Abstract
The fruits of Barringtonia racemosa are traditionally used in Indian medicine for the treatment of pain and inflammatory conditions. In this study, a fraction of ethyl acetate extract of fruits of B. racemosa (BREAF) was investigated for anti-inflammatory activity in experimental models of acute and chronic inflammation. Activity against acute inflammation was evaluated in inflammogens induced rat paw edema models. Whereas, effect in chronic inflammation was evaluated in cotton pellet granuloma and oxazolone induced delayed type hypersensitivity (DTH) model in mice. The BREAF exhibited dose dependent anti-inflammatory activity in both acute and chronic models at oral doses of 5, 10 and 20 mg/kg. BREAF inhibited both phases of carrageenan induced rat paw inflammation. The reduction in paw inflammation by BREAF was also evident in histamine and serotonin induced inflammation in rats. Effect of BREAF on DTH indicates inhibition of immune mediated inflammation. The reduction in cotton pellet granuloma by BREAF treatment shows inhibition of proliferative changes associated with chronic inflammation. Analysis of BREAF after chromatographic separations showed presence of bartogenic acid as a major constituent. Hence, it is proposed that anti-inflammatory effects of BREAF can be partially attributed to its bartogenic acid content. The minute doses at which this fraction shows anti-inflammatory effects emphasizes the need for further investigations on its efficacy in the immuno-inflammatory conditions.
Keywords: Barringtonia racemosa, lecythidaceae, bartogenic acid, anti-inflammatory, delayed type hypersensitivity
Abbreviations: BREAF, Barringtonia racemosa ethyl acetate fraction; DTH, Delayed type hypersensitivity; p.o., Per oral
Graphical abstract
1. Introduction
Inflammatory diseases are globally identified cause of morbidity among the population.1 Inflammation is a natural protective response of the body to tissue injury caused by chemical, mechanical or thermal stimuli, trauma, microbial agents or autoimmune diseases.1, 2, 3 Acute inflammatory response keeps the integrity of organisms through activation of immune cells.4 Inflammatory response is a complex process mediated through variety of cellular pathways and activated complement factors. Although, acute inflammation is a protective response of the body, if unresolved, it leads to painful conditions, like rheumatoid arthritis, inflammatory bowel diseases, asthma, allergy, atherosclerosis, immune-inflammatory ailments and even neoplastic transformation.5, 6, 7 Thus, persistent inflammation is vital factor in the development and progression of chronic diseases.8
Steroidal and non-steroidal anti-inflammatory drugs (NSAIDs) are globally practiced for the treatment of acute inflammatory disorders. However, their use is either associated with adverse effects or they are ineffective in the treatment of chronic inflammatory disorders, including rheumatoid arthritis.1, 9, 10 The long term use of NSAIDs is associated with gastric ulcer, bleeding, renal dysfunction, kidney damage, bronchospasm, cardiac abnormalities, bone marrow depression, retention of salts and water etc.3, 9, 11, 12 Therefore, it is a clinical necessity to recognize the more efficacious and safer drugs for the prevention and treatment of inflammatory diseases.12, 13 In contrast to the limitations of NSAIDs, natural products have a more favorable pharmacological profile accompanied by lower toxicities. Additionally, natural products are biocompatible and cost effective alternatives for the treatment of various inflammatory diseases.14, 15, 16 Anti-inflammatory drug discovery from plants is the most productive and rational strategy for the identification of novel drug candidates.9 India has a great legacy of various medicinal plants which are useful as alternative medicines against various diseases. There is a great opportunity to develop novel anti-inflammatory drugs through the integration of traditional knowledge and indigenous resources.1 Earlier evidence suggested that plant derived natural products exert their anti-inflammatory effects through the modulation of key inflammatory mediators, effects on pro-inflammatory molecule expression like cyclooxygenase (COX), nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-10 (IL-10) and other cytokines.12, 17
The various parts of Barringtonia racemosa (B. racemosa) are known to possess multiple biological activities.18 Extracts prepared from different parts of B. racemosa possess analgesic, antitumor and antimicrobial activities.19, 20, 21 The aqueous bark extract of B. racemosa exerted significant and dose-dependent antinociceptive activity in experimental animals. This activity is attributed to the presence of opioids or opiodiomimetics as well as phenolics and steroidal constituents in B. racemosa.22 Anti-oxidant and anti-inflammatory effects of B. racemosa leaves are attributed to its lycopene content.23 This extract exerted in vitro nitric oxide synthase inhibitory and antioxidant activity in RAW cells.23 Methanolic, ethanolic and boiling water extracts of B. racemosa leaves, sticks and barks at the concentration of 50 mg/mL were found to possess antifungal activity against Fusarium sp., Tricoderma koningii, Penicillium sp., Ganoderma tropicum, Ganoderma lucidum, Aspergillus sp. and Rhizopus sp.24 The extracts obtained from the aerial parts of this plant demonstrated in vitro antioxidant activity.25 B. racemosa leaves demonstrated higher antioxidant activities than the stems, owing to its antioxidant content. This plant is proposed as a potential source of natural antioxidants.26 Previously, we reported the protective effects of bartogenic acid isolated from the fruits of B. racemosa in complete Freund's adjuvant induced arthritis model in rats.27 Present study aimed to evaluate the anti-inflammatory activity of most active fraction of B. racemosa fruits in different models of acute and chronic inflammation. Recent review summarized the pharmacological activities of B. racemosa.28 The crude ethanolic extract of B. racemosa (125, 250 and 500 mg/kg, p.o.) was evaluated in carrageenan and formalin induced paw edema model.29 Another study reported the antioxidant activity of crude ethanolic bark extract of B. racemosa in DPPH assay, analgesic activity in acetic acid induced writhing model, anti-diarrheal activity in castor oil induced diarrhea model and antibacterial activity by disc diffusion assay. The extract was tested in-vivo at the oral doses of 250 and 500 mg/kg.30 Although, these earlier reports highlighted the anti-inflammatory potential of B. racemosa, these studies were either performed using the crude extracts at higher doses or restricted to acute model of inflammation. As per our understanding, there is no systematic study reporting the in-vivo efficacy of characterized B. racemosa fraction in animal models of acute, chronic and immune inflammation.
The present study involved anti-inflammatory evaluation of fraction, isolated from an ethyl acetate extract of B. racemosa fruits (BREAF), at the lower oral doses of 5, 10 and 20 mg/kg in experimental models of acute and chronic inflammation. Acute anti-inflammatory activity was studied in carrageenan, histamine and serotonin induced paw edema models. The cotton pellet assay and oxazolone induced contact dermatitis model were executed to study the effects of BREAF in chronic inflammation and delayed type hypersensitivity respectively.
