Abstract
BACKGROUND
Preterm infants with RDS given inositol had reduced BPD, death and severe ROP. We assessed the safety and pharmacokinetics(PK) of daily inositol to select a dose providing serum levels previously associated with benefit, and to learn if accumulation occurred when administered throughout the normal period of retinal vascularization.
METHODS
Infants ≤29wks GA (n=122, 14 centers) were randomized and treated with placebo or inositol at 10, 40 or 80mg/kg/day. Intravenous administration converted to enteral when feedings were established, and continued to the first of 10 weeks, 34weeks PMA or discharge. Serum collection employed a sparse sampling population PK design. Inositol urine losses and feeding intakes were measured. Safety was prospectively monitored.
RESULTS
At 80mg/kg/day mean serum levels reached 140mg/L, similar to Hallman’s findings. Levels declined after 2 weeks, converging in all groups by 6 wks. Analyses showed a mean volume of distribution 0.657 L/kg, clearance 0.058 L/kg/hr, and half-life 7.90 hr. Adverse events and co-morbidities were fewer in the inositol groups, but not significantly so.
CONCLUSIONS
Multiple dose inositol at 80mg/kg/day was not associated with increased adverse events, achieves previously effective serum levels, and is appropriate for investigation in a Phase 3 trial.
INTRODUCTION
Retinopathy of Prematurity is a common problem worldwide among preterm infants, often leading to vision impairment or blindness(1). Hallman reported two trials of postnatal inositol treatment of preterms with RDS to support phosphatidylinositol in surfactant synthesis, and both trials demonstrated improved RDS and a lower incidence of death or BPD, and ROP(2,3). Inositol is an important component of surfactant, and essential intracellularly as phosphoinositides. Howlett concluded in a Cochrane meta-analysis of inositol in preterm infants, “that a multi-center, randomized controlled trial of appropriate size is warranted to confirm these findings”(4). We reported the PK of a single dose of IV inositol in preterm infants at doses of 60mg/kg or 120mg/kg, and found the half-life was 5.22hr, with large urine losses, particularly in the first 12hr after dosing(5). Our 3 goals were to identify a daily dose to achieve serum levels similar to those reported by Hallman, [170mg/L (994μmole/L) at 8–9 days for infants given 160mg/kg/day, and an approximate mean value over the first week of life of 135mg/L (750μmole/L) when receiving 80mg/kg/day(2,6)]; to learn if divided doses would reduce urine losses; and to assure safety with up to 10 weeks of treatment. We examined the safety and PK of inositol given at 3 dose levels compared to placebo for up to 10wks, both IV and enteral (#NCT01030575). This time frame was chosen to support inositol levels throughout the post-preterm delivery period when most retinal vessel growth normally occurs within the high inositol, in-utero environment(7,8).
RESULTS
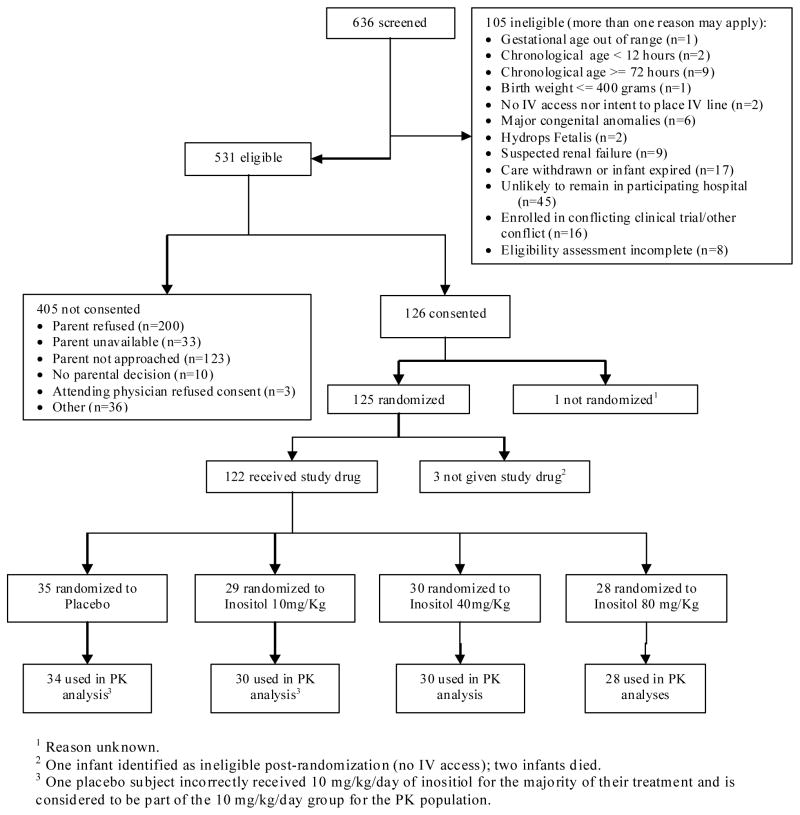
From January to October 2010, 125 infants ≤29weeks gestation were randomized and 122 received treatment during the time for the designed 96 infants to complete the protocol (Figure 1). The mean gestation was 26 weeks, and baseline characteristics were similar across groups (Table 1). Study drug was received for 42–51 days, and 43–57% of doses were IV. The number of missed or held doses was similar across groups (average of 1 to 3 per subject).
Figure 1. Consort Flow Sheet of Study Subjects.
1Reason unknown. 2One infant identified as ineligible post-randomization; two infants died. 3One placebo subject incorrectly received 10mg/kg/day of inositol for 7 days before it was discovered and stopped and is considered to be part of the 10m/kg/day group only for the PK analysis. However, other data from this subject are analyzed, as randomized, with the placebo infants.
Table 1.
