Abstract
Objective
HIV-Seropositive patients have higher risk of HPV infection even on anti-retroviral therapy. Infection with high-risk HPV genotypes can cause dysplasia leading to cancer. This study assessed HPV at different anatomical sites in HIV-seropositive individuals and factors associated with anal squamous intraepithelial lesions (ASIL).
Methods
Specimens were obtained from multiple anatomical sites for each participant in conjunction with routine screening for anal dysplasia. Female specimens included cervical and anal cytologies and oral wash. Male specimens included anal cytologies, oral wash, and exfoliated cells from penile head, penile shaft, scrotum, and from uncircumcised subjects, inner foreskin. Demographic and clinical characteristics were recorded. Following DNA extraction, HIV DNA copy was assessed by qPCR; HPV was genotyped. Statistical analyses included calculation of odds ratios (OR) and 95% confidence intervals (CI), t-tests or Mann-Whitney tests.
Results
Males were more likely to have ASIL: 29/50 (58%) compared to 1/11 females (9%) (OR=13.81, 95% CI: 1.64–116.32). HPV 6 or 11 in anal specimens was significantly associated with ASIL (OR= 6.29, 95% CI: 1.49–26.44). Number of HPV genotypes in anal specimens was also significant: ASIL+ (3.4 ± 3.1) versus ASIL− (1.6 ± 3.1) (p=0.009). Among 44 males, HPV was detected from at least one anatomical site for 33 participants (75%): 27 anus (61%), 19 oral wash (44%), 17 penile shaft (39%), 11 scrotum (26%), 10 penile head (23%), 0 foreskin. Detection of HPV in penile shaft specimens was significantly associated with ASIL (OR=6.79, 95% CI: 1.57–29.36) as was number of HPV genotypes in penile shaft specimens: ASIL+ (2.4 ± 4.0) versus ASIL− (0.6 ± 1.7) (p=0.025).
Only 1/11 females had ASIL; only 1/11 females had cervical dysplasia: OR was not estimable due to small numbers.
Conclusions
Males were more prone to ASIL than females. HPV at anal as well as non-anal sites may be indicative of ASIL.
Keywords: Human papillomavirus, Anal cancer, Anal dysplasia, Human immunodeficiency virus, HPV, HIV
INTRODUCTION
Individuals infected with human immunodeficiency virus type 1 (HIV) are at increased risk for human papillomavirus (HPV) infection and for anal dysplasia/cancer [1–4]. Presence of multiple HPV genotypes as well as presence of HPV at other anatomical sites potentially increase the risk for anal dysplasia/cancer [1,2,5,6]. While high-risk HPV genotypes are found in more than 90% of anal cancers among HIV-infected patients, there may be additional factors that lead to anal dysplasia/cancer [6,7]. Co-infection with HIV and HPV in the anal canal and presence of HPV at other anatomical sites may support ongoing exposure to HPV and/or HIV-related immune suppression, leading to increased risk for dysplasia/cancer. Recent data suggest that the continued persistence of HIV DNA in circulating monocytes in patients treated with combination antiretroviral therapy (cART) leads to progression of HIV disease itself and other HIV-associated complications, which may include HPV infection and associated anal neoplasia [8–11]. The objective of this study was to evaluate specimens from various anatomical sites of the same HIV-positive individual for HPV genotypes in relation to anal squamous intraepithelial lesions (ASIL). We hypothesized that ASIL would be associated with presence of HPV at multiple anatomical sites.
