Abstract
Skin cancer is extremely common, and melanoma causes about 80% of skin cancer deaths. In fact, melanoma kills over 50 thousand people around the world each year, and these numbers are rising. Clearly, standard treatments are not effectively treating melanoma, and alternative therapies are needed to address this problem. Hibiscus tea has been noted to have medicinal properties, including anticancer effects. Extracts from Hibiscus have been shown to inhibit the growth of a variety of cancer cells. In particular, recent studies found that polyphenols extracted from Hibiscus sabdariffa by organic solvents can inhibit melanoma cell growth. However, effects of aqueous extracts from Hibiscus rosa-sinesis flowers, which are commonly used to make traditional medicinal beverages, have not been examined on melanoma cells. Here, we report that aqueous H. rosa-sinesis flower extract contains compounds that inhibit melanoma cell growth in a dose dependent manner at concentrations that did not affect the growth of nontransformed cells. In addition, these extracts contain low molecular weight growth inhibitory compounds below 3 kD in size that combine with larger compounds to more effectively inhibit melanoma cell growth. Future work should identify these compounds, and evaluate their potential to prevent and treat melanoma and other cancers.
Keywords: Melanoma, Hibiscus, Tea, Cancer, Cell growth
Graphical abstract
1. Introduction
Skin cancer is the most common form of cancer and its incidence is rising. Melanoma causes about 80% of all skin cancer deaths, and is notoriously resistant to current treatments. Less than 25% of metastatic melanomas respond to existing therapies.1, 2 In addition, melanoma often reoccurs after initial response, and these cases can be extremely aggressive. Patients with advanced melanoma have a median survival of less than 1 year, and a 3-year survival rate of only 10–15%.3
Unfortunately, over 40 years of work and clinical trials with alkylating agents (e.g. DTIC), taxanes (e.g. taxol), signaling inhibitors (e.g. tamoxifen), and cytokines (e.g. interluekins and interferons) has not significantly increased melanoma survival rates.3, 4 In fact, melanoma kills over 50,000 people around the world each year.5 Alternative methods are clearly needed to prevent and treat melanoma.
Hibiscus rosa-sinensis is a flowering plant native to tropical Asia.6 Hibiscus is commonly consumed in teas made from its flowers, leaves, and roots. In addition to casual consumption, Hibiscus is also used as an herbal medicine to treat hypertension, cholesterol production, and cancer progression.7
Reports indicate that hibiscus extracts can inhibit the growth of cancer cell types including mammary carcinoma,8 leukemia,9 and melanoma.10 For example, recent studies found that Hibiscus polyphenols inhibit melanoma cell growth and viability.10 However, while H. rosa-sinensis flowers are commonly used to make medicinal tea,11, 12 these previous studies were performed with organic solvent extracts of leaves from different strains, namely Hibiscus sabdariffa.10
Here, we examined the effects of aqueous H. rosa-sinensis flower extract on melanoma cell growth. Results from these studies indicate that this extract contains components that inhibit melanoma cell growth. However, these data also suggest that more than one component is responsible for this effect, and these components act together to produce an optimal response. Thus, H. rosa-sinensis flowers may offer a source of products that can be used to prevent or treat melanoma, possibly in combination with other therapies.
2. Materials and methods
2.1. Hibiscus extract preparation
Dried H. rosa-sinensis flowers were incubated with 10 volumes (w/v) of boiling water for 20 min and cooled to room temperature. This 10% solution was then clarified by centrifugation, sterilized by filtration through 0.2 micron filters (Millipore), frozen, and lyophilized to dryness. This dried extract was suspended in water to a final concentration of 50 mg/ml.
2.2. Extract fractionation
To examine molecular weights of bioactive compounds, extract was fractionated as previously described.13 Briefly, components greater than 50 kD were concentrated over centrifugal membranes with a 50 kD nominal molecular weight pore size (EMD Millipore Amicon UFC5050). Filtrates were then concentrated over centrifugal membranes with a 3 kD nominal molecular weight pore size (EMD Millipore Amicon UFC5003) to concentrate components between 3 and 50 kD, and obtain material below 3 kD as filtrates. Size fractionation was verified by SDS-PAGE on 18% gels stained with SilverQuest dye (Invitrogen LC6070). Concentrated material, filtrate, and unfractionated extract were diluted in cell culture medium to achieve final concentrations equivalent to 4 mg/ml of original unfractionated extract.