2. Materials and methods
2.1. Chemicals and biochemicals
Drug sample of diclofenac sodium (purity > 98%) was obtained as a gift from Kirti Pharmachem, Nasik, India. Serotonin (RM 1825-1G; purity ≥ 98%) and dexamethasone (RM 4185-1G; purity > 98%) were purchased from Himedia Laboratories Pvt. Ltd. Mumbai, India. Carrageenan (λ) (C-3889-5G), histamine (H-7375; purity > 98%), 4-ethoxymethylene-2-phenyloxazolone (oxazolone, E-0753-1G; purity ≥ 90%) and cyproheptidine (C-6022; purity ≥ 98%) were purchased from Sigma Aldrich, USA. Other chemicals and solvents used in the extraction, fractionation and chromatographic separations were of analytical grade.
2.2. Plant material
Fruits of B. racemosa Roxb. (Lecythidaceae), collected from the sea cost of Konkan, Maharashtra were purchased from the local vendor. The specimen was authenticated by the experts of Botanical Survey of India, Pune, India and the specimen was deposited (sample reference: voucher no. 74843). An authentic marker of bartogenic acid was generously supplied by Dr. Mangala Gowri, Senior Scientist, IICT, Hyderabad, India.
2.3. Bioassay guided extraction and isolation of BREAF
The BREAF was isolated according to the procedure described by Gowri et al31 with some modifications. Dried fruits of B. racemosa were coarsely powdered and 500 g of material was defatted with petroleum ether (60–80°, 3 × 1 L), followed by extraction with methanol (3 × 1.5 L, each time) at room temperature for 3 days. For efficient extraction, the earlier solvent was replaced by an equal amount of fresh solvent at the end of each day. After filtration and concentration under reduced pressure, this process yielded 32.3 g of methanol extract. This extract was subjected to evaluation of anti-inflammatory activity in carrageenan induced rat paw edema model where it showed anti-inflammatory activity. Furthermore, methanol extract (15 g) was fractionated into n-butanol (6.2 g) and ethyl acetate (5.6 g) fractions and evaluated for the activity as mentioned earlier. Ethyl acetate extract displayed higher anti-inflammatory potency than methanol or n-butanol extracts. Hence, ethyl acetate extract (5 g) was subjected to further fractionation on silica gel column (3 × 90 cm, flow rate 2 mL/min), using ethyl acetate (1.4 L). Fractions of 100 mL were collected. Elution was monitored by TLC runs of each fraction using methanol as a solvent. The developed TLC plates were sprayed with p-anisaldehyde-sulfuric acid followed by heating at 110 °C for visualization. The fractions 8–15 were combined to get a triterpenoids enriched fraction called BREAF (0.9 g). BREAF was further methodically investigated for detailed anti-inflammatory activity. Part of this fraction (BREAF, 0.3 g) was subjected to chromatography on pre-coated reverse phase silica gel plate (Merck), using a mixture of acetonitrile: water (85:15, v/v) as eluent. High performance thin layer chromatography (HPTLC) of fraction revealed three spots, comprising of one intense blue spot having Rf = 0.68 resembling to bartogenic acid marker. To identify the composition and purity of BREAF, it was subjected to LC-ESI/MS analysis.
2.4. Experimental animals and drug administration
Healthy male Wistar rats (150–200 g) and male C57BL/6 mice (25–30 g) were used in the present study. Animals were maintained in polypropylene cages at 22 ± 2 °C with free access to food (commercially available standard pellet feed – Amrut Laboratory Animal Feed, Pune, India) and water. All the experimental procedures were approved by the Institutional Animal Ethics Committee (Reg. No. 651/PO/ReBi/S/02/CPCSEA) constituted under ‘Prevention of Cruelty to the Animals Act- 1960’, Government of India. For all the experimental models, each treatment group contained six animals (n = 6). The drug samples (including BREAF, diclofenac sodium, cyproheptidine and dexamethasone) were orally administered to animals as solutions in 5% tween-80 in water. Animals in the control group received appropriate volumes of dosing vehicle.
2.5. Anti-inflammatory screening
2.5.1. Carrageenan – induced paw edema
The effect of BREAF in acute inflammation was evaluated in Wistar rats, according to an earlier report.32 Carrageenan (0.1 mL of 1% suspension in normal saline) was injected into the subplantar region of right hind paw of each rat. The standard drug (diclofenac sodium 10 mg/kg, p.o.) and BREAF (5, 10 and 20 mg/kg, p.o.) were administered to respective groups, 1 h prior to the carrageenan injection. Paw volumes were measured using digital plethysmometer (Ugo Basile, 7140, Italy) before and at 1, 2 and 3 h after carrageenan injection. The percentage rise in paw volume was calculated by following formula.33, 34
| % Rise = (Vt − V0)/V0 × 100 |
Where, Vt = Paw volume at time t; V0 = Initial paw volume.
2.5.2. Histamine – induced paw edema
The right hind paw edema in rats was induced by subplantar injection of 0.1 mL of histamine in saline (1 mg/mL).35, 36 The paw volumes were measured using plethysmometer before histamine injection and at 1 h interval for 3 h. All the animals received respective treatments as in earlier model, 1 h before histamine injection. In this model, cyproheptidine (10 mg/kg, p.o.) was used as a reference standard.37, 38
2.5.3. Serotonin (5-HT) induced paw edema
In this model, paw edema was induced by subplanter injection of 0.1 mL of 5-HT solution (1 mg/mL) prepared in normal saline. The study groups and treatments were similar to that of histamine induced paw edema model. The paw volume was measured before and at 30, 60, 90, 120, 150 and 180 min after 5-HT injection.36, 37
2.5.4. Cotton pellet induced granuloma in mice
The effects of BREAF on chronic inflammation was evaluated through cotton pellet induced granuloma test in male C57BL/6 mice.39 Mice were divided into five groups, each containing six animals. Under ether anesthesia, sterile cotton pellets weighing 10 ± 1 mg were implanted subcutaneously and bilaterally in the groin region of mice through a single surgical blade incision.40, 41 Prior to implantation, the cotton pellets were soaked in 0.2 mL of water for injection containing 0.13 mg streptomycin and 0.1 mg penicillin. The standard drug treated group received dexamethasone (1 mg/kg/day, p.o.) for 7 days. Other three groups received BREAF (5, 10 and 20 mg/kg/day, p.o.) for 7 days starting from the day of pellet implantation.42 On 8th day mice were sacrificed by an overdose of anesthetics. The granulomas were carefully dissected out, weighed and dried at 60 °C to constant weight. The increase in wet and dry weight of pellets was recorded as a measure of granuloma formation. Percentage protection from granuloma development was calculated using following formula:
| % Protection = [(Mean granuloma weight in control group − Mean granuloma weight in test group)/(Mean granuloma weight in control group)] × 100 |
2.5.5. Oxazolone induced delayed type hypersensitivity (DTH) in mice
Effect of BREAF on oxazolone induced delayed type hypersensitivity in mice was investigated according to reported method.42, 43 C57BL/6 mice were sensitized by epicutaneous application of 25 μL mixture of acetone and olive oil (4:1) containing 2% oxazolone, on the shaved skin of abdomen. Mice were divided into five groups of six mice each and assigned to treatments as given in the cotton pellet granuloma model stated above. Six days after sensitization, all the mice were challenged on both sides of the right ears with 10 μL of 0.5% oxazolone solution in acetone and olive oil mixture. The left and right ear thickness was measured at 24 h after challenge using a digital Vernier caliper (Mitutoyo, Japan).44 The inhibitory effects of BREAF and dexamethasone on DTH reaction were determined by comparing the intensity of DTH reaction in these groups with that of the control group.42 The intensity of DTH reaction was calculated as follows:
| DTH intensity = [(Right ear thickness − Left ear thickness)/Right ear thickness] × 100 |
After the measurement of ear thickness, mice were sacrificed by an overdose of anesthetics and ears were dissected for histological examinations. The histological sections of ears were stained according to the routine hematoxylin-eosin staining protocol. Thymus and spleen were dissected from each mouse and weighed.43, 45, 46
2.6. Statistical analysis
Results are expressed as mean ± SEM and statistical significance of difference in the central tendencies of treatment groups was determined by one-way ANOVA followed by Dunnett's multiple comparison test. P < 0.05 was considered statistically significant.
