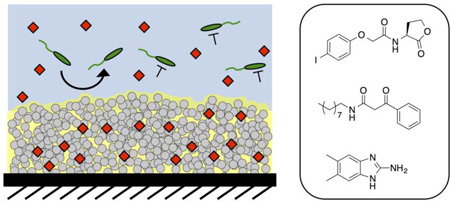
Abstract
Surfaces that can both prevent bacterial biofouling and inhibit the expression of virulence phenotypes in surrounding planktonic bacteria are of interest in a broad range of contexts. Here, we report new slippery-liquid infused porous surfaces (SLIPS) that resist bacterial colonization (owing to inherent ‘slippery’ surface character) and also attenuate virulence phenotypes in non-adherent cells by gradually releasing small-molecule quorum sensing inhibitors (QSIs). QSIs active against Pseudomonas aeruginosa can be loaded into SLIPS without loss of their slippery and anti-fouling properties, and imbedded agents can be released into surrounding media over hours to days depending on the structures of the loaded agent. This controlled-release approach is useful for inhibiting virulence factor production and can also inhibit bacterial biofilm formation on nearby, non-SLIPS-coated surfaces. Finally, we demonstrate that this approach is compatible with the simultaneous release of more than one type of QSI, enabling greater control over virulence and suggesting new opportunities to tune the anti-fouling properties of these slippery surfaces.
Keywords: Anti-Biofouling, Anti-Virulence, Biofilms, Controlled Release, Quorum Sensing, Slippery Surfaces
Graphical abstract
Introduction
Surface-associated fouling by bacteria is a common and persistent challenge facing the use of biomedical devices, industrial equipment, and many consumer products.1,2 The development of strategies that can slow or prevent microbial colonization and biofilm formation on surfaces is thus an important element in the design of materials and coatings intended for use in wet environments.3,4 The work reported here was motivated broadly by the recent development of slippery liquid-infused porous surfaces (or ‘SLIPS’),5–11 which have many physical properties and behaviors that render them well suited for the design of anti-biofouling surfaces. These ‘slippery’ materials consist of a porous or textured surface infused with a viscous liquid (e.g., perfluorinated lubricants,5,11,12 silicone oil,8,13,14 etc.). This general design maintains the infused liquid as a thin, dynamic film at the surface, creating a hydrophobic or omniphobic interface that allows other fluids and substances to easily slide or ‘slip’ off the surface with sliding angles as low as 2°.5,9,15 Several recent reports reveal SLIPS to be a promising platform for the development of new anti-biofouling interfaces for biological and environmental applications.9,14,16–21 Indeed, SLIPS have been reported to resist fouling by a broad range of organisms, including clinically important bacterial14,16,17,21 and fungal21 pathogens, marine barnacle cyprids,19 and mammalian cells.18,20,21
Slippery character is the conditio sine qua non of a SLIPS-coated surface, yet this essential quality only allows SLIPS to prevent fouling by organisms on the surfaces to which these coatings are physically applied. Conventional SLIPS-coated surfaces, for example, cannot prevent bacteria from colonizing other nearby (non-SLIPS-coated) surfaces. In addition, conventional SLIPS are not designed to either kill or retard the growth of bacteria—cells that are prevented from adhering to SLIPS-coated surfaces remain alive in the surrounding medium. SLIPS also do not currently have inherent mechanisms through which they can prevent these non-adherent (or ‘planktonic’) bacteria from producing toxins or engaging in other virulent behaviors, including forming biofilms on nearby unprotected surfaces.
To address these issues and develop new slippery anti-fouling surfaces that can also exert control over the behaviors of microorganisms in surrounding media, we recently reported a novel controlled release-based approach to the design of multifunctional SLIPS that prevent biofouling by pathogenic fungal and bacterial cells and kill planktonic microorganisms in surrounding media.21 In that work, we leveraged the properties of a porous polymer matrix and an infused silicone oil phase to sustain the long-term release of the model small-molecule microbicidal agent triclosan. That study demonstrated that triclosan could be readily incorporated into SLIPS without impacting their anti-fouling properties, and that the slow release of triclosan could kill planktonic Candida albicans effectively and improve the overall antifouling and antifungal properties.21 Because triclosan is a broad-spectrum antibiotic, it is likely that this approach could also be used to design multi-functional SLIPS that also kill planktonic bacteria. We note, however, that the use of triclosan and other cytotoxic drugs (e.g., antibiotics) have several disadvantages in applied contexts, perhaps most notably the fact that the widespread use of these agents has led to evolved resistance in many clinically relevant pathogens.22
The work reported here sought to further develop the potential of SLIPS as reservoirs for the controlled release of active agents, with a focus on the design of multifunctional and chemical-eluting SLIPS capable of attenuating the colonization and virulence of planktonic bacteria through non-biocidal means (e.g., by sustaining the release of active agents that do not kill bacteria, but instead attenuate virulent behaviors by targeting non-essential pathways). Such ‘anti-virulence’ strategies have attracted considerable interest over the past decade as the incidence of bacterial resistance has increased.23–25 One promising target for potential anti-virulence approaches is bacterial quorum sensing (QS) circuits.26 QS is a small molecule-based communication system used by a range of bacteria to coordinate the expression of genes encoding group-beneficial behaviors when a threshold population density (i.e., a ‘quorum’) is reached in a given environment.27–29 In many common pathogens, such as the Gram-negative bacterium Pseudomonas aeruginosa, QS systems control the production of excreted virulence factors and the formation of biofilms, but are non-essential for cell growth—targeting these systems thus presents a basis for the development of ‘non-biocidal’ approaches to controlling bacterial virulence.26,30–32 Over the last 10 years, our laboratory33–38 and others30–32,39,40 have developed suites of potent small molecule inhibitors of QS (QSIs) that are active in P. aeruginosa and other pathogens, and that represent valuable chemical tools to test such anti-virulence approaches.
As part of a broader effort to explore and exploit the potential therapeutic value of QSIs, we41–45 and others46–55 have reported strategies for the encapsulation or integration of QSIs and other anti-virulence agents into polymer-based materials or onto inorganic surfaces. These past studies have yielded many different approaches to the release of anti-virulence agents, but they have relied, in large measure, on materials and tactics that do not inherently prevent biofouling on the surface of an object or device (apart from the activities of the released inhibitors). Here, we demonstrate that the polymer and oil phases of polymer-based SLIPS can be exploited to load and control the release of synthetic small molecules that inhibit or modulate QS in P. aeruginosa. We demonstrate that these QSIs can be loaded into SLIPS without affecting slippery or anti-fouling properties, and that the agents remain biologically active, enabling QSI-loaded SLIPS to both prevent bacterial surface colonization and attenuate the production of key excreted virulence factors in planktonic cultures of this pathogen. These liquid-infused materials can also be designed to release multiple QSIs that target multiple different QS circuits simultaneously. Further, we demonstrate that these polymer-based SLIPS, which are inherently resistant to the formation of P. aeruginosa biofilms on their own surfaces, can also release non-bactericidal, small molecule biofilm inhibitors that confer robust protection against the formation of biofilms on other nearby unprotected (non-SLIPS-coated) surfaces. Our results suggest the basis of new non-bactericidal approaches to the design and protection of anti-fouling surfaces that could circumvent issues of evolved resistance associated with the use of traditional antibiotics. More broadly, this work also advances new approaches to the integration of controlled release strategies with SLIPS-based technologies that could improve the properties of these versatile and inherently anti-fouling, oil-infused surfaces in a range of other contexts.
