Abstract
Oxaliplatin-induced peripheral neuropathy characterized especially as cold dysesthesia is a major dose-limiting side effect of the drug and is very difficult to control. In the present study, we examined whether the traditional herbal formulation Shakuyakukanzoto (SKT: 芍藥甘草湯Sháo Yào Gān Cǎo Tāng) could relieve oxaliplatin-induced cold dysesthesia in mice. The inhibitory mechanisms were also investigated. Repetitive administration of SKT (0.1–1.0 g/kg) starting from the day after oxaliplatin injection inhibited cold dysesthesia in a dose-dependent manner. Our previous report has shown that the mRNA expression of transient receptor potential melastatin 8 (TRPM8), characterized as a cold-sensing cation channel, is increased in the dorsal root ganglia of mice treated with oxaliplatin. In addition, TRPM8 antagonist TC-I 2014 (10 and 30 mg/kg) also attenuated cold dysesthesia in oxaliplatin-treated mice. Taken together, it is suggested that TRPM8 is involved in the cold dysesthesia induced by oxaliplatin. Repetitive administration of SKT inhibited the mRNA expression of TRPM8 induced by oxaliplatin in the dorsal root ganglia. These results suggested that prophylactic repetitive administration of SKT is effective in preventing the exacerbation of oxaliplatin-induced cold dysesthesia by inhibiting the mRNA expression of TRPM8 in the dorsal root ganglia.
Keywords: Oxaliplatin, Shakuyakukanzoto, TRPM8, Cold dysesthesia, Dorsal root ganglia
Graphical abstract
1. Introduction
Oxaliplatin is a third-generation platinum-based chemotherapeutic drug and is mainly used for the treatment of colorectal cancer. However, oxaliplatin produces numerous side effects (e.g., acute neurotoxicity and chronic peripheral neuropathy) that the regulation has extremely difficult.1 Although cold dysesthesia is a characteristic symptom in oxaliplatin-induced peripheral neuropathy,2 its underlying mechanisms are not completely understood. Oxalate, a metabolite of oxaliplatin, has been reported to be involved in cold dysesthesia.3, 4 Our previous report has also been shown that transient receptor potential melastatin 8 (TRPM8), characterized as a cold-sensing cation channel5, 6, 7, 8 is involved in oxaliplatin-induced cold dysesthesia.9, 10
In oxaliplatin-treated patients, several drugs (e.g., calcium gluconate/magnesium sulfate, glutathione, carbamazepine, gabapentin, amifostine, acetyl-l-carnitine and α-lipoic acid) are used for the management of the peripheral neuropathy.3, 11 Although the above drugs are effective against mild neuropathy,3, 11 the regulation is still difficult.
Shakuyakukanzoto (SKT: 芍藥甘草湯Sháo Yào Gān Cǎo Tāng) is a traditional herbal formulation consisting of two herbal medicines: Paeoniae Radix (芍藥 Sháo Yào) and Glycyrrhizae Radix (甘草 Gān Cǎo). SKT is effective in treating muscle pain, muscle spasms, joint pain, and numbness in patients.12, 13, 14, 15 In rodents (rats and mice), SKT inhibits chemotherapy-induced mechanical allodynia.16 However, it is unknown whether SKT is effective in oxaliplatin-induced cold dysesthesia. Thus, in this study, we investigated the effects of SKT on oxaliplatin-induced cold allodynia in mice.
2. Materials and methods
2.1. Animals
Male C57BL/6Ncr mice (6 week old at the start of the experiment; Japan SLC Ltd, Hamamatsu, Japan) were used. Five animals were housed per cage under controlled temperature (21–23 °C), humidity (45–65%), and light (7:00 AM to 7:00 PM) conditions. Food and water were available ad libitum. Procedures were approved by the Committee for Animal Experiments at the University of Toyama.