3. Results
3.1. Bartogenic acid identified as major constituent of BREAF
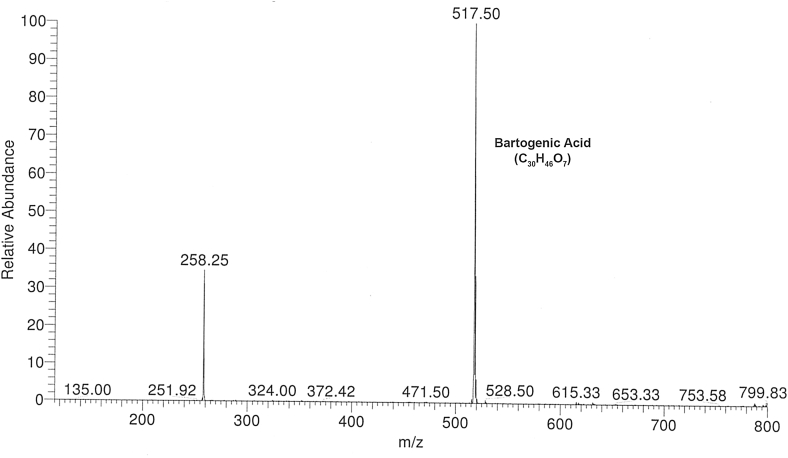
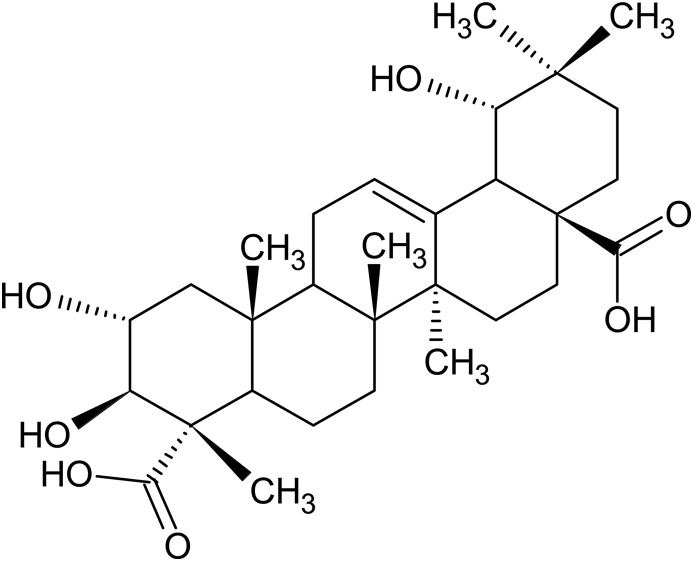
The isolated BREAF was obtained as an amorphous powder which on HPTLC showed three spots including one intense blue spot having Rf = 0.68 matching with bartogenic acid marker. The LC-ESI-MS analysis revealed, molecular ion peak at m/z 517.5 [M−H]− (Fig. 1), corresponding to molecular formula C30H46O7 which is in fine agreement with previously reported data of bartogenic acid.31, 47, 48 The content of bartogenic acid in BREAF was found to be 78.57 %. The HPTLC and LC-MS analysis confirmed that bartogenic acid (Fig. 2) is a major component of BREAF.
Fig. 1.
LC-ESI-MS spectrum of BREAF isolated from Barringtonia racemosa fruits.
Fig. 2.
Structure of bartogenic acid (2α, 3β, 19β-trihydroxyolean-12-en-23, 28-dioic acid).
3.2. BREAF exhibited acute anti-inflammatory action
3.2.1. Carrageenan induced paw edema
Results of different inflammogen induced paw edema models are represented as percentage (%) rise in paw volume at the peak of inflammation in Table 1. Anti-inflammatory effect of BREAF in carrageenan induced paw edema model was dose dependent and diclofenac was used as reference drug. The maximum rise in paw volume was observed after 3 h of carrageenan injection in vehicle treated group. BREAF at the tested doses of 5, 10 and 20 mg/kg, significantly inhibited both phases of inflammation induced by carrageenan in a dose-dependent manner. However, effect of BREAF was less potent than corresponding standard diclofenac.
Table 1.
Effect of BREAF on inflammogens induced rise in rat paw edema.
| Treatment group | Carrageenan (% Rise in paw volume) |
Histamine (% Rise in paw volume) |
Serotonin (% Rise in paw volume) |
|
|---|---|---|---|---|
| 1 h | 3 h | 1 h | 1.5 h | |
| Control | 31.0 ± 1.1 | 50.0 ± 1.9 | 18.0 ± 0.9 | 37.0 ± 0.9 |
| BREAF (5 mg/kg, p.o.) | 26.0 ± 1.7* | 44.0 ± 1.6** | 13.0 ± 1.8* | 29.0 ± 1.9** |
| BREAF (10 mg/kg, p.o.) | 20.0 ± 1.1** | 33.0 ± 2.1** | 12.0 ± 1.5* | 27.0 ± 1.0** |
| BREAF (20 mg/kg, p.o.) | 13.0 ± 0.7** | 28.0 ± 1.3** | 11.0 ± 1.1** | 24.0 ± 1.6** |
| Diclofenac (10 mg/kg, p.o.) | 8.5 ± 0.5** | 14.0 ± 1.5** | – | – |
| Cyproheptidine (10 mg/kg, p.o.) | – | – | 7.9 ± 0.7** | 22.0 ± 1.2** |
Data represented in mean ± SEM, (n = 6).
*p < 0.05, **p < 0.01 compared with control group.