2.3. Cell culture
B16, NIH3T3, and LA25 cells were maintained in DMEM (Hyclone SH30021) supplemented with 25 mM HEPES (Hyclone SH3027) and 10% FBS (Seradigm 1400-500) at 37 °C in 5% CO2 and 100% humidity as described.14, 15, 16, 17, 18 B16 and NIH3T3 cells were plated at 5000 cells/well in standard 24 well culture plates and allowed to adhere to plates for 24 h. Different concentrations of aqueous plant extract were then added and cells were incubated for an additional 72 h. LA25 cells were grown overnight at non permissive temperature (40 °C) before being incubated for 24 h at permissive (33 °C) and nonpermissive (40 °C) temperatures with or without hibiscus extract, and then stained with Trypan blue to distinguish living and dead cells. Cells were analyzed on an inverted Zeiss Axiovert microscope and counted from images with the aid of Zeiss Axiovision software as previously described.14, 15 Cells treated with fractionated extracts were trypsinized and counted with a Coulter counter as previously described.13, 15 Statistics were analyzed with Graphpad Prism Software version 5 as previously described.14, 15
2.4. Western blotting
Protein extracted from LA25 cells grown at permissive (33 °C) and nonpermissive (40 °C) temperature was analyzed by Western blotting to detect total v-Src (Millipore 05-185), active Src (phosphorylated at tyrosine 416) (Millipore 04-857), and ß-actin (Sigma A1978) as previously described.16, 17, 18
3. Results
3.1. H. rosa-sinensis flower extract inhibits melanoma cell growth
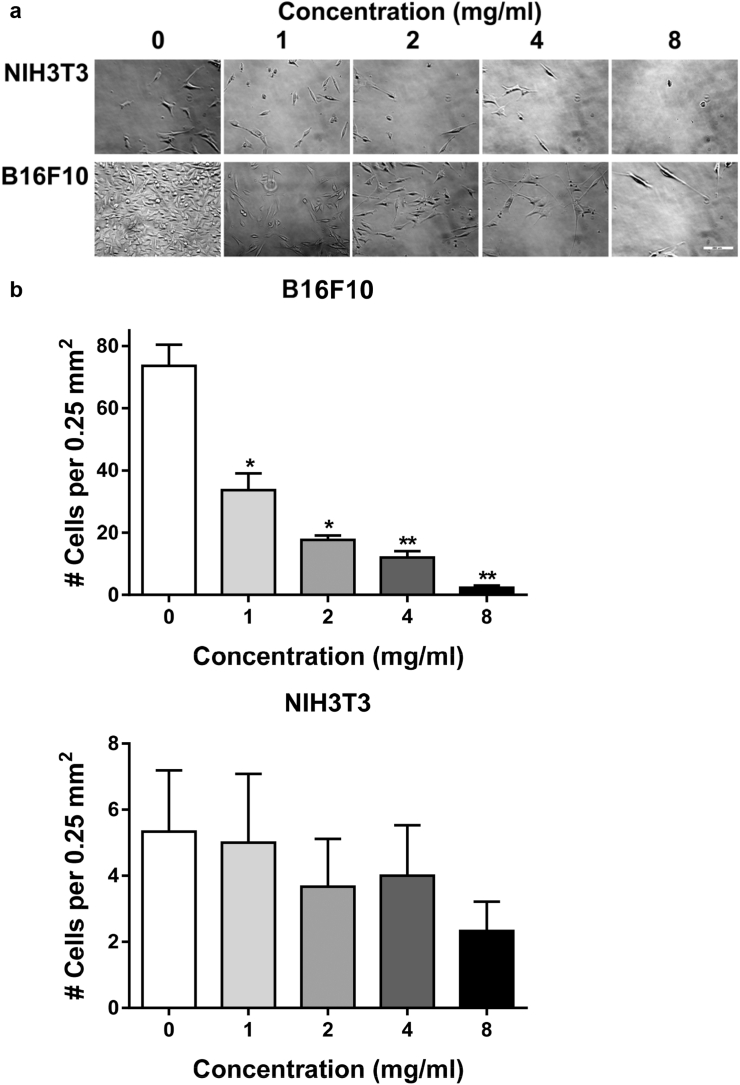
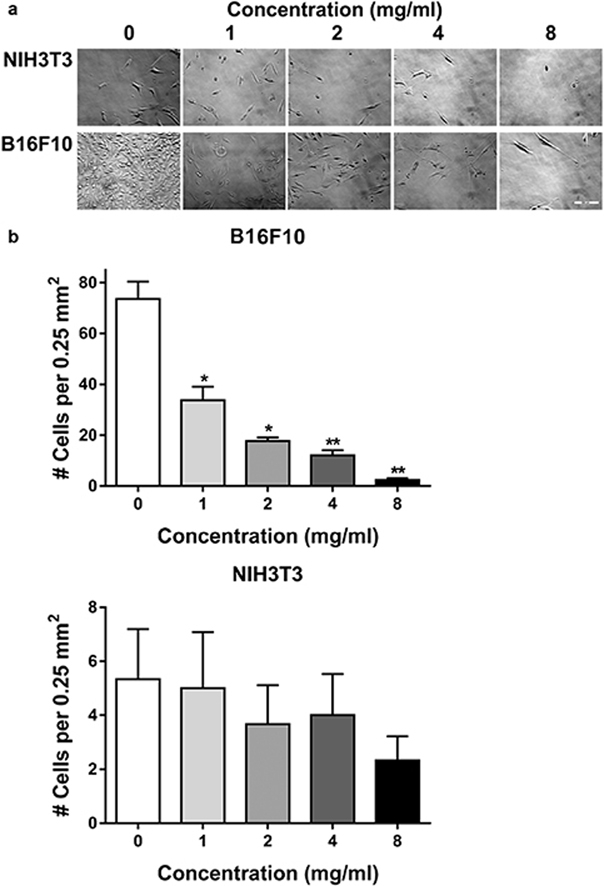