2.2. Drugs
Oxaliplatin was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was dissolved in 5% glucose. Oxaliplatin was injected intraperitoneally; the dose (3 mg/kg) was selected from our previously published report17 and was in accordance with the recommended clinical dose of 85 mg/m2 body surface area, which corresponds to a dose of 2.4 mg/kg in a person of 170 cm body height and 60 kg weight. Dried extracts of SKT (Lot. No. 2020068010) were obtained from Tsumura and Co. Ltd (Tokyo, Japan) and were dissolved in 5% gum arabic. SKT or the vehicle was administered orally once daily on day 1 and 2 of oxaliplatin injection. 3-[7-(Trifluoromethyl)-5-[2-(trifluoromethyl)phenyl]-1H-benzimidazol-2-yl]-1-oxa-2-azaspiro[4.5]dec-2-ene (TC-I 2014) (Tocris Bioscience, Bristol, UK) was dissolved in 20% 2-hdroxypropyl-β-cyclodextrin and was administered orally on day 3 of oxaliplatin injection.18
2.3. Behavioral experiments for the evaluation of cold dysesthesia
Mice were placed individually in a plastic cage (110 × 180 × 150 mm) with a wire mesh bottom. After an acclimation of at least 60 min, acetone (10 μl) was applied to the plantar skin. The escape response was observed immediately after acetone application in not only oxaliplatin-treated mice but also healthy mice. Thus, this response was not evaluated. The reaction following it was scored as follows: 0, no response; 1, lifting of the hind paw; 2, flinching or licking of the hind paw. The stimulus was applied 2 times to each hind paw at intervals of more than 20 s, and the average (total 4 times/mouse) served as a response score.
2.4. Reverse transcriptase and polymerase chain reaction (RT-PCR)
The animals were anesthetized with pentobarbital (80 mg/kg, intraperitoneal) and transcardially perfused with phosphate-buffered saline (PBS). The dorsal root ganglia (L4–L5 levels) was removed, immediately frozen in liquid nitrogen, and stored at −80 °C until assay. Total RNA was extracted with TRIzol® regent (Thermo Fisher Sci. Inc., Waltham, MA, USA) and treated with DNase I (Takara Bio Inc., Shiga, Japan). RT-PCR assay of TRPM8 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed as described.18 The sequences of primers were as follows: TRPM8, 5′-ggctggagatgagattgtgag-3′ (sense) and 5′-gctgaagtgggtggagaaga-3′ (antisense); glyceraldehyde-3-phosphate dehydrogenase, 5′-ccaaggtcatccatgacaac-3′ (sense) and 5′-ttactccttggaggccacgt-3′ (antisense). The expression levels of mRNA were analyzed with NIH Image software (National Institute of Health, Bethesda, Maryland, USA). The mRNA expression level of TRPM8 in each sample was normalized to that of GAPDH and was then further normalized to its expression in the vehicle control.
2.5. Statistical analysis
All data are present as the mean and standard error of the mean. Statistical significance was analyzed using two-way repeated measures analysis of variance (ANOVA) and post hock Holm–Šidák multiple comparisons test or one-way ANOVA and post-hoc Dunnett's test. P < 0.05 was considered statistically significant.