3.2.2. Histamine induced paw edema
BREAF demonstrated significant anti-inflammatory activity at the tested doses after 1 h of histamine injection. The rise in paw edema was 18%, 11% and 7.9% in control, BREAF (20 mg/kg) and cyproheptidine treated groups respectively (Table 1). Although, BREAF showed significant (P < 0.05) anti-inflammatory activity at 5 and 10 mg/kg dose, more significant (P < 0.01) results were obtained at the higher tested dose (20 mg/kg) of BREAF. Percentage rise in paw edema was prominently lower in all the groups treated with BREAF and cyproheptidine as compared to control group. Moreover, inhibition of paw edema by BREAF was observed to be dose dependent.
3.2.3. Serotonin (5-HT) induced paw edema
Acute anti-inflammatory activity of BREAF was evaluated by 5-HT induced rat paw edema test and results are presented in Table 1. The inceased paw volume after 1.5 h of 5-HT administration was significantly (P < 0.01) inhibited by BREAF. In case of 5-HT induced inflammation, the anti-inflammatory effect of BREAF were statistically significant, but less potent than that of cyproheptidine (10 mg/kg, p.o.). BREAF, at all the tested doses showed significant (P < 0.01) and dose dependent anti-inflammatory activity against 5-HT induced rat paw edema test (Table 1).
3.3. BREAF exhibited chronic anti-inflammatory action
The chronic anti-inflammatory effect of BREAF was studied at the doses of 5, 10 and 20 mg/kg, by using cotton pellet granuloma test in mice and results are presented in Table 2. The edematous and proliferative deposits around implants were reduced in BREAF (5, 10, 20 mg/kg, p.o.) and dexamethasone (1 mg/kg, p.o.) treated groups. BREAF treatment significantly (P < 0.001) reduced both dry and wet weights of the granuloma. Statistically significant reduction was recorded at all the tested doses of 5 (P < 0.05), 10 and 20 mg/kg (P < 0.01) of BREAF. The BREAF demonstrated dose dependent decrease in granuloma weights with highest activity observed at the dose of 20 mg/kg, p.o. which was still less than the effect of standard drug dexamethasone.
Table 2.
Chronic anti-inflammatory activity of BREAF in cotton pellet granuloma test in mice.
| Group | Wet weight (mg) | Dry weight (mg) |
|---|---|---|
| Control | 157.0 ± 10.0 | 74.0 ± 4.6 |
| BREAF (5 mg/kg, p.o.) | 129.0 ± 8.1* (17.8) | 59.0 ± 3.2* (20.2) |
| BREAF (10 mg/kg, p.o.) | 122.0 ± 3.4** (22.2) | 56.0 ± 3.1** (24.3) |
| BREAF (20 mg/kg, p.o.) | 113.0 ± 5.5** (28.0) | 49.0 ± 3.2** (33.7) |
| Dexamethasone (1 mg/kg, p.o.) | 106.0 ± 5.5** (32.4) | 43.0 ± 3.5** (41.8) |
Values in parenthesis indicate % protection in respective groups.
Data represented in mean ± SEM, (n = 6).
*p < 0.05 and **p < 0.01 compared with control group.
3.4. BREAF suppressed oxazolone induced DTH in mice
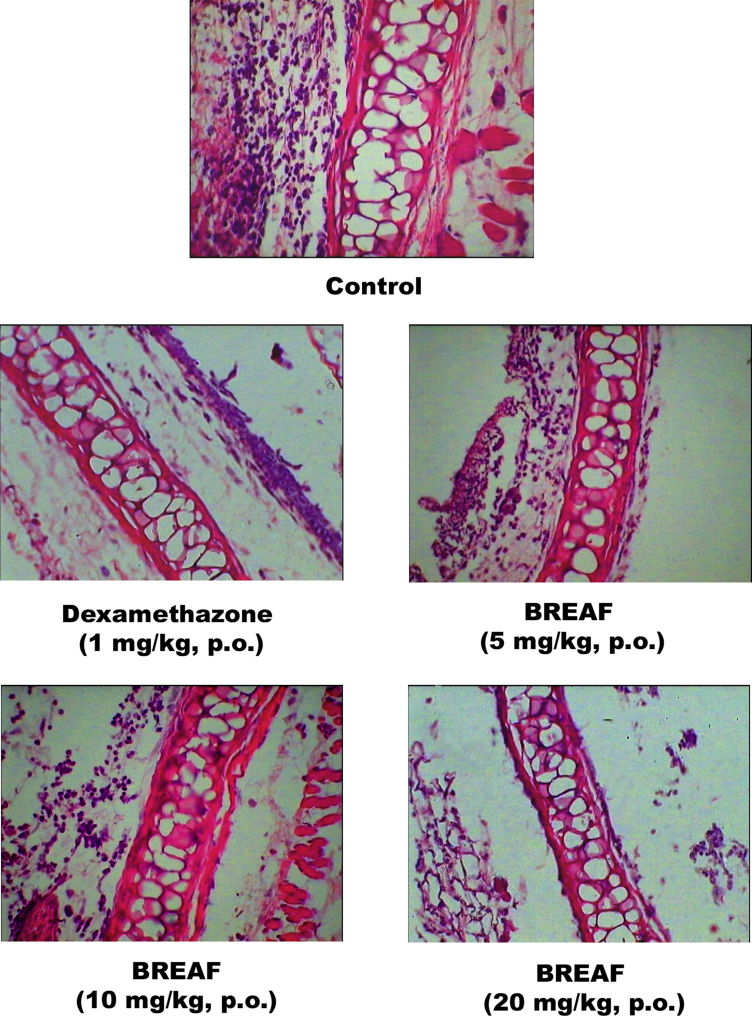
The effect of BREAF on oxazolone induced mouse ear edema (delayed type hypersensitivity) is shown in Table 3. BREAF treatment reduced the ear thickness and intensity of DTH in a dose dependent manner. Inhibition of DTH was also evident in the histopathology of ear sections (Fig. 3). Histological changes induced by oxazolone like acanthosis, spongiosis, papillomatosis, exocytosis, edema and inflammatory lesions were significantly inhibited by BREAF treatments at all the tested doses. The effects of BREAF (20 mg/kg, p.o.) and Dexamethasone (1 mg/kg, p.o.) were comparable as determined by blinded evaluation of histology slides by two different observers. Furthermore, there was a significant decrease in the relative weights of thymus and spleens in the mice treated with BREAF and dexamethasone. However, this effect on lymphoid organ weights was more potent in dexamethasone treated group as compared with BREAF treated groups.
Table 3.