Results and Discussion
Fabrication and loading of QSIs into nanoporous, multilayer-based SLIPS
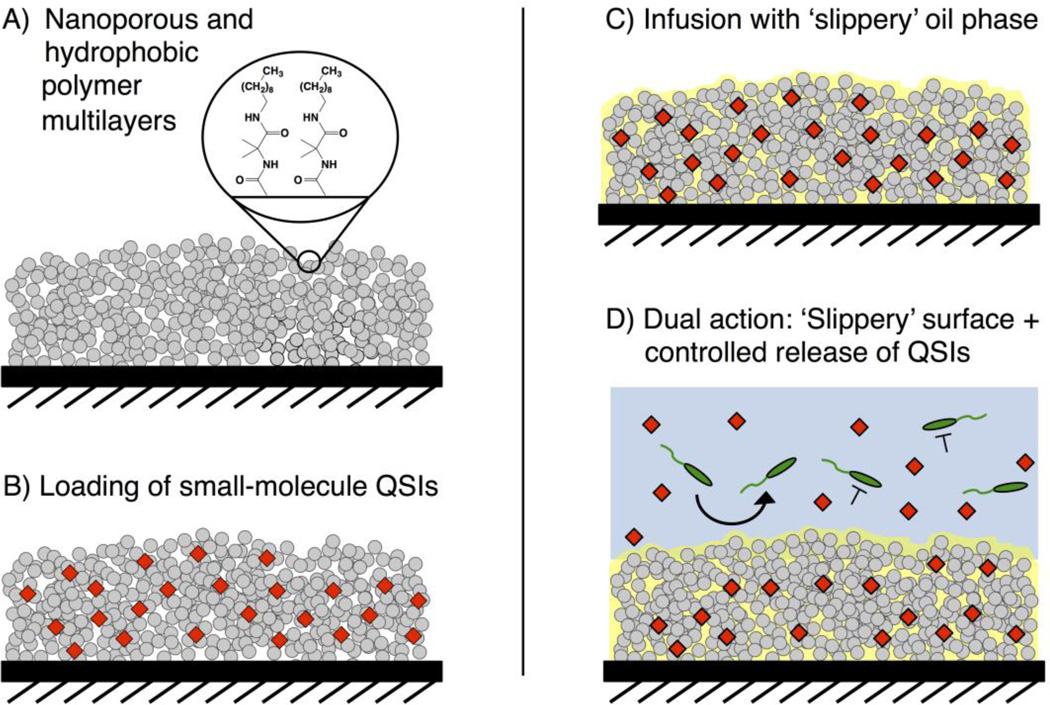
The SLIPS used in this study were constructed by the infusion of silicone oil into nanoporous and covalently-crosslinked polymer multilayers fabricated by the reactive layer-by-layer assembly of poly(vinyl-4,4-dimethylazlactone) (PVDMA) and branched poly(ethyleneimine (PEI) on planar glass substrates.13,21,56–58 After film fabrication, these reactive multilayers were treated with n-decylamine to functionalize residual azlactones remaining in the films with hydrophobic alkyl groups (Figure 1A)13,21,57 and render them more chemically compatible with silicone oil.13,21 Infusion of silicone oil into the decylamine-functionalized multilayers yielded SLIPS that exhibited water droplet sliding angles of ≤ 10°, in agreement with our past studies (Table 1).13,21
Figure 1.
Schematic illustration showing the fabrication of the QSI-loaded SLIPS used in this study. (A) Reactive and nanoporous polymer multilayers (grey) are functionalized with n-decylamine to render them hydrophobic. (B) Small molecule QSIs (red) are loaded into the multilayers by adding an acetone solution of the agents to dried films and allowing the solvent to evaporate. (C) Silicone oil is infused into the multilayers. (D) The loaded SLIPS gradually release QSIs into aqueous solution; the SLIPS prevent the colonization of bacteria directly on the coated surface while the released QSI modulate the behaviors of nearby planktonic bacteria.
Table 1.
Slide Velocities for Compound-Loaded SLIPS.
| Loaded Compound |
Slide Velocity (mm/s)a |
|---|---|
| None | 7.6 ± 0.3 |
| E22 | 6.9 ± 0.3 |
| V-06-018 | 6.6 ± 0.6 |
| E22 + V-06-018 | 4.3 ± 0.2 |
| DMABI | 7.4 ± 0.2 |
Values are the mean and standard error of six independent replicates. Experiments were performed by placing droplets of water (10 µL) on SLIPS-coated surfaces held at angles of 10° and measuring the times required to slide a fixed distance (see Methods for details).
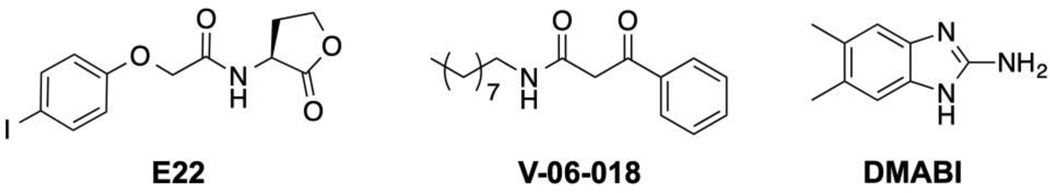
We selected two P. aeruginosa QSIs [E22 (an acyl l-homoserine lactone (AHL)-based antagonist of the RhlR QS receptor35,36) and V-06-018 (a non-AHL-based antagonist of the LasR QS receptor)34,59] and DMABI (a potent biofilm inhibitor that indirectly modulates QS in P. aeruginosa60) for this study because they represent some of the most potent and/or well-characterized QS modulators known (structures shown in Figure 2). QSI- and DMABI-loaded SLIPS were prepared by applying small volumes of dilute QSI or DMABI solutions in acetone to each side of the multilayer-coated substrates (prior to the infusion of oil, see Methods; Figure 1B). The acetone solutions quickly wet the entirety of the nanoporous coatings and, upon evaporation, left the loaded compounds adsorbed within the multilayers. A small excess of silicone oil was then pipetted onto both sides of the compound-loaded coatings and allowed to infuse and spread across the entirety of the surface (Figure 1C). We adopted this approach to loading (e.g., as opposed to an alternative approach in which dry multilayers were infused directly with silicone oil containing dissolved compound) on the basis of our past results using triclosan,21 and because this approach enables more precise control over the amount of compound loaded in ways that are not limited by the solubility of a given compound in the oil phase. All QSI- or DMABI-loaded SLIPS used in this study contained 240 nmol or 300 nmol of compound per substrate, respectively. We confirmed that the loading of these small molecules did not impact the slippery properties of the resulting oil-infused multilayers by placing 10 µL droplets of water on compound-loaded SLIPS held at a tilt angle of 10° and measuring the sliding velocities of the droplets. As revealed by the results shown in Table 1, SLIPS loaded with E22, V-06-018, DMABI, or a 1:1 ratio of both E22 and V-06-018 (at a loading of 120 nmol each) did not have a substantial impact on droplet sliding velocities relative to unloaded SLIPs.
Figure 2.
Structures of the small molecule anti-virulence agents used in this study.
