increased transcription of Hsp72 mRNA, in both males and females, at the onset and culmination of discrete and repeated bouts of exercise-heat stress. Gibson et al.7 reported an increase in Hsp72 mRNA pre to post on D1 (1.9 ± 0.6-fold, 4.9 ± 1.1-fold), D5 (2.3 ± 0.8-fold, 5.3 ± 2.5-fold) and D10 (2.1 ± 0.7-fold, 4.3 ± 1.3-fold) during a 10 d controlled hyperthermia HA protocol in male participants. The data in the current study demonstrates females have a comparable magnitude of response to males. Accordingly, this data provides mechanistic support for practitioners prescribing controlled hyperthermia HA for female athletes. Sustained increases in Hsp72 mRNA throughout the HA, further demonstrates the continued stimulation of the pathways responsible for thermotolerance, i.e. the equality of HSR, in both males and females.
Previously, a greater HSP72 increase has been reported in males compared to females16,20; however, these studies measured the protein (HSP) whereas the current study measured the gene (Hsp mRNA). Interestingly, the current data contradicts Paroo et al.29 findings, who reported a sex specific HSR at the level of protein and mRNA; with male rats having a significantly higher HSP70 (200% of control) and Hsp70 mRNA (+900% of control) response following 60 min of exercise at 70% V̇O2 max when compared with females (HSP70 = 100% of control; Hsp70 mRNA = 450% of control). Paroo et al.29 did however provide mechanistic evidence for an interaction between HSP70, Hsp70 mRNA and estrogen. Ovariectomized female animals treated with a placebo demonstrated equivalent increases in HSP72 (+150% of control) and Hsp72 mRNA (+1,200% of control) to males, while endogenous estrogen returned the typical inhibited female HSR response (HSP70 100% of control; Hsp70 mRNA 300% of control). Methodologically, Paroo et al.29 implemented the northern blotting technique which is less sensitive than the RT- QPCR technique used in the current study, potentially explaining the non-significantly increased Hsp72 mRNA.
Elevated estrogen affords cellular protection17 and thus, this cytoprotective pathway may inhibit changes in HSP72 translation.18 It is likely, that estrogen most greatly mediates post Hsp72 mRNA transcriptional changes which inhibit the translation of HSP72. The mechanism by which estrogen attenuates HSR may be mediated through its indirect antioxidant properties by stabilizing cellular membranes and attenuating oxidative stress; such an effect could protect thermal sensitive cells against exercise-induced damage, and thereby result in a blunted HSP72 response.29 The lack of observed difference between males and females in the current study, may be a result of low estrogen concentrations, which may not have been sufficient to exert an antioxidant effect.
Limitations
Future work should involve the measurement of HSP72 protein alongside Hsp72 mRNA across the course of controlled hyperthermia HA in males and females, to help underpin the true effect of sex on the HSR; the absence of which is a limitation of the present study. Furthermore, estrogen is reported to have a dose dependent inhibition of HSP72 expression at the transcription level.17 Future work should investigate the HSR, and subsequent HSP72 and Hsp72 mRNA response to discrete and repeated bouts of exercise-heat stress in high and low estrogen conditions and in post-menopausal women, who naturally have lower estrogen concentrations. This information would help practitioners implement controlled hyperthermia HA strategies that ensure an optimal stimulus for cellular adaptation.
Conclusion
Males and females have equal Hsp72 mRNA expression throughout 10 d of controlled hyperthermia HA. This suggests that there are no differences in the endogenous criteria to transcribe Hsp72 mRNA via the HSR between males and females. Differences in basal HSP72 observed elsewhere are therefore likely to result from inhibited protein translation, potentially due to the influence of estrogen.
Abbreviations
- BM
Body mass
- CT
Cycle threshold
- HA
Heat acclimation
- HR
Heart rate; Hsp72 mRNA Heat shock protein 72 messenger ribonucleic acid
- HSP
Heat shock protein
- HSR
Heat shock response
- RH
Relative humidity
- RT- QPCR
Reverse transcription polymerase chain reaction
- Tre
Rectal temperature
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The author would like to thank the volunteers for their participation in this investigation.