Effect of BREAF on the oxazolone-induced DTH in C57BL6 mice.
| Treatment | Intensity of DTH at 24 h | Thymus weight (mg) | Spleen weight (mg) |
|---|---|---|---|
| Control | 64.0 ± 2.7 | 41.0 ± 1.8 | 168.0 ± 14.0 |
| BREAF (5 mg/kg, p.o.) | 52.0 ± 2.1* | 38.0 ± 1.5 | 137.0 ± 2.4 |
| BREAF (10 mg/kg, p.o.) | 51.0 ± 3.6** | 34.0 ± 1.5* | 128.0 ± 6.2* |
| BREAF (20 mg/kg, p.o.) | 46.0 ± 2.8** | 27.0 ± 2.2** | 119.0 ± 8.8** |
| Dexamethasone (1 mg/kg, p.o.) | 41.0 ± 1.8** | 25.0 ± 1.6** | 106.0 ± 6.3** |
Data represented in mean ± SEM, (n = 6).
*p < 0.05 and **p < 0.01 compared with control group.
Fig. 3.
BREAF protected mice from oxazolone induced contact dermatitis related histological alterations.
4. Discussion and conclusion
In the present study, BREAF was evaluated for its effects in experimental models of acute inflammation, chronic inflammation and DTH. Lambda (λ) carrageenan, is a natural product derived from red seaweeds belonging to a family of linear sulfated polysaccharides. Intraplanter injection of carrageenan leads to the development of inflammatory response characterized by well known classical signs, including increased volume of injected paw, redness, heat and local hypersensitivity.49, 50 Carrageenan induced paw edema is a standard model of acute inflammation, which has been accepted as an useful phlogistic tool for investigating new anti-inflammatory drugs.51, 52 Earlier studies reported that carrageenan induced inflammatory response is mediated through multiple mechanisms like release of eicosanoids, cytokines, chemokines, mast cell derived products, neuropeptides, transcription factors and involvement of neutrophil migration.50 Carrageenan induces biphasic inflammation comprising of first phase that occurs within an hour of the carrageenan injection.32 This early phase is associated with histamine, serotonin and bradykinin liberation. While the late phase occurs within 1 h and lasts for 3 h or more; it is associated with formation of prostaglandins.53, 54 In the present study, BREAF effectively reduced both phases of inflammation indicating that it inhibits the synthesis and effects of mediators like histamine and serotonin. Carrageenan-induced paw edema is efficiently controlled with the arachidonate cyclooxygenase (COX) inhibitors due to its COX-dependent mechanism, therefore, results of current study signifies the possibility that BREAF has arachidonate COX inhibitory property.55, 56 To study the involvement of acute phase mediators, effect of BREAF was separately evaluated using histamine and serotonin induced rat paw inflammation model. Inflammation induced by exogenous histamine and serotonin was also inhibited by BREAF. This indicates inhibitory effect of BREAF on the receptors or signal transduction related to these mediators rather than inhibition of their synthesis. Histamine and serotonin increases vascular permeability and acts additively with prostaglandins to induce edema.57, 58 BREAF probably inhibits histamine and serotonin induced increase in vascular permeability and thereby inhibits inflammation. Effect of BREAF on second phase of carrageenan induced inflammation could be either due to inhibition of the effects of endogenous arachidonic acid derivatives or inhibition of their synthesis. In the present study, exact step in arachidonic acid cascade that is affected by BREAF is not elucidated. In the histamine and 5-HT induced inflammation model, we used cyproheptidine (10 mg/kg, p. o.) as a standard drug whereas in carrageenan induced inflammation diclofenac (10 mg/kg, p.o.) was used. The highest tested dose of BREAF (20 mg/kg, p.o.) had less potent anti-inflammatory activity as compared to these reference standards.
Histamine is a vital inflammatory mediator and histamine induced inflammation is a good model for the study of acute anti-inflammatory drugs.59 Histamine is a potent vasodilator substance which leads to increased vascular permeability and attract neutrophils at the target site.58, 60 It results in increased blood flow leading to swelling and redness of inflamed area.59 Due to transient action of histamine, inflammation decreases quickly after its induction, suggesting the role of histamine in initiation of early inflammatory response.61 BREAF decreased this early inflammatory response induced by histamine. It signifies that anti-inflammatory activity of BREAF is probably assisted by its anti-histamine activity.38 5-HT (serotonin) is a main inflammatory mediator which induces inflammation by initiating vasodilation and increasing vascular permeability.60, 62 In this study, transient increase in rat paw volume was observed after serotonin injection.36 The ability of BREAF to inhibit 5-HT induced inflammation implies the contribution of anti-serotonin activity of BREAF behind its anti-inflammatory activity. Thus, it can be proposed that BREAF exerts its anti-inflammatory effects by inhibiting the action of inflammatory mediators including histamine, serotonin and prostaglandins.
The cotton pellet induced granuloma formation is a characteristic feature of an established chronic inflammatory reaction.56 It is a common test used for the evaluation of transudative, exudative and proliferative phases of chronic inflammation.41, 63 The response to subcutaneously implanted cotton pellet can be divided into transudative and proliferative phases. The cotton pellet granuloma formation response is characterized by three phases. Initial phase is early response which occurs within 3 h after cotton pellet implantation and characterized by increased vascular permeability which leads to leakage of fluid from blood vessels. The exudative phase continues from 3 h to 72 h after cotton pellet implantation, which is denoted by protein leakage around granuloma which occurs as a result of repairing mechanism towards altered vascular permeability. The proliferative phase lasts from 3 days to 6 days, which is characterized by the development of granuloma tissue as a consequence of pro-inflammatory mediator release.34, 63 Increase in wet weight of the cotton pellet represents transudative phase, whereas proliferative phase is represented by increase in dry weight of granuloma.60 Granuloma tissue formation is characterized by an increase in number of fibroblasts, synthesis of collagen and mucopolysaccharide followed by penetration of proliferating fibroblasts into exudate which ultimately leads to formation of vascularized mass.40 The process of granuloma formation occurs due to release of proinflammatory mediators, lyosomal enzymes and reactive oxygen species. BREAF demonstrated reduction in both wet and dry weight of granuloma (Table 3). It reflects its efficacy to inhibit the proliferative phase of inflammatory process.62 Bartogenic acid is major active constituent present in BREAF and belongs to the class of pentacyclic triterpenoids which are known to possess variety of useful pharmacological actions. Pentacyclic triterpenoids like bartogenic acid have structural resemblance with steroids. BREAF demonstrated its effects in transudative and poliferative phases of granulaoma formation. Therefore, it seems that BREAF has steroid like effects in chronic inflammation. The ability of BREAF (10 and 20 mg/kg, p.o.) to significantly (P < 0.01) inhibit the cotton pellet granuloma formation designated anti-inflammatory activity of BREAF during chronic phase of inflammation. These effects could be due to the increased levels of anti-inflammatory cytokines and decreased production of proinflammatory mediators like myeloperoxidase, nitric oxide and some interleukins.41
The cytokines released by activated T lymphocytes promote activation of macrophages, which initiates an acute inflammatory response through the release of TNF-α, IL-1, chemokines, prostaglandins and leukotrienes.44 Oxazolone is a strong contact sensitizer that induces delayed-type hypersensitivity (DTH) resembling human allergic contact dermatitis. Oxazolone induces skin sensitization, sustained ear swelling, marked polymorphonuclear and T lymphocyte infiltration by increasing CD8+ T-lymphocyte activity.44, 64 Additionally, TRPV1 receptors have a protective role in the development of oxazolone induced contact dermatitis.43 In this model corticosteroids reduced the DTH induced swelling as well as levels of eicosanoids.46 Our results revealed that BREAF is able to reduce the intensity of DTH reaction. Its effect was similar to the effects of dexamethasone but less potent. BREAF inhibited the histological changes such as acanthosis, spongiosis, papillomatosis, exocytosis and particularly edema and inflammatory lesions, which were observed in DTH reaction induced by oxazolone. Histopathological changes in BREAF and dexamethasone treated group were less severe as compared with changes observed in control group animals. This effect of BREAF on DTH can be attributed to inhibition of activities of arachidonate metabolites such as leukotrienes and prostaglandins in the tissues.This observation is in congruence with the results of carrageenan induced inflammation where BREAF inhibited second phase of inflammation that involves the same mediators including leukotrienes and prostaglandins. BREAF also reduced thymus and spleen weights and this effect was comparable with that of standard drug, dexamethasone. Such a decrease in thymus weight is an index of suppression of immune response. These results denote the possible efficacy of BREAF in conditions like rheumatoid arthritis.