Characterization of the release of QS modulators from SLIPS
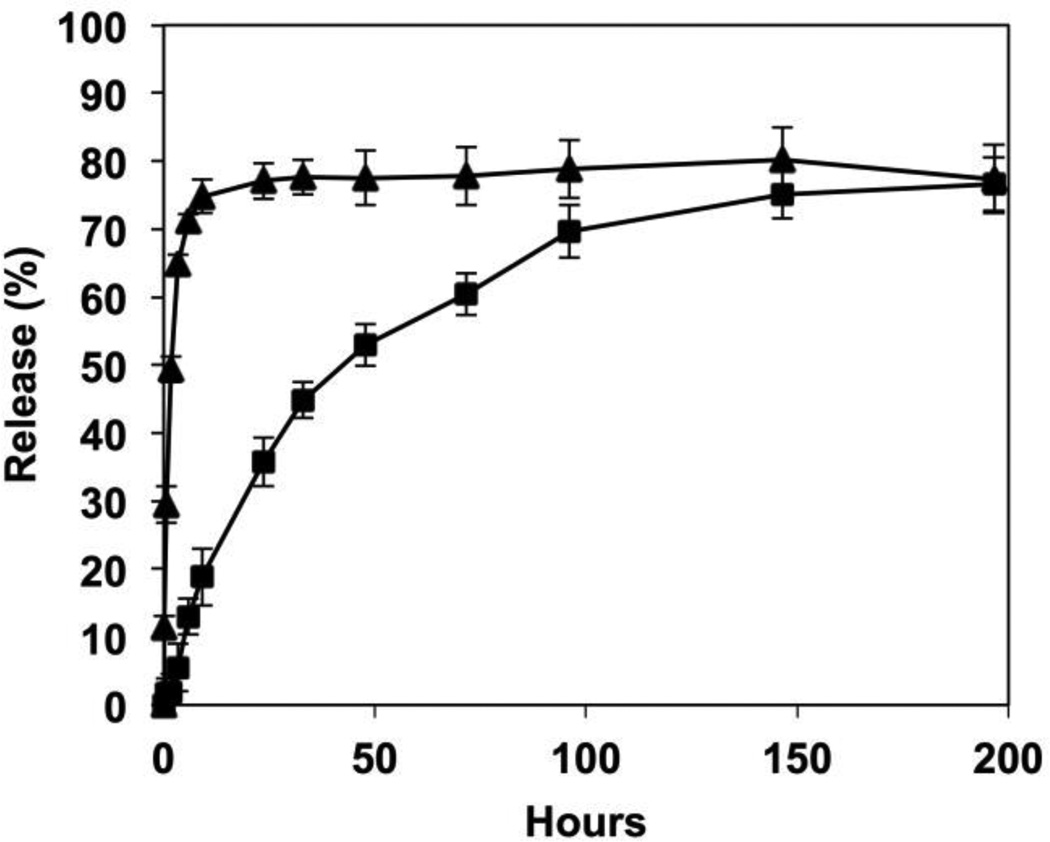
We incubated compound-loaded SLIPS in phosphate-buffered saline (PBS) to characterize the release of the imbedded QSIs into the surrounding medium under physiologically relevant conditions (37 °C; pH = 7.4). For these studies, we used SLIPS loaded with E22 and DMABI because the relatively strong UV absorbance of these compounds permitted facile monitoring of release by UV/vis spectrophotometry (the release of V-06-018 was not evaluated because its UV absorbance was obscured by the presence of silicone oil). As shown in Figure 3, compound E22 was released into surrounding buffer relatively quickly, with approximately 75% of the total amount loaded released over the first 12 hours of incubation. No further compound was released over an additional 190 hours of incubation. In contrast to the relatively rapid release exhibited by these AHL-loaded SLIPS, coatings loaded with the 2-aminobenzimidazole-based biofilm inhibitor DMABI released their contents much more slowly, with approximately 40% of the loaded compound released after the first 24 hours, and an additional ~40% released over the next 150 hours; see Figure 3).
Figure 3.
Plot showing percent release versus time for the release of compounds E22 (triangles) and DMABI (squares) from SLIPS-coated surfaces incubated in PBS buffer at 37 °C. Data points represent the mean of four replicates. The percentage of each compound released was calculated based on the total amount of compound initially loaded.
It is clear from these results that the structures of the loaded compounds can have a significant influence on rates and extents of release from the SLIPS, likely a result of differences in the interactions of these compounds with the porous polymer matrix and differences in the extent to which the compounds partition into the silicone oil phase. Additional studies are underway to understand more broadly the factors that lead to these large differences in release profiles, as well as the extent to which changes in film structure and the properties of the infused oil phase can be exploited to tune the release profiles of these and other agents more broadly. The time scales and the amounts of QSIs and DMABI released by the materials reported here (Figure 3) were more than sufficient to demonstrate robust proofs of concept in all biological studies described below.
Release of QSIs from SLIPS attenuates P. aeruginosa pyocyanin production
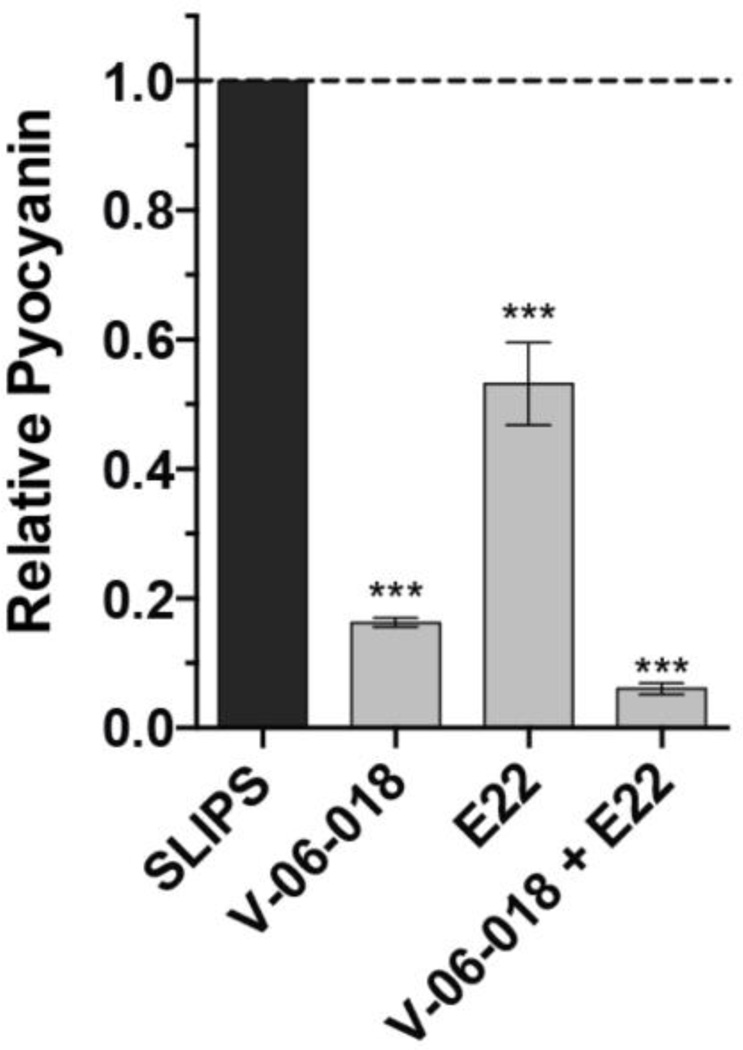
To characterize the biological activities of QSI-loaded SLIPS, we monitored production of the redox-active virulence factor pyocyanin by P. aeruginosa.61 Pyocyanin production is controlled, in part, by the QS receptors LasR and RhlR in P. aeruginosa,35,38 and can be attenuated by both V-06-018 (a LasR antagonist) and E22 (an RhlR antagonist). We grew wild-type P. aeruginosa (PAO1) in the presence of SLIPS substrates loaded with either V-06-018 or E22 (in amounts designed to yield approximately 100 µM of compound in the assay culture upon full release) and quantified pyocyanin production after 17 hours of shaking incubation (see Materials and Methods). As shown in Figure 4, SLIPS loaded with V-06-018 and E22 inhibited pyocyanin production by approximately 80% and 45%, respectively. These values are equivalent to the levels of pyocyanin inhibition observed when these compounds are administered exogenously,38 indicating that both of these compounds are released from the SLIPS-coated surfaces in intact, biologically-active forms and in concentrations sufficient to inhibit QS under these assay conditions. Although not investigated specifically as part of this study, we note that the overall strategy used here, in which encapsulated payloads are stored within a polymer matrix infused with a hydrophobic and water-immiscible oil—and are, thus, largely protected from contact with bulk water until they diffuse across the oil/water interface—could prove useful for the prolonged release of active agents that hydrolyze or decompose readily upon contact with water. For instance, it is well known that AHLs hydrolyze relatively rapidly in aqueous media and that the ring-opened forms are inactive;37,41,62 as such, we anticipate that SLIPS containing AHLs (such as E22) could provide means for extending their effective half-lives in water, and thus extend their utility as QSIs.
