Abstract
Objective
To investigate wound healing, antimicrobial and antioxidant activity of leaf extract of Pongamia Pinnata.
Materials and methods
Methanolic extracts of P. pinnata leaf were studied for wound healing efficiency, and was assessed by the rate of wound contraction, tensile strength, breaking strength, hydroxyproline and hexosamine content, along with its effect on pro-inflammatory and anti-inflammatory cytokines was assessed using excision and incision model of wound repair in Wistar rats. Antimicrobial activity against ten microorganisms was also assessed. In vivo antioxidant activity was performed to understand the mechanism of wound healing potency.
Results
The results indicated that P. pinnata extract has potent wound healing capacity as evident from the wound contraction and increased tensile strength. Hydroxyproline and hexosamine expression were also well correlated with the healing pattern observed. extract exhibited significant antimicrobial activity, Staphylococcus aureus, Staphylococcus pyogenes, Staphylococcus epidermidis, Escherichia coli, Micrococcus luteus, Enterobacter aerogenes, Salmonella typhi, Pseudomonas aeruginosa, Candida albicans, Aspergillus niger also indicate that P. pinnata posses potent antioxidant activity by inhibition lipid peroxidation, reduce glutathione, superoxide dismutase level and increases catalase activity. During early wound healing phase TNF-α and IL-6 level were found to be up-regulated by P. pinnata treatment.
Conclusion
Increased wound contraction and tensile strength, augmented hydroxyproline and hexosamine content, antioxidative activity and moderate antimicrobial activity support the early wound healing exhibited by P. pinnata. Induction in cytokine production may be one of the mechanisms in accelerating the wound healing. Results suggest that P. pinnata may be useful in tropical management of wound healing.
Keywords: Pongamia pinnata, Wound healing, Pro-inflammatory cytokines, Anti-inflammatory cytokines, Antimicrobial activity antioxidant
Graphical abstract
1. Introduction
Pongamia pinnata (Fabaceae), commonly known as Karanj (Hindi), Arbre de pongolote (French), bangkong (Indonesian, Javanese), it is a fast growing deciduous tree up to 20 meters tall that is thought to have originated in India and is found throughout Asia, Indonesia and into northern Australia. The leaves are a soft, shiny burgundy in early summer and mature to a glossy, deep green as the season progresses. Small clusters of white, purple, and pink flowers blossom on their branches throughout the year, maturing into brown seed pods. Ethno medically, this plant are used in folk remedies for treating Wounds, inflammations, piles, ulcers and skin infection and it contains potent anti-inflammatory and ulcerogenic effect.1 Studies have revealed the presence of different classes of major chemical components reported from P. pinnata are alkaloids demethoxy-kanugin, gamatay, glabrin, glabrosaponin, kaempferol, kanjone, kanugin, karangin, neoglabrin, pinnatin, pongamol, pongapin, quercitin, saponin, b-sitosterol, and tannin & syringyl groups (phytochemicals).2, 3, 4, 5, 6, 7
Wound healing consists of an orderly progression of events that re-establish the integrity of the damaged tissue: inflammatory, proliferation and remodeling stages.8 The inflammation stage begins immediately after injury, first with vasoconstriction that favours homeostasis and releases inflammation mediators. The proliferative phase is characterized by granulation tissue proliferation formed mainly by fibroblast and the angiogenesis process. The remodeling stage is characterized by reformulations and improvement in the components of the collagen fiber that increases the tensile strength.9 Healing accomplished by the release of eicosanoids, prostaglandins, leukotrienes and reactive oxygen species (ROS). Among these, ROS plays a vital role in healing and serve as cellular messengers that drive numerous aspects of molecular and cell biology. ROS is produced in high amounts at wound site as defense mechanism against invading bacteria. At the same time, the process of wound healing may be hampered by the presence of free radicals, which can damage wound surrounding cells, or by microbial infection.10 Due to poor hygienic condition and infection of pathogenic micro-organism creates difficulties to manage wound infection and presently wide range of antibiotics are being used for treating wound infection but due to their adverse effect and antibiotic resistance is now paying attention towards natural biologically active herbal compounds as an alternative medicine11, 12, 13, 14.
The purpose of the present study deals with evaluation of wound healing and antimicrobial potential of P. pinnata methanolic extracts against pathogenic micro-organism. The antioxidant & induction of pro-inflammatory and anti-inflammatory cytokines was also studied to understand the mechanism behind wound healing activity.
2. Materials and methods
2.1. Plant material
Plant materials were collected from forest nursery of Minor Forest Produce Processing & Research Center (MFPPARC) Bhopal, M.P. India. The herbarium of the plant was identified and authenticated by Dr. Suman Mishra Scientist Plant taxonomy MFPPARC, Bhopal. The voucher specimen MPCA-0041 was deposited to the Central Herbarium of Minor Forest produce processing & Research center, Bhopal M.P. India.