MATERIALS AND METHODS
During a 12-month period, men and women, 18–65 years of age, were either self-referred or referred by community physicians for anal dysplasia/cancer screening in collaboration with the Hawaii Center for AIDS, University of Hawaii (UH), and UH Cancer Center. Subjects were included if HIV-positive regardless of previous history of HPV infection, anal dysplasia/cancer, or related treatment. Participants provided written consent in accordance with UH Institutional Review Board policy. Two anal cytology specimens were collected with a Dacron swab [12] and stored in ThinPrep collection medium (Hologic, Inc., Bedford, MA). One anal specimen was processed by a CLIA-certified clinical laboratory with cytopathology reviewed and reported by the same experienced cytopathologist (JK) according to the Bethesda system: anal cytology was evaluated, using criteria and terminology adapted from standardized cervical cytology screening [6,13–19]. The other anal specimen was assayed for HPV and HIV DNA. Anal cytology specimens were assessed for adequacy and categorized as 1) Negative (ASIL−) if no cellular changes could be detected or if cellular changes were caused by inflammation or reparative process or 2) Positive for anal squamous intraepithelial lesions (ASIL+) if abnormal cytological changes were found, including high-grade squamous intraepithelial lesions (HSIL), low-grade squamous intraepithelial lesions (LSIL), atypical squamous cells of undetermined significance (ASC-US), and atypical squamous cells which cannot exclude HSIL (ASC-H). Oral wash specimens were collected according to methods previously established [19]. From male participants, exfoliated cells from the penile glans/coronal sulcus, penile shaft, and scrotum were collected consecutively from each site and placed in separate collection vials as previously described [19]. Inner foreskin specimens were collected from uncircumcised subjects. Cervical cytology specimens were also obtained from female participants however no vaginal or vulva samplings were performed. Other data obtained per informed consent included plasma HIV RNA viral load and nadir CD4 cell count as well as age, gender, and ethnicity. Not all specimens and data were collected from all participants. DNA from each specimen was extracted using QIAamp DNA Micro Kit (Qiagen Inc, Valencia, CA) and analyzed for presence or absence of HPV DNA by PCR using a modified version of the PGMY09/PGMY11 primer system [20]. β-globin-positive and HPV DNA-positive specimens were genotyped using the Linear Array HPV Genotyping Test to detect 37 different HPV types (Roche Molecular Diagnostics, Pleasanton, CA), including 13 high-risk types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68). Anal specimens had HIV DNA copy number quantified by real-time PCR as previously published [9]. Primers and probes included HIV gag (forward 5’-GAC ATC AAG CAG CCA TGC AA-3’; reverse 5’-CTC ATC TGG CCT GGT GCA AT-3’) and β−globin (forward 5’-AGG GCC TCA CCA CCA ACT TC; reverse 5’-TCA CTA GCA ACC