Figure 4.
Plot showing the inhibition of pyocyanin production by QSIs released from SLIPS-coated surfaces. P. aeruginosa cultures were grown in the presence of SLIPS loaded with the indicated compounds and the final amount of pyocyanin in the culture was quantified after 17 hours of incubation. Compounds were loaded at levels estimated to give final released concentrations of 100 µM (for experiments involving a single loaded compound) or 50 µM (for experiments involving two loaded compounds). Error bars represent the standard error of three independent replicates (n = 3). *** = p < 0.0005.
We recently demonstrated that cocktails of compounds simultaneously targeting multiple QS circuits in P. aeruginosa could result in greater attenuation of virulence factor production (as compared to levels attenuated upon the administration of a single compound targeting a single QS circuit).38 To explore the potential of our polymer-based SLIPS to promote the simultaneous release of two different active agents, and develop SLIPS-coated surfaces that attenuate QS more strongly than those described above, we prepared SLIPS loaded with both V-06-018 and E22 (in amounts designed to give approximately 50 µM of each compound in the assay culture upon full release) and quantified pyocyanin production when the compounds were released simultaneously. We observed over 90% inhibition of pyocyanin production using this dual-release approach (Figure 4). We note that this dual-QSI release approach allows for lower loadings of each individual agent and promotes levels of inhibition greater than those exhibited when either agent is used alone because it targets both RhlR and LasR QS receptors simultaneously. The ease with which these QSIs (and other acetone-soluble agents) can be loaded into these nanoporous multilayers, without necessitating any changes to the fabrication process, suggests that this basic approach should be general. This result is significant, as it indicates that these SLIPS systems should be appropriate for use in other applications that would benefit from the simultaneous release of single or multiple different active agents.
We note that the SLIPS-coated substrates emerging from these in situ virulence factor production experiments remained anti-fouling to bacteria, but exhibited water droplet sliding angles higher than those that were measured prior to incubation with bacteria (e.g., droplets of water required tilt angles of ~30° or more to slide freely, as compared to sliding angles of < 10° for substrates prior to incubation as shown in Table 1), suggesting that some oil may have been lost from the SLIPS during those experiments. Additional control experiments demonstrated this decrease in droplet sliding angles to result from incubation at the high densities of bacteria required for these pyocyanin assays (incubation under static conditions at lower densities of bacteria or shaking in the absence of bacteria did not affect droplet sliding angles). It has been reported in past studies that exposure to high shear forces (induced by flow, etc.) can promote the leaching of the infused liquid phases of SLIPS in ways that can impact their slippery properties.63,64 It is also possible in this particular context that the presence of amphiphilic molecules produced by the P. aeruginosa cultures (i.e., rhamnolipid and other bio-surfactants)65 could help promote the extraction of small amounts of oil over the course of these experiments. In support of this proposition, we note that sliding angles could be restored to values of 10° or less by adding small amounts of silicone oil to the surface of the coatings (e.g., by pipette; past reports also demonstrate that the gradual loss of oil can be addressed through the design of porous substrates with vascular networks that can continually replenish lost oil).64 Despite this increase in water droplet sliding angles, the results of additional experiments described below demonstrate that these compound-loaded SLIPS-coated substrates remain anti-fouling to bacteria and can prevent the formation of bacterial biofilms.
DMABI-Loaded SLIPS reduce biofilm formation on surrounding uncoated surfaces
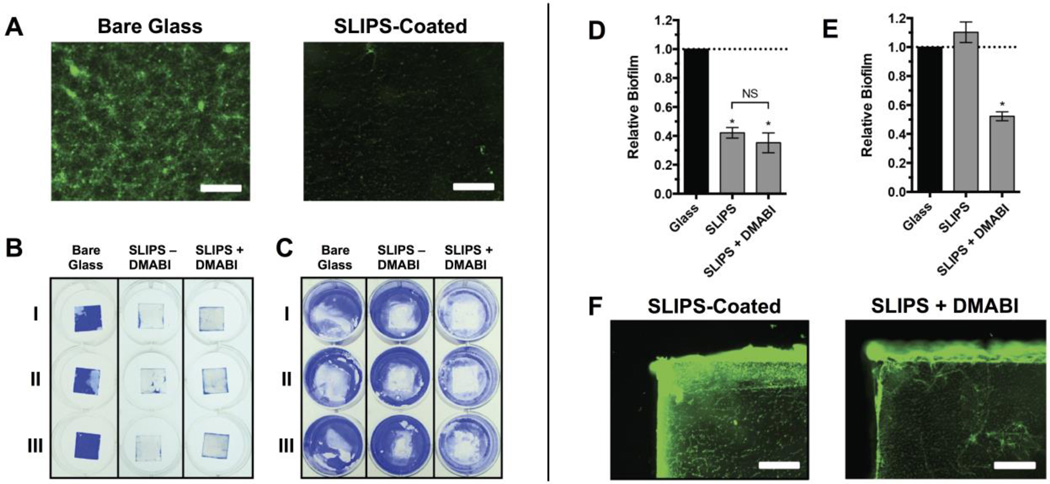
As described above, the native silicone oil-infused SLIPS used in this study have been demonstrated to resist the formation of P. aeruginosa biofilms under static culture conditions (e.g., in the absence of added agents).21 To demonstrate the potential of our controlled-release SLIPS to also prevent biofilm formation in surrounding environments—and, thus, also confer measures of anti-biofouling protection to nearby surfaces that are not SLIPS-coated—we also performed studies using SLIPS loaded with the anti-biofilm agent DMABI. For these studies, we cultured P. aeruginosa in 12-well microtiter plates containing DMABI-loaded SLIPS substrates in the well bottoms (such that they were completely submerged in media). After 24 hours of incubation, the amount of surface-attached biofilm on both the SLIPS substrates and the surrounding (uncoated) areas of the well bottoms were characterized by fluorescence microscopy and by staining with crystal violet (CV; see Materials and Methods). The SLIPS substrates were highly resistant to biofilm attachment, as expected from our past studies.21 We observed no biofilm over the entire central region of the coated substrates by fluorescence microscopy, and little to no CV staining on the SLIPS surface (Figure 5A–B). However, when we quantified the amount of CV on the SLIPS by UV/vis spectrophotometry, we observed more CV staining than expected by visual inspection of the substrates (corresponding to an approximately 50% reduction in staining relative to the uncoated control; Figure 5D). We determined, using fluorescence microscopy, that this residual staining was a result of the presence of biofilm near the uncoated edges of the SLIPS-coated substrates (Figure 5F), and not a result of biofilm on the SLIPS surfaces themselves. These uncoated edges are a result of the manner in which the SLIPS-coated substrates were prepared for these proof-of-concept studies (e.g., by the fracture of larger ‘parent’ SLIPS-coated surfaces into smaller ‘daughter’ chips; see Materials and Methods for details) and are not an inherent limitation of the SLIPS surfaces themselves.
Figure 5.