Baseline Characteristics and Demographics
| Characteristic | Statistics | Inositol Dose group (mg/kg/day) | a P-value | |||
|---|---|---|---|---|---|---|
| 0 (N=35) | 10 (N=29) | 40 (N=30) | 80 (N=28) | |||
| GA (weeks) | Mean (SD) | 26.5 (0.3) | 26.6(0.3) | 26.7 (0.3) | 26.7 (0.4) | 0.95 |
| Lower GA stratum | 23–26 week n (%) | 19 (54%) | 15 (52%) | 16 (53%) | 14 (50%) | 0.99 |
| Birth weight (grams) | Mean (SD) | 884 (38) | 897 (51) | 939 (45) | 921 (54) | 0.83 |
| Head circumference cm | Mean (SD) | 23.5 (0.4) | 24.1 (0.4) | 25.1 (0.5) | 24.6 (0.4) | 0.10 |
| Gender | Female | 17 (49%) | 15 (52%) | 14 (47%) | 16 (57%) | 0.87 |
| Race | N. American Native | 0 | 3 (10%) | 0 | 0 | 0.12 |
| Asian | 0 | 1 (3%) | 1 (3%) | 0 | ||
| Black | 18 (51%) | 15 (52%) | 12 (40%) | 13 (46%) | ||
| More Than Once Race | 0 | 1 (3%) | 0 | 0 | ||
| White | 17 (49%) | 9 (31%) | 17 (57%) | 15 (54%) | ||
| Ethnicity | Hispanic or Latino | 9 (26%) | 3 (10%) | 7 (23%) | 8 (29%) | 0.35 |
| Antenatal steroids | 32 (91%) | 24 (83%) | 24 (80%) | 26 (93%) | 0.37 | |
| Chorioamnionitis | 5 (14%) | 4 (14%) | 4 (13%) | 3 (11%) | 0.97 | |
| Cesarean Delivery | 21 (60%) | 16 (55%) | 19 (63%) | 14 (50%) | 0.75 | |
| Early onset sepsis | 0 | 0 | 1 (3%) | 0 | 0.38 | |
| Apgar-1 minute | Median (range) | 3 (1–9) | 5 (1–8) | 3 (1–8) | 5 (1–8) | 0.97 |
| Apgar-5 minute | Median (range) | 7 (1–9) | 8 (1–9) | 7 (1–8) | 7 (3–9) | 0.97 |
P-values calculated by testing the null hypothesis of equality across all 4 treatment groups using ANOVA techniques for continuous measures, Mantel-Haenszel mean score tests using modified ridit scores for ordinal measures, and chi-square or Fisher’s exact test for nominal measures.
Safety Outcomes
At least one adverse event of moderate or greater severity occurred in 104 infants, and the average number/subject (5.5 to 5.7) was similar across treatment groups (Supplementary Table S1 (online)). No specific type of event occurred more frequently in the inositol groups compared to the placebo group. Per protocol, inositol doses were held for severe oliguria (renal losses of inositol are large enough that oliguria could have led to high serum inositol levels). This occurred in 5 infants on placebo and 2, 4, and 3 infants in the 10, 40 and 80mg/kg/day groups, respectively. Of these, 8 infants resumed study drug after recovery, 4 discontinued study drug permanently (1, placebo; 1, 10mg/kg/d; and 2, 80mg/kg/d), and 2 expired (both in the 40mg/kg/d group). Comparing adverse event rates in placebo vs all inositol, or across the dose groups, p-values were all >0.05 with most >0.10.
Serious adverse events (severe, life threatening or fatal) were common in this population (Table 2), but no specific types occurred more frequently in the inositol groups compared to placebo. Clinical diagnoses are listed in Table 3, and all 15 deaths occurred in the 23–26 week GA stratum. Infection was reported as a primary cause of death in the 40mg/kg group for 17% of subjects; compared to 0–3% as the cause of death for other dose groups (p<0.01 for comparing across all dose groups). No diagnoses had p-values <0.05 when comparing across the treatment groups (Table 3). However, intraventricular hemorrhage (any IVH, as well as grades III/IV) occurred more frequently in the 0 and 40 groups than in the 10 and 80 groups (p=0.05 for IVH III/IV). Failing the discharge hearing screening in either ear occurred more often in the 40 or 80mg/kg/day inositol groups (20% and 14%, respectively) than in the 0 and 10mg/kg/day groups (4%), (p=0.25).
Table 2.
Notable Adverse Events through 7 days post last dose of study drug
| Category | Preferred Term | Inositol Dose group (mg/kg/day) | P-values | ||||
|---|---|---|---|---|---|---|---|
| 0 (N=35) | 10 (N=29) | 40 (N=30) | 80 (N=28) | INS vsa. Placebo | Across Dosesb | ||
| Any | Any | 33 (94%) | 25 (86%) | 27 (90%) | 24 (86%) | 0.34 | 0.65 |
| Cardiopulmonary | Poor perfusion or hypotension | 10 (29%) | 7 (24%) | 5 (17%) | 7 (25%) | 0.48 | 0.74 |
| Gastrointestinal | Elevated liver enzymes | 1 (3%) | 4 (14%) | 1 (3%) | 1 (4%) | 0.67 | 0.33 |
| Hematologic | Anemia | 16 (46%) | 8 (28%) | 14 (47%) | 11 (39%) | 0.54 | 0.42 |
| Neutropenia | 3 (9%) | 6 (21%) | 3 (10%) | 4 (14%) | 0.55 | 0.52 | |
| Thrombocytopenia | 3 (9%) | 7 (24%) | 5 (17%) | 3 (11%) | 0.27 | 0.34 | |
| Thrombocytosis | 8(23%) | 10 (34%) | 4 (13%) | 6 (21%) | 1.00 | 0.31 | |
| Metabolic | Hyperglycemia | 8 (23%) | 4 (14%) | 4 (13%) | 3 (11%) | 0.18 | 0.63 |
| Other | 7 (20%) | 5 (17%) | 4 (13%) | 2 (7%) | 0.40 | 0.53 | |
| Renal | Proteinuria | 3 (9%) | 0 (0%) | 0 (0%) | 1 (4%) | 0.07 | 0.21 |
| Oliguria | 6 (17%) | 7 (24%) | 4 (13%) | 4 (14%) | 1.00 | 0.72 | |
| Respiratory | Apnea | 11 (31%) | 6 (21%) | 10 (33%) | 13 (46%) | 1.00 | 0.23 |
P-values calculated by testing the null hypothesis of equality between aplacebo and all active doses combined, and bseparately across all 4 treatment groups using Fisher’s exact tests.
