Abstract
Yokukansan, a traditional Japanese herbal medicine, has been considered to be a novel alternative treatment for several neurological diseases such as neurodegenerative disorders, as well as neurosis, insomnia, and behavioral and psychological symptoms in Alzheimer's disease. Moreover, it has been shown that yokukansan has antidepressant-like and pain-relieving effects in animal models. Recently, several studies have shown that yokukansan has a neuroprotective effect. In this study, we focused on whether or no yokukansan influences cell proliferation related to cell-cycle progression by using B65 neuroblastoma cells derived from monoaminergic neurons. Under treatment with yokukansan, the proliferation rate of B65 neuroblastoma cells significantly increased in a dose-dependent manner. In particular, a proliferative effect was observed after treatment with yokukansan for 48 h and 72 h. Moreover, among seven medicinal herbs that comprise yokukansan, both Bupleuri Radix and Glycyrrhize Radix also enhanced the proliferation of B65 neuroblastoma cells. We assessed the effect of yokukansan on p44/42 mitogen-activated protein kinase (MAPK) phosphorylation in B65 neuroblastoma cells, and found that yokukansan increased p44/42 MAPK phosphorylation after treatment for 48 h. In contrast, neither Bupleuri Radix nor Glycyrrhize Radix altered the level of p44/42 MAPK phosphorylation, although they did increase cell proliferation. Our findings suggest that yokukansan has a cell-proliferative due to both Bupleuri Radix and Glycyrrhize Radix, and this is unrelated to the p44/42 MAPK signaling cascade.
Keywords: Yokukansan, Cell proliferation, B65 neuroblastoma, Bupleuri Radix, Glycyrrhize Radix
Abbreviations: BPSD, Behavioral and psychological symptoms of dementia; ALR, Atractylodis lanceae Rhizoma; PR, Poria; CR, Cnidii Rhizoma; UR, Uncariae Uncis cum Ramulus; AR, Angelicae Radix; BR, Bupleuri Radix; GR, Glycyrrhize Radix; MAPK, Mitogen-activated protein kinase; 5-HT, Serotonin; SSRI, Selective serotonin reuptake inhibitor
Graphical abstract
1. Introduction
Yokukansan is a traditional Japanese herbal medicine. It originated from the traditional Chinese herbal medicine Yi-Gan San, which was modified to create a unique Japanese herbal medicine called “Kampo”. Yokukansan is composed of seven medicinal herbs: Atractylodis lanceae Rhizoma (ALR), Poria (PR), Cnidii Rhizoma (CR), Uncariae Uncis Cum Ramulus (UR), Angelicae Radix (AR), Bupleuri Radix (BR) and Glycyrrhize Radix (GR). Since ancient times, this herbal medicine has been used to treat patients with symptoms such as nervousness, short temper, irritability and sleeplessness in infants and young children.
Recently, clinical evidence regarding the effectiveness of yokukansan has been reported. In particular, it has been demonstrated that yokukansan can improve dementia and several psychiatric conditions.1 Several clinical studies have shown that the administration of yokukansan could ameliorate the symptoms of behavioral and psychological symptoms of dementia (BPSD) in Alzheimer's disease.2, 3 Moreover, yokukansan could also improve neuropsychiatric symptoms associated with Parkinson's diseases, including hallucinations, anxiety and apathy without severe adverse events or worsening of Parkinsonism.4 According to the clinical studies of yokukansan mentioned above, yokukansan may have multiple components that are effective at treating various central nervous system diseases. As a mechanism of the ameliorative effect against various central nervous system diseases, it is has been considered that yokukansan has neuroprotective effects. Several studies have demonstrated that yokukansan has a neuroprotective effect against glutamate-induced excitotoxicity in cultured cells,5, 6 and this neuroprotective effect involves the modification of gene expression of cystine/glutamate antiporter system Xc−.7 We also previously reported that yokukansan showed a neuroprotective effect against cytotoxicity induced by corticosterone on mouse hippocampal neurons.8 In contrast, it has been demonstrated, albeit in an animal experiment, that yokukansan had antidepressive and antinociceptive effects on behavioral despair and acetic acid-induced writhing in mice.9 Another animal behavioral experiment revealed that yokukansan showed anxiolytic effects against experienced aversive stress in rat.10 Based on this evidence, it is reasonable to consider that yokukansan might contain some components that can improve nervousness, depression and anxiety disorder. Actually, several studies have stated that yokukansan contains components that act as agonists or antagonists against several receptors.11, 12 It has been reported that the anxiolytic or antidepressant effects of yokukansan in rats that have been exposed to experienced-aversive stress might be caused via serotonin 5-HT1A receptor agonism.10, 13 Therefore since 5-HT1A agonist has an antidepressant-like effect, the antidepressant-like effect induced by yokukansan may be due to stimulation of 5-HT1A receptor. Moreover, chronic stress decreased the number of neural stem cells in the subventricular zone, and an antidepressant, such as selective serotonin reuptake inhibitor (SSRI), attenuated the corticosteroid- or chronic stress-induced decrease in the viability and proliferation of neuroblastoma or neural stem cells.14, 15 Furthermore, it has been suggested that the finding that 5-HT1A agonists increased cell proliferation in the adult central nervous system may be related to antidepressive mechanisms.16 These various findings suggest that some component(s) in yokukansan, which has an antidepressive effect, may enhance cell proliferation which is related to cell-cycle progression in neurogenesis or cell survival.
In this study, we investigated whether or not yokukansan has a cell-proliferative effect and the mechanism that underlies this effect by using B65 neuroblastoma cell derived from monoaminergic neurons.