2.2. Processing of leafs for extraction & phytochemical screening
500 g of withered leafs were washed vigorously with tap water to remove soil and dust. These leafs were left in the shade to dry for 15–20 days. All dried leafs were chopped into small fragments and reduced into fine powder with mortar and pestle. Powdered samples were extracted at room temperature thrice with methanol for 48 h on an orbital shaker to make methanolic extracts and concentrated using rotary evaporator (Buchi Instruments R-210) at a reduced pressure and at < 40 °C.
P. pinnata methanolic extract was taken up for biological activity along with screening for the presence of different phytochemical constitutents viz. alkaloids (Dragendorff's test), saponins (foam formation), flavonoids (using magnesium and dil. HCl), and terpenes and steroids (Liebermann–Burchard's test), glycosides (Molisch's reagent), fixed oil (spot test), proteins (ninhydrine test), tannins (5% ferric chloride test) according to standard methods14, 15, 16, 17, 18.
2.3. Cultures
Bacterial (Staphylococcus aureus, Staphylococcus pyogenes, Staphylococcus epidermidis, Escherichia coli, Micrococcus luteus, Enterobacter aerogenes, Salmonella typhi, Pseudomonas aeruginosa) and fungal cultures (Candida albicans, Aspergillus niger) were used. These were obtained from the repository of Vindhya Herbal Testing & Research Laboratory Bhopal, Madhya Pradesh, India.
2.4. Experimental animals
Wistar rats weighing 160–180 g were acclimatized for a week prior to the initiation of the experiment in standard stainless steel cages & maintained in the animal house under laboratory conditions (temperature 22 ± 2 °C, relative humidity 60–70%, and 12 h–12 h light–dark cycle). They fed with balanced diet purchased from Agro Corporation Private Limited, Bangalore, India, and water adlibitum (Principles of Laboratory Animal Care NIH publication no, 85-23, revised 1985). The animal experiment was performed according to the departmental ethical committee guidelines.
2.5. Antimicrobial activity
The antimicrobial activity was evaluated by the agar diffusion method. Bacteria were cultured overnight at 37 °C in Mueller Hinton Broth (MHB, Difco) and fungi at 28 °C for 72 h in Potato Dextrose Broth (PDB, Difco) and used as inoculums. The suspension of 100 μl of inoculums containing 108 CFU/ml of bacteria and 104 spore/ml of fungi spread on Mueller Hinton Agar (MHA) and Potato Dextrose Agar (PDA) medium, respectively.
The disc (6 mm in diameter) was impregnated with 10 μl of 100 mg/ml (1 mg/disc) P. pinnata methanolic extract and DMSO placed on seeded agar. Streptomycin (10 μg/disc), and tetracycline (10 μg/disc) were used as positive controls for bacteria and fluconazole (10 μg/disc), ketoconazole (10 μg/disc) for fungi. MIC values were also studied for microorganisms, which were determined as sensitive to leaf extracts in disc diffusion assay. Sterile filter paper discs (6 mm in diameter) containing 2.5–1000 μg/disc of all the components were placed on the surface of a medium. MIC was defined as the lowest concentration of extract that inhibited visible growth on agar.
2.6. Wound healing activity
Excision and incision wound healing models were used to evaluate the wound healing activity of P. pinnata. Animals were divided into three groups of six animals (n = 6) each as follows:
Group I: control group treated with dimethyl sulfoxide DMSO.
Group II: standard group treated with Vitamin E (100 mg/kg body weight) after being suspended in vehicle (0.5% sodium carboxymethyl cellulose suspension in distilled water).
Group III: test group treated with P. pinnata leaf (100 mg/kg body weight) extract in vehicle.
Test drugs were orally administered once a day in an equivalent volume of 10 ml/kg body weight of the animal for 19 days.
2.6.1. Incision wound model
All rats were anesthetized with anesthetic ether, then a paravertebral long incision of 4 cm length was made through the skin and cutaneous muscle at a distance about 1.5 cm from the middle on right side of the depilated back. Afterward, wound was closed by means of interrupted sutures placed at equidistant points of 0.5 cm intervals using sterile surgical thread (No. 000) and a curved needle (No. 11).19, 20 The sutures were removed on the 7th day. Wound breaking strength (WBS) was measured on the 10th post-wounding day.
Wound breaking strength: Briefly, wounded anesthetized rats were secured on operation table, and then a line was drawn on 3 mm away of either side of the wound.21 Line was gripped with forceps in a manner that both ends were opposing each other. One side of forceps was connected to a freely suspended light weight graduated polypropylene container through a string run over to a pulley. Increasing water level gradually increased the weight and pull wound edge away from fixed end, when wound just opened up, the weight of water was recorded. Average of three different recorded readings for incision wound taken as an individual value of breaking strength. Tensile strength was also calculated using the following formula:
2.6.2. Excision wound model
For excision wound study, rats were anesthetized with anesthetic ether and depilated at the dorsal thoracic region. A circular piece of full thickness (500 mm2) was cut off from a pre-determined area on the dorsal back of the rats.19, 20 The wound area was measured immediately by placing a transparent paper over the wound and tracing it out, area of this impression was calculated using graph sheet. The same protocol was followed on every 4th day to calculate the percentage of wound contraction.