Table 3.
Clinical Diagnoses Through Hospital discharge
| Dose group (mg/kg/day) | |||||
|---|---|---|---|---|---|
|
| |||||
| Co-morbidities | 0 (N=35) | 10 (N=29) | 40 (N=30) | 80 (N=28) | P-valueb |
| Death (through NRN statusa) | 6 (17%) | 2 (7%) | 6 (20%) | 1 (4%) | 0.16 |
| BPD (O2 at 36 weeks PMA or prior death from BPD) | 11 (38%) | 7 (26%) | 7 (30%) | 8 (30%) | 0.81 |
| Respiratory Distress Syndrome | 34 (97%) | 29 (100%) | 30 (100%) | 27 (96%) | 0.72 |
| PDA | 13 (37%) | 14 (48%) | 14 (47%) | 10 (36%) | 0.68 |
| PDA (received surgery) | 3 (9%) | 3 (10%) | 1 (3%) | 3 (11%) | 0.75 |
| c IVH (any) | 13 (38%) | 4 (14%) | 10 (34%) | 5 (18%) | 0.08 |
| IVH (grade III/IV) | 10 (29%) | 2 (7%) | 6 (21%) | 2 (7%) | 0.05 |
| Seizures (Rx for >72 hrs) | 2 (6%) | 0 | 1 (3%) | 0 | 0.62 |
| Cystic areas in parenchyma (within 28 days of birth) | 2 (15%) | 0 | 1 (10%) | 1 (14%) | 1.00 |
| Sepsis (early onset) | 0 | 0 | 1 (3%) | 0 | 0.71 |
| Sepsis (late onset) | 4 (11%) | 6 (21%) | 7 (23%) | 5 (18%) | 0.63 |
| d NEC (suspected or proven) | 5 (14%) | 1 (3%) | 4 (13%) | 1 (4%) | 0.28 |
| NEC (requiring surgery) | 3 (9%) | 0 | 2 (7%) | 0 | 0.17 |
| Spontaneous e GI perforation | 2 (6%) | 0 | 2 (7%) | 1 (4%) | 0.72 |
| Severe ROPf | 5/27(9%) | 3/26(12%) | 2/24 (8%) | 2/23(9%) | 0.72 |
| Hearing screen failed (either ear) | 1 (4%) | 1 (4%) | 4 (20%) | 3 (14%) | 0.25 |
NRN status = Neonatal Research Network definition: age of earliest of death, discharge, transfer, or 120 days after birth.
P-values calculated by testing the null hypothesis of equality across all 4 treatment groups using Fisher’s exact tests.
IVH intraventricular hemorrhage,
NEC necrotizing enterocolitis,
GI gastrointestinal,
Retinopathy of Prematurity meeting criteria for treatment or treated with laser, cryotherapy or anti-VEGF injection among those who survived for evaluation. With the 6 adjudicated ROP outcomes included, rates were similar (17%, 11%, 8% and 8% respectively). The denominators for BPD are all enrolled.
Severe ROP meeting criteria for surgery, or receiving intervention for ROP occurred among the surviving infants examined in 19% of the placebo group and in 12%, 8%, and 9% of the 10, 40, and 80mg/kg/day inositol groups, respectively (p=0.72). The planned Phase 3 study primary outcome of meeting criteria for ROP surgery, or death before ROP outcome, among those infants eligible for that trial (<280/7 weeks GA) was highest in the placebo group and lower in the inositol groups (44%, 23%, 36%, and 19%, for the placebo, 10, 40, and 80mg/kg/day groups, respectively, p=0.29). Results including the six adjudicated ROP outcomes were similar: 41%, 22%, 36% and 16%, respectively.
Growth (weight, head circumference, length) was examined using z-scores to adjust for PMA, and these parameters did not significantly differ across groups. An average of 15 concomitant medications were received while on study drug (range 3 to 34) and the number of courses of medication was similar across the 4 dose groups (average 19 courses, range 3–67). The 10 most frequent concomitant drug exposures by study group did not reveal a particular pattern (Supplementary Table S2 (online)).