3. Results
3.1. Effects of prophylactic administration of SKT on oxaliplatin-induced cold dysesthesia
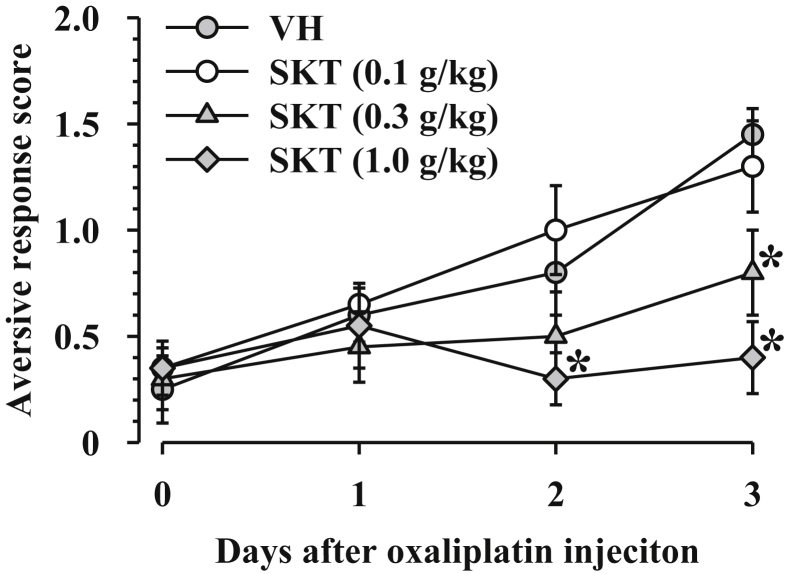
A single intraperitoneal injection of oxaliplatin (3 mg/kg) induces cold dysesthesia, which peaks on day 3.9 Thus, in the present study, we therefore evaluated the cold dysesthesia until day 3 after oxaliplatin injection. Repetitive administration of SKT (0.1–1.0 g/kg) did not affect the initial development of cold dysesthesia after oxaliplatin administration, but SKT did not significantly and dose-dependently inhibit the exacerbation of the dysesthesia from day 2 after oxaliplatin injection, in comparison with the vehicle (Fig. 1). Two-way repeated measures ANOVA revealed a significant difference with prophylactic SKT (F3,16 = 4.292, P < 0.05) and a significant interaction between oxaliplatin treatment and time (F9,48 = 2.344, P < 0.05).
Fig. 1.
Effects of prophylactic Shakuyakukanzoto administration on oxaliplatin-induced cool dysesthesia in mice. Mice were given an intraperitoneal injection of oxaliplatin (3 mg/kg) on day 0. Shakuyakukanzoto (SKT) or the vehicle (VH: 5% gum arabic) was administered orally once daily on day 1 and 2 of oxaliplatin injection. The evaluation of cold dysesthesia was performed before SKT or VH administration. Data are presented as mean and standard error of the mean (n = 5). *P < 0.05 compared to VH (post-hoc Holm-Šidák multiple comparisons).
3.2. Effect of TRPM8 antagonist TC-I 2014 on oxaliplatin-induced cold dysesthesia
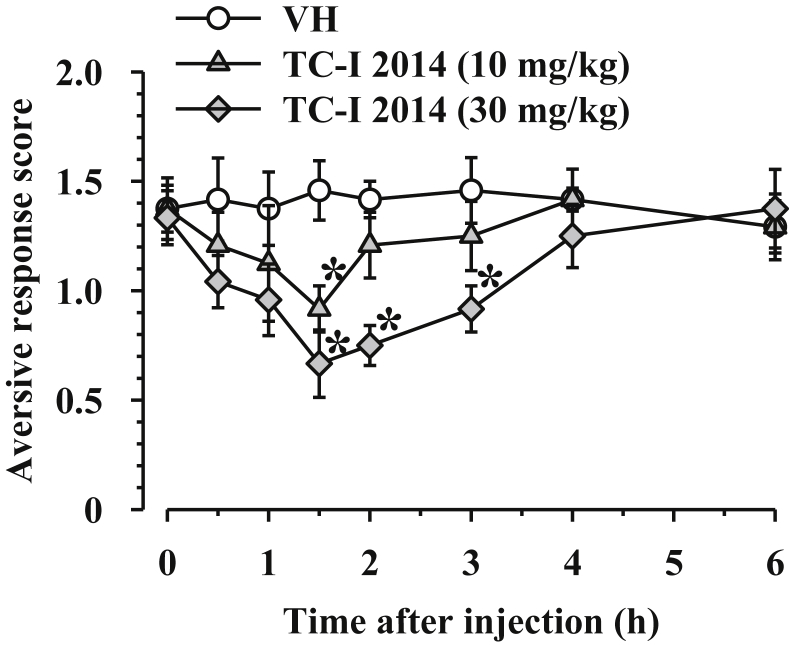
Our previous report has shown that the mRNA expression of TRPM8 in the dorsal root ganglia was observed to peak on day 3 after oxaliplatin injection, and may be involved in oxaliplatin-induced cold dysesthesia.9, 10 Thus, this study examined whether TRPM8 was involved in oxaliplatin-induced cold dysesthesia using TRPM8 antagonist TC-I 2014. Oral administration of TRPM8 antagonist TC-I 2014 (10 and 30 mg/kg) inhibited the cold dysesthesia in a dose-dependent manner (Fig. 2). Two-way repeated measures ANOVA revealed a significant difference with TC-I 2014 (F2,15 = 8.404, P < 0.01).