TCA AAC AGA CAC C-3’) primers; and VIC-labeled HIV gag (5’ACC ATC AAT GAG GAA GCT GCA GAA TGG GA-3’) and FAM-labeled β-globin (5’-CTC CTG AGG AGA AGT CTG CCG TTA CTG CC-3’) probes. Controls included OM10.1 cells, each carrying a single, integrated HIV provirus; and water. Assays were performed in triplicate. Resulting data were analyzed using StepOne Plus software (Thermo Fisher Scientific, Waltham, MA). Copy numbers of each target gene were calculated based on the standard curve, and HIV DNA copy numbers per 1 × 106 cells were determined.
Statistical analyses were conducted by JABSOM Biostatistics & Quantitative Health Sciences. Mean age of participant subsets were compared using t-tests. Odds ratios (OR), 95% confidence intervals (CI), and p-values were calculated to investigate associations between anal cytology results (ASIL+ or ASIL−) and patient characteristics as well as HPV at various anatomical sites. For analyses, specimens were designated high-risk HPV-positive if one or more of 13 high-risk HPV genotypes (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) was detected. The numbers of HPV genotypes or high-risk HPV genotypes per specimen were compared according to anal cytology results (ASIL+ or ASIL−) using Mann-Whitney tests. Kappa coefficient and McNemar’s test p-value were used to assess co-incident HPV genotypes.
RESULTS
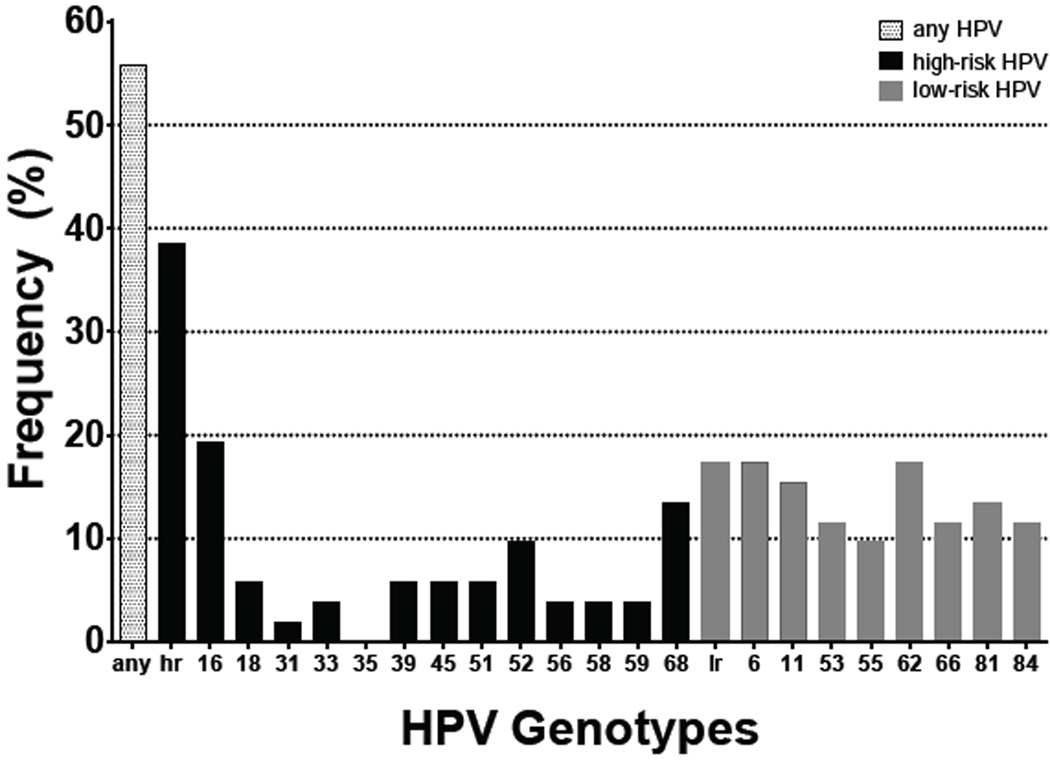
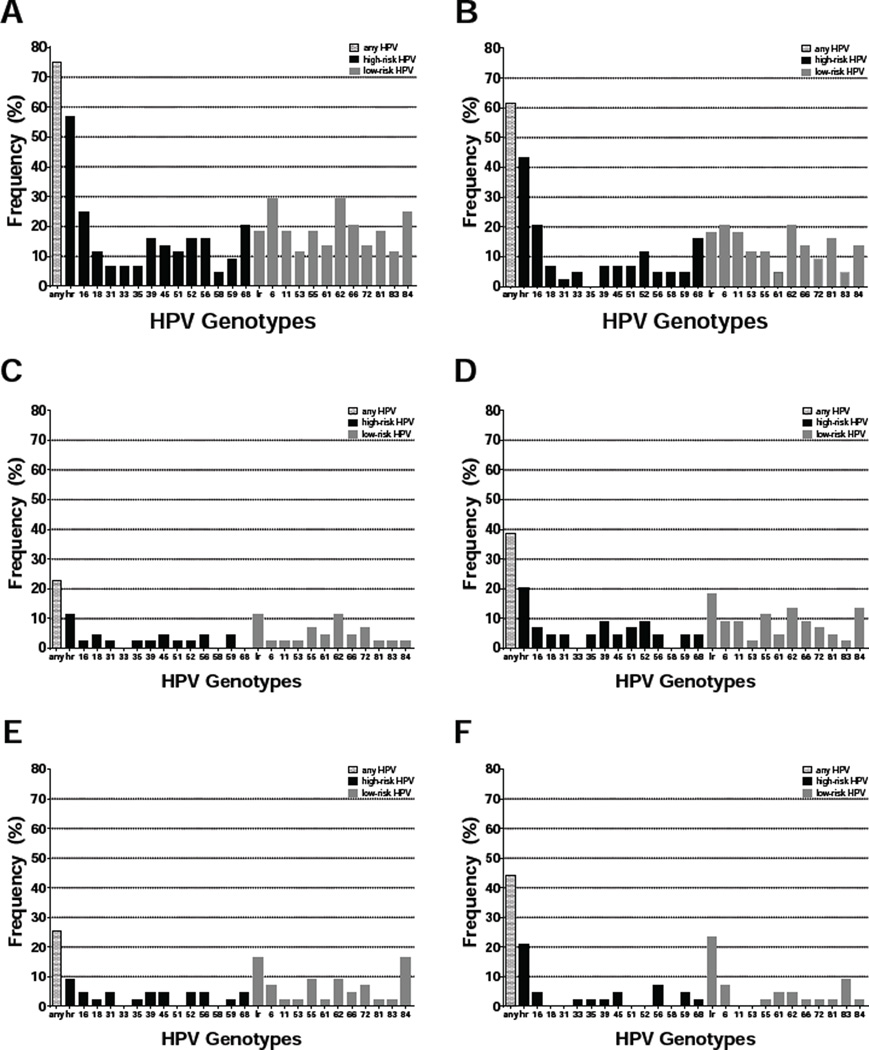
Out of 61 participants (50 males and 11 females) enrolled in the study, 30 (49%) presented with ASIL. Data was not available to determine severity of atypical cytology. None of the participants were previously diagnosed with a relevant cancer. Mean age with standard deviation was similar for ASIL+ (50.1 ± 8.1) compared to ASIL− (47.4 ± 8.8) participants (p=0.275) as well as males (48.8 ± 9.1) compared to females (49.4 ± 5.2) (p=0.735) (Table 1). Men had significantly higher odds of presenting with ASIL: 29/50 (58%) compared to 1/11 (9%) women (OR=13.81, 95% CI: 1.64–116.32). Race/ethnicity was not a notable factor for ASIL: 13/27 (48%) non-White versus 17/34 (50%) White participants (OR=0.93, 95% CI: 0.38–2.55). All participants were HIV-seropositive and on combination antiretroviral therapy (cART), with 41/48 males (85%) and 9/9 females (100%) exhibiting undetectable plasma HIV levels. Data regarding length of HIV infection and cART use was not collected for this study. Participants with detectable viral levels had higher odds of presenting with ASIL though not statistically significant (OR=2.93, 95% CI: 0.52–16.58) (Table 1). Likewise, nadir CD4 ≤ 200 cells/mm3 conferred higher odds of ASIL but not statistically significant (OR=2.30, 95% CI: 0.80–6.61). Detectable HIV DNA in anal specimens was not associated with ASIL (OR=0.91, 95% CI: 0.31–2.64). Among 52 anal specimens collected and assayed, 29 (56%) were positive for any HPV; 20 (38%) were positive for one or more high-risk HPV genotypes (Figure 1). Genotypes detected most frequently in anal specimens included HPV 6 (17%) and 11 (15%), which are associated with genital warts; HPV 16 (19%), 52 (10%), and 68 (13%), which are high-risk types associated with cancer; as well as low-risk types like HPV 53 (12%), 55 (10%), 62 (17%), 66 (12%), 81 (13%), and 84 (12%). Other HPV genotypes were detected at frequencies below 10%. HPV in anal specimens conferred at least two-fold higher odds of ASIL (Table 2). Detection of HPV 6 or 11 in anal specimens was significantly associated with ASIL (OR=6.29, 95% CI: 1.49–26.44) while detection of any HPV approached statistical significance (OR=2.65, 95% CI: 0.86–8.24). The number of HPV genotypes detected in anal specimens was also significantly different for ASIL+ (3.4 ± 3.1) versus ASIL− (1.6 ± 3.1) participants (p=0.009). Multiple anatomical site specimens were collected from 44 males: 25 (57%) were ASIL+ while 19 (43%) were ASIL−. HPV was detected in these specimens as follows: 27/44 (61%) anal, 19/43 (44%) oral wash, 17/44 (39%) penile shaft, 11/43 (26%) scrotum, 10/44 (23%) penile head, and 0/7 foreskin. Overall, 33/44 (75%) males had at least one HPV+ specimen. HPV was detectable at more anatomical sites among ASIL+ (1.3 ± 1.8) versus ASIL− (0.6 ± 1.1) males (p=0.045). While 33/44 (75%) males were positive for any HPV across all anatomical sites, 25 (57%) were positive for one or more high-risk HPV genotypes (Figure 2). Across all specimens, genotypes detected most frequently among male participants included HPV 6 (30%) and 11 (18%), which are associated with genital warts; HPV 16 (25%), 18 (11%), 39 (16%), 45 (14%), 51 (11%), 52 (16%), 56 (16%), and 68 (20%), which are high-risk types associated with cancer; as well as low-risk types like HPV 53 (11%), 55 (18%), 61 (14%), 62 (30%), 66 (20%), 72 (14%), 81 (18%), 83 (11%) and 84 (25%). Other HPV genotypes were detected at frequencies below 10%. In relation to ASIL, the number of unique HPV genotypes detected across all anatomical sites per participant approached statistical significance: ASIL+ (5.3 ± 5.4) compared to ASIL− (2.6 ± 4.2) males (p=0.054). For all but one association between male specimen site and HPV variable tested, detectable HPV corresponded with increased odds of ASIL (Table 3). In particular, detection of HPV 16 or 18 proffered at least two-fold higher odds of ASIL across specimen types. Interestingly, penile shaft specimens displayed OR>3 across HPV variables. Any HPV in penile shaft specimens was significantly associated with ASIL (OR=6.79, 95% CI: 1.57–29.36) while detection of HPV 6 or 11 in anal specimens approached statistical significance (OR=4.19, 95% CI: 0.97–18.12). The number of HPV genotypes detected at some male specimen sites also differed according to ASIL status: significant for anal (p=0.0496) and penile shaft (p=0.025) specimens and approaching significance for scrotum (p=0.087) specimens (Table 4). Of 11 females enrolled in the study, only one presented with ASIL, and only one presented with cervical dysplasia. Odds ratios, 95% confidence intervals, and p-values were not estimable due to small numbers.