Serum Inositol
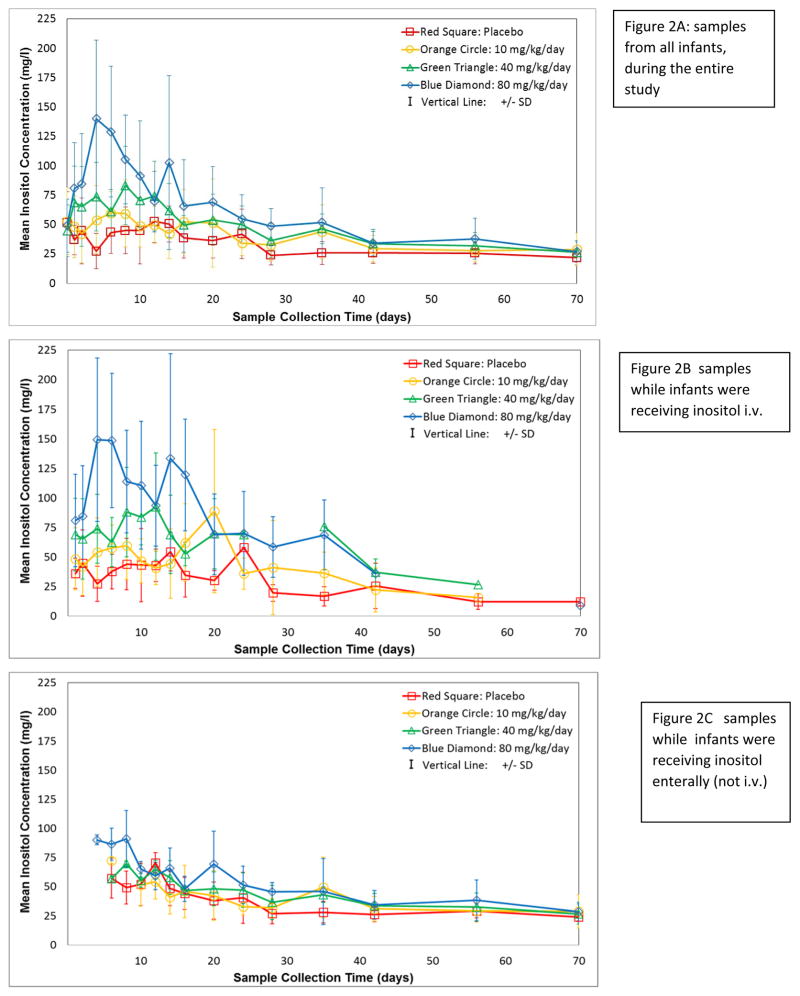
Mean serum levels were elevated in a dose-related manner in the early weeks (Figure 2, panel A); however by 6 weeks, the differences were minimal. To explore an effect of changing from IV to enteral drug, serum levels were plotted separately for samples obtained while infants were on IV drug (Figure 2, panel B), and while on enteral drug (Figure 2, panel C). Serum levels continued to decrease with age with either drug route, and once IV doses ended, mean levels over 75mg/L (416μM) were rare.
Figure 2. Serum Inositol Levels.
Mean±SD, by dose, clustered by days on study. Symbols: 80mg/kg/day = blue diamond, 40mg/kg/day = green triangle, 10mg/kg/day = orange circle, placebo = red square. Panel A includes all samples; panel B values only while subjects were receiving IV doses; panel C values obtained only when subjects were receiving enteral dosing. Timed samples were collected within scheduled windows (see Methods), plus additional scavenged laboratory residual samples as available and if exact timing after the previous dose was known for the sample. For presentation, collection days are clustered in mean values to simplify display: Study Day 0=baseline before 1st infusion; Day 2= 1st sample after 1st infusion; day 3=3rd study day; day 4=4–5d; day 6=6–7d; day 8=8–9d; day 10=10–11d; day 12=12–13d; day 14=14–15d; day 16=16–18d; day 20=19–22d; day 24=23–26d; day 28=27–31d; day 35d=32–38d; day 42=39–48d; day 56=49–63d; day 70=64–77d. Plotting only peak values, or only trough levels did not assist in displaying the data.
Inositol intake from feedings (calculated from the measured inositol levels and daily volumes of each type of feeding) rose from an average of 4mg/kg/day in week 1, to 40 – 50mg/kg/day by week 6, and did not differ significantly across groups (data available from authors). There was no evidence of inositol accumulation in the serum with continued treatment at 80mg/kg/day, despite the additional intake of inositol from full enteral feeds.
Pharmacokinetics
The PK analysis initially considered a three-part model with components for the (i) combined effects of endogenous synthesis of inositol and inositol from feeding, (ii) initial IV administration and (iii) the shift to enteral administration. The enteral administration portion of the analysis used a multiple-administration, first-order absorption with linear elimination model including terms for bioavailability and a lag time prior to the start of absorption. However, it was not possible to estimate the third part of the model related to enteral administration. As noted in relation to Figure 2, it appears that as an infant matures, and is more likely to receive enteral inositol, the serum concentration is less affected by exogenous administration. The remainder of the PK analysis focused on the first two parts of the inositol serum concentration model.
The final PK analysis used data from two sources. The data from the current study were limited to observations obtained prior to the first enteral administration of inositol (Figure 2, panel B). The data thus correspond to observations related to the multiple IV administrations and are referred to as the multiple-administration dataset. The single-dose data previously analyzed were included in parts of the analysis and will be referred to as the single-administration dataset(5). Both studies were conducted by the same investigators in the same research network using protocols consistent across both studies except for the repeated dosing.
For both datasets, a constant variance for the residual error fit best. Also, the relationships between the random effects were graphically studied by plotting uVi vs. uCli, uVi vs. uRi and uCli vs. uRi for all infants (see definitions in Methods section). A strong linear relationship was observed between the random-effects estimates for clearance (Cl) and endogenous production rate (R) with no apparent relationship between the other two combinations of random effects. The random effects were then modeled only with the correlation between Cl and R.
Table 4 presents the Pop-PK estimates for the IV administration model including the apparent endogenous infusion rate. Derived values for the elimination rate, the half-life, and the apparent concentration associated with endogenous synthesis are also shown. This is done for three available sets of data, the single IV administration column as previously published, the multiple IV administration column from fitting the model to the new multiple-administration dataset, and the last column from fitting the model to the combined datasets(5). The three sets of results are very consistent, with the combined results intermediate to the single and multiple-administration results. The half-life estimates range from the 5.22hr for the single-administration data to 7.90hr for the multiple-administration data, with the combined data estimate being 6.31hr. The random effect variance and correlation estimates are shown in Table 5 for the combination single-and multiple-administration data. Plots of the actual versus individual predicted values were examined (not shown) and the values were well aligned, indicating the model provided a good fit to the data. In addition, plots comparing the individual predicted residuals versus the actual values did not indicate any major model deficiencies.
Table 4.