The area of wound at the time of wounding was considered as 100%. Number of days required for falling of eschar without any residual raw wound was recorded to estimate the period of epithelialization.22
2.7. Estimation of pro-inflammatory (IL-6, TNF-alpha) and anti-inflammatory cytokine (IL-10) induction
Blood samples were collected from all the animals of each group at different time intervals, i.e., 24 h and 8th day after wound formation. Pro-inflammatory cytokines (IL-6 and TNF-α) and anti-inflammatory cytokines (IL-10) were estimated by performing enzyme linked immunosorbent assay (ELISA) using IL-6 and TNF-alpha and IL-10 (Invitrogen) ELISA kits. Assays were performed according to the manufacturer's instructions. Concentration of cytokines was determined in pg/ml by plotting the graph for standard. All the experiments were performed in triplicate to ensure the observations.
2.8. Estimation of hydroxyproline and hexosamine
On day 4, 8 and 16 of the post surgery of excision, a piece of skin from the healed wound area was collected and analyzed for hydroxyproline content, which is basic constituent of collagen. Tissues were dried in a hot air oven at 60–70 °C to constant weight and were hydrolyzed in 6 N HCl at 130 °C for 4 h in sealed tube. The hydrolysate was neutralized to pH 7.0 and was subjected to Chloramine T oxidation for 20 min, the reaction was terminated by addition of 0.4 M per chloric acid and color was developed with the help of Ehrlich reagent at 60 °C (Woessner, 1961) and measured at 557 nm using UV/Vis spectrophotometer (Shimadzu). For estimation of hexosamine, the weighed granulation tissues were hydrolyzed in 6 N HCl for 8 h at 98 °C, neutralized to pH 7 with 4 N NaOH and diluted with Milli-Q water. Hexosamine contents of granulation tissues were estimated with minor modifications.23 The diluted solution was mixed with acetyl acetone solution and heated to 96 °C for 40 min. The mixture was cooled and 96% ethanol was added, followed by r-dimethylamino-benzaldehyde solution (Ehrlich's reagent). The solution was thoroughly mixed, kept at room temperature for 1 h and the absorbance was measured at 530 nm using a double beam UV/Vis spectrophotometer (Shimadzu). The amount of hexosamine was determined by comparing with a standard curve. Hexosamine content has been expressed as mg/g dry tissue weight.24, 25
2.9. In vivo estimation of antioxidant activity
To evaluate antioxidant activity, rats were divided into two groups of control and experimental rats. Experimental rats received 1.25 ml of (100 mg/kg b.w.) of leaf extract, while control rats received DMSO. Tissue homogenate (10%) was prepared with 0.15 M KCl and centrifuged at 8000 rpm for 10 min. The cell free supernatant was used for the antioxidative enzyme assay. The extent of lipid peroxidation (LPO) was determined by analyzing the levels of thiobarbituric acid reactive substances (TBARS).26 Endogenous antioxidant status was evaluated by estimating the levels of reduced glutathione (GSH)27 and activities of superoxide dismutase (SOD) by utilizing the standard technique. Catalase (CAT) was assayed by the method of.28
2.10. Statistical analysis
Calculations and statistics were performed using GraphPad 5.0 software (GraphPad Software Inc., La Jolla, CA). The results were analyzed using one-way analysis of variance (ANOVA) with post hoc Scheffe's test. Significance was defined as P < 0.05. Results are presented as mean ± the standard error of the mean (S.E.M.).
3. Results
3.1. Phytochemical screening
The results of preliminary phytochemical screening of the methanolic extract of P. pinnata leaf are tabulated in Table 1, the results revealed the presence of alkaloids, glycosides, steroids, flavonoids, and proteins.
Table 1.
Preliminary phytochemical screening of the Pongamia pinnata leaf extract.
| Extracts | Alkaloids | Glycosides | Tannins | Steroids | Terpenes | Flavonoids | Proteins |
|---|---|---|---|---|---|---|---|
| Pongamina pinnata leaf | + | + | – | + | – | + | + |
3.2. Sensitivity test
The methanol extracts from leafs of P. pinnata has shown inhibition effects on the growth of the entire microorganism tested, but their potential of inhibition was varied from one organism to another. P. pinnata has shown inhibition diameter from 9.30 to 16.44 mm. S. aureus was most sensitive to P. pinnata followed by E. coli Candida abicans, E. aerogenes, P. aeruginosa, S. pyogenes, S. typhi, A. niger, S. epidermidis, Microccus leteus and the MICs ranged from 100 to 400 (μg/disc) the lowest MIC observed was 100 for C. albicans.