2. Materials and methods
2.1. Materials
Yokukansan is composed of seven dried medicinal herbs: 4.0 g of ALR, 4.0 g of PC, 3.0 g of CR, 3.0 g of UR, 3.0 g of AR, 2.0 g of BR and 1.5 g of GR. These herbs are registered in the Pharmacopeia of Japan ver. 16. The powdered water extracts of yokukansan and each individual medicinal herb used in this study were manufactured according to previously reported,8, 17 and supplied by Tsumura & Co. (Tokyo, Japan). A three-dimensional high-performance liquid chromatography (3D-HPLC) profile of representative batches of yokukansan and each individual medicinal herb which were provided by Tsumura & Co. are shown in Fig. 1. All other materials for experiments, including reagents, drugs, antibodies and media were purchased from the companies indicated in each description.
Fig. 1.
Three-dimensional HPLC profiles of yokukansan, UR, BR, AR, CR, ALR, GR and PR. Each peak of yokukansan and each individual medicinal herb comprising yokukansan in the HPLC profile was identified by comparison of the retention times and UV spectra (200–400 nm) of chemically defined standard compounds.
2.2. Cell culture
B65 neuroblastoma (DS Pharma Biomedical, Osaka, Japan) is a rat ethyl nitrosourea-induced tumor cell line. Briefly, B65 neuroblastoma cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with a high glucose concentration (Wako, Osaka, Japan) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Biowest, Nuaillé, France), 100 u/mL penicillin and 100 μg/mL streptomycin (Wako, Osaka, Japan) in a humidified incubator under a 95%/5% mixture of air and CO2. Cells were generally passaged every 5 days.
2.3. Cell viability assay
The proliferation of B65 neuroblastoma cells was analyzed by a WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium] assay using a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). Once cells became confluent, they were plated into 96-well microplates at a density of 5 × 104/mL (5 × 103/well). To apply yokukansan or each individual medicinal herb to B65 neuroblastoma cells, they were first suspended in culture media. The solution was then centrifuged for 5 min at 15,000 × g to remove insoluble residue. To avoid denaturing the effective component, the solution of cells dissolved in culture media was prepared immediately before every experiment. After centrifugation, the supernatant was used in all experiments. At 24, 48 and 72 h of culture with yokukansan, the individual medicinal herbs or chemical compounds, the cells were incubated with WST-8 solution at 37 °C for 2 h. The spectrophotometric absorbance of WST-8-formazan produced by dehydrogenase activity in living cells was measured at a wavelength of 450 nm using a VersaMax (Molecular Devices, Tokyo, Japan). Statistical analysis was performed by using absolute absorbance values.
2.4. Western blotting
Protein samples were obtained from B65 neuroblastoma cells that had been exposed to various concentrations of yokukansan or individual medicinal herbs for 24, 48 and 72 h. For the preparation of protein extract from B65 neuroblastoma cells, the cells were washed with ice-cold homogenizing buffer (pH7.4; 250 mM sucrose, 2 mM EDTA 2Na, 10 mM EGTA and 20 mM Tris-HCl), and centrifuged to obtain a cell pellet. The cell pellet was treated with lysis buffer (homogenizing buffer containing 1% Triton X-100 and a protease inhibitor cocktail) and sonicated on ice. After the sample was centrifuged, the supernatant was collected as a total protein extract. For western blotting, protein extracts were placed on SDS-polyacrylamide gel, transferred to a polyvinylidene difluoride membrane (BioRad, Hercules, CA, U.S.A) and immunoblotted with anti-p44/42 mitogen-activated protein kinase (MAPK) polyclonal antibody (Cell Signaling Technology, Danvers, MA, U.S.A., 1:1000), anti-phospho-p44/42 MAPK polyclonal antibody (Cell Signaling Technology, Danvers, MA, U.S.A., 1:1000) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (Millipore, Billerica, MA, U.S.A., 1:5000). The blots were developed with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA, U.S.A., 1:20000) and visualized by chemiluminescence using an ECL prime Western Blotting Detection System (Amersham, Piscataway, NJ, USA) and Western BLoT Quant HRP Substrate (TaKaRa, Ootsu, Japan).
2.5. [3H]Serotonin uptake assay
Approximately 4 × 104 B65 neuroblastoma cells were divided equally into 24-well plates. [3H]Serotonin (5-HT) uptake assays were carried out 72 h after passaging of cells. In brief, the culture medium was removed and then cells were incubated for 15 min at 37 °C in the presence of 100 nM [3H]5-HT. The uptake of [3H]5-HT was stopped by washing the cells. After cells were lysed, the radioactivity of cell extracts was measured as the total [3H]5-HT uptake by cells. In contrast, the radioactivity of cell extracts that were obtained from cells incubated with 10 μM fluvoxamine was considered to be nonspecific [3H]5-HT uptake by cells. The net [3H]5-HT uptake by cells was calculated as the difference between total [3H]5-HT uptake and nonspecific [3H]5-HT uptake.
2.6. Statistics
The results are presented as the mean ± S.D. A statistical analysis was performed using the Dunnett multiple comparisons test. A p value < 0.05 was considered to be statistically significant.
3. Results
3.1. Serotonin uptake by B65 neuroblastoma cells
First, we investigated whether or not B65 neuroblastoma cells can uptake 5-HT, since they are considered to be monoaminergic as well as dopaminargic neurons. As shown in Fig. 2, a [3H]5-HT-uptake assay showed that B65 neuroblastoma cells can uptake 5-HT. The uptake of [3H]5-HT by cells, including via both 5-HT transporter and other mechanisms, was 96.17 ± 3.00 cpm/μg. In contrast, the uptake of [3H]5-HT by cells treated with the SSRI fluvoxamine was 21.07 ± 1.40 cpm/μg, which means that this amount of [3H]5-HT uptake did not occur through 5-HT transporter. The net uptake of [3H]5-HT was 71.03 ± 2.98 cpm/μg. This finding strongly supported the notion that B65 neuroblastoma cells have some of the same features as serotonergic neurons and it is reasonable to use these cells to examine antidepressive effects.