An aqueous extract of H. rosa-sinensis flowers was prepared according to basic recipes used to make tea. Effects of this extract on cell growth were examined after sterilization by filtration. As shown in Fig. 1, this extract inhibited melanoma cell growth in a dose dependent manner. This extract reduced melanoma cell growth by 2 fold at 1 mg/ml, and 4 fold at 2 mg/ml.
Fig. 1.
Hibiscus extract inhibits melanoma cell growth. (a) B16F10 melanoma cells and nontransformed NIH3T3 fibroblasts (5,000 cells per well) were grown overnight before being incubated for 72 h with indicated concentrations of hibiscus extract and photographed (bar = 280 microns). (b) Cells were counted and shown as the number of cells in a 500 × 500 micron area in the center of each well (mean + SEM, n = 3). Single and double asterisks denote p < 0.05 and p < 0.01 compared to untreated control cells, respectively.
Interestingly, this extract preferentially inhibited growth of melanoma cells over nontransformed fibroblasts. Although there did seem to be a trend for fibroblast growth inhibition, this effect was not significant, even at concentrations up to 8 mg/ml (see Fig. 1). These data are consistent with recent studies indicating that H. sabdariffa leaf extracts contain polyphenols that specifically inhibit the growth of melanoma cells, but not nontransformed cells.10
3.2. Bioactive components of H. rosa-sinensis flower extract can be fractionated by size
The aqueous H. rosa-sinensis flower extract was fractionated to examine the nature of its bioactive components. Centrifugal filtration was used to obtain products greater than 50 kD, between 3 kD and 50 kD, and less than 3 kD. The effect of these fractions on melanoma cell growth was then examined at an original concentration of 4 mg/ml, which potently inhibited melanoma cell growth (Fig. 1).