3.3. Wound healing activity
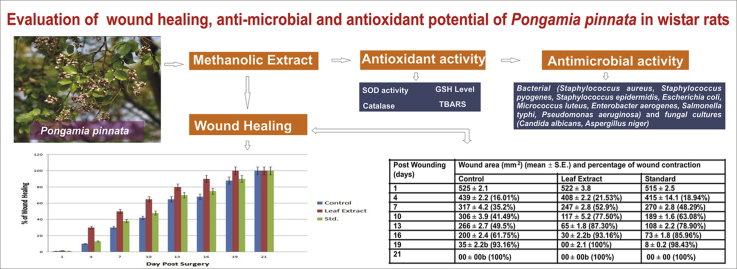
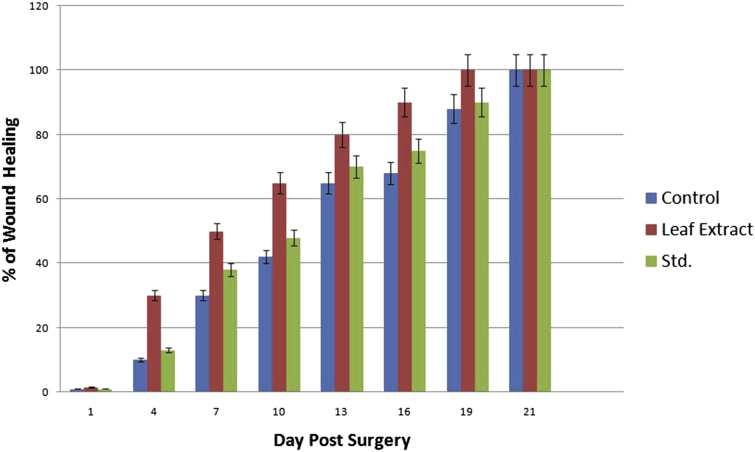
A significant increases in wound healing was observed in P. pinnata treated groups in comparison to control group Table 2 records the reduction of wound area of different groups over the period of 21 days. The area of wound was measured on the 1, 4, 7, 10, 13, 16, 19 and 21 days of post surgery in all groups. A very high significant rate of closure of wound was observed between the 4th day to 10th day. Mean wound area of P. pinnata treated group on the 16th day post surgery was 30 mm2 and in DMSO treated group 200 mm2. Leaf extract has significant wound healing activity (p < 0.05) while standard groups treated with vitamin E has shown gradual closure of wound but complete wound closure was observed by 19th day of post surgery in all the treated groups and by 21st day in control. The period of epithelialisation was found to be 19th day for P. pinnata treated animals (Fig. 1). The upper layer of wound was surgically removed and subjected to histological studies. Histological examination of the haematoxylin and eosin stained tissue of the rat wounds treated with P. pinnata and Vitamin has lead to reduce scar formation and enhance fibroblast proliferation, angiogenesis, keratinization and epithelialization as compared to vehicle treated group or control group.
Table 2.
Effect of Pongamia pinnata on wound contraction by excision wound model (N = 6).
| Post wounding (days) | Wound area (mm2) (mean ± S.E.) and percentage of wound contraction |
||
|---|---|---|---|
| Control | Leaf extract | Standard | |
| 1 | 525 ± 2.1 | 522 ± 3.8 | 515 ± 2.5 |
| 4 | 439 ± 2.2 (16.01%) | 408 ± 2.2 (21.53%) | 415 ± 14.1 (18.94%) |
| 7 | 317 ± 4.2 (35.2%) | 247 ± 2.8 (52.9%) | 270 ± 2.8 (48.29%) |
| 10 | 306 ± 3.9 (41.49%) | 117 ± 5.2 (77.50%) | 189 ± 1.6 (63.08%) |
| 13 | 266 ± 2.7 (49.5%) | 65 ± 1.8 (87.30%) | 108 ± 2.2 (78.90%) |
| 16 | 200 ± 2.4 (61.75%) | 30 ± 2.2b (93.16%) | 73 ± 1.8 (85.96%) |
| 19 | 35 ± 2.2b (93.16%) | 00 ± 2.1 (100%) | 8 ± 0.2 (98.43%) |
| 21 | 00 ± 00b (100%) | 00 ± 00b (100%) | 00 ± 00 (100%) |
p < 0.05. Statistically significant difference in comparison with control group.
Fig. 1.
Showing results of different experimental treatments and % wound healing area during the days post surgery.
3.4. Measurement of tensile strength
Tensile strength of healing skin treated & different test group was measured on 10th day of post surgery. Tensile strength for P. pinnata treated animals was recorded as 4.20 ± 7.6 g/mm2 (p < 0.05), while the control (DMSO) group was 2.65 ± 6.2 g/mm2 & tensile strength of standard (Vitamin) group was observed to be 3.55 ± 6.8 g/mm2 this observation confirms that the leaf extract of P. pinnata possesses excellent wound healing property so far as strength of wound healing tissue is concerned. Breaking strength of P. pinnata treated group was 687 ± 31.15 g while that of control group and standard (Vitamin E) group was 329 ± 14.12 g and 495 ± 14.8 g respectively. Animals treated with P. pinnata extract have shown higher breaking strength (P < 0.001) than other groups.