Funding
Funding and support was obtained from the University of Brighton and the University of Bedfordshire.
References
- [1].Taylor NS. Human heat adaptation. Compr Physiol 2014; 4:325-65; PMID:24692142; http://dx.doi.org/ 10.1002/cphy.c130022. [DOI] [PubMed] [Google Scholar]
- [2].Horowitz M. Heat acclimation, epigenetics, and cytoprotection memory. Compr Physiol 2014; 4:199-230; PMID:24692139; http://dx.doi.org/ 10.1002/cphy.c130025. [DOI] [PubMed] [Google Scholar]
- [3].Sawka MN, Leon LR, Montain SJ, Sonna LA. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr Physiol 2011; 1:1883-928; PMID:23733692. [DOI] [PubMed] [Google Scholar]
- [4].Périard JD, Racinais S, Sawka MN. Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports. Scand J Med Sci Sports 2015; 25:20-38; http://dx.doi.org/ 10.1111/sms.12408. [DOI] [PubMed] [Google Scholar]
- [5].Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol 1997; 83:1413-7; PMID:9375300. [DOI] [PubMed] [Google Scholar]
- [6].McClung JP, Hasday JD, He JR, Montain SJ, Cheuvront SN, Sawka MN, et al.. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol 2008; 294:R185-191; PMID:17977914. [DOI] [PubMed] [Google Scholar]
- [7].Gibson OR, Mee JA, Taylor L, Tuttle JA, Watt PW, Maxwell NS. Isothermic and fixed-intensity heat acclimation methods elicit equal increases in Hsp72 mRNA. Scand J Med Sci Sports 2015; 25:259-68; PMID:25943677; http://dx.doi.org/ 10.1111/sms.12430. [DOI] [PubMed] [Google Scholar]
- [8].Gibson OR, Turner G, Tuttle JA, Taylor L, Watt PW, Maxwell NS. Heat Acclimation attenuates physiological strain and the Hsp72, but not Hsp90α mRNA response to acute normobaric hypoxia. J Appl Physiol 2015; 119:889-99; PMID:26205540; http://dx.doi.org/ 10.1152/japplphysiol.00332.2015. [DOI] [PubMed] [Google Scholar]
- [9].Lee BJ, Miller A, James RS, Thake CD. Cross acclimation between heat and hypoxia: Heat acclimation improves cellular tolerance and exercise performance in acute normobaric hypoxia. Front Physiol 2016; 7:1-15; PMID:26858649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Senf SM, Howard TM, Ahn B, Ferreira LF, Judge AR. Loss of the Inducible Hsp70 Delays the Inflammatory Response to Skeletal Muscle Injury and Severely Impairs Muscle Regeneration. PLoS One 2013; 8:e62687; PMID:23626847; http://dx.doi.org/ 10.1371/journal.pone.0062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Drew BG, Ribas V, Le JA, Henstridge DC, Phun J, Zhou Z, et al.. HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes 2014; 63:1488-505; PMID:24379352; http://dx.doi.org/ 10.2337/db13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee SH, Kwon HM, Kim YJ, Lee KM, Kim M, Yoon BW. Effects of Hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke 2004; 35:2195-9; PMID:15243143; http://dx.doi.org/ 10.1161/01.STR.0000136150.73891.14. [DOI] [PubMed] [Google Scholar]
- [13].Kuennen M, Gillum T, Dokladny K, Bedrick E, Schneider S. Thermotolerance and heat acclimation may share a common mechanism in humans. Am J Physiol Regul Integr Comp Physiol 2011; 1:524-33; http://dx.doi.org/ 10.1152/ajpregu.00039.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moran DS, Eli-Berchoer L, Heled Y, Mendel L, Schocina M, Horowitz M. Heat intolerance: does gene transcription contribute?. J Appl Physiol 2006; 100:1370-6; PMID:16357068; http://dx.doi.org/ 10.1152/japplphysiol.01261.2005. [DOI] [PubMed] [Google Scholar]