The present investigation revealed that bartogenic acid enriched fraction obtained from the fruits of B. racemosa possesses potent anti-inflammatory activity in acute and chronic models of inflammation. Additionally, BREAF possesses inhibitory effect on cell mediated immunity as evident from its inhibition of oxazolone induced DTH. Considering efficacy of BREAF at doses below 20 mg/kg, p.o., it is proposed that BREAF is an effective anti-inflammatory compound having a protective role in immuno-inflammatory conditions. As bartogenic acid was confirmed to be a major constituent of BREAF, observed anti-inflammatory effects can be attributed, at least partially, to its bartogenic acid content. Further studies are essential to comprehend the mechanisms of action of BREAF behind these effects. However, present investigation elucidated the pharmacological actions of BREAF and highlighted possible use of bartogenic acid rich fraction for treating immune-inflammatory conditions. The present investigation provides scientific evidence for anti-inflammatory activity of B. racemosa fruits and validate its use in the traditional medicine. This study suggests that bartogenic acid is a potential candidate for the treatment of inflammation related diseases. Further studies are essential to comprehend the anti-inflammatory mechanisms of action of BREAF.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
Authors are thankful to Dr. P. Mangala Gowri and Dr. J. Madhusudhana Rao of Indian Institute of Chemical Technology, Hyderabad (India) for generous supply of a sample of Bartogenic acid. Authors wish to extend their gratitude to Dr. Manish Desai (Pathologist) for his guidance in histopathological studies.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Kalpesh R. Patil, Email: kalpeshpharma20@gmail.com.
Chandragouda R. Patil, Email: pchandragouda@yahoo.com.
References
- 1.Dewanjee S., Dua T.K., Sahu R. Potential anti-inflammatory effect of Leea macrophylla Roxb. leaves: a wild edible plant. Food Chem Toxicol. 2013;59:514–520. doi: 10.1016/j.fct.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Mothana R.A. Anti-inflammatory, antinociceptive and antioxidant activities of the endemic Soqotraen Boswellia elongata Balf. f. and Jatropha unicostata Balf. f. in different experimental models. Food Chem Toxicol. 2011;49:2594–2599. doi: 10.1016/j.fct.2011.06.079. [DOI] [PubMed] [Google Scholar]
- 3.Saleem U., Ahmad B., Ahmad M., Hussain K., Bukhari N.I. Anti-nociceptive, anti-inflammatory and anti-pyretic activities of latex and leaves methanol extract of Euphorbia helioscopia. Asian Pac J Trop Dis. 2015;5:322–328. [Google Scholar]
- 4.Li Y.-Y., Huang S.-S., Lee M.-M., Deng J.-S., Huang G.-J. Anti-inflammatory activities of cardamonin from Alpinia katsumadai through heme oxygenase-1 induction and inhibition of NF-κB and MAPK signaling pathway in the carrageenan-induced paw edema. Int Immunopharmacol. 2015;25:332–339. doi: 10.1016/j.intimp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Debnath S., Ghosh S., Hazra B. Inhibitory effect of Nymphaea pubescens Willd. flower extract on carrageenan-induced inflammation and CCl 4-induced hepatotoxicity in rats. Food Chem Toxicol. 2013;59:485–491. doi: 10.1016/j.fct.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Babu N.P., Pandikumar P., Ignacimuthu S. Anti-inflammatory activity of Albizia lebbeck Benth., an ethnomedicinal plant, in acute and chronic animal models of inflammation. J Ethnopharmacol. 2009;125:356–360. doi: 10.1016/j.jep.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 7.Fangkrathok N., Junlatat J., Sripanidkulchai B. In vivo and in vitro anti-inflammatory activity of Lentinus polychrous extract. J Ethnopharmacol. 2013;147:631–637. doi: 10.1016/j.jep.2013.03.055. [DOI] [PubMed] [Google Scholar]
- 8.Ganguly A., Al Mahmud Z., Uddin M.M.N., Rahman S.A. In-vivo anti-inflammatory and anti-pyretic activities of Manilkara zapota leaves in albino Wistar rats. Asian Pac J Trop Dis. 2013;3:301–307. [Google Scholar]
- 9.Sofidiya M.O., Imeh E., Ezeani C., Aigbe F.R., Akindele A.J. Antinociceptive and anti-inflammatory activities of ethanolic extract of Alafia barteri. Rev Bras Farm. 2014;24:348–354. [Google Scholar]
- 10.Kumari K., Weerakoon T., Handunnetti S., Samarasinghe K., Suresh T. Anti-inflammatory activity of dried flower extracts of Aegle marmelos in Wistar rats. J Ethnopharmacol. 2014;151:1202–1208. doi: 10.1016/j.jep.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 11.Qandil A.M. Prodrugs of nonsteroidal anti-inflammatory drugs (NSAIDs), more than meets the eye: a critical review. Int J Mol Sci. 2012;13:17244–17274. doi: 10.3390/ijms131217244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Oliveira R.G., Mahon C.P.A.N., Ascêncio P.G.M., Ascêncio S.D., Balogun S.O., de Oliveira Martins D.T. Evaluation of anti-inflammatory activity of hydroethanolic extract of Dilodendron bipinnatum Radlk. J Ethnopharmacol. 2014;155:387–395. doi: 10.1016/j.jep.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 13.Hur S.J., Kang S.H., Jung H.S. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr Res. 2012;32:801–816. doi: 10.1016/j.nutres.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Jamuna S., Karthika K., Paulsamy S., Thenmozhi K., Kathiravan S., Venkatesh R. Confertin and scopoletin from leaf and root extracts of Hypochaeris radicata have anti-inflammatory and antioxidant activities. Ind Crops Prod. 2015;70:221–230. [Google Scholar]