Population pharmacokinetic parameter estimates for a typical infant (fixed effects)
| Parameter | Units | Estimates (Standard Error) | ||
|---|---|---|---|---|
| Single IV Administration Dataset | Multiple IV Administration Dataset | Combined Dataset | ||
| Model Parameters | ||||
| V – volume | l/kg | 0.5115 (0.0345) | 0.6572 (0.0707) | 0.5610 (0.0341) |
| Cl – clearance | (l/kg)/h | 0.0679 (0.0064) | 0.0577 (0.0061) | 0.0616 (0.0048) |
| R – endogenous infusion rate | (mg/kg)/h | 2.666 (0.2762) | 2.369 (0.3151) | 2.449 (0.2336) |
| Standard deviation of residual error | mg/l | 18.71 (1.048) | 24.77 (0.971) | 22.96 (0.739) |
| Derived Values | ||||
| k – elimination rate (=Cl/V) | 1/h | 0.1327 (0.0154) | 0.0878 (0.0137) | 0.1098 (0.0109) |
| t1/2 – half=life (=.693/k) | h | 5.22 (0.605) | 7.90 (1.229) | 6.31 (0.631) |
| E – concentration due to endogenous infusion (=R/Cl) | mg/l | 39.26 (1.655) | 41.06 (1.777) | 40.71 (1.255) |
Table 5.
Population pharmacokinetic random effect variances and correlations for the combined dataset
| Volume (uV) | Clearance (uCl) | Endogenous infusion rate (uR) | |
|---|---|---|---|
| Volume (uV) | 0.1181 | -- | -- |
| Clearance (uCl) | 0.0a | 0.3508 | -- |
| Endogenous infusion rate (uR) | 0.0a | 0.9349 | 0.4899 |
Random effect variances are displayed on the diagonal and correlations between the random effects on the off diagonal.
Correlation set to 0.0 (zero) based on review of plots of uVi vs. uCli and uVi vs. uRi.
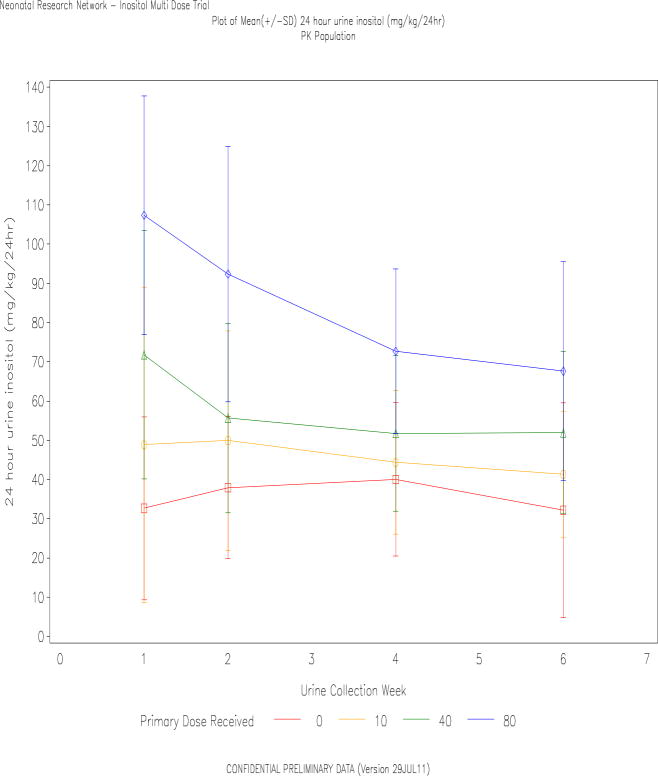
Twenty four hour urine inositol losses were determined at the end of weeks 1, 2, 4 and 5 to 6 (Figure 3). At week one, mean urine losses were close to, or greater than the dose received, despite dividing doses q 12hr to lower peak serum levels. Week 1 excretion rates in the 80 mg/kg group were similar to the observed 24hr excretion following a single dose of 120mg/kg in week 1, previously published(5). At all ages, the mean inositol excretion was highest in the 80mg/kg/day group, falling from 107mg/kg/24hr at week 1, to 68mg/kg/24hr at weeks 5–6. There was no evidence of a diuretic effect of inositol as urine volumes measured between 3 and 5 ml/kg/hr and did not vary significantly by group (data not shown).
Figure 3. Inositol Urine Losses.
Inositol in the urine from each diaper (Concentration × Volume) was summed over 24hr to determine the urine losses, at weekly intervals. Data are mean±SD, and slightly offset for better visualization. Symbols: Square=placebo, circle=10mg/kg/day, triangle=40mg/kg/day and diamond=80mg/kg/day.
DISCUSSION
Inositol at 80mg/kg/day in low gestation infants was effective in reaching serum levels similar to those achieved during previous trials, and importantly, these levels did not continue to rise with dosing throughout the period of rapid retinal vascular development up to 10 weeks (approximately 34 weeks PMA)(9). There was no significant evidence of harm at any dose during the study, but prospective monitoring of hearing and infection should be conducted in future trials. Although not statistically significant with these small sample sizes, several co-morbidities appeared less frequent in the treated groups, which is reassuring in consideration of a Phase 3 trial.
The PK were best described by a 1-compartment multiple dose IV infusion model with linear elimination combined with apparent endogenous production for the periods of time when infants were receiving IV administration. While an expanded model was considered that included both IV infusion and enteral administration, we were unable to get a single model to converge for both the IV and enteral portions of the PK study. Brown, et. al. reported the turnover rate of inositol using dual labeled stable isotopes to be approximately 150–250mg/kg day in 33–34 weeks GA infants, a value consistent with the data that inositol is endogenously synthesized, as well as catabolized in the kidney(10,11). The inositol oxidase enzyme, unique to the renal cortex, catabolizes inositol to glucuronic acid and becomes active in the weeks after birth in the term newborn, likely contributing our finding of decreasing inositol in the urine despite ongoing treatment plus increasing inositol from feeds(12).