3.3. Bioactive components of H. rosa-sinensis flower extract work together to inhibit melanoma cell growth
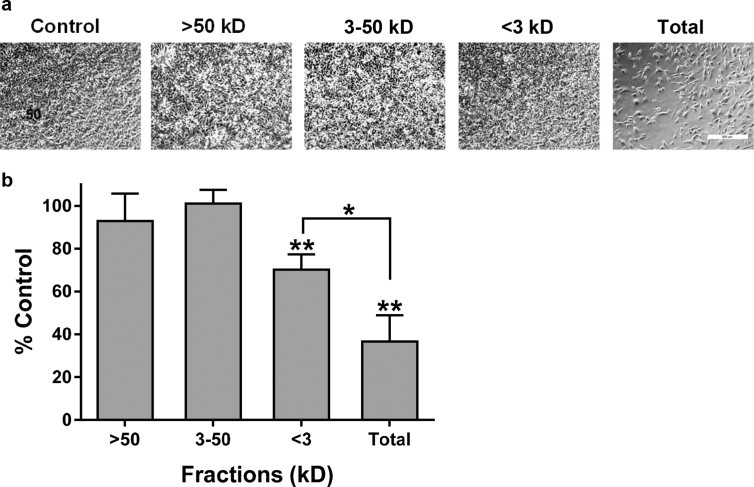
The combined fractions of this extract inhibited melanoma cell growth by over 60% as shown in Fig. 2. In addition, low molecular weight components below 3 kD also inhibited cell growth, but to a significantly smaller extent of about 30%. Moreover, compounds larger than 3 kD did not inhibit melanoma cell growth. Taken together, these data suggest that while low molecular weight compounds in H. rosa-sinensis flower extract can inhibit melanoma cell growth to some extent, they combine with other higher molecular weight compounds to double their effectiveness.
Fig. 2.
Hibiscus extract contains multiple bioactive components. (a)B16F10 melanoma cells (5,000 cells per well) were grown overnight before being incubated for 72 h with no extract (Control), unfractionated hibiscus extract (Total), or fractions containing components of hibiscus extract greater than 50 kD (>50 kD), between 3 and 50 kD (3–50 kD), or less than 3 kD (<3 kD) and photographed (bar = 350 microns). (b) Cells were counted and shown as percent of controls (mean + SEM, n = 3). Single and double asterisks denote p < 0.05 compared to control cells treated without extract and p < 0.1 between cells treated with total extract or fractions with components less than 3 kD as indicated.
3.4. Hibiscus extract inhibits Src transformed cell growth, but not oncogenic Src kinase activity
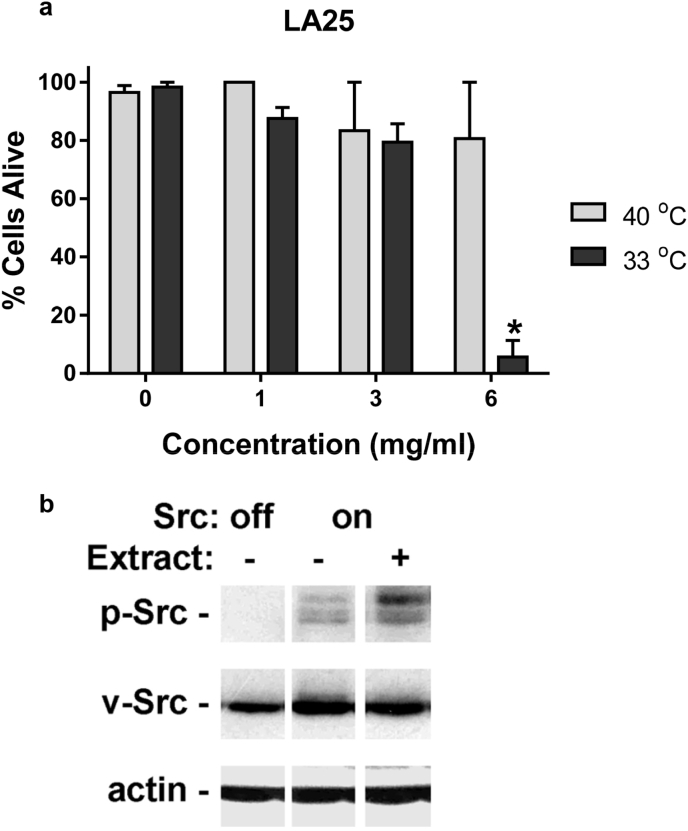
The Src kinase has been implicated in melanoma progression,19, 20, 21 and is being pursued as a potential chemotherapeutic target.22, 23 We utilized LA25 cells transformed by a temperature sensitive oncogenic Src kinase construct to investigate the effects of hibiscus extract on Src transformed cells and kinase activity. As shown in Fig. 3a, hibiscus extract was preferentially toxic to these cells transformed by Src kinase when grown at permissive temperature. However, as shown in Fig. 3b, hibiscus treatment did not inhibit Src kinase activity in these cells. These data suggest that hibiscus extract contains compounds that affect elements downstream of the Src kinase, or unrelated pathways that control tumor cell viability.