SLIPS loaded with DMABI resist fouling on the substrate surface and inhibit biofilm formation on surrounding uncoated surfaces. Glass or SLIPS-coated substrates were submerged in P. aeruginosa cultures at the bottoms of the wells of 12-well microtiter plates and incubated for 24 h. Substrates were then removed and the attached biofilms were stained with either SYTO 9 or CV. (A) Representative fluorescence microscopy images of P. aeruginosa biofilm near the center of glass (left) or SLIPS-coated (right) substrates. (B) Representative image of CV-stained biofilms attached to glass substrates, SLIPS without DMABI, or SLIPS loaded with DMABI. (C) Representative image of CV staining of the bottoms of the wells of the 12-well microtiter plate (after the removal of the substrates shown in panel A). (D) Quantification of biofilm formed on the glass and SLIPS substrates shown in panel B. (E) Quantification of biofilm formed on the surrounding well bottoms shown in panel C, showing a reduction of biofilm in wells that contained DMABI-loaded SLIPS. (F) Representative fluorescence microscopy images of biofilm formed on the exposed glass edges of SLIPS substrates without loaded DMABI (left) and with loaded DMABI (right). Scale bars in A and F are 400 µm. Error bars in D and E represent the standard error of three independent experiments (n = 3). * = p <0.05. NS = no statistical difference.
As expected, native (unloaded) SLIPS had no significant influence on the formation of P. aeruginosa biofilms in regions of the surrounding (uncoated) well bottoms in these studies (Figure 5C,E). DMABI-loaded SLIPS, however, prevented biofilm attachment on the SLIPS-coated surface by CV staining (Figure 5B,D) and inhibited biofilm formation on the surrounding uncoated well bottoms by approximately 50% (Figure 5C,E)—a result that we attribute to the gradual release of imbedded DMABI into surrounding media (consistent with results shown in Figure 3). It is likely that the levels of inhibition observed on uncoated well bottoms in these experiments (and the observation of persistent biofilm on the uncoated edges of the SLIPS-coated substrates, as noted above) could be improved further by increasing the loading of DMABI or tuning the rate at which it is released through modifications to the structure of the polymer matrix or the properties of the infused oil (the extended release profile shown in Figure 3, for example, suggests that DMABI was only likely to have been partially released from these silicone oil-infused SLIPS over the course of these short-term experiments). The modular nature of this SLIPS-based approach should facilitate further optimization of these materials for use in specific applications and environments.
Summary and Conclusions
Materials and surface coatings that are resistant to bacterial colonization and that can simultaneously inhibit bacterial virulence phenotypes on and around their surfaces would be useful in a range of biomedical, environmental, and industrial contexts. Materials that can accomplish both of these important tasks without impacting bacterial growth would be particularly valuable, as they would also have the potential to avoid serious problems associated with the development of evolved resistance that currently plague traditional bactericidal approaches. The work reported here takes a step toward addressing these materials and anti-microbial challenges by developing new slippery and anti-fouling oil-infused surfaces that can be used as a robust platforms for the controlled release or delivery of small-molecule QSIs and biofilm inhibitors. Our results demonstrate that this novel approach can significantly (i) reduce production of a virulence factor by planktonic bacteria in the vicinity of the surface and (ii) reduce the biofilm burden on the surface of the material itself and on surrounding non-SLIPS-coated surfaces. Our results also demonstrate that this controlled-release SLIPs approach can be used to load and release of combinations or ‘cocktails’ of these agents that may be more effective than any single antibiotic or QSI alone.
The methods used to fabricate these slippery coatings can be used to coat topologically complex substrates, including tubing, filters, or implants,13,21 and should thus allow for protection against surface fouling and facilitate the local, controlled delivery of anti-virulence agents directly to sites endemic to bacterial colonization in medical devices and/or industrial equipment. The modular nature of these SLIPS also provides opportunities to tune the slippery and controlled release properties of these coatings though changes in the structure of the polymer matrix, the physicochemical properties of the infused oil phase, and the solubilities and structures of the small-molecule agents that are loaded. We anticipate that the approaches and new strategies reported here could thus form the basis of a general and multi-functional materials platform that should prove useful for combating bacterial biofouling and virulence via non-biocidal pathways in a range of important fundamental and applied contexts.
Materials and Methods
Materials and General Considerations
All chemicals were purchased from Sigma-Aldrich, unless indicated otherwise, and used without further purification. 2-Vinyl-4,4-dimethylazlactone (VDMA) was a gift from Dr. Steven M. Heilmann (3M Corporation, Minneapolis, MN). Poly(2-vinyl-4,4-dimethylazlactone) (PVDMA) was synthesized as described previously.66 Glass microscope slides were purchased from Fisher Scientific (Pittsburgh, PA). Dimethyl-2-aminobenzamidazole (DMABI)60 and compounds E2267 and V-06-01839 were synthesized as previously reported. Compressed air used to dry samples was filtered through a 0.2 µm membrane syringe filter. UV/vis measurements were made using a Beckman Coulter DU520 UV/vis spectrophotometer (Fullerton, CA). Fluorescence microscopy images were acquired using an Olympus IX70 microscope and analyzed using the Metavue version V7.7.8.0 software package (Molecular Devices). Absorbance measurements in biological assays were made using a Biotek Synergy 2 plate reader running Gen 5 software (version 1.05).
Fabrication of Polymer Multilayers
Prior to film fabrication, glass microscope slides were cut into 0.8 cm wide strips and scored in 1 cm long segments. Covalently crosslinked and nanoporous polymer multilayers composed of PVDMA and branched poly(ethyleneimine) (PEI; MW ~25,000), referred to from hereon as PEI/PVDMA multilayers, were fabricated on the glass substrates using a covalent/reactive layer-by-layer assembly process, as previously described.13,21,58 Briefly, the substrates were submerged iteratively in the following solutions for 20 s each: (i) PEI (20 mM in acetone with respect to the polymer repeat unit); (ii) two acetone rinses; (iii) PVDMA (20 mM in acetone with respect to the polymer repeat unit); (iv) two additional acetone rinses. This cycle was repeated 35 times. Polymer solution volumes were maintained with fresh acetone to compensate for evaporation and maintain polymer concentration during the dipping process. After fabrication, films were functionalized and rendered hydrophobic by immersion in a 20 mM solution of n-decylamine in THF overnight at room temperature. Functionalized films were then rinsed and dried using compressed, filtered air. Film-coated substrates were then fragmented along the pre-fabrication scores to produce 0.8 cm × 1.0 cm samples.
Small Molecule Loading, Oil Infusion, and Characterization of Release
A 10-µL aliquot of an acetone solution of a small-molecule agent (12.0 mM in compounds E22 or V-06-018 for individual release experiments; 6.0 mM of both compound E22 and compound V-06-018 for dual release experiments; 15.0 mM for experiments using DMABI) was applied to the top side of a film-coated substrate, allowed to dry, and repeated on the coating on the opposite side of the substrate. Immediately prior to use, loaded films were infused with silicone oil (for melting point and boiling point apparatuses; Sigma-Aldrich) by placing a 2.25 µL droplet of oil on each side and allowing the oil to spread over the entire surface. Excess oil was removed from the surface using tissue paper. For release experiments, small-molecule-loaded and oil-infused films were incubated at 37 °C in 1.0 mL of PBS buffer (pH = 7.4). At designated time points, substrates were removed from the incubation buffer and placed in fresh buffer before returning to the incubator. The release of the loaded agents into the incubation buffer was characterized using a UV/vis spectrophotometer.
Bacterial Strains and Growth Conditions
All media and reagents for bacterial culture were purchased from Sigma-Aldrich, unless indicated otherwise. Wild-type P. aeruginosa strain PAO168 was generously donated by Prof. Barbara Iglewski (University of Rochester). Overnight cultures of bacteria were grown in Luria-Bertani (LB) medium at 37 °C with shaking at 200 rpm. Freezer stocks of bacterial strains were maintained at −80 °C in 1:1 LB:glycerol. MOPS Glutamate medium was prepared as described by Mellbye et al.69 The assay medium was prepared prior to each experiment by diluting 10× MOPS buffer (500 mM MOPS, 40 mM tricine, 500 mM NaCl, 10 mM K2HSO4, 500 µM MgCl2, 100 µM CaCl2, 3 µM (NH4)6Mo7O24, 400 µM H3BO3, 30 µM Co(OAc)2, 10 µM CuSO4, 80 µM MnSO4, 10 µM ZnSO4, pH 7.0, filter sterilized) into sterile 18 MΩ water. To this working solution, a sterile 10× stock solution of l-glutamate (250 mM) and sterile 100× stock solutions of K2HPO4 (400 mM), FeSO4 (500 µM), and NH4Cl (1.5 M) were added in appropriate amounts.