Fig. 2.
Serotonin uptake in B65 neuroblastoma cells. [3H]5-HT uptake assays were carried out 72 h after passaging of cells. [3H]5-HT was measured when cells were incubated for 15 min at 37 °C in the presence of 100 nM [3H]5-HT. Shaded column shows the raw uptake of [3H]5-HT by B65 neuroblastoma cells. On the other hand, open column indicates the net uptake of [3H]5-HT by B65 neuroblastoma cells, which was calculated by subtracting the uptake of [3H]5-HT cultured with 10 μM fluvoxamine from the raw uptake of [3H]5-HT. Columns show means ± S.D (n = 4).
3.2. Cell proliferative effect of yokukansan on B65 neuroblastoma cells
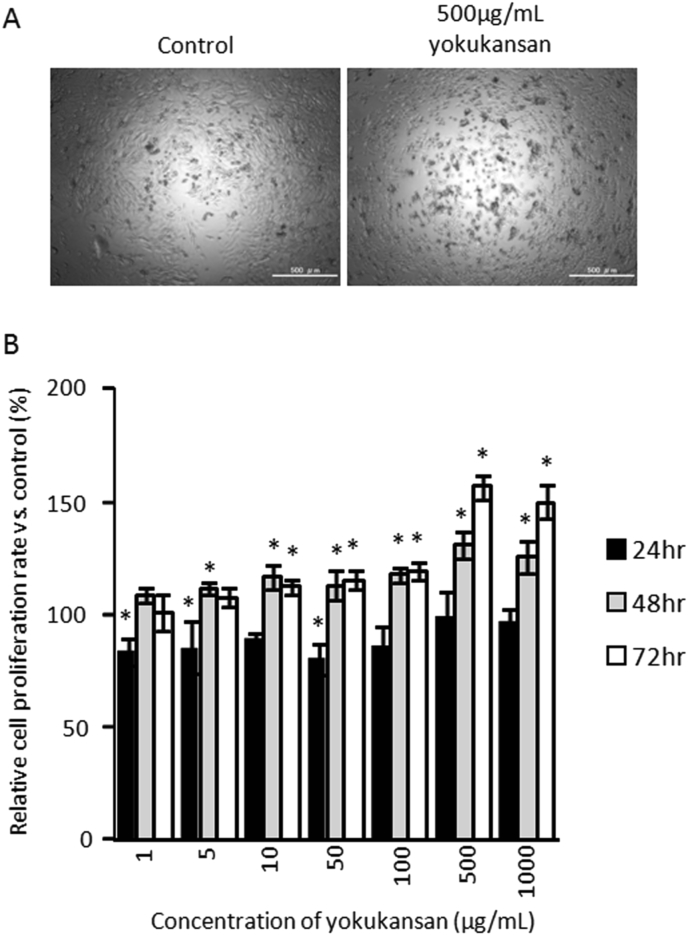
Since antidepressants, such as 5-HT1A agonist or SSRI, have been demonstrated to have cell proliferative effects on neural stem cells or neuroblastoma cells derived from monoaminergic neurons, we investigated whether or not yokukansan has a cell proliferative effect on B65 neuroblastoma cells. The cell proliferation rate was slightly but significantly decreased after treatment with more than 10 μg/mL of yokukansan for 24 h, compared with the control condition without yokukansan. However, after treatment with yokukansan for 48 h and 72 h, the cell proliferation rate significantly increased in a dose- and time-dependent manner (Fig. 3A and B).
Fig. 3.
Cell proliferative effect of yokukansan on B65 neuroblastoma cells. (A) Images of B65 neuroblastoma cells cultured with and without 500 μg/mL yokukansan. Pictures were taken after treatment with 500 μg/mL yokukansan for 48hr. The scale bars represent 500 μm. (B) WST-8 cell proliferation assay of B65 neuroblastoma cells cultured with or without various concentrations of yokukansan after treatment for 24, 48 and 72 h. The vertical axis shows the proliferation rate relative to that in the control without yokukansan. The histogram shows means ± S.D (n = 3). When the absolute absorbance value was statistically different from control without yokukansan, it was considered to be statistically significant (*; p < 0.05 vs. control absolute absorbance value).