Fig. 3.
Hibiscus extract inhibits Src transformed cell growth, but not oncogenic Src kinase activity. (a) LA25 cells were grown overnight at nonpermissive temperature (40 °C) before being incubated for 24 h at permissive (33 °C) and nonpermissive (40 °C) with concentrations of hibiscus extract as indicated. Cells were then stained with Trypan blue, and examined to measure cytotoxicity. Data are shown as the percent of live cells (mean + SEM, n = 3). (b) Total (v-Src) and active (p-Src) were detected by Western blotting from cells grown at nonpermissive temperature (Src off) and permissive temperature (Src on) with and without 4 mg/ml hibiscus extract as indicated.
4. Discussion
Hibiscus has been consumed for many centuries. In particular, flowers from H. rosa-sinensis are used to prepare tea. This beverage is purported to have medicinal properties that ameliorate conditions including cardiac hypertension and cancer.7
Results from this study indicate that H. rosa-sinensis tea extracts contain compounds that inhibit melanoma cell growth at concentrations that do not affect nontransformed cells. These data are consistent with recent studies indicating that polyphenols from H. sabdariffa inhibit melanoma cell growth.10 Although the polyphenols examined in previous studies were extracted with organic solvents, they may also be present in aqueous extracts used in traditional beverages.
Our results indicate that aqueous H. rosa-sinensis flower extract may also contain low molecular weight compounds that inhibit melanoma cell growth. These may also be polyphenols such as those obtained from leaves of different strains as previously reported.10 However, our results also indicate that these compounds actually work with other higher molecular weight compounds to more effectively inhibit melanoma cell growth in a manner that does not inhibit Src kinase activity. Future work should identify these components and evaluate their potential to prevent and treat melanoma, and possibly other cancers. It should be particularly important to determine if these compounds can potentiate the effects of other treatments including traditional cytotoxic therapies, and more targeted agents including BRAF and CTLA4 blockers which target oncogenic serine kinase signaling events and immunomodulation, respectively.3, 4
Conflict of interests
None declared.
Acknowledgments
This work was funded in part by support from the Osteopathic Heritage Foundation and the Graduate School of Biomedical Sciences at Rowan University.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Jilaveanu L.B., Aziz S.A., Kluger H.M. Chemotherapy and biologic therapies for melanoma: do they work? Clin Dermatol. 2009;27:614–625. doi: 10.1016/j.clindermatol.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Ho W.L., Comber H., Hill A.D., Murphy G.M. Malignant melanoma and breast carcinoma: a bidirectional correlation. Ir J Med Sci. 2009;180:901–903. doi: 10.1007/s11845-009-0297-5. [DOI] [PubMed] [Google Scholar]
- 3.Eggermont A.M., Robert C. New drugs in melanoma: it's a whole new world. Eur J Cancer. 2011;47:2150–2157. doi: 10.1016/j.ejca.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty K.T. Throwing the kitchen sink at melanoma drug development. Pigment Cell Melanoma Res. 2012;25:543–544. doi: 10.1111/j.1755-148X.2012.01038.x. [DOI] [PubMed] [Google Scholar]
- 5.Scarlett W.L. Ultraviolet radiation: sun exposure, tanning beds, and vitamin D levels. What you need to know and how to decrease the risk of skin cancer. J Am Osteopath Assoc. 2003;103:371–375. [PubMed] [Google Scholar]
- 6.Purushothaman A., Nandhakumar E., Sachdanandam P. Phytochemical analysis and anticancer capacity of Shemamruthaa, a herbal formulation against DMBA- induced mammary carcinoma in rats. Asian Pac J Trop Med. 2013;6:925–933. doi: 10.1016/S1995-7645(13)60166-2. [DOI] [PubMed] [Google Scholar]