Pyocyanin Assay Protocol
The amount of pyocyanin in P. aeruginosa culture supernatants was measured following the protocol of O’Loughlin et al. with modifications.39 A 10 mL overnight culture of P. aeruginosa PAO1 was grown for 16 h. An inoculating culture was prepared by diluting the overnight culture 1:100 into freshly prepared MOPS Glutamate medium, and 2 mL aliquots of this subculture were added to each test tube (0.5% DMSO). SLIPS-coated surfaces (sterilized by UV irradiation for 20 min in a biological safety cabinet) were placed in each tube, and the cultures were grown for 17 h at 37 °C with shaking incubation at 200 rpm. The final cell density was measured by reading absorbance at 600 nm (OD600). Relative pyocyanin levels were measured by first pelleting 1.5 mL of well-mixed, aerated, culture at 4,000g for 10 min, transferring 200 µL of the resulting supernatant to a clear, plastic 96-well microtiter plate, and reading absorbance at 695 nm. Media background absorbance (measured from a “no bacteria” control) was subtracted, the resulting values were normalized by dividing by the final OD600, and the data were plotted relative to an unloaded positive SLIPS control.
P. aeruginosa Biofilm Growth and Crystal Violet Staining Assay Protocol
Biofilm formation by P. aeruginosa was quantified by crystal violet (CV) staining following the protocol of Frei et al. with modifications.60 A 10 mL overnight culture of P. aeruginosa PAO1 was grown for 16 h. An inoculating subculture was prepared by centrifugation of the overnight culture at 4,000g for 10 min, followed by resuspension of the cell pellet in an amount of fresh M9+ medium (see Frei et al.60 for full details of this medium) supplemented with 5% (v/v) LB to effect a 1:10 dilution (v/v) of the overnight culture. Glass substrates were placed into the wells of a 12-well microtiter plate (Costar 3737) and sterilized by UV irradiation for 20 min in a biological safety cabinet. Subculture was added to each well in 2 mL aliquots, and the plates were incubated under static conditions at 37 °C for 24 h. Substrates were removed from the wells using forceps, gently dabbed on a paper towel to remove excess liquid, and placed in a new 12-well plate. Spent culture medium was removed from the wells by inverting the assay plate over a basin, and the attached biofilm was washed once with 1 mL of PBS. The substrates and assay plate were allowed to dry in a 37 °C incubator for at least 8 h. The substrates and well bottoms were stained with 1 mL of a CV solution (0.1% CV (w/v) in 95:5 water:ethanol) for 10 min. Excess CV stain was removed by washing twice with 1 mL of water, and the substrates and plate were dried at 37 °C for at least 4 h. CV stain absorbed by the attached biofilm was quantified by re-solubilizing the stain in 1 mL (wells) or 0.5 mL (substrates) 30% (v/v) acetic acid, transferring 200 µL of this solution to a clear 96-well microtiter plate (Costar 3370), and measuring absorbance at 590 nm.
Characterization of Biofilms Using Fluorescence Microscopy
Biofilms attached to glass substrates were imaged by fluorescence microscopy using the above biofilm growth protocol with the following modifications. After incubation, substrates were gently removed from the assay medium using forceps, washed once by dipping into PBS, and stained with SYTO 9 (Invitrogen) according to the manufacturer’s protocol. Excess staining solution was removed by dabbing on a paper towel, and the substrates were covered by 400 µL of PBS in a 24-well plate. Biofilms were then imaged using an Olympus IX71 fluorescence microscope.
Acknowledgments
Financial support for this work was provided by the Office of Naval Research (N00014-16-1-2185) and the National Science Foundation through a grant provided to the UW-Madison Materials Research Science and Engineering Center (MRSEC; DMR-1121288). The authors acknowledge the use of instrumentation supported by the NSF (DMR-1121288).
References
- 1.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 2.Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F. Resistance of bacterial biofilms to disinfectants: a review. Biofouling. 2011;27:1017–1032. doi: 10.1080/08927014.2011.626899. [DOI] [PubMed] [Google Scholar]
- 3.Busscher HJ, van der Mei HC, Subbiahdoss G, Jutte PC, van den Dungen J, Zaat SAJ, Schultz MJ, Grainger DW. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012;4:153rv110. doi: 10.1126/scitranslmed.3004528. [DOI] [PubMed] [Google Scholar]
- 4.Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34:8533–8554. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 5.Wong TS, Kang SH, Tang SKY, Smythe EJ, Hatton BD, Grinthal A, Aizenberg J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature. 2011;477:443–447. doi: 10.1038/nature10447. [DOI] [PubMed] [Google Scholar]
- 6.Yao X, Hu YH, Grinthal A, Wong TS, Mahadevan L, Aizenberg J. Adaptive fluid-infused porous films with tunable transparency and wettability. Nat. Mater. 2013;12:529–534. doi: 10.1038/nmat3598. [DOI] [PubMed] [Google Scholar]
- 7.Liu HL, Zhang PC, Liu MJ, Wang ST, Jiang L. Organogel-based thin films for self-cleaning on various surfaces. Adv. Mater. 2013;25:4477–4481. doi: 10.1002/adma.201301289. [DOI] [PubMed] [Google Scholar]
- 8.Smith JD, Dhiman R, Anand S, Reza-Garduno E, Cohen RE, McKinley GH, Varanasi KK. Droplet mobility on lubricant-impregnated surfaces. Soft Matter. 2013;9:1772–1780. [Google Scholar]
- 9.Grinthal A, Aizenberg J. Mobile interfaces: Liquids as a perfect structural material for multifunctional, antifouling surfaces. Chem. Mater. 2014;26:698–708. [Google Scholar]
- 10.Leslie DC, Waterhouse A, Berthet JB, Valentin TM, Watters AL, Jain A, Kim P, Hatton BD, Nedder A, Donovan K, Super EH, Howell C, Johnson CP, Vu TL, Bolgen DE, Rifai S, Hansen AR, Aizenberg M, Super M, Aizenberg J, Ingber DE. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 2014;32:1134–1140. doi: 10.1038/nbt.3020. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JP, Wang AQ, Seeger S. Nepenthes Pitcher Inspired Anti-Wetting Silicone Nanofilaments Coatings: Preparation, Unique Anti-Wetting and Self-Cleaning Behaviors. Adv. Funct. Mater. 2014;24:1074–1080. [Google Scholar]
- 12.Glavan AC, Martinez RV, Subramaniam AB, Yoon HJ, Nunes RMD, Lange H, Thuo MM, Whitesides GM. Omniphobic "R-F Paper" Produced by Silanization of Paper with Fluoroalkyltrichlorosilanes. Adv. Funct. Mater. 2014;24:60–70. [Google Scholar]
- 13.Manna U, Lynn DM. Fabrication of liquid-infused surfaces using reactive polymer multilayers: Principles for manipulating the behaviors and mobilities of aqueous fluids on slippery liquid interfaces. Adv. Mater. 2015;27:3007–3012. doi: 10.1002/adma.201500893. [DOI] [PubMed] [Google Scholar]
- 14.MacCallum N, Howell C, Kim P, Sun D, Friedlander R, Ranisau J, Ahanotu O, Lin JJ, Vena A, Hatton B, Wong TS, Aizenberg J. Liquid-Infused Silicone As a Biofouling-Free Medical Material. ACS Biomater. Sci. Eng. 2015;1:43–51. doi: 10.1021/ab5000578. [DOI] [PubMed] [Google Scholar]