3.3. Cell proliferative effects of extracts of the medicinal herbs that comprise yokukansan on B65 neuroblastoma cells
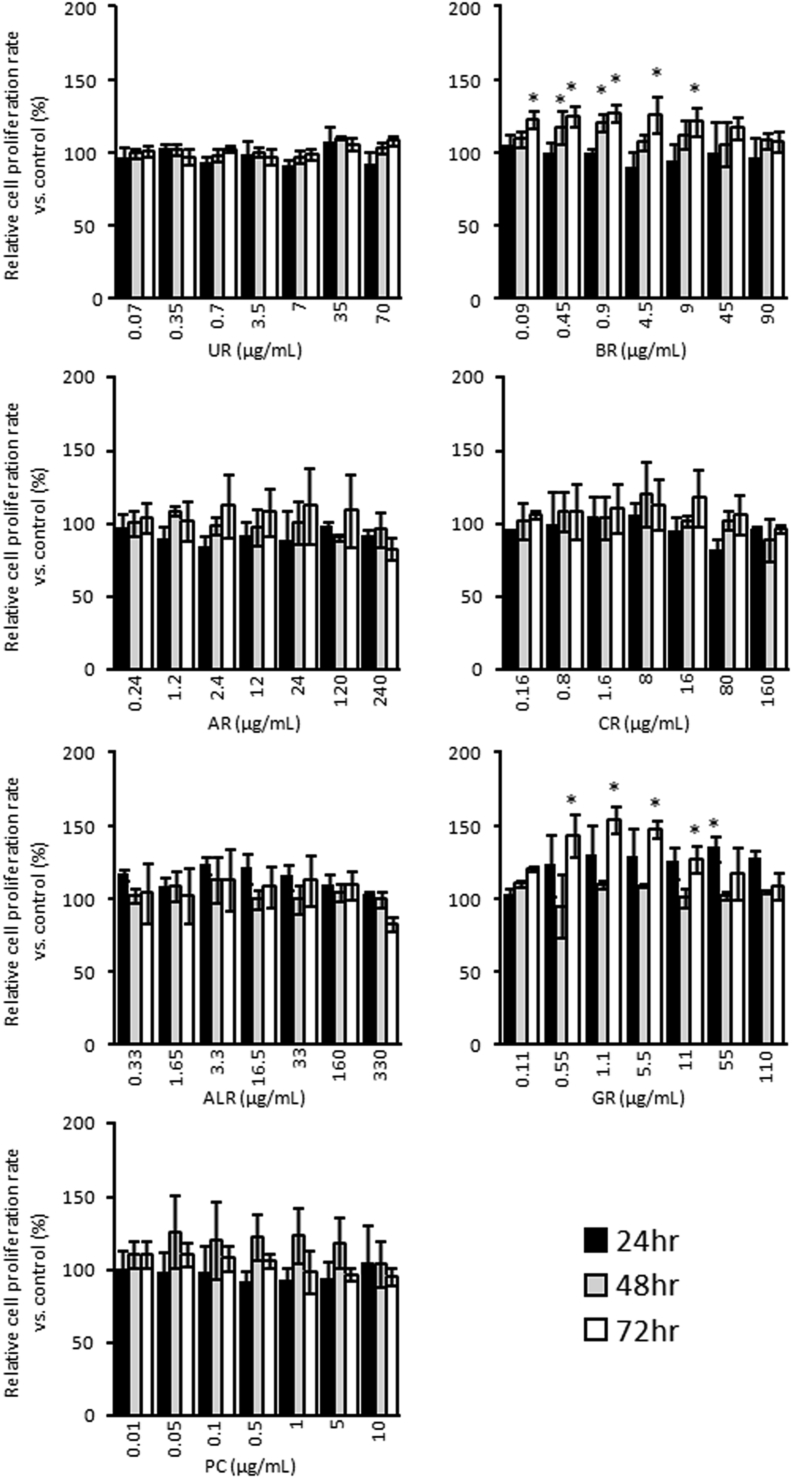
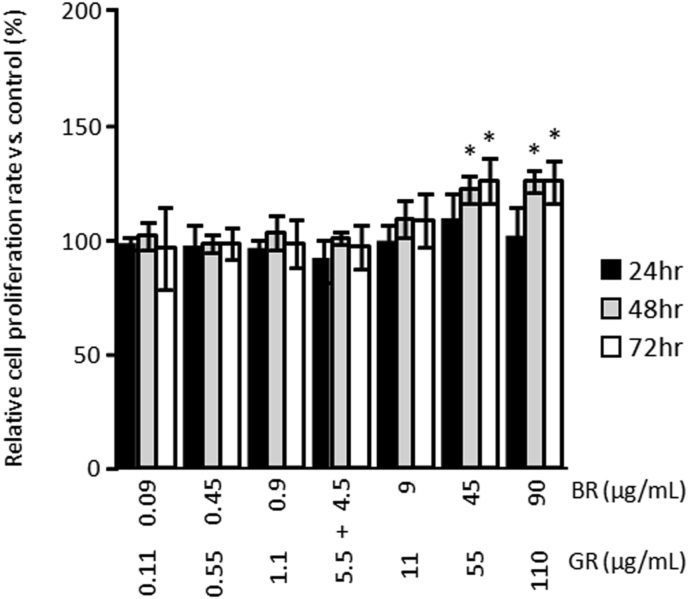
Since yokukansan was shown to have a cell proliferative effect on B65 neuroblastoma cells, we sought to identify which of its constituent medicinal herbs are responsible for this effect. In the present study, it is reasonable to consider that the component that produces the cell proliferative effect is water-soluble because it should dissolve in the culture medium. Therefore, we calculated the concentration of each medicinal herb extract from the composition of the powdered water extract from the crude drug component in yokukansan, and show these concentrations in the right-most column in Table 1. Fig. 4 shows the cell proliferative effect of each medicinal herb extract from the crude drugs that comprise yokukansan. The extracts of two of the seven constituent medicinal herbs (BR and GR) slightly, but significantly, enhanced cell proliferation. Under treatment with BR, cell proliferative effect was observed at relatively low concentrations, which were between 0.09 to 0.18 μg/mL of BR, after treatment for 48 h and 72 h. The relative cell proliferation rate of 0.09–9 μg/mL BR increased, and the relative cell proliferation rate of 0.9 μg/mL BR increased to 122.28 ± 5.70 % compared to that of the control after treatment for 72 h. In addition, GR had a cell proliferative effect at a mid-range concentration, and this effect was significant after treatment with GR for 72 h. In particular, the relative cell proliferation rate of 0.55–5.5 μg/mL GR increased, and the relative cell proliferation rate of 1.1 μg/mL GR increased to 154.01 ± 9.01 % compared to that in the control after treatment for 72 h (Fig. 4). On the other hand, neither UR, AR, CR ALR nor PC significantly increased cell proliferation (Fig. 4). We also investigated whether or not treatment with a combination of BR and GR increased the proliferation of B65 neuroblastoma cells. As shown in Fig. 5, similar to treatment with BR and GR individually, treatment with the combination of 45 μg/mL BR and 55 μg/mL GR for 48 h and 72 h increased the proliferation of B65 neuroblastoma cells to 122.16 ± 5.97 % and 125.32 ± 4.77 %, respectively (Fig. 5). In addition, treatment with the combination of 90 μg/mL BR and 110 μg/mL GR for 48 h and 72 h also increased the proliferation of B65 neuroblastoma cells to 126.22 ± 9.87 % and 125.99 ± 8.99 %, respectively (Fig. 5).