3.5. Pro-inflammatory cytokine (TNF-alpha and IL-6) Production
3.5.1. TNF-α
The results of TNF-α production are tabulated in Table 3. The production of TNF-α was augmented in P. pinnata fed rats as compared to control rats and its level increased during 12–48 h. After the induction of wound, the level of TNF-α in control DMSO groups (1st day: 147.4 ± 23.9 pg/ml; 8th day: 254.4 ± 62.9 pg/ml) was significantly higher than that in the standard (Vitamin E) group (1st day: 13.5 ± 2.8 pg/ml; 8th day: 129.5 ± 20.9 pg/ml). The level of TNF-α in P. pinnata treated group (151.0 ± 23.8 pg/ml) was higher than that in standard group (13.5 ± 2.8 pg/ml) on day 1. On day 8, the level of TNF-α in P. pinnata treated group (8th day: 159.3 ± 32.5 pg/ml) was significantly (P < 0.05) lower than that in the group treated with control (8th day: 254.4 ± 62.9 pg/ml) and was different from that in standard group (129.5 ± 20.9 pg/ml).
Table 3.
Effect of Pongamia pinnata on pro-inflammatory and anti-inflammatory cytokines.
| Cytokines | Day post surgery | Sample |
||
|---|---|---|---|---|
| DMSO | Pongamia pinnata leaf | Vitamin E | ||
| TNF-alpha (pg/ml) | 1st day | 147.4 ± 23.9 | 151.0 ± 23.8 | 13.5 ± 2.8 |
| 8th day | 254.4 ± 62.9 | 159.3 ± 32.5 | 129.5 ± 20.9 | |
| IL-6 (pg/ml) | 1st day | 81.8 ± 22.31 | 87.0 ± 9.8 | 24.0 ± 18.5 |
| 8th day | 92.5 ± 24.8 | 72.0 ± 22.8 | 22.9 ± 30.2 | |
| IL-10 (pg/ml) | 1st day | 389.6 ± 155.3 | 1078.1 ± 169.2 | 885.5 ± 220.6 |
| 8th day | 595.7 ± 114.9 | 899.0 ± 272.3 | 811.9 ± 220.3 | |
3.5.2. IL-6
Productions of IL-6 are tabulated in Table 3. In P. pinnata treated rats after 24 h post surgery was found to be increased while on 8th post surgery day it was decreased significantly (1st day: 87.0 ± 9.8 pg/ml; 8th day: 72.0 ± 22.8 pg/ml). In control group IL-6 level increased on 8th post surgery day (1st day: 81.8 ± 22.31 pg/ml; 8th day: 92.5 ± 24.8 pg/ml) and the value observed in the standard group was considerably low on 8th post surgery day (1st day: 24.0 ± 18.5 pg/ml; 8th day: 22.9 ± 30.2 pg/ml).
3.6. Anti-inflammatory cytokine (IL-10) production during healing
The results of IL-10 production on different time intervals are tabulated in (The mean ± S.E.) Table 3 after induction of wound the level of IL-10 in P. pinnata treated group was found to be higher (1st day: 1078.1 ± 169.2 pg/ml; 8th day: 899.0 ± 272.3 pg/ml) and in DMSO treated group (1st day: 389.6 ± 155.3 pg/ml; 8th day: 595.7 ± 114.9 pg/ml) was significantly lower than that of standard (1st day: 885.5 ± 220.6 pg/ml; 8th day: 811.9 ± 220.3 pg/ml) treated group.
3.7. Determination of hydroxyproline and hexosamine content
The hydroxyproline and hexosamine content of granulation tissue various post surgery days are given in Table 4. A significant increase in both hydroxyproline and hexosamine content was observed in P. pinnata treated groups than control groups. There is gradual increase in hydroxyproline content on standard and leaf extract treated group in different days but in control a slow gradual increase were observed till 16th day. Throughout the course of healing, hydroxyproline and hexosamine content were found to be more in all treated groups than control group, which are important constituent of extracellular matrix for healing. These compounds are good marker for wound healing.
Table 4.