Table 1.
Demographic and Clinical Characteristics of Participants and Association with ASIL.
| Anal Cytology, n | |||||
|---|---|---|---|---|---|
| Variable | ASIL+ | ASIL− | OR | 95% CI | p-value |
| Age (yrs.), Mean ± SD | 50.1 ± 8.1 | 47.7 ± 8.8 | 0.275 | ||
| Gender (n=61) | |||||
| Male | 29 | 21 | 13.81 | 1.64–116.32 | 0.016 |
| Female | 1 | 10 | |||
| Race/Ethnicity (n=61) | |||||
| White | 17 | 17 | |||
| Asian | 4 | 5 | |||
| Native Hawaiian | 3 | 5 | |||
| Native American | 3 | 2 | |||
| African-American | 2 | 2 | |||
| Hispanic | 1 | 0 | |||
| Nadir CD4 (n=58)* | |||||
| ≤ 200 cells/mm3 | 19 | 12 | 2.30 | 0.80–6.61 | 0.121 |
| > 200 cells/mm3 | 11 | 16 | |||
| Plasma HIV Viral Load (n=58)* | |||||
| Detectable | 5 | 2 | 2.93 | 0.52–16.58 | 0.223 |
| Not detectable | 23 | 27 | |||
| Anal HIV DNA (n=59)* | |||||
| Detectable | 10 | 11 | 0.91 | 0.31–2.64 | 0.861 |
| Not detectable | 19 | 19 | |||
ASIL = anal squamous intraepithelial lesion
OR = odds ratio
95% CI = 95% confidence interval
SD = standard deviation
Data not available for all participants
Figure 1.
Percent frequency of HPV in anal specimens (n=52, male and female) containing any detectable HPV (any), one or more high-risk HPV genotypes (hr), only low-risk HPV genotypes (lr), or specific HPV genotypes, including 13 high-risk types associated with cancer as well as low-risk types 6 and 11, associated with genital warts, and others appearing at high frequency.
Table 2.
Association between HPV in Anal Specimens (n=52) and ASIL.
| Anal Cytology, n | |||||
|---|---|---|---|---|---|
| Variable | ASIL+ | ASIL− | OR | 95% CI | p-value |
| Any HPV | |||||
| Detectable | 17 | 12 | 2.65 | 0.86–8.24 | 0.091 |
| Not detectable | 8 | 15 | |||
| Any high-risk HPV | |||||
| Detectable | 12 | 8 | 2.19 | 0.70–6.85 | 0.177 |
| Not detectable | 13 | 19 | |||
| HPV 6 or 11 | |||||
| Detectable | 11 | 3 | 6.29 | 1.49–26.44 | 0.012 |
| Not detectable | 14 | 24 | |||
| HPV 16 or 18 | |||||
| Detectable | 7 | 4 | 2.24 | 0.57–8.84 | 0.251 |
| Not detectable | 18 | 23 | |||
| # HPV Genotypes, Mean ± SD | 3.4 ± 3.1 | 1.6 ± 3.1 | 0.009 | ||
| # High-risk HPV Genotypes, Mean ± SD | 1.2 ± 1.5 | 0.5 ± 1.2 | 0.105 | ||
ASIL -Anal Squamous Intraepithelial Lesion
OR - Odds Ratio
95% CI = 95% confidence interval
SD - Standard Deviation
Figure 2.
Percent frequency of HPV in male specimens containing any detectable HPV (any), one or more high-risk HPV genotypes (hr), only low-risk HPV genotypes (lr), or specific HPV genotypes, including 13 high-risk types associated with cancer as well as low-risk types 6 and 11, associated with genital warts, and others appearing at high frequency: A - all sites (n=44), B - anus (n=44), C - penile head (n=44), D - penile shaft (n=44), E - scrotum (n=43), F - oral wash (n=43).
Table 3.