Table 1.
Composition rate of powdered water extract from crud medicinal herb component in yokukansan.
| Crude medicinal herb name | Raw weight of crud medicinal herb component (g) | Obtained powdered water extract (g) | Yield of powdered water extract (%) | Composition rate of crud medicinal herb component in yokukansan (g) | Composition rate of powdered water extact from crude medicinal herb component in yokukansan (mg/100 mg yokukansan) |
|---|---|---|---|---|---|
| Uncariae Uncis cum Ramulus (UR) | 476.5 | 41.8 | 8.8 | 3.0 | 66.0 (≈70) |
| Bupleuri Radix (BR) | 589.0 | 103.1 | 17.5 | 2.0 | 87.8 (≈90) |
| Angelicae Radix (AR) | 424.1 | 136.9 | 32.3 | 3.0 | 243.1 (≈240) |
| Cnidii Rhizoma (CR) | 502.2 | 103.6 | 20.6 | 3.0 | 155.4 (≈160) |
| Atractyloids lanceae Rhizoma (ALR) | 500.0 | 165.1 | 33.0 | 4.0 | 331.4 (≈330) |
| Glychrrhizae Radix (GR) | 500.0 | 141.3 | 28.3 | 1.5 | 106.3 (≈110) |
| Poria (PR) | 615.8 | 6.2 | 1.0 | 4.0 | 10.0 (≈10) |
Fig. 4.
Cell proliferative effects of extracts of the medicinal herbs comprising yokukansan on B65 neuroblastoma cells. WST-8 cell proliferation assay of B65 neuroblastoma cells cultured with various concentrations of UR, BR, AR, CR, ALR, GR and PC after treatment for 24, 48 and 72 h. The vertical axis shows the proliferation rate relative to that in the control without the test compounds. The histogram shows means ± S.D (n = 3). When the absolute absorbance value was statistically different from control without each medicinal herbs comprising yokukansan, it was considered to be statistically significant (*; p < 0.05 vs. control absolute absorbance value).
Fig. 5.
Cell proliferative effect of the combination of BR and GR on B65 neuroblastoma cells. WST-8 cell proliferation assay of B65 neuroblastoma cells cultured with or without various concentrations of the combination of BR and GR after treatment for 24, 48 and 72 h. The vertical axis shows the proliferation rate relative to that in the control without these compounds. The histogram shows means ± S.D (n = 3). When the absolute absorbance value was statistically different from control without BR and GR, it was considered to be statistically significant (*; p < 0.05 vs. control absolute absorbance value).
3.4. Effects of yokukansan, Bupleuri Radix and Glycyrrhize Radix on p44/42 mitogen-activated protein kinase phosphorylation
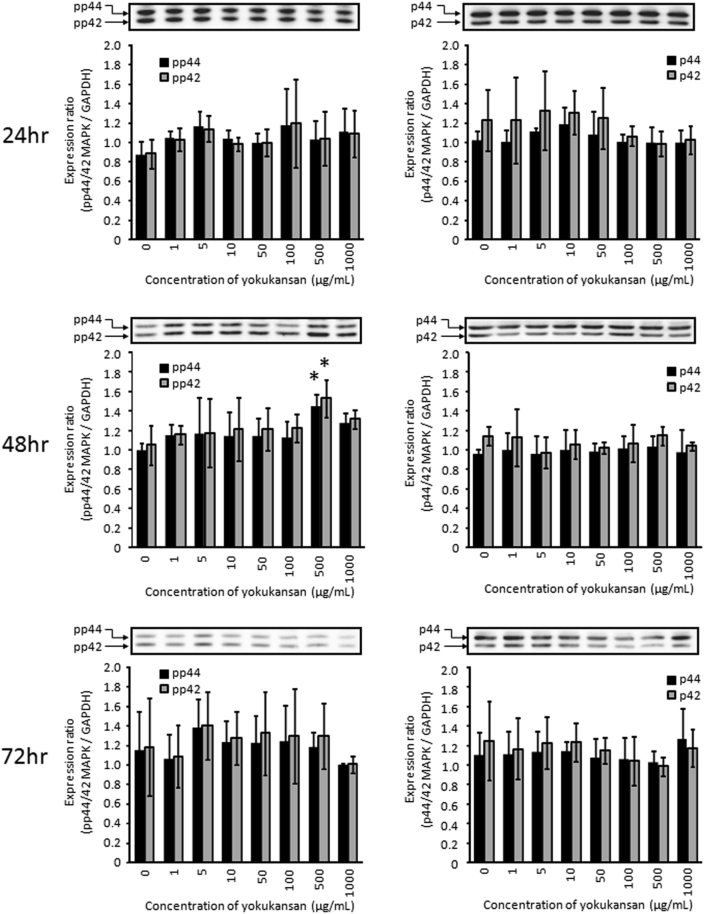
The phosphorylation of p44/42 MAKP plays a role in cell survival, proliferation and differentiation, and this phosphorylation usually occurs acutely, within approximately 30 min. First, the acute change in p44/42 MAPK phosphorylation was investigated by western blotting analysis of B65 neuroblastoma cells. Under treatment with various concentrations of yokukansan for 30 min, there were no changes in the expression levels of phosphorylated-p44/42 MAPK. The expression levels of p44/42 MAPK also did not change under treatment with various concentrations of yokukansan (Fig. 6). Sustainable p44/42 MAPK phosphorylation was also investigated, and we found that the expression level of phosphorylated-p44/42 MAPK was significantly increased by treatment with 500 μg/mL yokukansan for 48 h (Fig. 7). In contrast, there were no significant changes in p44/42 MAPK phosphorylation under various other culture conditions, although the expression levels of phosphorylated-p44/42 MAPK tended to increase (Fig. 7). The expression levels of p44/42 MAPK also did not change under treatment with various concentrations of yokukansan.