- [15].Silver JT, Noble EG. Regulation of survival gene hsp70. Cell Stress Chaperones 2012; 17:1-9; PMID:21874533; http://dx.doi.org/ 10.1007/s12192-011-0290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morton JP, Holloway K, Woods P, Cable NT, Burniston J, Evans L, et al.. Exercise training-induced gender-specific heat shock protein adaptations in human skeletal muscle. Muscle Nerve 2009; 39:230-3; PMID:19058194; http://dx.doi.org/ 10.1002/mus.21182. [DOI] [PubMed] [Google Scholar]
- [17].Shinohara T, Takahashi N, Ooie T, Ichinose M, Hara M, Yonemochi H, et al.. Estrogen inhibits hyperthermia-induced expression of heat-shock protein 72 and cardioprotection against ischemia/reperfusion injury in female rat heart. J Mol Cell Cardiol 2004; 37:1053-61; PMID:15522282; http://dx.doi.org/ 10.1016/j.yjmcc.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [18].Bombardier E, Vigna C, Iqbal S, Tiidus PM, Tupling AR. Effects of ovarian sex hormones and downhill running on fiber-type-specific HSP70 expression in rat soleus. J Appl Physiol 2009; 106:2009-15; PMID:19359608; http://dx.doi.org/ 10.1152/japplphysiol.91573.2008. [DOI] [PubMed] [Google Scholar]
- [19].Ogita H, Node K, Kitakaze M. The role of estrogen and estrogen-related drugs in cardiovascular diseases. Curr Drug Metab 2003; 4:497-504; PMID:14683477; http://dx.doi.org/ 10.2174/1389200033489271. [DOI] [PubMed] [Google Scholar]
- [20].Gillum T, Kuennen M, Gourley C, Dokladny K, Schneider S, Moseley P. Sex differences in heat shock protein 72 expression in peripheral blood mononuclear cells to acute exercise in the heat. Int J Endocrinol Metab 2013; 11:8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mee JA, Gibson OR, Doust J, Maxwell NS. A comparison of males and females' temporal patterning to short- and long-term heat acclimation. Scand J Med Sci Sports 2015; 25:250-8; PMID:25943676; http://dx.doi.org/ 10.1111/sms.12417. [DOI] [PubMed] [Google Scholar]
- [22].Winget CM, DeRoshia CW, Holey DC. Circadian rythms and athletic performance. Med Sci Sport Exerc 1985; 17:498-516; http://dx.doi.org/ 10.1249/00005768-198510000-00002. [DOI] [PubMed] [Google Scholar]
- [23].Taylor L, Midgley AW, Chrismas B, Hilman AR, Madden LA, Vince RV, et al.. Daily hypoxia increases basal monocyte HSP72 expression in healthy human subjects. Amino Acids 2011; 40:393-401; PMID:20552383; http://dx.doi.org/ 10.1007/s00726-010-0644-x. [DOI] [PubMed] [Google Scholar]
- [24].Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sport Exerc 2007; 39:377-90; http://dx.doi.org/ 10.1249/01.mss.0000272779.34140.3b. [DOI] [PubMed] [Google Scholar]
- [25].Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Acclimatization to heat in man by controlled elevation of body temperature. J Physiol 1963; 166:530-47; PMID:13959046; http://dx.doi.org/ 10.1113/jphysiol.1963.sp007121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gibson OR, Mee JA, Tuttle JA, Taylor L, Watt PW, Maxwell NS. Isothermic and fixed intensity heat acclimation methods induce similar heat adaptation following short and long-term timescales. J Therm Biol 2015; 49–50:55-65; PMID:25774027. [DOI] [PubMed] [Google Scholar]
- [27].Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162:156-9; PMID:2440339; http://dx.doi.org/ 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- [28].Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 2008; 3:1101-8; PMID:18546601; http://dx.doi.org/ 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- [29].Paroo Z, Dipchand ES, Noble EG. Estrogen attenuates postexercise HSP70 expression in skeletal muscle. Am J Physiol Cell Physiol 2002; 282:C245-51; PMID:11788335; http://dx.doi.org/ 10.1152/ajpcell.00336.2001. [DOI] [PubMed] [Google Scholar]