- 15.Sunny S, Vogel N, Howell C, Vu TL, Aizenberg J. Lubricant-Infused Nanoparticulate Coatings Assembled by Layer-by-Layer Deposition. Adv. Funct. Mater. 2014;24:6658–6667. [Google Scholar]
- 16.Epstein AK, Wong TS, Belisle RA, Boggs EM, Aizenberg J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. U. SA. 2012;109:13182–13187. doi: 10.1073/pnas.1201973109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JS, Kleintschek T, Rieder A, Cheng Y, Baumbach T, Obst U, Schwartz T, Levkin PA. Hydrophobic liquid-infused porous polymer surfaces for antibacterial applications. ACS Appl. Mater. Inter. 2013;5:6704–6711. doi: 10.1021/am401532z. [DOI] [PubMed] [Google Scholar]
- 18.Ueda E, Levkin PA. Micropatterning hydrophobic liquid on a porous polymer surface for long-term selective cell-repellency. Adv. Healthcare Mater. 2013;2:1425–1429. doi: 10.1002/adhm.201300073. [DOI] [PubMed] [Google Scholar]
- 19.Xiao LL, Li JS, Mieszkin S, Di Fino A, Clare AS, Callow ME, Callow JA, Grunze M, Rosenhahn A, Levkin PA. Slippery liquid-infused porous surfaces showing marine antibiofouling properties. ACS Appl. Mater. Inter. 2013;5:10074–10080. doi: 10.1021/am402635p. [DOI] [PubMed] [Google Scholar]
- 20.Leslie DC, Waterhouse A, Berthet JB, Valentin TM, Watters AL, Jain A, Kim P, Hatton BD, Nedder A, Donovan K, Super EH, Howell C, Johnson CP, Vu TL, Bolgen DE, Rifai S, Hansen AR, Aizenberg M, Super M, Aizenberg J, Ingber DE. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 2014;32:1134–1140. doi: 10.1038/nbt.3020. [DOI] [PubMed] [Google Scholar]
- 21.Manna U, Raman N, Welsh MA, Palecek SP, Blackwell HE, Lynn DM. Slippery liquid-infused porous surfaces that prevent microbial surface fouling and kill non-adherent pathogens in surrounding media: A controlled release approach. Adv. Funct. Mater. 2016;26 doi: 10.1002/adfm.201505522. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh CT, Wright GD. Antimicrobials. Curr. Opin. Microbiol. 2009;12:473–475. doi: 10.1016/j.mib.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 24.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Disc. 2010;9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 25.Allen RC, Popat R, Diggle SP, Brown SP. Targeting virulence: can we make evolution-proof drugs? Nat. Rev. Microbiol. 2014;12:300–308. doi: 10.1038/nrmicro3232. [DOI] [PubMed] [Google Scholar]
- 26.Njoroge J, Sperandio V. Jamming bacterial communication: New approaches for the treatment of infectious diseases. EMBO Molecular Medicine. 2009;1:201–210. doi: 10.1002/emmm.200900032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuster M, Sexton DJ, Diggle SP, Greenberg EP. Acyl-Homoserine Lactone Quorum Sensing: From Evolution to Application. In: Gottesman S, editor. Annual Review of Microbiology, Vol 67. 2013. pp. 43–63. [DOI] [PubMed] [Google Scholar]
- 30.Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor perspectives in medicine. 2012;2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amara N, Krom BP, Kaufmann GF, Meijler MM. Macromolecular Inhibition of Quorum Sensing: Enzymes, Antibodies, and Beyond. Chem. Rev. 2011;111:195–208. doi: 10.1021/cr100101c. [DOI] [PubMed] [Google Scholar]
- 32.Galloway W, Hodgkinson JT, Bovvden S, Welch M, Spring DR. Applications of small molecule activators and inhibitors of quorum sensing in Gram-negative bacteria. Trends Microbiol. 2012;20:449–458. doi: 10.1016/j.tim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Geske GD, O'Neill JC, Miller DM, Mattmann ME, Blackwell HE. Modulation of bacterial quorum sensing with synthetic ligands: systematic evaluation of N-acylated homoserine lactones in multiple species and new insights into their mechanisms of action. J. Am. Chem. Soc. 2007;129:13613–13625. doi: 10.1021/ja074135h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore JD, Rossi FM, Welsh MA, Nyffeler KE, Blackwell HE. A Comparative Analysis of Synthetic Quorum Sensing Modulators in Pseudomonas aeruginosa: New Insights into Mechanism, Active Efflux Susceptibility, Phenotypic Response, and Next-Generation Ligand Design. J. Am. Chem. Soc. 2015;137:14626–14639. doi: 10.1021/jacs.5b06728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welsh MA, Eibergen NR, Moore JD, Blackwell HE. Small Molecule Disruption of Quorum Sensing Cross-Regulation in Pseudomonas aeruginosa Causes Major and Unexpected Alterations to Virulence Phenotypes. J. Am. Chem. Soc. 2015;137:1510–1519. doi: 10.1021/ja5110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eibergen NR, Moore JD, Mattmann ME, Blackwell H. Potent and Selective Modulation of the RhlR Quorum Sensing Receptor by Using Non-native Ligands: An Emerging Target for Virulence Control in Pseudomonas aeruginosa. Chembiochem. 2015;16:2348–2356. doi: 10.1002/cbic.201500357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Reilly MC, Blackwell HE. Structure-Based Design and Biological Evaluation of Triphenyl Scaffold-Based Hybrid Compounds as Hydrolytically Stable Modulators of a LuxR-Type Quorum Sensing Receptor. ACS Infect. Dis. 2016;2:32–38. doi: 10.1021/acsinfecdis.5b00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsh MA, Blackwell HE. Chemical genetics reveals environment-specific roles for quorum sensing circuits in Pseudomonas aeruginosa. Cell Chem. Biol. 2016;23:361–369. doi: 10.1016/j.chembiol.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. U. SA. 2013;110:17981–17986. doi: 10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morkunas B, Galloway WRJD, Wright M, Ibbeson BM, Hodgkinson JT, O'Connell KMG, Bartolucci N, Della Valle M, Welch M, Spring DR. Inhibition of the production of the Pseudomonas aeruginosa virulence factor pyocyanin in wild-type cells by quorum sensing autoinducer-mimics. Org Biomol Chem. 2012;10:8452–8464. doi: 10.1039/c2ob26501j. [DOI] [PubMed] [Google Scholar]
- 41.Breitbach AS, Broderick AH, Jewell CM, Gunasekaran S, Lin Q, Lynn DM, Blackwell HE. Surface-mediated release of a synthetic small-molecule modulator of bacterial quorum sensing: Gradual release enhances activity. Chem. Commun. 2011;47:370–372. doi: 10.1039/c0cc02316g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manna U, Kratochvil MJ, Lynn DM. Superhydrophobic Polymer Multilayers that Promote the Extended, Long-Term Release of Embedded Water-Soluble Agents. Adv. Mater. 2013;25:6405–6409. doi: 10.1002/adma.201302561. [DOI] [PubMed] [Google Scholar]
- 43.Broderick AH, Breitbach AS, Frei R, Blackwell HE, Lynn DM. Surface-Mediated Release of a Small-Molecule Modulator of Bacterial Biofilm Formation: A Non-Bactericidal Approach to Inhibiting Biofilm Formation in Pseudomonas aeruginosa. Adv. Healthcare Mater. 2013;2:993–1000. doi: 10.1002/adhm.201200334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broderick AH, Stacy DM, Tal-Gan Y, Kratochvil MJ, Blackwell HE, Lynn DM. Surface Coatings that Promote Rapid Release of Peptide-Based AgrC Inhibitors for Attenuation of Quorum Sensing in Staphylococcus aureus. Adv. Healthcare Mater. 2014;3:97–105. doi: 10.1002/adhm.201300119. [DOI] [PubMed] [Google Scholar]