Fig. 2.
Effect of TRPM8 TC-I 2014 on oxaliplatin-induced cool dysesthesia in mice. Mice were given an intraperitoneal injection of oxaliplatin (3 mg/kg) on day 0. TC-I 2014 or the vehicle (VH: 20% 2-hdroxypropyl-β-cyclodextrin) was administered orally on day 3 of oxaliplatin injection. Following the evaluation of cold dysesthesia was performed before TC-I 2014 or VH administration, the dysesthesia was evaluated over time after the administration of them. Data are presented as mean and standard error of the mean (n = 6). *P < 0.05 compared to VH (post-hoc Holm-Šidák multiple comparisons).
3.3. Effects of prophylactic administration of SKT on the expression of TRPM8 mRNA in dorsal root ganglia of mice treated with oxaliplatin
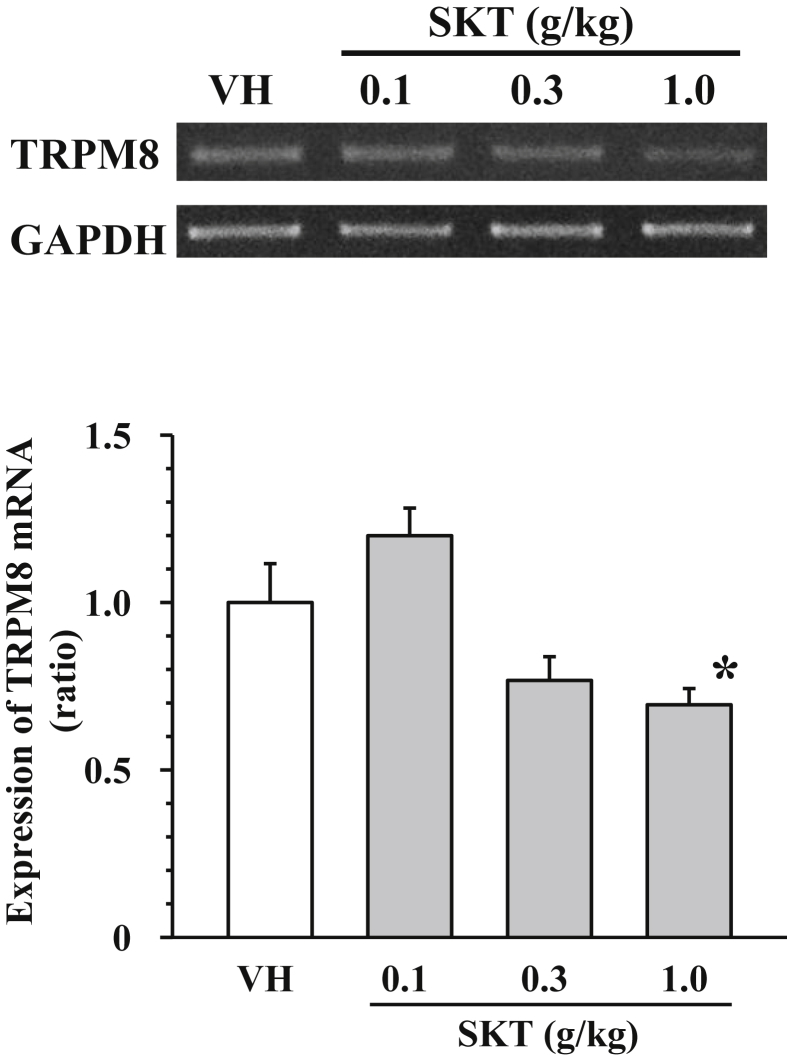
It has been demonstrated that TRPM8 is involved in oxaliplatin-induced cold dysesthesia based on the present results and our previous reports.9, 10 In addition, single administration of SKT does not inhibit oxaliplatin-induced cold dysesthesia, suggesting that SKT does not have the antagonistic activity of TRPM8.19 We therefore examined the effect of repetitive administration of SKT (0.1–1.0 g/kg) on the expression of TRPM8 mRNA in the dorsal root ganglia20 of mice treated with oxaliplatin. Repetitive administration of SKT (1.0 g/kg) significantly inhibited the expression of TRPM8 mRNA, compared with mice administered the vehicle (Fig. 3). Although the administration of SKT at higher concentrations (0.3 g/kg) had a tendency to decrease the mRNA expression, SKT at lower concentrations (0.1 g/kg) did not have that effect (Fig. 3).