Hexosamine and hydroxyproline content of granulation on different days of healing.
| Treatments |
Hexosamine (mg/100 mg of tissue) |
Hydroxyproline (mg/g tissue) |
||||
|---|---|---|---|---|---|---|
| 4th day | 8th day | 16th day | 4th day | 8th day | 16th day | |
| DMSO | 0.27 ± 0.40 | 0.45 ± 0.04 | 0.64 ± 0.09 | 19.8 ± 4.11 | 31.6 ± 1.08 | 42.8 ± 1.21 |
| Pongamia pinnata leaf | 0.39 ± 0.03* | 0.73 ± 0.02* | 0.82 ± 0.01* | 38.8 ± 1.13* | 53.2 ± 2.10* | 75.8 ± 2.27* |
| Vitamin E | 0.43 ± 0.08 | 0.81 ± 0.06 | 0.89 ± 0.03 | 41.9 ± 1.09 | 59.9 ± 4.00 | 81.5 ± 2.23 |
3.8. Antioxidant property
The results for in vivo antioxidant potential of P. pinnata were given in Table 5. It is indicated that it possess potent antioxidant activity by inhibiting lipid peroxidation, reduced glutathione levels while increasing SOD activity and CAT activity This validates the potent wound healing activity shown by P. pinnata leaf extract.
Table 5.
Antioxidant activity of methanolic extract of Pongamia pinnata.
| Sample | SOD activity (units/g protein) | GSH level (μg/mg) | Catalase (k/s/mg protein) | TBARS (nmoles/mg protein) |
|---|---|---|---|---|
| DMSO | 1.73 ± 0.15 | 0.02 ± 0.01 | 0.005 ± 0.004 | 1.33 ± 0.15 |
| Pongamia pinnata leaf | 4.30 ± 0.42a | 0.28 ± 0.04a | 0.277 ± 0.011a | 1.19 ± 0.47a |
| Vitamin E | 4.87 ± 0.03 | 0.32 ± 0.02 | 0.329 ± 0.016 | 1.6 ± 0.03 |
SOD, superoxide dismutase; GSH, reduced glutathione; CAT, catalase; TBARS, thiobarbituric acid reactive substances values are mean ± S.E.M. of six replicates.
P < 0.05 as compared to control.
4. Discussion
Treatment of P. pinnata on wounded animals produce significant wound healing activity. Wound healing is homeostasis process in which repithelialization, granulation tissue formation and remodeling of the extracellular matrix. Healing process takes place by immunological activities of victim itself and does not require much help, but various risk factors such as infection and week immunity delay in healing has brought attention to promote this process.29, 30
All the parameters studied and observed were significantly affecting wound healing activity of the leaf extract. A significant antimicrobial activity has been observed. In our opinion the compound present in the crude extract are responsible for the effective antimicrobial activity. The results of our study showed that P. pinnata treatment significantly increased the level of antioxidant enzymes the increased level of superoxide dismutase (SOD) leads to dimutation of superoxide radicals and prevent further generation of free radicals like peroxnitrite and hydroxyl radicals. An increase in intracellular thiol based antioxidant GSH was also observed GSH serves to detoxify the damaging radicals either by directly scavenging them or by acting as a co substrate in the glutathione peroxidase (GPx)-catalyzed reduction of hydrogen peroxide and lipid peroxides. The antioxidant activity may be due to potent-radical-scavenging activity of the phenolics present in the extract. The activity of phenolics is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers. They may also have a metal chelating potential.31 The synergistic effect of both antimicrobial and antioxidant activity accelerated the wound healing process.8, 32, 33
P. pinnata treatment produced significant wound healing activity, which may be due to its angiogenic and mitogenic potential. Its prohealing activity was marked, as all the parameters observed were significantly affected. A healing tissue synthesizes collagen, which is a constituent of growing cell. Concentration of hydroxyproline is a measure of concentration of collagen. Higher the concentration of hydroxyproline indicates faster rate of healing wound. Biochemical analysis showed increased hydroxyproline content, which is a reflection of increased cellular proliferation and there by increased collagen synthesis. Increased hexosamine content reflects the stabilization of collagen molecules by enhancing electrostatic and ionic interactions.20 Collagen not only confers strength and integrity to the tissue matrix but also plays an important role in homeostasis and in epithelialization at the latter phase of healing.25 Hence enhanced synthesis of hydroxyproline and hexosamine-treated rats provide strength to repaired tissue and also healing pattern. The result showed potent wound healing capacity as evident from the wound contraction; increased tensile strength and increased biochemical parameters in healing tissue have thus validated the ethnotherapeutic claim.
In the inflammatory phase of wound healing, pro-inflammatory cytokines (TNF-α and IL-6,) known to play a major role by enhancing angiogenesis.34, 35 Our study revealed that during the wound healing TNF-α and IL-6 level was detected after 12 h & 24 h of wound infliction and it increased to reach at maximum after 24 h in P. pinnata fed animals. The level of TNF-α and IL-6 declined after 48 h in P. pinnata fed animals. There are reports (Siqueira et al, 2010) that TNF-α inhibits collagen formation and hydroxyproline production which are essential for the final part of proliferative phase in wound healing, but the low level of TNF-α and IL-6 after 48 h till 8th day did not interfere with collagen formation and hydroxyproline production. This supports the present finding that TNF-α and IL-6 increased up to 24 h of wounding and down regulated after 48 h.