Association between HPV at Male Specimen Sites and ASIL.
| Specimen Site, OR (95% CI) | |||||
|---|---|---|---|---|---|
| Variable | Anus (n=44) |
Penile Head (n=44) |
Penile Shaft (n=44) |
Scrotum (n=43) |
Oral Wash (n=43) |
| Any HPV | 1.91 (0.56–6.55) |
4.00 (0.74–21.66) |
6.79* (1.57–29.36) |
2.67 (0.60–11.92) |
1.71 (0.50–5.86) |
| Any high-risk HPV | 1.58 (0.47–5.35) |
3.43 (0.35–33.52) |
3.31 (0.60–18.20) |
2.57 (0.25–26.94) |
1.78 (0.38–8.30) |
| HPV 6 or 11 | 4.19° (0.97–18.12) |
0.75 (0.04–12.82) |
5.68 (0.62–51.97) |
1.64 (0.14–19.54) |
1.64 (0.14–19.54) |
| HPV 16 or 18 | 2.07 (0.46–9.40) |
4.15 (0.19–91.66) |
3.43 (0.35–33.52) |
6.35 (0.31–130.87) |
4.33 (0.20–96.84) |
ASIL-Anal Squamous Intraepithelial Lesion
OR-Odds Ratio
95% CI = 95% confidence interval
Statistically significant (p=0.010)
Approaching statistical significance (p=0.055)
Table 4.
Association between Number of HPV Genotypes at Male Specimen Sites and ASIL.
| # HPV Genotypes, Mean ± SD |
|||
|---|---|---|---|
| Specimen Site | ASIL+ | ASIL− | p-value |
| Anus (n=44) | 3.4 ± 3.1 | 1.9 ± 3.6 | 0.0496 |
| Penile Head (n=44) | 1.2 ± 2.9 | 0.5 ± 1.8 | 0.367 |
| Penile Shaft (n=44) | 2.4 ± 4.0 | 0.6 ± 1.7 | 0.025 |
| Scrotum (n=43) | 1.7 ± 3.6 | 0.5 ± 2.1 | 0.087 |
| Oral Wash (n=43) | 0.8 ± 1.3 | 0.5 ± 0.8 | 0.635 |
ASIL - Anal Squamous Intraepithelial Lesion
SD - Standard Deviation
DISCUSSION
Among our participants, men presented with ASIL at a higher frequency than women. Male participants were predominantly men who have sex with men (MSM), whose behavioral factors place them at higher risk for anal dysplasia/cancer [21] though such risk factors were not assessed in this study. Consistent with literature, female participants presented with lower rates of anal and cervical dysplasia. ASIL did not differ by race/ethnicity in our diverse group of participants. Previous studies have linked low CD4 cell counts to risk for ASIL. Bertisch et al. identified significant associations between anal cancer and low CD4 counts at nadir, at anal cancer diagnosis, and particularly 6–7 years before diagnosis [22]. While nadir CD4 and plasma HIV viral levels increased the odds of ASIL among our participants, the results were not statistically significant, perhaps owing to the small sample size. In the current cART era with effective reduction of HIV RNA to undetectable levels, presence of both HPV and residual HIV effects could establish a local, chronic inflammatory and metaplastic environment conducive to dysplastic expansion [9,23]. Contrary to expectations, detectable HIV DNA in anal specimens was not associated with ASIL. Analysis of HPV in anal specimens did produce some results consistent with current literature and knowledge. HIV-seropositive patients in our study exhibited a high frequency of HPV DNA present in anal specimens, with HPV 16 as the most frequently occurring genotype. Like previous studies, detectable anal HPV and the number of anal HPV genotypes was associated with ASIL [5]. Our results also showed a high degree of association between HPV 6 or 11 in anal specimens and ASIL. Further analysis demonstrated only slight agreement between anal specimens that were positive for HPV 6 or 11 and those positive for high-risk HPV (kappa=0.20, McNemar’s test p-value=0.11). Therefore, association between HPV 6 or 11 and ASIL is unlikely to be due to co-incidence of HPV 6 or 11 with high-risk HPV genotypes. Although HPV 6 and 11 are known for causing genital warts but not cancer, the pathologist’s finding of abnormal cellular changes may correspond to condyloma triggered by these genotypes. More surprising, however, were the associations between HPV in non-anal male specimens and ASIL. All but one association tested displayed higher odds of ASIL among males with HPV at non-anal sites. The association was particularly significant for detectable HPV in penile shaft specimens and less so for oral wash specimens; the variability among sites was corroborated by analysis of association between number of HPV genotypes at specimen site and ASIL. Although less well-studied, especially among HIV-positive MSM, penile HPV infection and penile intraepithelial neoplasia (PIN) have been recorded at lower rates than anal HPV infection and anal intraepithelial neoplasia (AIN) [24]. In a study of 263 HIV-positive MSM in Germany, Kreuter et al., reported 156 (59%) cases of AIN but only 11 (4%) of PIN [25]. Moreover, only 