Fig. 3.
Effects of prophylactic Shakuyakukanzoto administration on the expression of TRPM8 mRNA in the dorsal root ganglia of mice treated with oxaliplatin. Mice were given an intraperitoneal injection of oxaliplatin (3 mg/kg) on day 0. Shakuyakukanzoto (SKT) or the vehicle (VH: 5% gum arabic) was administered orally once daily on day 1 and 2 of oxaliplatin injection. The extraction of mRNA on day 3 of oxaliplatin injection was performed after behavioral evaluation. The mRNA expression level of TRPM8 in each sample was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and further normalized to its expression level in the vehicle control. Upper panel: a typical example of the mRNA expression levels of TRPM8 and GAPDH. Lower panel: The mRNA expression level of TRPM8 normalized with GAPDH. Data are presented as mean and standard error of the mean (n = 5). *P < 0.05 compared to vehicle (post-hoc Dunnett's test).
4. Discussion
Prophylactic repetitive administration of SKT prevented the development of cold dysesthesia in mice treated with oxaliplatin. In our preliminary experiments, prophylactic repetitive administration, but not single administration, inhibited mechanical allodynia in mice.19 Therefore, SKT would need to be administered repetitively to produce an anti-dysesthesia activity, suggesting that the regulation of gene expression related in cold dysesthesia is involved in SKT's mechanism of action.