- 45.Kratochvil MJ, Tal-Gan Y, Yang T, Blackwell HE, Lynn DM. Nanoporous Superhydrophobic Coatings that Promote the Extended Release of Water-Labile Quorum Sensing Inhibitors and Enable Long-Term Modulation of Quorum Sensing in Staphylococcus aureus. ACS Biomater. Sci. Eng. 2015;1:1039–1049. doi: 10.1021/acsbiomaterials.5b00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hume EBH, Baveja J, Muir BW, Schubert TL, Kumar N, Kjelleberg S, Griesser HJ, Thissen H, Read R, Poole-Warren LA, Schindhelm K, Willcox MDP. The control of Staphylococcus epidermidis biofilm formation and in vivo infection rates by covalently bound furanones. Biomaterials. 2004;25:5023–5030. doi: 10.1016/j.biomaterials.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 47.Baveja JK, Wilcox MDP, Hume EBH, Kumar N, Odell R, Poole-Warren LA. Furanones as potential anti-bacterial coatings on biomaterials. Biomaterials. 2004;25:5003–5012. doi: 10.1016/j.biomaterials.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 48.Melander C, Moeller PDR, Ballard TE, Richards JJ, Huigens RW, III, Cavanagh J. Evaluation of dihydrooroidin as an antifouling additive in marine paint. International Biodeterioration & Biodegradation. 2009;63:529–532. doi: 10.1016/j.ibiod.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho KKK, Cole N, Chen R, Willcox MDP, Rice SA, Kumar N. Characterisation and in vitro activities of surface attached dihydropyrrol-2-ones against Gram-negative and Gram-positive bacteria. Biofouling. 2010;26:913–921. doi: 10.1080/08927014.2010.531463. [DOI] [PubMed] [Google Scholar]
- 50.Nowatzki PJ, Koepsel RR, Stoodley P, Min K, Harper A, Murata H, Donfack J, Hortelano ER, Ehrlich GD, Russell AJ. Salicylic acid-releasing polyurethane acrylate polymers as anti-biofilm urological catheter coatings. Acta Biomater. 2012;8:1869–1880. doi: 10.1016/j.actbio.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 51.Gomes J, Grunau A, Lawrence AK, Eberl L, Gademann K. Bioinspired, releasable quorum sensing modulators. Chem. Commun. 2013;49:155–157. doi: 10.1039/c2cc37287h. [DOI] [PubMed] [Google Scholar]
- 52.Ho KKK, Chen R, Willcox MDP, Rice SA, Cole N, Iskander G, Kumar N. Quorum sensing inhibitory activities of surface immobilized antibacterial dihydropyrrolones via click chemistry. Biomaterials. 2014;35:2336–2345. doi: 10.1016/j.biomaterials.2013.11.072. [DOI] [PubMed] [Google Scholar]
- 53.Nafee N, Husari A, Maurer CK, Lu C, de Rossi C, Steinbach A, Hartmann RW, Lehr C-M, Schneider M. Antibiotic-free nanotherapeutics: Ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J. Control. Release. 2014;192:131–140. doi: 10.1016/j.jconrel.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 54.Shenderovich J, Feldman M, Kirmayer D, Al-Quntar A, Steinberg D, Lavy E, Friedman M. Local sustained-release delivery systems of the antibiofilm agent thiazolidinedione-8 for prevention of catheter-associated urinary tract infections. Int. J. Pharm. 2015;485:164–170. doi: 10.1016/j.ijpharm.2015.02.067. [DOI] [PubMed] [Google Scholar]
- 55.Lu HD, Spiegel AC, Hurley A, Perez LJ, Maisel K, Ensign LM, Hanes J, Bassler BL, Semmelhack MF, Prud'homme RK. Modulating Vibrio cholerae Quorum-Sensing-Controlled Communication Using Autoinducer-Loaded Nanoparticles. Nano Lett. 2015;15:2235–2241. doi: 10.1021/acs.nanolett.5b00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buck ME, Zhang J, Lynn DM. Layer-by-layer assembly of reactive ultrathin films mediated by click-type reactions of poly(2-alkenyl azlactone)s. Adv. Mater. 2007;19:3951–3955. [Google Scholar]
- 57.Manna U, Broderick AH, Lynn DM. Chemical Patterning and Physical Refinement of Reactive Superhydrophobic Surfaces. Adv. Mater. 2012;24:4291–4295. doi: 10.1002/adma.201200903. [DOI] [PubMed] [Google Scholar]
- 58.Manna U, Lynn DM. Synthetic Surfaces with Robust and Tunable Underwater Superoleophobicity. Adv. Funct. Mater. 2015;25:1672–1681. [Google Scholar]
- 59.Muh U, Schuster M, Heim R, Singh A, Olson ER, Greenberg EP. Novel Pseudomonas aeruginosa quorum-sensing inhibitors identified in an ultra-high-throughput screen. Antimicrob. Agents Chemother. 2006;50:3674–3679. doi: 10.1128/AAC.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frei R, Breitbach AS, Blackwell HE. 2-Aminobenzimidazole derivatives strongly inhibit and disperse Pseudomonas aeruginosa biofilms. Angew. Chem. Int. Ed. 2012;51:5226–5229. doi: 10.1002/anie.201109258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rada B, Leto TL. Pyocyanin effects on respiratory epithelium: relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. 2013;21:73–81. doi: 10.1016/j.tim.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glansdorp FG, Thomas GL, Lee JJK, Dutton JM, Salmond GPC, Welch M, Spring DR. Synthesis and stability of small molecule probes for Pseudomonas aeruginosa quorum sensing modulation. Org. Biomol. Chem. 2004;2:3329–3336. doi: 10.1039/B412802H. [DOI] [PubMed] [Google Scholar]
- 63.Howell C, Vu TL, Johnson CP, Hou X, Ahanotu O, Alvarenga J, Leslie DC, Uzun O, Waterhouse A, Kim P, Super M, Aizenberg M, Ingber DE, Aizenberg J. Stability of Surface-Immobilized Lubricant Interfaces under Flow. Chem. Mater. 2015;27:1792–1800. [Google Scholar]
- 64.Howell C, Vu TL, Lin JJ, Kolle S, Juthani N, Watson E, Weaver JC, Alvarenga J, Aizenberg J. Self-Replenishing Vascularized Fouling-Release Surfaces. ACS Appl. Mater. Inter. 2014;6:13299–13307. doi: 10.1021/am503150y. [DOI] [PubMed] [Google Scholar]
- 65.Koch AK, Kappeli O, Fiechter A, Reiser J. Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J. Bacteriol. 1991;173:4212–4219. doi: 10.1128/jb.173.13.4212-4219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buck ME, Schwartz SC, Lynn DM. Superhydrophobic thin films fabricated by reactive layer-by-layer assembly of azlactone-functionalized polymers. Chem. Mater. 2010;22:6319–6327. doi: 10.1021/cm102115e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geske GD, Mattmann ME, Blackwell HE. Evaluation of a focused library of N-aryl L-homoserine lactones reveals a new set of potent quorum sensing modulators. Bioorg. Med. Chem. Lett. 2008;18:5978–5981. doi: 10.1016/j.bmcl.2008.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holloway BW. Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 1955;13:572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- 69.Mellbye B, Schuster M. Physiological framework for the regulation of quorum sensing-dependent public goods in Pseudomonas aeruginosa. J. Bacteriol. 2014;196:1155–1164. doi: 10.1128/JB.01223-13. [DOI] [PMC free article] [PubMed] [Google Scholar]