In this study, P. pinnata elevated the level of IL-10 production it is an anti-inflammatory cytokine produced by various cells including macrophages and T lymphocyte. The level on day 1 (24 h) and day 8 after induction of wound was observed. Healing of wound has been associated with a decrease in pro-inflammatory cytokines. IL-10 appears to influence the wound-healing environment by decreasing the expression of pro-inflammatory/profibrotic mediators, resulting in decreased recruitment of inflammatory cells to the wound (Ribbons et al, 1997). In addition, the mild anti-inflammatory effects of IL-10 may be due to the suppression of TNF-α production. Bodger et al (1997) showed that mucosal secretion of IL-10 and TNF-α is increased during wound healing. IL-10 may be protective and can limit tissue damage caused by inflammation. Therefore, elevation of IL-10 can down-regulate TNF-α production in macrophage leading to increased wound healing.
Treatment with P. pinnata increased the serum IL-10 concentration, in the same time down regulates TNF-α and IL-6. Finding suggested that P. pinnata extract regulate anti-inflammatory and pro-inflammatory cytokines and ultimately systemic immune pathways attached with them. It is also possible that a molecule regulated by ingredients of P. pinnata extract has regulated IL-10. Howsoever, IL10 has potent role in macrophage deactivation, blocking the induced synthesis of TNF-α, IL-1, IL-6, IL-8, and GMCSF by human monocytes.
Hence the synergistic effect of antimicrobial, antioxidant activity and immune response of anti inflammatory & pro inflammatory cytokines with cells of immune system (monocytes and macrophages) were significantly in wound healing process.
5. Conclusion
The present studies results confirm that potent significant wound healing activity of P. Pinnata. Wound contraction, increased tensile strength, increased hydroxyproline and hexosamine content, modulation of pro inflammatory and anti inflammatory cytokine, moderate antimicrobial activity and in vivo antioxidant activity explains the reputed wound healing observed. The exact mechanisms and the active principles remain to be investigated.
Conflicts of interest
The authors state that they have no conflicts of interest to declare.
Acknowledgements
The authors are grateful to the Vindhya Herbal Testing & Research Laboratory & Department of Microbiology, Barkatullah University, Bhopal M.P. for laboratory support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Srinivasan K., Muruganandan S., Lal J., Chandra S., Tandan S.K., Prakash V.R. Evaluation of anti-inflammatory activity of Pongamia pinnata leaves in rats. J Ethnopharmacol. 2001;78:151–157. doi: 10.1016/s0378-8741(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S., Meera B., Kalidhar S.B. A review of the chemistry and biological activity of Pongamia pinnata. J Med Aromat Plant Sci. 2003;25:411–465. [Google Scholar]
- 3.Ayyanar M., Ignacimuthu S. Herbal medicines for wound healing among tribal people in Southern India: ethnobotanical and scientific evidences. Int J Appl Res Nat Prod. 2009;2:29–42. [Google Scholar]
- 4.Badole S.L., Bodhankar S.L. Anti hyperglycemic activity of Pongamia pinnata stem bark in diabetic mice. Pharma Biol. 2008;46:900–905. [Google Scholar]
- 5.Chopade V.V., Thankar A.N., Pande A.N. Pongamia pinnata: phytochemical constituents, traditional uses and pharmacological properties: a review. Int J Green Pharm. 2008;2:72–75. [Google Scholar]
- 6.Al Muqarrabun L.M., Ahmat N., Ruzaina S.A., Ismail N.H., Sahidin I. Medicinal uses, phytochemistry and pharmacology of Pongamia pinnata (L.) Pierre: a review. J Ethnopharmacol. 2013;150:395–420. doi: 10.1016/j.jep.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Sajid Z.I., Anwar F., Shabir G., Rasul G., Khalid M. Alkharfy, Gilani AH. Antioxidant, antimicrobial properties and phenolics of different Solvent extracts from bark, leaves and seeds of Pongamia pinnata (L.) Pierre. Molecules. 2012;17:3917–3932. doi: 10.3390/molecules17043917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokane D.D., More R.Y., Kale M.B., Nehete M.N., Mehendale P.C., Gadgoli C.H. Evaluation of wound healing activity of root of Mimosa pudica. J Ethnopharmacol. 2009;124:311–315. doi: 10.1016/j.jep.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 9.Varoglu E., Seven B., Gumustekin K., Aktas O., Sahin A., Dane S. The effects of vitamin E and selenium on blood flow to experimental skin burns in rats using the 133Xe clearance technique. Cent Eur J Med. 2010;5:219–223. [Google Scholar]