Fig. 6.
Effect of yokukansan on the acute expression of phosphorylated and total p44/42 MAPK in B65 neuroblastoma cells. The protein expression of phosphorylated and total p44/42 MAPK by B65 neuroblastoma cells cultured with or without various concentrations of yokukansan after treatment for 30 min. The relative expressions of both phosphorylated and total p44/42 MAPK are shown as the relative ratio on the basis of the expression of GAPDH. The histogram shows means ± S.D (n = 3, *; p < 0.05 vs. control).
Fig. 7.
Effect of yokukansan on the sustainable expression of phosphorylated and total p44/42 MAPK in B65 neuroblastoma cells. The protein expression of phosphorylated and total p44/42 MAPK by B65 neuroblastoma cells cultured with or without various concentrations of yokukansan after treatment for 24, 48 and 72 h. The relative expressions of both phosphorylated and total p44/42 MAPK are shown as the relative ratio on the basis of the expression of GAPDH. The histogram shows means ± S.D (n = 5–6, *; p < 0.05 vs. control).
Moreover, to clarify whether the phosphorylation of p44/42 MAPK under treatment with yokukansan results from some components of BR or GR, western blotting analysis was performed under the same condition as for yokukansan treatment. Unexpectedly, neither BR nor GR affected the phosphorylation of p44/42 MAPK under various culture conditions, although both BR and GR, like yokukansan, had a cell proliferative effect (Fig. 8, Fig. 9).
Fig. 8.
Effect of BR on the sustainable expression of phosphorylated and total p44/42 MAPK in B65 neuroblastoma cells. The protein expression of phosphorylated and total p44/42 MAPK by B65 neuroblastoma cells cultured with or without various concentrations of BR after treatment for 24, 48 and 72 h. The relative expressions of both phosphorylated and total p44/42 MAPK are shown as the relative ratio on the basis of the expression of GAPDH. The histogram shows means ± S.D (n = 3–6, *; p < 0.05 vs. control).
Fig. 9.
Effect of GR on the sustainable expression of phosphorylated and total p44/42 MAPK in B65 neuroblastoma cells. The protein expression of phosphorylated and total p44/42 MAPK by B65 neuroblastoma cells cultured with or without various concentrations of GR after treatment for 24, 48 and 72 h. The relative expressions of both phosphorylated and total p44/42 MAPK are shown as the relative ratio on the basis of the expression of GAPDH. The histogram shows means ± S.D (n = 5–9, *; p < 0.05 vs. control).
4. Discussion
Based on various clinical studies which showed that yokukansan is effective in treating some neurological diseases, such as BPSD,1 several studies have demonstrated that yokukansan possesses a cell proliferative effect, cell protective effect or neurotrophic effect in various cell lines derived from neurons or neurons in primarily culture.8, 18 In animal studies, it has been reported that treatment with yokukansan could ameliorate anxiety-like behavior and alleviate emotional abnormality in mice.13, 19 Recently, several studies have indicated that depression is caused by an adult neurogenesis disturbance in the central nervous system. Actually, it has been demonstrated that adult neurogenesis in the hippocampus is disturbed by stress stimuli or glucocorticoid which is known to be released under stressful conditions,20 and this disturbance is restored by treatment with an antidepressant.21 In addition, yokukansan has the potential to increase hippocampal neurogenesis associated with the suppression of activated microglia.22 Furthermore, several studies related to pharmacokinetics of yokukansan have demonstrated that several compounds contained to yokukansan migrate to both plasma and brain after oral administration in in vivo experiments.23, 24 According to these studies, it is reasonable to consider that yokukansan and its compounds would distribute to the brain by oral administration and be effective for the treatment of depression or anxiety disorder and the mechanism may be similar to those of antidepressants. However, most in vitro studies have been insufficient to explain the effect of yokukansan on neuronal function because the neuronal cells used in those studies either were unknown or were neuroblastoma cells derived from dopaminergic, but not serotonergic, neurons. In this respect, B65 neuroblastoma are advantageous because they can uptake 5-HT, as shown to Fig. 2, which means that B65 neuroblastoma cells have the same features as serotonergic neurons.
First, we investigated whether yokukansan, like antidepressants, has a cell proliferative effect on B65 neuroblastoma cells. When B65 neuroblastoma cells were treated with yokukansan, cell proliferation increased in a dose-dependent manner. Since antidepressants recovered the proliferation of SH-SY5Y neuroblastoma cells that had been suppressed by treatment with dexamethasone,14 the enhancement of the proliferative effect of yokukansan on B65 neuroblastoma cells may indicate that the action mechanism of yokukansan resembles that of antidepressants. Moreover, antidepressants propagated the expression of brain-derived neurotrophic factor mRNA in a time-dependent manner.25 These findings suggest that yokukansan may increase neurogenesis and potentiate the production of neurotrophic factor from neurons.