A single intraperitoneal injection of oxaliplatin increases the mRNA expression of TRPM8 in the dorsal root ganglia of mice and peaks at day3 after the injection.9, 10 TRP ankyrin 1 (TRPA1) also is known as a cold-sensing TRP channel.7 TRPM8 and TRPA1 are activated at temperature below 25 °C and 17 °C, respectively.7 In this study, we used acetone for the cold stimulus. In our preliminary experiment, the application of acetone to the plantar skin decreased the temperature from 28.67 ± 0.10 °C to 24.46 ± 0.24 °C (Andoh, Mizoguchi and Kuraishi: unpublished observation). In addition to our previous report,10 TRPM8 antagonist TC-I 2014 was observed to inhibit oxaliplatin-induced cold dysesthesia in mice. Taken together, TRPM8 is suggested to be involved in the cold dysesthesia evaluated in this study. However, we do not deny the involvement of TRPA1 in cold dysesthesia induced by oxaliplatin, as other recent studies have shown that TRPA1 is involved in oxaliplatin-induced cold dysesthesia.21, 22
TRPM8 mRNA is expressed in the dorsal root ganglia, but not the brain, spinal cord, heart, skin and muscle.6 Repetitive administration of SKT inhibited the increase of TRPM8 mRNA in the dorsal root ganglia of mice treated with oxaliplatin. However, the inhibitory mechanisms of SKT on TRPM8 mRNA expression are still unclear. cAMP response element-binding, c-Myc, myocyte enhancer factor 2, etc. are the reported transcription factors for the regulation of TRPM8 mRNA expression.23 There are no reports on the regulation of the above transcription factors by SKT. Hidaka et al.16 have reported that the component Shakuyaku, but not Kanzo, plays an important role in the regulation of mechanical allodynia. The main component of Shakuyaku is paeoniflorin. Oxaliplatin increases intracellular Ca2+ ions in primary cultures of dorsal root ganglion neurons,24 resulting in a greater chance that CREB may be phosphorylated and the activate transcription.25 In contrast, paeoniflorin inhibits the phosphorylation of CREB26 through the inhibition of an increase in intracellular Ca2+ ions.27 In addition, paeoniflorin activates the A1 adenosine receptor, which inhibits the activity of adenylyl cyclase.28 Thus, the decrease in the concentration of cAMP induced by paeoniflorin may also be involved in the inhibition of CREB phosphorylation. However, there is no evidence of the regulation of other transcription factors by paeoniflorin and other components of Shakuyaku. These issues will be the focus of our future study, as SKT may inhibit the mRNA expression of TRPM8 through the regulation of the activation of transcription factors including CREB.
5. Conclusion
Prophylactic repetitive administration of SKT prevents the exacerbation of oxaliplatin-induced cold dysesthesia. Thus, SKT may be useful for the prevention of oxaliplatin-induced peripheral neuropathy. The underlying mechanism of the anti-cold dysesthesia action of SKT may involve the regulation of the expression of TRPM8.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
This study is supported in part by the contract research fund on Wakan-yaku and Biotechnology from Toyama Prefectural Government.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Park S.B., Goldstein D., Krishnan A.V. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63:419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 2.Quasthoff S., Hartung H.P. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 3.Pasetto L.M., D'Andrea M.R., Rossi E., Monfardini S. Oxaliplatin-related neurotoxicity: how and why? Crit Rev Oncol Hematol. 2006;59:159–168. doi: 10.1016/j.critrevonc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai M., Egashira N., Kawashiri T., Yano T., Ikesue H., Oishi R. Oxaliplatin-induced neuropathy in the rat: involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain. 2009;147:165–174. doi: 10.1016/j.pain.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 5.McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 6.Peier A.M., Moqrich A., Hergarden A.C. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 7.Tominaga M., Caterina M.J. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- 8.Story G.M. The emerging role of TRP channels in mechanisms of temperature and pain sensation. Curr Neuropharmacol. 2006;4:183–196. doi: 10.2174/157015906778019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauchan P., Andoh T., Kato A., Kuraishi Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci Lett. 2009;458:93–95. doi: 10.1016/j.neulet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Andoh T., Mizoguchi S., Gauchan P., Yakura T., Kuraishi Y. Involvement of increased expression of transient receptor potential melastatin 8 in acute cold allodynia induced by oxaliplatin in mice. Eur J Pain Sup. 2010;4:47. [Google Scholar]