- 7.Da-Costa-Rocha I., Bonnlaender B., Sievers H., Pischel I., Heinrich M. Hibiscus sabdariffa L. – a phytochemical and pharmacological review. Food Chem. 2014;165:424–443. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Hsu R.J., Hsu Y.C., Chen S.P. The triterpenoids of Hibiscus syriacus induce apoptosis and inhibit cell migration in breast cancer cells. BMC Complement Altern Med. 2015;15:65. doi: 10.1186/s12906-015-0592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai T.C., Huang H.P., Chang Y.C., Wang C.J. An anthocyanin-rich extract from Hibiscus sabdariffa linnaeus inhibits N-nitrosomethylurea-induced leukemia in rats. J Agric Food Chem. 2014;62:1572–1580. doi: 10.1021/jf405235j. [DOI] [PubMed] [Google Scholar]
- 10.Chiu C.T., Hsuan S.W., Lin H.H., Hsu C.C., Chou F.P., Chen J.H. Hibiscus sabdariffa leaf polyphenolic extract induces human melanoma cell death, apoptosis, and autophagy. J Food Sci. 2015;80:H649–H658. doi: 10.1111/1750-3841.12790. [DOI] [PubMed] [Google Scholar]
- 11.Mishra N., Tandon V.L., Gupta R. Immunomodulation by Hibiscus rosa-sinensis: effect on the humoral and cellular immune response of Mus musculus. Pak J Biol Sci. 2012;15:277–283. doi: 10.3923/pjbs.2012.277.283. [DOI] [PubMed] [Google Scholar]
- 12.Jadhav V.M., Thorat R.M., Kadam V.J., Sathe N.S. Traditional medicinal uses of Hibiscus rosa-sinensis. J Pharm Res. 2009;2:1220–1222. [Google Scholar]
- 13.Goldberg G.S., Bechberger J.F., Tajima Y. Connexin43 suppresses MFG-E8 while inducing contact growth inhibition of glioma cells. Cancer Res. 2000;60:6018–6026. [PubMed] [Google Scholar]
- 14.Ochoa-Alvarez J.A., Krishnan H., Pastorino J.G. Antibody and lectin target podoplanin to inhibit oral squamous carcinoma cell migration and viability by distinct mechanisms. Oncotarget. 2015;6:9045–9060. doi: 10.18632/oncotarget.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochoa-Alvarez J.A., Krishnan H., Shen Y. Plant lectin can target receptors containing sialic acid, exemplified by podoplanin, to inhibit transformed cell growth and migration. PLoS One. 2012;7:e41845. doi: 10.1371/journal.pone.0041845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnan H., Ochoa-Alvarez J.A., Shen Y. Serines in the intracellular tail of podoplanin (PDPN) regulate cell motility. J Biol Chem. 2013;288:12215–12221. doi: 10.1074/jbc.C112.446823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Y., Chen C.S., Ichikawa H., Goldberg G.S. SRC induces podoplanin expression to promote cell migration. J Biol Chem. 2010;285:9649–9656. doi: 10.1074/jbc.M109.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Y., Khusial P.R., Li X., Ichikawa H., Moreno A.P., Goldberg G.S. Src utilizes Cas to block gap junctional communication mediated by connexin43. J Biol Chem. 2007;282:18914–18921. doi: 10.1074/jbc.M608980200. [DOI] [PubMed] [Google Scholar]
- 19.Schraw W., Richmond A. Melanoma growth stimulatory activity signaling through the class II interleukin-8 receptor enhances the tyrosine phosphorylation of Crk-associated substrate, p130, and a 70-kilodalton protein. Biochemistry. 1995;34:13760–13767. doi: 10.1021/bi00042a006. [DOI] [PubMed] [Google Scholar]
- 20.Summy J.M., Gallick G.E. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 21.Qi J., Wang J., Romanyuk O., Siu C.H. Involvement of Src family kinases in N-cadherin phosphorylation and beta-catenin dissociation during transendothelial migration of melanoma cells. Mol Biol Cell. 2006;17:1261–1272. doi: 10.1091/mbc.E05-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montero J.C., Seoane S., Ocana A., Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res. 2011;17:5546–5552. doi: 10.1158/1078-0432.CCR-10-2616. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan H., Miller W.T., Goldberg G.S. SRC points the way to biomarkers and chemotherapeutic targets. Genes Cancer. 2012;3:426–435. doi: 10.1177/1947601912458583. [DOI] [PMC free article] [PubMed] [Google Scholar]