Next, we also considered that yokukansan may affect the phosphorylation of p44/42 MAPK because imipramine attenuated the decrease in p44/42 MAPK phosphorylation induced by dexamethasone.14 Moreover, the fact that the phosphorylation of p44/42 MAPK is important for cell-cycle progression, such as cell proliferation, survival and neuronal differentiation, should let us investigate the expression level of phosphorylated-p44/42 MAPK.26 When we investigated the effect of yokukansan on expression levels of phosphorylated-p44/42 MAPK in B65 neuroblastoma cells, the transient elevation of phosphorylated-p44/42 MAPK expression was observed after treatment with yokukansan for 48 h. This result indicated that yokukansan may affect the cell signaling cascade in a similar manner as antidepressants.
Furthermore, we tried to identify which medicinal herb(s) contained the component with the cell proliferative effect and p44/42 MAPK-phosphorylated effect on B65 neuroblastoma cells. BR and GR each significantly increased the proliferation of B65 neuroblastoma cells. This result was unexpected because some reports have stated that both BR and GR contain anti-proliferative compounds.27, 28 However, another study demonstrated that BR has a cell proliferative effect.29 Several kampo prescriptions such as hochuekkito or shosaikoto have not been reported that they have the effect to enhance cell proliferation which is related to cell-cycle progression although both of them also contains BR and GR like yokukansan. Considering that kampo prescription composes various medicial herbs, some of components contained to hochuekkito or shosaikoto may mask the effect of BR and GR to enhance cell proliferation.
These differences may be due to the cell types or the method used to prepare the medicinal herb extract. Furthermore, we explored whether BR or GR, like yokukansan, also increased the expression level of phosphorylated-p44/42 MAPK. Surprisingly, neither BR nor GR affected the expression level of phosphorylated-p44/42 MAPK. These findings indicate that the cell proliferative effect of yokukansan does not correlate with the phosphorylation of p44/42 MAPK. It is possible that multiple components of yokukansan which have biological activity affect cell-cycle progression, either with or without interaction with each other.
5. Conclusion
Yokukansan and its medicinal herb extracts, BR and GR, influenced the proliferation of B65 neuroblastoma cells. Moreover, yokukansan transiently enhanced the expression level of phosphorylated-p44/42 MAPK. These findings suggest that BR and GR may be main component to enhance cell viability and act as an activator, like neurotrophic factor, to improve neuronal function under malfunction of the central nervous system, such as in depression or anxiety disorder. Further studies will be needed to clarify the molecular mechanism by which yokukansan has an antidepressive effect.
Funding
This work was supported in part by grants from Japan Society for the Promotion of Science, (Grant-in-Aid for Scientific Research (C), 26460703, 2014) and Japan Society for the Promotion of Science, (Grant-in-Aid for Young Scientists (B), 15K18878, 2015).
Conflict of interest statement
None to declare.
Acknowledgments
We thank Yoko Hiroki, Megumi Kuriya and Aya Kobe for their help with the experiments, and the members of the Department of Pharmacotherapeutics, School of Pharmacy, International University of Health and Welfare, for their helpful discussions throughout this study.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Okamoto H., Iyo M., Ueda K., Han C., Hirasaki Y., Namiki T. Yokukan-san: a review of the evidence for use of this Kampo herbal formula in dementia and psychiatric conditions. Neuropsychiatr Dis Treat. 2014;10:1727–1742. doi: 10.2147/NDT.S65257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monji A., Takita M., Samejima T. Effect of yokukansan on the behavioral and psychological symptoms of dementia in elderly patients with Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:308–311. doi: 10.1016/j.pnpbp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi Y., Ishida Y., Inoue T. Treatment of behavioral and psychological symptoms of Alzheimer-type dementia with yokukansan in clinical practice. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:541–545. doi: 10.1016/j.pnpbp.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Hatano T., Hattori N., Kawanabe T., Group, Y. P. s. D. S An exploratory study of the efficacy and safety of yokukansan for neuropsychiatric symptoms in patients with Parkinson's disease. J Neural Transm. 2014;121:275–281. doi: 10.1007/s00702-013-1105-y. [DOI] [PubMed] [Google Scholar]
- 5.Kawakami Z., Kanno H., Ueki T. Neuroprotective effects of yokukansan, a traditional Japanese medicine, on glutamate-mediated excitotoxicity in cultured cells. Neuroscience. 2009;159:1397–1407. doi: 10.1016/j.neuroscience.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami Z., Kanno H., Ikarashi Y., Kase Y. Yokukansan, a kampo medicine, protects against glutamate cytotoxicity due to oxidative stress in PC12 cells. J Ethnopharmacol. 2011;134:74–81. doi: 10.1016/j.jep.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 7.Kanno H., Kawakami Z., Mizoguchi K., Ikarashi Y., Kase Y. Yokukansan, a kampo medicine, protects PC12 cells from glutamate-induced death by augmenting gene expression of cystine/glutamate antiporter system Xc−. PLoS One. 2014;9:e116275. doi: 10.1371/journal.pone.0116275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakatani Y., Tsuji M., Amano T. Neuroprotective effect of yokukansan against cytotoxicity induced by corticosterone on mouse hippocampal neurons. Phytomedicine. 