63% of penile cancers are attributable to HPV infection compared to 91% of anal cancers [26]. In light of this evidence, the high-degree of association between penile shaft HPV and ASIL in our study is unlikely to be due to co-incident penile dysplasia. Our discovery linking HPV at non-anal sites to ASIL may reflect autoinoculation -- transfer of HPV from the anus to other sites by the participant himself -- or behavioral factors among the predominantly MSM participants resulting in HPV introduction across multiple anatomical sites. Nevertheless, these results suggest that HPV at non-anal sites may also serve as indicators for ASIL among HIV-positive males. Previous studies of women have described strong concordance of HPV and dysplasia between cervical and anal sites as well as probable autoinoculation between the two [27–29]. Only one HIV-positive female in our study presented with ASIL, and another presented with cervical dysplasia. Associations between cervical HPV and dysplasia with anal HPV and dysplasia could not be estimated due to small sample size. Few studies have reported on the clinical significance of HPV in non-anal sites with respect to ASIL risk in HIV-seropositive individuals. Rather, studies have reported on the natural history or presence of HPV in multiple genital sites irrespective of ASIL [6,7]. Our cross-sectional study reports for the first time that presence of HPV at multiple sites is associated with ASIL among HIV-positive males. Because HIV-seropositive individuals have an increased risk for anal dysplasia/cancer [1,3,30], studies that demonstrate HPV presence at non-anal sites may have important implications for diagnosis of anal dysplasia and for better understanding risk factors leading to anal cancer in the setting of HIV. Our study was limited by the number of participants enrolled after referral for routine anal dysplasia screening and by cross-sectional design. Future investigations warrant enrollment of larger numbers of patients to assess the relationship between ASIL and HPV at non-anal sites among men and women in the context of HIV.
Acknowledgments
The study was supported by grants U56CA096254, P20RR011091, U54CA143727, U01CA121947, U54RR026136, U54MD007584, G12MD007601, and P20GM103466 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. A special thanks is extended to the participants of the study.
B.Y. Hernandez has received consultation fees from Merck, Inc. for work unrelated to this study.
REFERENCES
- 1.Goldstone SE, Moshier E. Detection of oncogenic human papillomavirus impacts anal screening guidelines in men who have sex with men. Dis Colon Rectum. 2010;53:1135–1142. doi: 10.1007/DCR.0b013e3181e10842. [DOI] [PubMed] [Google Scholar]
- 2.Kreuter A, Brockmeyer NH, Altmeyer P, Wieland U. German Competence Network HIV/AIDS. Anal intraepithelial neoplasia in HIV infection. J Dtsch Dermatol Ges. 2008;6:925–934. doi: 10.1111/j.1610-0387.2008.06737.x. [DOI] [PubMed] [Google Scholar]
- 3.Palefsky JM, Holly EA, Efirdc JT, Da Costa M, Jay N, Berry JM, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19:1407–1414. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 4.Salit IE, Lytwyn A, Raboud J, Sano M, Chong S, Diong C, et al. The role of cytology (Pap tests) and human papillomavirus testing in anal cancer screening. AIDS. 2010;24:1307–1313. doi: 10.1097/QAD.0b013e328339e592. [DOI] [PubMed] [Google Scholar]
- 5.del Amo J, Gonzalez C, Geskus RB, Torres M, Del Romero J, Viciana P, et al. What drives the number of high-risk human papillomavirus types in the anal canal in HIV-positive men who have sex with men? J Infect Dis. 2013;207:1235–1241. doi: 10.1093/infdis/jit028. [DOI] [PubMed] [Google Scholar]
- 6.van Rijn VM, Mooij SH, Mollers M, Snijders PJ, Speksnijder AG, King AJ, et al. Anal, penile, and oral high-risk HPV infections and HPV seropositivity in HIV-positive and HIV-negative men who have sex with men. PLoS One. 2014;9:92208. doi: 10.1371/journal.pone.0092208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Videla S, Darwich L, Canadas MP, Coll J, Pinol M, Garcia-Cuyas F, et al. Natural history of human papillomavirus infections involving anal, penile, and oral sites among HIV-positive men. Sex Transm Dis. 2013;40:3–10. doi: 10.1097/OLQ.0b013e31827e87bd. [DOI] [PubMed] [Google Scholar]