- 15.Liu X., Yang B., Zhang L., Lu Y., Gong M., Tian J. An in vivo and in vitro assessment of the anti-inflammatory, antinociceptive, and immunomodulatory activities of Clematis terniflora DC. extract, participation of aurantiamide acetate. J Ethnopharmacol. 2015;169:287–294. doi: 10.1016/j.jep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Uddin G., Rauf A., Siddiqui B.S., Muhammad N., Khan A., Shah S.U.A. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine. 2014;21:954–959. doi: 10.1016/j.phymed.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Bellik Y., Boukraâ L., Alzahrani H.A. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: an update. Molecules. 2012;18:322–353. doi: 10.3390/molecules18010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadkarni K.M. Popular Prakashan; Bombay: 1976. Indian Materia Medica with Ayurvedic, Unani-tibbi, Siddha, Allopathic, Homeopathic, Naturopathic & Home Remedies, Appendices & Indexes. [Google Scholar]
- 19.Khan S., Jabbar A., Hasan C., Rashid M. Antibacterial activity of Barringtonia racemosa. Fitoterapia. 2001;72:162–164. doi: 10.1016/s0367-326x(00)00264-1. [DOI] [PubMed] [Google Scholar]
- 20.Khan M., Omoloso A. Antibacterial, antifungal activities of Barringtonia asiatica. Fitoterapia. 2002;73:255–260. doi: 10.1016/s0367-326x(02)00067-9. [DOI] [PubMed] [Google Scholar]
- 21.Thomas T.J., Panikkar B., Subramoniam A., Nair M.K., Panikkar K. Antitumour property and toxicity of Barringtonia racemosa Roxb seed extract in mice. J Ethnopharmacol. 2002;82:223–227. doi: 10.1016/s0378-8741(02)00074-0. [DOI] [PubMed] [Google Scholar]
- 22.Deraniyagala S., Ratnasooriya W., Goonasekara C. Antinociceptive effect and toxicological study of the aqueous bark extract of Barringtonia racemosa on rats. J Ethnopharmacol. 2003;86:21–26. doi: 10.1016/s0378-8741(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 23.Behbahani M., Ali A.M., Muse R., Mohd N.B. Anti-oxidant and anti-inflammatory activities of leaves of Barringtonia racemosa. J Med Plants Res. 2007;1:095–102. [Google Scholar]
- 24.Hussin N., Muse R., Ahmad S. Antifungal activity of extracts and phenolic compounds from Barringtonia racemosa L. (Lecythidaceae) Afr J Biotechnol. 2009;8:2835–2842. [Google Scholar]
- 25.Muse R., Ramli J., Ahmad S., Mahmood M. Antioxidant activities of different aerial parts of putat (Barringtonia racemosa L.) Malays J Biochem Mol Biol. 2008;16:15–19. [Google Scholar]
- 26.Kong K.W., Mat-Junit S., Aminudin N., Ismail A., Abdul-Aziz A. Antioxidant activities and polyphenolics from the shoots of Barringtonia racemosa (L.) Spreng in a polar to apolar medium system. Food Chem. 2012;134:324–332. [Google Scholar]
- 27.Patil K.R., Patil C.R., Jadhav R.B., Mahajan V.K., Patil P.R., Gaikwad P.S. Anti-arthritic activity of bartogenic acid isolated from fruits of Barringtonia racemosa Roxb. (Lecythidaceae) Evid Based Complement Altern Med. 2011:2011. doi: 10.1093/ecam/nep148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osman N.I., Sidik N.J., Awal A. Pharmacological activities of Barringtonia racemosa L. (Putat), a tropical medicinal plant species. J Pharm Sci Res. 2015;7:185–188. [Google Scholar]
- 29.Shikha P., Latha P., Suja S. Anti-inflammatory and analgesic activity of Barringtonia racemosa Roxb. fruits. Indian J Nat Prod. 2010;1:356–361. [Google Scholar]
- 30.Saha S., Subrahmanyam E., Kodangala C., Mandal S.C., Shastry S.C. Evaluation of antinociceptive and anti-inflammatory activities of extract and fractions of Eugenia jambolana root bark and isolation of phytoconstituents. Rev Bras Farm. 2013;23:651–661. [Google Scholar]
- 31.Gowri P.M., Tiwari A.K., Ali A.Z., Rao J.M. Inhibition of α-glucosidase and amylase by bartogenic acid isolated from Barringtonia racemosa Roxb. seeds. Phytother Res. 2007;21:796–799. doi: 10.1002/ptr.2176. [DOI] [PubMed] [Google Scholar]
- 32.Winter C.A., Risley E.A., Nuss G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 33.Duwiejua M., Woode E., Obiri D. Pseudo-akuammigine, an alkaloid from Picralima nitida seeds, has anti-inflammatory and analgesic actions in rats. J Ethnopharmacol. 2002;81:73–79. doi: 10.1016/s0378-8741(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 34.Pingsusaen P., Kunanusorn P., Khonsung P., Chiranthanut N., Panthong A., Rujjanawate C. Investigation of anti-inflammatory, antinociceptive and antipyretic activities of Stahlianthus involucratus rhizome ethanol extract. J Ethnopharmacol. 2015;162:199–206. doi: 10.1016/j.jep.2014.10.060. [DOI] [PubMed] [Google Scholar]
- 35.Bani S., Chand D., Suri K., Suri O., Sharma O. Antiinflammatory effects of an ethyl acetate extract of Euphorbia royleana. Phytother Res. 1996;10:285–291. [Google Scholar]
- 36.Baravalia Y., Vaghasiya Y., Chanda S. Brine shrimp cytotoxicity, anti-inflammatory and analgesic properties of Woodfordia fruticosa Kurz flowers. Iran J Pharm Res IJPR. 2012;11:851. [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal M., Ghosh M., Nagori B., Sasmal D. Analgesic and anti-inflammatory studies of cyclopeptide alkaloid fraction of leaves of Ziziyphus nummularia. Saudi J Biol Sci. 2013;20:365–371. doi: 10.1016/j.sjbs.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maran S., Subramanium P. Evaluation of anti-inflammatory and analgesic activities of methanolic leaf extract of the endangered tree species, Hildegardia populifolia (Roxb.) Schott and Endl. Int J Green Pharm. 2015;9:125–130. [Google Scholar]
- 39.Samy R.P., Gopalakrishnakone P., Houghton P., Thwin M.M., Ignacimuthu S. Effect of aqueous extract of Tragia involucrata Linn. on acute and subacute inflammation. Phytother Res. 2006;20:310–312. doi: 10.1002/ptr.1845. [DOI] [PubMed] [Google Scholar]
- 40.Hosseinzadeh H., Haddadkhodaparast M.H., Arash A.R. Antinociceptive, antiinflammatory and acute toxicity effects of Salvia lerifolia Benth. Seed extract in mice and rats. Phytother Res. 2003;17:422–425. doi: 10.1002/ptr.1154. [DOI] [PubMed] [Google Scholar]