- 11.Saif M.W., Reardon J. Management of oxaliplatin-induced peripheral neuropathy. Ther Clin Risk Manag. 2005;1:249–258. [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto K., Hoshiai H., Noda K. Effects of shakuyaku-kanzo-to on muscle pain from combination chemotherapy with paclitaxel and carboplatin. Gynecol Oncol. 2001;81:333–334. doi: 10.1006/gyno.2001.6168. [DOI] [PubMed] [Google Scholar]
- 13.Hyodo T., Taira T., Kumakura M. The immediate effect of Shakuyaku-kanzo-to, traditional Japanese herbal medicine, for muscular cramps during maintenance hemodialysis. Nephron. 2002;90:240. doi: 10.1159/000049057. [DOI] [PubMed] [Google Scholar]
- 14.Hinoshita F., Ogura Y., Suzuki Y. Effect of orally administered shao-yao-gan-cao-tang (Shakuyaku-kanzo-to) on muscle cramps in maintenance hemodialysis patients: a preliminary study. Am J Chin Med. 2003;31:445–453. doi: 10.1142/S0192415X03001144. [DOI] [PubMed] [Google Scholar]
- 15.Hosokawa A., Ogawa K., Ando T. Preventive effect of traditional Japanese medicine on neurotoxicity of FOLFOX for metastatic colorectal cancer: a multicenter retrospective study. Anticancer Res. 2012;32:2545–2550. [PubMed] [Google Scholar]
- 16.Hidaka T., Shima T., Nagira K. Herbal medicine Shakuyaku-kanzo-to reduces paclitaxel-induced painful peripheral neuropathy in mice. Eur J Pain. 2009;13:22–27. doi: 10.1016/j.ejpain.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Gauchan P., Andoh T., Kato A., Sasaki A., Kuraishi Y. Effects of the prostaglandin E1 analog limaprost on mechanical allodynia caused by chemotherapeutic agents in mice. J Pharmacol Sci. 2009;109:469–472. doi: 10.1254/jphs.08325sc. [DOI] [PubMed] [Google Scholar]
- 18.Parks D.J., Parsons W.H., Colburn R.W. Design and optimization of benzimidazole-containing transient receptor potential melastatin 8 (TRPM8) antagonists. J Med Chem. 2011;54:233–247. doi: 10.1021/jm101075v. [DOI] [PubMed] [Google Scholar]
- 19.Andoh T. 2011. Regulation of chemotherapy-induced peripheral neuropathy using traditional medicines; p. 5. 4th International Symposium on Scientific Research of Traditional Medicine – Basic and Clinical research on Traditional Medicine – supple. [Google Scholar]
- 20.Kobayashi K., Fukuoka T., Obata K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 21.Nassini R., Gees M., Harrison S. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–1631. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 22.Zhao M., Isami K., Nakamura S., Shirakawa H., Nakagawa T., Kaneko S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol Pain. 2012;8:55. doi: 10.1186/1744-8069-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L., Barritt G.J. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr Relat Cancer. 2006;13:27–38. doi: 10.1677/erc.1.01093. [DOI] [PubMed] [Google Scholar]
- 24.Shirahama M., Ushio S., Egashira N. Inhibition of Ca2+/calmodulin-dependent protein kinase II reverses oxaliplatin-induced mechanical allodynia in rats. Mol Pain. 2012;8:26. doi: 10.1186/1744-8069-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson P.I., Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu Rev Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X.J., Chen H.L., Li Z. Analgesic effect of paeoniflorin in rats with neonatal maternal separation-induced visceral hyperalgesia is mediated through adenosine A(1) receptor by inhibiting the extracellular signal-regulated protein kinase (ERK) pathway. Pharmacol Biochem Behav. 2009;94:88–97. doi: 10.1016/j.pbb.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Tsai T.Y., Wu S.N., Liu Y.C., Wu A.Z., Tsai Y.C. Inhibitory action of L-type Ca2+ current by paeoniflorin, a major constituent of peony root, in NG108-15 neuronal cells. Eur J Pharmacol. 2005;523:16–24. doi: 10.1016/j.ejphar.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Liu H.Q., Zhang W.Y., Luo X.T., Ye Y., Zhu X.Z. Paeoniflorin attenuates neuroinflammation and dopaminergic neurodegeneration in the MPTP model of Parkinson's disease by activation of adenosine A1 receptor. Br J Pharmacol. 2006;148:314–325. doi: 10.1038/sj.bjp.0706732. [DOI] [PMC free article] [PubMed] [Google Scholar]