2014;21:1458–1465. doi: 10.1016/j.phymed.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Koshikawa N., Imai T., Takahashi I., Yamauchi M., Sawada S., Kansaku A. Effects of Hochu-ekki-to, Yoku-kan-san and Saiko-ka-ryukotsu-borei-to on behavioral despair and acetic acid-induced writhing in mice. Methods Find Exp Clin Pharmacol. 1998;20:47–51. doi: 10.1358/mf.1998.20.1.485631. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi T., Tsujimatsu A., Kumamoto H. Anxiolytic effects of yokukansan, a traditional Japanese medicine, via serotonin 5-HT1A receptors on anxiety-related behaviors in rats experienced aversive stress. J Ethnopharmacol. 2012;143:533–539. doi: 10.1016/j.jep.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Nishi M., Noriko H.H., Kawata M. Intranuclear dynamics of corticosteroid receptors and effects of proteasomal activity in cultured hippocampal neural cells. Neurosci Lett. 2011;494:65–69. doi: 10.1016/j.neulet.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami Z., Ikarashi Y., Kase Y. Isoliquiritigenin is a novel NMDA receptor antagonist in kampo medicine yokukansan. Cell Mol Neurobiol. 2011;31:1203–1212. doi: 10.1007/s10571-011-9722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuji M., Takeuchi T., Miyagawa K. Yokukansan, a traditional Japanese herbal medicine, alleviates the emotional abnormality induced by maladaptation to stress in mice. Phytomedicine. 2014;21:363–371. doi: 10.1016/j.phymed.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Leskiewicz M., Jantas D., Regulska M. Antidepressants attenuate the dexamethasone-induced decrease in viability and proliferation of human neuroblastoma SH-SY5Y cells: a involvement of extracellular regulated kinase (ERK1/2) Neurochem Int. 2013;63:354–362. doi: 10.1016/j.neuint.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Hitoshi S., Maruta N., Higashi M., Kumar A., Kato N., Ikenaka K. Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress. J Neurosci Res. 2007;85:3574–3585. doi: 10.1002/jnr.21455. [DOI] [PubMed] [Google Scholar]
- 16.Arnold S.A., Hagg T. Serotonin 1A receptor agonist increases species- and region-selective adult CNS proliferation, but not through CNTF. Neuropharmacology. 2012;63:1238–1247. doi: 10.1016/j.neuropharm.2012.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata K., Yokoyama E., Yamazaki T. Effects of yokukansan on behavioral and psychological symptoms of vascular dementia: an open-label trial. Phytomedicine. 2012;19:524–528. doi: 10.1016/j.phymed.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa Y., Watanabe S., Okuyama S., Nakajima M. Neurotrophic effect of citrus auraptene: neuritogenic activity in PC12 cells. Int J Mol Sci. 2012;13:5338–5347. doi: 10.3390/ijms13055338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogami A., Sakata Y., Uchida N. Effects of yokukansan on anxiety-like behavior in a rat model of cerebrovascular dementia. J Nat Med. 2011;65:275–281. doi: 10.1007/s11418-010-0487-5. [DOI] [PubMed] [Google Scholar]
- 20.Cameron H.A., Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 21.Qiu G., Helmeste D.M., Samaranayake A.N. Modulation of the suppressive effect of corticosterone on adult rat hippocampal cell proliferation by paroxetine. Neurosci Bull. 2007;23:131–136. doi: 10.1007/s12264-007-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuya M., Miyaoka T., Tsumori T. Yokukansan promotes hippocampal neurogenesis associated with the suppression of activated microglia in Gunn rat. J Neuroinflammation. 2013;10:145. doi: 10.1186/1742-2094-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kushida H., Fukutake M., Tabuchi M. Simultaneous quantitative analyses of indole and oxindole alkaloids of Uncaria Hook in rat plasma and brain after oral administration of the traditional Japanese medicine Yokukansan using high-performance liquid chromatography with tandem mass spectrometry. Biomed Chromatogr. 2013;27:1647–1656. doi: 10.1002/bmc.2974. [DOI] [PubMed] [Google Scholar]
- 24.Kitagawa H., Munekage M., Ichikawa K. Pharmacokinetics of active components of Yokukansan, a traditional japanese herbal medicine after a single oral administration to healthy japanese volunteers: a cross-over, randomized study. PLoS One. 2015;10:e0131165. doi: 10.1371/journal.pone.0131165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnici L., Tiraboschi E., Tardito D., Musazzi L., Racagni G., Popoli M. Time-dependent biphasic modulation of human BDNF by antidepressants in neuroblastoma cells. BMC Neurosci. 2008;9:61. doi: 10.1186/1471-2202-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohno M., Pouyssegur J. Pharmacological inhibitors of the ERK signaling pathway: application as anticancer drugs. Prog Cell Cycle Res. 2003;5:219–224. [PubMed] [Google Scholar]
- 27.Hsu Y.L., Kuo P.L., Weng T.C., Yen M.H., Chiang L.C., Lin C.C. The antiproliferative activity of saponin-enriched fraction from Bupleurum Kaoi is through Fas-dependent apoptotic pathway in human non-small cell lung cancer A549 cells. Biol Pharm Bull. 2004;27:1112–1115. doi: 10.1248/bpb.27.1112. [DOI] [PubMed] [Google Scholar]
- 28.Li S., Zhu J.H., Cao L.P. Growth inhibitory in vitro effects of glycyrrhizic acid in U251 glioblastoma cell line. Neurol Sci. 2014;35:1115–1120. doi: 10.1007/s10072-014-1661-4. [DOI] [PubMed] [Google Scholar]
- 29.Seo M.K., Cho H.Y., Lee C.H. Antioxidant and proliferative activities of Bupleuri Radix extract against serum deprivation in SH-SY5Y cells. Psychiatry Investig. 2013;10:81–88. doi: 10.4306/pi.2013.10.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]