Inositol is necessary for phosphatidylinositol surfactant synthesis, which predominates over phosphatidylglycerol in preterm infants. Infants unable to receive human milk or formula feedings experience falling serum inositol levels. While supplementation may improve RDS and reduce both BPD and ROP, the effect on ROP was unexpected, and it is possible inositol was sufficiently effective in reducing pulmonary morbidity that it lowered the risk for ROP as a secondary effect by reducing oxygen exposure(2,3). However, phosphoinositides, inositol polyphosphates and inositol phosphoglycerols serve as signaling molecules in a number of intracellular events, and are essential factors in the chain of mediators leading to vascular growth, including VEGF, and insulin-like growth factor (IGF-1) (7,8,13). Therefore, inositol or its derivatives may be rate limiting in the roles of VEGF, IGF-1, or other factors critical to retinal vascular development which normally occurs in-utero from 14 to 36 weeks gestation. If inositol is permissive for sustaining the health of retinal endothelial cells during this time period, it may explain why infants with low inositol levels in the early neonatal period are at higher risk for ROP(14). Metabolism of inositol is complex and not fully understood, changing in utero, with birth, and affected by enteral intake and complex endogenous controls that are likely developmentally regulated(7). Nonetheless, in early trials, inositol treatment appears safe and beneficial in preterm infants.
CONCLUSIONS
These data add to the evidence that inositol at doses up to 80mg/kg/day for 7–10 weeks is well tolerated and does not increase adverse events. As recommended in the Cochrane Review, the data on inositol supplementation warrants a large Phase 3 clinical trial to test its safety and efficacy to improve survival without severe ROP(4).
METHODS
Study Design
A randomized double-masked Phase 2 clinical trial was conducted in 14 centers of the Eunice Kennedy Shriver NICHD Neonatal Research Network(NRN). The study design was approved by the FDA and registered on clinicaltrials.gov (#NCT01030575). The protocol was approved by the NRN Data Safety Monitoring Committee and the IRB from each institution, and each subject’s parent or guardian provided written informed consent.
Population
Subjects were 230/7 to 296/7 weeks GA who weighed at least 400g, and could receive study drug by 72hr after birth. Exclusions included death before 12hrs, major congenital anomalies, severe oliguria, or a moribund state. Eligible infants were randomized and stratified within center by gestational strata (230/7–266/7 vs. 270/7–296/7wks), to placebo or one of 3 daily doses of inositol.
Study Drug
Myo-inositol 5% Injection was provided by Abbott Nutrition, Columbus, OH as an isotonic, preservative and pyrogen-free, sterile, 5% solution of myo-Inositol IV at neutral pH. Doses were 10, 40, or 80mg/kg/day, divided q12hr given over 20minutes. Placebo was 5% glucose USP for IV infusion and dispensed at the equivalent various volumes to maintain masking. Randomization was by central computer, communicated to the research pharmacists who prepared doses of inositol or placebo in the pharmacy, dispensed as unit doses. Thus research and clinical personnel, and families remained masked to treatment assignment. When enteral feedings reached at least 100mL/kg/day (or the infant no longer was receiving IV fluids), the study drug was given enterally as the same formulation, at the same per kg dose. Study drug continued until 10 weeks chronologic age, 34 weeks PMA, death, or discharge.
Individual infants contributed 8–10 scheduled serum samples over the 10 weeks (Supplementary Design Table S3 (online)). Sampling times were assigned so that approximately 8 samples were collected in each of the specified time frames for each dose group. Collection times were divided within each time window to collect peak, trough, and mid-dose samples. The time of each sample and the starting time of the preceding drug infusion were recorded. Additional measurements from scavenged serum or plasma with known times of collection (left over from laboratory studies ordered for usual care) were also processed, if available, with consent.
A 24hr urine collection was obtained at the end of weeks 1, 2, 4, and 6 (or 5 if being discharged). The change of diaper weight (dry to wet) was used as the best estimate of void volume where one gram=one ml. Urine was expressed from each wet diaper separately during the collection period, and an aliquot frozen(15). The time of each diaper-on and diaper-off were recorded.
Enteral feeds
Volume and sources (human milk, fortified human milk, or specific formulas) were recorded daily until intake was 100ml/kg/day for at least 7 days, and then were recorded weekly. Inositol concentrations were sampled from human milk actually given to the infant in the same week, unless insufficient volume was available. Formula inositol concentrations were measured twice from the stock of each type of formula the infant received, and re-assayed if the formula was then fortified. Inositol from milk feedings was calculated by summing the daily inositol intake from the volume ingested of each milk, multiplied by the measured inositol concentration for that milk.
Assay
myo-Inositol was measured with a validated assay on 25 or 50μl samples of serum, plasma, urine and milks, utilizing a multiple-column, multiple mobile phase liquid chromatographic system with electrochemical detection(15).
Clinical Outcomes
Adverse events were prospectively monitored from 24hrs prior to study drug until 7 days following the final dose (unless discharged sooner), and judged according to a neonatal toxicity table developed for the study. Concomitant medications were recorded from 24hr prior to the start of study drug until 7 days following its final dose, unless the infant was discharged sooner. Weight, length, and head circumferences were measured prospectively.
Retinopathy outcomes were determined from the clinical eye examinations(16). The primary examining ophthalmologist at each center was trained and certified on the International Classification of ROP as used in 2006(17). An unfavorable outcome was defined as either Type 1 ROP or worse, in either eye, or surgical intervention for severe ROP in either eye(18). A favorable ROP outcome was assigned if the retinal vessels progressed to full vascularization in both eyes without meeting criteria for severe ROP, or if on two consecutive examinations the retinal vessels were in zone III(16). Examinations were continued, if necessary, up to 55 weeks PMA (3 months after term due date)(19,20). Infants who did not meet either criterion had all available examinations reviewed by an adjudication committee.
Co-morbidities were recorded prospectively using the established definitions of the NRN Generic Database Protocol(21). At 18–22 months corrected age, infants received a set of standardized examinations of neurologic function and development according to the NRN Follow Up Protocol (to be reported separately)(22).
Statistical Analyses
Sample size
Enrollment was continued until at least 48 infants in each of the two GA strata had completed a minimum of 28 days on study drug and contributed at least 5 serum samples. Infants were enrolled and randomized during the time when waiting to document that 96 infants had completed the protocol. All infants beginning treatment were permitted to complete the protocol, and all available data are included in the final analyses. Population PK studies typically target 6–8 samples at each of the time points to describe the change in serum concentrations(23,24). No formal power calculations were conducted because no formal hypotheses were to be tested in this Phase 2 study, and the analyses are exploratory and descriptive in nature.