- 10.Houghton P.J., Hylands P.J., Mensahb A.Y., Hensel A., Deters A.M. In vitro tests and ethnopharmacological investigations: wound healing as an example. J Ethnopharmacol. 2005;100:100–107. doi: 10.1016/j.jep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Mertz P., Ovington L. Wound healing microbiology. Dermatol Clin. 1993;11:739. [PubMed] [Google Scholar]
- 12.Essawi T., Srour M. Screening of some Palestinian medicinal plants for antibacterial activity. J Ethnopharmacol. 2000;70:343–349. doi: 10.1016/s0378-8741(99)00187-7. [DOI] [PubMed] [Google Scholar]
- 13.Peterson L.R. Squeezing the antibiotic balloon: the impact of antimicrobial classes on emerging resistance. Clin Microbiol Inf. 2005;11:4–16. doi: 10.1111/j.1469-0691.2005.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falagas M.E., Bliziotis I.A. Drug-resistant gram-negative bacteria: the dawn of the post-antibiotic era? Int J Antimicrob Agents. 2007;4:191–198. doi: 10.1016/j.ijantimicag.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Sofowora E.A. Wiley; Chichester: 1982. Medicinal Plants and Traditional Medicine in Africa; p. 256. [Google Scholar]
- 16.Trease G.E., Evans W.C. ELSB Baillere Tindal; Oxford: 1987. A Text Book of Pharmacognosy; p. 1055. [Google Scholar]
- 17.Khandelwal K.R. 19th ed. Nirali Prakashan Publishers; Pune, India: 2008. Practical Pharmacognosy Techniques and Experiments. [Google Scholar]
- 18.Udupa A.L., Kulkarni D.R., Udupa S.L. Effect of Tridax procumbens extracts on wound healing. Int J Pharmacog. 1995;33:37–40. [Google Scholar]
- 19.Kumar B., Vijayakumar M., Govindarajan R., Pushpangadan P. Approaches to wound healing–exploring medicinal plants of India. J Ethnopharmacol. 2007;114:103–113. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Nayak B.S., Raju S.S., Eversley M., Ramsubhag A. Evaluation of wound healing activity of Lantana camara L.: a preclinical study. Phytothera Res. 2009;23:241–245. doi: 10.1002/ptr.2599. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee P., Mukherjee K., Kumar Rajesh M., Pal M., Saha B.P. Evaluation of wound healing activity of some herbal formulations. Phytothera Res. 2003;17:265–268. doi: 10.1002/ptr.931. [DOI] [PubMed] [Google Scholar]
- 22.Morton J.J., Malone M.H. Evaluation of vulnerary activity by open wound procedure in rats. Arch Int Pharmacodyn Ther. 1972;196:117–120. [PubMed] [Google Scholar]
- 23.Johansen P.G., Marshall R.D., Neuberger A. Carbohydrates in protein. 2. The hexose, hexosamine, acetyl and amide-nitrogen content of hen's-egg albumin. Biochem J. 1960;77:239–247. doi: 10.1042/bj0770239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurung S., Basnet N.S. Wound healing properties of Carica papaya latex: In vivo evaluation in mice burn model. J Ethnopharmacol. 2009;121:338–341. doi: 10.1016/j.jep.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Suntar I.P., Akkol E.K., Yilmazer D. Investigations on the in-vivo wound healing potential of Hypericum perforatum L. J Ethnopharmacol. 2010;127:468–477. doi: 10.1016/j.jep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Niehaus W.G., Samuelsson B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 27.Ellman G.L. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Sinha K.A. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 29.Muthusamy S.K., Kirubanandan S., Sripriya, Sehgal P.K. Triphala promotes healing of infected full-thickness dermal wound. J Surg Res. 2008;144:94–101. doi: 10.1016/j.jss.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 30.Nayak B.S., Anderson M., Pereire P. Evaluation of wound healing potential of Catharanthus roseus leaf extract in rats. Fitoterpia. 2007;78:540–544. doi: 10.1016/j.fitote.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Rice Evans C.A., Miller N.J., Bolwell P.G., Bramley P.M., Pridham J.B. The relative antioxidant activities of plant derived polyphenolic flavonoids. Free Radic Res. 1995;22(4):375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 32.Akkol E.K., Koca U., Pesin I., Yilmazer D., Toker G., Yesilada E. Exploring the wound healing activity of Arnebia densiflora (Nordm.) Ledeb. by in vivo models. J Ethnopharmacol. 2009;124(1):137–141. doi: 10.1016/j.jep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Deshmukh P.T., Fernandes J., Atul A., Toppo E. Wound healing activity of Calotropis gigantea root bark in rats. J Ethnopharmacol. 2009;125(1):178–181. doi: 10.1016/j.jep.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg LZ, de la Torre J. Wound healing, growth factors, Available at (2006) http://www.emedicine.com/plastic/TOPIC457.HTM.
- 35.Pattanayaka S.P., Sunitab P. Wound healing, anti-microbial and antioxidant potential of Dendrophthoe falcata (L.f) Ettingsh. J Ethnopharmacol. 2008;120(2):241–247. doi: 10.1016/j.jep.2008.08.019. [DOI] [PubMed] [Google Scholar]