- 41.Boonyarikpunchai W., Sukrong S., Towiwat P. Antinociceptive and anti-inflammatory effects of rosmarinic acid isolated from Thunbergia laurifolia Lindl. Pharmacol Biochem Behav. 2014;124:67–73. doi: 10.1016/j.pbb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Park E.K., Rhee H.I., Jung H.S. Antiinflammatory effects of a combined herbal preparation (RAH13) of Phellodendron amurense and Coptis chinensis in animal models of inflammation. Phytother Res. 2007;21:746–750. doi: 10.1002/ptr.2156. [DOI] [PubMed] [Google Scholar]
- 43.Bánvölgyi Á., Pálinkás L., Berki T. Evidence for a novel protective role of the vanilloid TRPV1 receptor in a cutaneous contact allergic dermatitis model. J Neuroimmunol. 2005;169:86–96. doi: 10.1016/j.jneuroim.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Rauh L.K., Horinouchi C.D., Loddi A.M. Effectiveness of Vernonia scorpioides ethanolic extract against skin inflammatory processes. J Ethnopharmacol. 2011;138:390–397. doi: 10.1016/j.jep.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Bas E., Recio M.C., Máñez S. New insight into the inhibition of the inflammatory response to experimental delayed-type hypersensitivity reactions in mice by scropolioside A. Eur J Pharmacol. 2007;555:199–210. doi: 10.1016/j.ejphar.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Bas E., Recio M.C., Abdallah M. Inhibition of the pro-inflammatory mediators' production and anti-inflammatory effect of the iridoid scrovalentinoside. J Ethnopharmacol. 2007;110:419–427. doi: 10.1016/j.jep.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 47.Subba Rao G., Prasanna S., Sashi Kumar V., Mallavarapu G.R. Bartogenic acid, a new triterpene acid from Barringtonia speciosa. Phytochemistry. 1981;20:333–334. [Google Scholar]
- 48.Arramon G., Saucier C., Colombani D., Glories Y. Identification of triterpene saponins in Quercus robur L. and Q. petraea Liebl. heartwood by LC-ESI/MS and NMR. Phytochem Anal. 2002;13:305–310. doi: 10.1002/pca.658. [DOI] [PubMed] [Google Scholar]
- 49.Nantel F., Denis D., Gordon R. Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Br J Pharmacol. 1999;128:853–859. doi: 10.1038/sj.bjp.0702866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meira N.A., Klein L.C., Jr., Rocha L.W. Anti-inflammatory and anti-hypersensitive effects of the crude extract, fractions and triterpenes obtained from Chrysophyllum cainito leaves in mice. J Ethnopharmacol. 2014;151:975–983. doi: 10.1016/j.jep.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Ziyan L., Yongmei Z., Nan Z., Ning T., Baolin L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta medica. 2007;73:221–226. doi: 10.1055/s-2007-967122. [DOI] [PubMed] [Google Scholar]
- 52.Yin Z.Z., Jin H.L., Yin X.Z., Li T.Z., Quan J.S., Jin Z.N. Effect of Boschniakia rossica on expression of GST-P, p53 and p21 ras proteins in early stage of chemical hepatocarcinogenesis and its anti-inflammatory activities in rats. World J Gastroenterol. 2000;6:812–818. doi: 10.3748/wjg.v6.i6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vinegar R., Schreiber W., Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 1969;166:96–103. [PubMed] [Google Scholar]
- 54.Wang S.-J., Tong Y., Lu S. Anti-inflammatory activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Planta Med. 2010;76:1492–1496. doi: 10.1055/s-0030-1249780. [DOI] [PubMed] [Google Scholar]
- 55.Panthong A., Kanjanapothi D., Taesotikul T., Wongcome T., Reutrakul V. Anti-inflammatory and antipyretic properties of Clerodendrum petasites S. Moore. J Ethnopharmacol. 2003;85:151–156. doi: 10.1016/s0378-8741(02)00368-9. [DOI] [PubMed] [Google Scholar]
- 56.Sengar N., Joshi A., Prasad S.K., Hemalatha S. Anti-inflammatory, analgesic and anti-pyretic activities of standardized root extract of Jasminum sambac. J Ethnopharmacol. 2015;160:140–148. doi: 10.1016/j.jep.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 57.Vasudevan M., Gunnam K.K., Parle M. Antinociceptive and anti-inflammatory effects of Thespesia populnea bark extract. J Ethnopharmacol. 2007;109:264–270. doi: 10.1016/j.jep.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 58.Singh B., Bani S., Gupta D., Chandan B., Kaul A. Anti-inflammatory activity of ‘TAF’an active fraction from the plant Barleria prionitis Linn. J Ethnopharmacol. 2003;85:187–193. doi: 10.1016/s0378-8741(02)00358-6. [DOI] [PubMed] [Google Scholar]
- 59.Ghosh S., Saha K., Dasgupta S., Gomes A. In vitro and in vivo anti-arthritic and anti-inflammatory activity of Bungarus fasciatus venom. J Toxins. 2015;2:5. [Google Scholar]
- 60.Damre A., Damre A., Saraf M. Evaluation of sesquiterpene lactone fraction of Saussurea lappa on transudative, exudative and proliferative phases of inflammation. Phytother Res. 2003;17:722–725. doi: 10.1002/ptr.1152. [DOI] [PubMed] [Google Scholar]
- 61.Gutierrez R.M.P. Evaluation of anti-inflammatory activity of the bark of Eysenhardtia polystachya in experimental animal models. Afr J Pharm Pharmacol. 2015;9:230–236. [Google Scholar]
- 62.Chattopadhyay D., Arunachalam G., Mandal A.B., Sur T.K., Mandal S.C., Bhattacharya S. Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J Ethnopharmacol. 2002;82:229–237. doi: 10.1016/s0378-8741(02)00165-4. [DOI] [PubMed] [Google Scholar]
- 63.Swingle K., Shideman F. Phases of the inflammatory response to subcutaneous implantation of a cotton pellet and their modification by certain antiinflammatory agents. J Pharmacol Exp Ther. 1972;183:226–234. [PubMed] [Google Scholar]
- 64.Fujii Y., Takeuchi H., Tanaka K., Sakuma S., Ohkubo Y., Mutoh S. Effects of FK506 (tacrolimus hydrate) on chronic oxazolone-induced dermatitis in rats. Eur J Pharmacol. 2002;456:115–121. doi: 10.1016/s0014-2999(02)02554-2. [DOI] [PubMed] [Google Scholar]