Data obtained within the study assessment windows were all used. While the primary analyses were conducted without imputation for missing ROP data due to loss to follow-up or indeterminate final status, additional exploratory analyses were conducted to assess the impact of the missing data on the estimates of ROP. Adjudication was conducted by a committee of three experienced ophthalmologists not involved with the study and masked to study group assignment. They were provided data on the infant’s GA, birth weight, and each available eye examination, including age (chronologic and PMA) at each exam. The final ROP status was judged separately in each eye as ‘probably favorable’, ‘probably unfavorable’ or ‘cannot be determined’, and the majority classification was assigned as the adjudicated outcome.
Baseline characteristics and co-morbidities for all randomized and treated infants were compared by testing the null hypothesis of equality across all 4 dose groups using ANOVA for continuous measures, Mantel-Haenszel mean score tests (using modified ridit scores) for ordinal measures, and chi-square or Fisher’s exact test for nominal measures.
Methods for Pharmacokinetic Analyses
In recognition of the relatively sparse sampling design for the collection of serum samples, population PK(Pop-PK) models were fit to the data using the nonlinear mixed effects approach in Monolix 3.2, manufacturer LIXOF, Antony, France. This approach accounts for the variability between infants in the model parameters, the correlation between measurements in the same infant at different occasions, as well the residual unexplained variability in serum concentrations(25). Two issues dictated the structure of the Pop-PK models that were considered for modeling the data from the study: 1) endogenous synthesis of inositol by the infants and inositol contained in feedings of human milk or infant formula; and 2) initial IV administration of inositol.
Endogenous synthesis and feeding intake of inositol were modeled in the same way as the single dose PK analyses(5). The steady state endogenous concentration for the ith infant is modeled as Ei = Ri/Cli where Ri is the apparent rate of inositol infusion due to the combination of endogenous synthesis and feeding, and Cli is the clearance. It is not possible to separate endogenous synthesis and feeding intake of inositol since enteral feeding intake was measured as the total amount fed over a day and not the amount fed at each occasion. In addition, as discussed in the Results section, the estimation of Ri will be from data from the period of time prior to the establishment of full enteral feeds.
As was used previously, the Pop-PK model for the initial IV administration period is a 1-compartment IV infusion model with linear elimination(5). For this study the model was expanded to account for multiple administrations of inositol rather than a single administration used with the previous single-dose study. The model for serum concentrations resulting from endogenous synthesis and feeding of inositol and from IV administration is then
where Ci(t) is the serum concentration for the ith infant at time t, in hours, with time measured since the start of the first IV administration. The time of the kth IV administration to the ith infant is tDik and T is the duration of the infusion period common to all infants and administrations (1/3hr). The summations are over the IV administrations for the ith infant up to time t with n such that tDin ≤ t < tDin+1. Di is the dosage administered to the ith infant at each administration in mg of inositol per kg of body weight. Vi is the apparent volume of distribution. Finally, εit is the residual error at time t.
The between infant variability in the Pop-PK model parameters, Ri, Cli, and Vi, is modeled using random effect variables (uR, uCl and uv) that approximate the individual trajectory over time of each infant’s serum inositol concentration. The random effects are assumed to be normally distributed with means of 0 (zero) and variances and correlations that will be estimated. For example, the clearance for the i-th infant is modeled as Cli = Cl × euCli where Cl is the fixed-effect common to all infants and uCli is the random effect unique to the i-th infant. Similarly for Ri and Vi. Thus, the three model parameters are log-normal. Individual specific parameter estimates were obtained as the conditional modes, or the maximum a posteriori, of the Bayes estimates of the parameters. The fixed effects, R, Cl and V, are the median, also modal, values of the parameters and are often called the typical values for the population from which each infant’s parameters are derived. The residual error, εit, is assumed to be uncorrelated with the random effects and normally distributed with mean 0 (zero) and variance that is estimated from the data. The quality of fit of the Pop-PK model was judged by visual examination of plots of observed vs. individual predicted concentrations and of residuals vs. individual predicted concentrations.
Supplementary Material
Acknowledgments
We are indebted to the families who consented to take part in the study, and to our medical, nursing, pharmacy, and research coordinator colleagues (See Supplemental Acknowledgments).
STATEMENT OF FINANCIAL SUPPORT
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network and the Pediatric Pharmacology Research Units Network, with co-funding from the National Eye Institute provided grant support for this trial, with additional support from the National Center for Research Resources, and the National Center for Advancing Translational Sciences, Bethesda, MD, USA. The following NRNetwork Centers and Institutions (with grant numbers) participated in the Inositol Multi-dose Study: Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904); Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80); Duke University School of Medicine, University Hospital, and Durham Regional Hospital (U10 HD40492, M01 RR30); Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, M01 RR39, UL1 TR454); Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children at Indiana University Health, and Wishard Health Services (U10 HD27856, M01 RR750); Stanford University (U10 HD27880); Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54); University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32); University of Iowa (U10 HD53109, M01 RR59, UL1 TR442); University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997); University of Rochester Medical Center, Golisano Children’s Hospital (U10 HD40521, M01 RR44); University of Texas Southwestern Medical Center, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633); University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, U10 HD45986, M01 RR64); Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385, U10 HD37261); Yale University, Yale-New Haven Children’s Hospital (U10 HD27871, UL1 RR24139, M01 RR125).
Footnotes
DISCLOSURES: The authors report no commercial, proprietary, or financial interest in any of the products described in this article, nor any conflicts of interest. NICHD is the sponsor of the study and holds the Investigational New Drug Application (IND). Abbott Nutrition Division, Abbott Laboratories, Columbus, OH, USA, supplied the inositol drug used in the study, and conducted the inositol assays on the milk and urine samples. Portions of this study were presented at the 2012 Pediatric Academic Societies Annual Meeting, Boston, Massachusetts, April 28-May 1, 2012. While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of the NICHD.