- 8.Shiramizu B, Ananworanich J, Chalermchai T, Siangphoe U, Troelstrup D, Shikuma C, et al. Failure to clear intra-monocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. J Neurovirol. 2012;18:69–73. doi: 10.1007/s13365-011-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, et al. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci. 2012;24:71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Re MC, Vitone F, Bon I, Schiavone P, Gibellini D. Meaning of DNA detection during the follow-up of HIV-1 infected patients: a brief review. New Microbiol. 2006;29:81–88. [PubMed] [Google Scholar]
- 11.Rouzioux C, Hubert JB, Burgard M, Deveau C, Goujard C, Bary M, et al. Early levels of HIV-1 DNA in peripheral blood mononuclear cells are predictive of disease progression independently of HIV-1 RNA levels and CD4+ T cell counts. J Infect Dis. 2005;192:46–55. doi: 10.1086/430610. [DOI] [PubMed] [Google Scholar]
- 12.Anal Dysplasia and Cancer. New York: New York State Department of Health AIDS Institute; New York State Department of Health AIDS Institute Office of the Medical Director, Johns Hopkins University Division of Infectious Diseases. [Google Scholar]
- 13.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 14.Darragh TM. Cervical human papillomavirus testing to triage borderline abnormal pap tests in HIV-coinfected women. AIDS. 2014;28:1696–1698. doi: 10.1097/QAD.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ ASCCP-Sponsored Consensus Conference. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 16.D’Souza G, Burk RD, Palefsky JM, Massad LS, Strickler HD, et al. WIHS HPV Working Group. Cervical human papillomavirus testing to triage borderline abnormal pap tests in HIV-coinfected women. AIDS. 2014;28:1696–1698. doi: 10.1097/QAD.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darragh TM, Birdsong GG. Anal Rectal Cytology. In: Solomon D, Nayar R, editors. The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria and Explanatory Notes. 2nd. New York: Springer; 2004. pp. 169–175. [Google Scholar]
- 18.Darragh TM, Winkler B. Anal cancer and cervical cancer screening: key differences. Cancer Cytopathol. 2011;119:5–19. doi: 10.1002/cncy.20126. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez BY, Wilkens LR, Unger ER, Steinau M, Markowitz L, Garvin K, et al. Evaluation of genital self-sampling methods for HPV detection in males. J Clin Virol. 2013;58:168–175. doi: 10.1016/j.jcv.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 20.Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlée F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Souza G, Wiley DJ, Li X, Chmiel JS, Margolick JB, Cranston RD, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008;48:491–499. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertisch B, Franceschi S, Lise M, Vernazza P, Keiser O, Schöni-Affolter F, et al. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol. 2013;178:877–884. doi: 10.1093/aje/kwt153. [DOI] [PubMed] [Google Scholar]
- 23.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 24.Kreuter A, Wieland U. Human papillomavirus-associated diseases in HIV-infected men who have sex with men. Curr Opin Infect Dis. 2009;22:109–114. doi: 10.1097/QCO.0b013e3283229fc8. [DOI] [PubMed] [Google Scholar]
- 25.Kreuter A, Brockmeyer NH, Weissenborn SJ, Gambichler T, Stücker M, Altmeyer P, et al. Penile intraepithelial neoplasia is frequent in HIVpositive men with anal dysplasia. below J Invest Dermatol. 2008;128:2316–2324. doi: 10.1038/jid.2008.72. [DOI] [PubMed] [Google Scholar]
- 26.Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107:djv086. doi: 10.1093/jnci/djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman MT, Shvetsov JYB, McDuffie K, Wilkens LR, Zhu X, Thompson PJ, et al. Sequential acquisition of human papillomavirus (HPV) infection of the anus and cervix: the Hawaii HPV Cohort Study. J Infect Dis. 2010;201:1331–1339. doi: 10.1086/651620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calore EE, Giaccio CM, Nadal SR. Prevalence of anal cytological abnormalities in women with positive cervical cytology. Diagn Cytopathol. 2011;39:323–327. doi: 10.1002/dc.21386. [DOI] [PubMed] [Google Scholar]
- 29.Moscicki AB, Schiffman M, Burchell A, Albero G, Giuliano AR, Goodman MT, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012;5:24–33. doi: 10.1016/j.vaccine.2012.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunleavey R. The role of viruses and sexual transmission in anal cancer. Nurs Times. 2005;101:38–41. [PubMed] [Google Scholar]