Ethical Oversight
The institutional review boards of each center approved the protocol, and written informed consent was obtained for each participant. An independent Data safety Monitoring committee approved the protocol and monitoring plan before the study began and monitored the accumulating safety data. The US Food and Drug Administration approved the protocol which was conducted under an IND, and the trial was registered with ClinicalTrials.gov (NCT01030575). Data collected at participating centers and inositol assay results were transmitted to the data coordinating center(DCC), RTI International, which stored, managed and analyzed it. Dr. Abhik Das (DCC Principal Investigator) and Dr. Tracy Nolen (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Drs. Rick Williams, Michael Goedecke, and Timothy Fennell had full access to all data in the study and were responsible for conducting the pharmacokinetic analyses.
References
- 1.Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Ped Res. 2013;74(Suppl):35–49. doi: 10.1038/pr.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallman M, Bry K, Hoppu K, Lappi M, Pohjavuori M. Inositol supplementation in premature infants with respiratory distress syndrome. New Engl J Med. 1992;326:1233–39. doi: 10.1056/NEJM199205073261901. [DOI] [PubMed] [Google Scholar]
- 3.Hallman M, Jarvenpaa AL, Pohjavuori M. Respiratory distress syndrome and inositol supplementation in preterm infants. Arch Dis Child. 1986;61:1076–83. doi: 10.1136/adc.61.11.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlett A, Ohlsson A, Plakkal N. Inositol in preterm infants at risk for or having respiratory distress syndrome. Cochrane Database Syst Rev, Neonatal Module. 2014 CD000366.pub3. [Google Scholar]
- 5.Phelps DL, Ward RM, Williams RL, et al. Pharmacokinetics and safety of a single intravenous dose of myo-inositol in preterm infants of 23–29 wk. Ped Res. 2013;74:721–29. doi: 10.1038/pr.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallman M, Arjomaa P, Hoppu K. Inositol supplementation in respiratory distress syndrome: relationship between serum concentration, renal excretion, and lung effluent phospholipids. J Pediatr. 1987;110:604–10. doi: 10.1016/s0022-3476(87)80561-9. [DOI] [PubMed] [Google Scholar]
- 7.Hallman M. Inositol during Perinatal Transition. NeoReviews. 2015;16:e84–e93. [Google Scholar]
- 8.Hellstrom A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–57. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Academy of Pediatrics Committee on Fetus and Newborn. Engle WA, Blackmon LR, et al. Age terminology during the perinatal period. Pediatrics. 2004;114:1362–64. doi: 10.1542/peds.2004-1915. [DOI] [PubMed] [Google Scholar]
- 10.Brown LD, Cheung A, Harwood JEF, Battaglia FC. Inositol and mannose metabolism in term and late preterm infants. J Nutr. 2009;139:1648–52. doi: 10.3945/jn.109.109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements RS., Jr The polyol pathway. A historical review. Drugs. 1986;32(Suppl 2):3–5. doi: 10.2165/00003495-198600322-00003. [DOI] [PubMed] [Google Scholar]
- 12.Bry K, Hallman M. Perinatal development of inositol synthesis and catabolism in rabbit kidney. Biol Neonate. 1991;60:249–57. doi: 10.1159/000243416. [DOI] [PubMed] [Google Scholar]
- 13.Xia P, Aiello LP, Ishii H, et al. Characterization of vascular endothelial growth factor’s effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J of Clin Invest. 1996;98:2018–26. doi: 10.1172/JCI119006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman CA, McVey J, Borne MJ, et al. Relationship between serum inositol concentration and development of retinopathy of prematurity: a prospective study. J Ped Ophthal Strab. 2000;37:79–86. doi: 10.3928/0191-3913-20000301-06. [DOI] [PubMed] [Google Scholar]
- 15.Schimpf KJ, Meek CC, Leff RD, Phelps DL, Schmitz DJ, Cordle CT. Quantification of myo-Inositol, 1,5-anhydro-D-sorbitol, and D-chiro-inositol using high performance liquid chromatography with electrochemical detection in very small volume clinical samples. Biomed Chromatogr. 2015;11:1629–36. doi: 10.1002/bmc.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Section on Ophthalmology American Academy of Pediatrics, American Academy of Ophthalmology, American Association of Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572–6. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]
- 17.International Committee for Classification of ROP. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthal. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 18.ETROP Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthal. 2003;121:1684–94. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds JD, Dobson V, Quinn GE, et al. Evidence-based screening criteria for retinopathy of prematurity: natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthal. 2002;120:1470–6. doi: 10.1001/archopht.120.11.1470. [DOI] [PubMed] [Google Scholar]
- 20.Ni Y-Q, Huang X, Xue K, et al. Natural involution of acute retinopathy of prematurity not requiring treatment: factors associated with the time course of involution. Invest Ophthal & Vis Sci. 2014;55:3165–70. doi: 10.1167/iovs.13-13744. [DOI] [PubMed] [Google Scholar]
- 21.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams-Chapman I, Bann CM, Das A, et al. Neurodevelopmental outcome of extremely low birth weight infants with candida infection. J Pediatr. 2013;163:961–7. doi: 10.1016/j.jpeds.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun H, Fadiran EO, Jones CD, et al. Population pharmacokinetics. A regulatory perspective. Clinical Pharmacokinetics. 1999;37:41–58. doi: 10.2165/00003088-199937010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Duffull S, Waterhouse T, Eccleston J. Some consideration on the design of population pharmacokinetic studies. J Pharmacokinet & Pharmacodyn. 2005;32:441–57. doi: 10.1007/s10928-005-0034-2. [DOI] [PubMed] [Google Scholar]
- 25.Bertrand J, Comets E, Mentre F. Comparison of model-based tests and selection strategies to detect genetic polymorphisms influencing pharmacokinetic parameters. J Biopharm Stat. 2008;18:1084–102. doi: 10.1080/10543400802369012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.