Abstract
Depending on the species, edible insects are highly nutritious and thus represent a noteworthy alternative food and feed source. The current work investigates the protein extractability and techno-functionality of insect flour fractions recovered from Tenebrio molitor and Hermetia illucens. T. molitor and H. illucens flours contained about 20% crude fat and 60% and 36 % crude protein, respectively. Defatting reduced the crude fat content to 2.8% (T. molitor) and 8.8% (H. illucens) and increased the crude protein content to 68% and 47%, respectively. To isolate proteins from the flours, protein solubility was optimized by varying the pH, the ionic strength, and the extraction temperature of the solvent. All products and by-products accumulated in the protein production process were characterized by composition, selected techno-functional properties, protein solubility, composition and structure as well as their microbial load.
Keywords: Food Science
1. Introduction
Recently, insects have gained more and more attention in Europe as an underexploited sustainable protein and nutrient source for food and feed. Their potential has been pointed out for example in two FAO publications (Durst et al., 2010; Van Huis et al., 2013) and several reviews (Barroso et al., 2014; Makkar et al., 2014; Nowak et al., 2016; Rumpold and Schlüter, 2013; Sánchez-Muros et al., 2014). A risk profile published by the EFSA (EFSA, 2015) has emphasized the numerous uncertainties and knowledge gaps regarding the use of insects and products thereof as food and feed. In addition, consumer acceptance is a major challenge. In general, western consumers may be reluctant to accept insects as a legitimate protein source because they have never played a substantial role in their food culture. In an exploratory research, 32 Italian consumers, aged 20–35 years, were interviewed in groups on their willingness to eat insect-based food products. It was discovered that this willingness depends on the presented form of the products (Balzan et al., 2016). This was confirmed by a Dutch study on meat replacers (Schösler et al., 2012) where the authors found that the consumer acceptance of insects in food products increased when insects were not visible in modified products indistinguishable from familiar ones. This suggests it is favorable to introduce insects to the human consumer in a masked form as powder, meal or fraction. However, supplementing food products with insect-based hemi-products/ingredients, proteins and fractions requires extensive knowledge on their properties. In case of proteins, these properties include, among others, solubility, amino acid profile, thermal stability and techno-functional properties as water and oil binding, gelling, foaming and emulsifying capacity. Separating extracted protein groups based on their solubility in solvents produces water-soluble and water-insoluble fractions, which can be used for specific applications in the food industry.
Extracting insect proteins for human food products – a process already being carried out – could be a useful way of increasing acceptability among wary consumers. There is little scientific data published on protein extraction from insects. Del Valle et al. (1982) performed a protein extraction from the Mexican fruit fly Anastrepha ludens with a maximum protein solubility at pH 10 and subsequent protein precipitation at pH 5. A protein concentrate with a protein content of 65.4% and a protein isolate with a protein content of 86.6% were obtained (based on dry matter, respectively). Investigation of the functional A. ludens protein properties resulted in considerably lower foaming and emulsion capacities compared to egg white protein. The solubility of the extracted fly proteins was highest at a pH of 10 (95%) and lowest at the isoelectric point at a pH of 5 (8%).
Yi et al. (2013) investigated the techno-functional properties of proteins from five insect species: Tenebrio molitor (larvae), Zophobas morio (larvae), Alphitobius diaperinus (larvae), Acheta domesticus (adult) and Blaptica dubia (adult). An aqueous protein extraction was performed. The protein purity based on dry matter ranged from 50–75%. It was observed that the insect proteins investigated had the ability to form gels depending on their concentration and on the pH having the potential to be used as gelling agents or texturizers in food (Yi et al., 2013). A comparison of three differently produced protein extracts from aphids using mass spectrometry and gel electrophoresis suggests that the protein extraction methods influence the properties of the extracted protein (Cilia et al., 2009). Mariod et al. (2011) extracted the protein gelatin from the two defatted, dried and ground Sudanese beetles Aspongubus viduatus and Agonoscelis pubescens. The applicability of the gelatin extracted from insects in comparison to commercially available gelatin as a stabilizer in the ice cream production was investigated and was rated as acceptable by a panel. In addition, there were no significant differences by the general preferences between ice cream produced with insect gelatin and produced with commercial gelatin (Mariod, 2013).
A targeted application of insect-derived ingredients in food formulae is facilitated by insect processing and protein extraction. For an industrial bio-fractionation, established processing chains for the production of high-quality and affordable proteins from traditional protein sources need to be adapted to meet the specific requirements of edible insects as a raw material. In this context and in contrast to the study published by Yi et al. (2013) who recovered soluble insect proteins by a simple aqueous extraction procedure and concluded that research is needed for developing further extraction and purification procedures, and for more detailed insight into functional properties, aim of the present study was to characterize the proteins from Tenebrio molitor and Hermetia illucens under varying extraction conditions (pH, ionic strength and temperature) and to investigate the composition and properties of recovered insect flour fractions. A further goal and differentiation to published studies was to identify the necessary process stages and extraction parameters required in order to maximize the yield of soluble insect proteins. For these purposes, the intermediate products T. molitor flour (T-F) and H. illucens flour (H-F), defatted T. molitor flour (T-DF) and H. illucens flour (H-DF), T. molitor high protein fraction (T-HPF) and T. molitor low protein fraction (T-LPF) were characterized for their techno-functional, microbial and protein properties. The effects of nonthermal processing steps applied and extraction conditions used on protein and selected techno-functional properties of insect intermediates were investigated to predict their application in food and feed processing.
2. Material and methods
Black soldier fly (Hermetica illucens) and yellow mealworm (T. molitor) larvae are among the most promising insects for industrial production in the western world. These two species were used in the present work in order to study the applicability of a cold wet process as a preparation for the protein extraction on a laboratory scale. The process steps chosen ensure a minimization of thermal effects for the production of insect flours containing proteins in its native form.
2.1. Processing of the T. molitor and H. illucens larvae
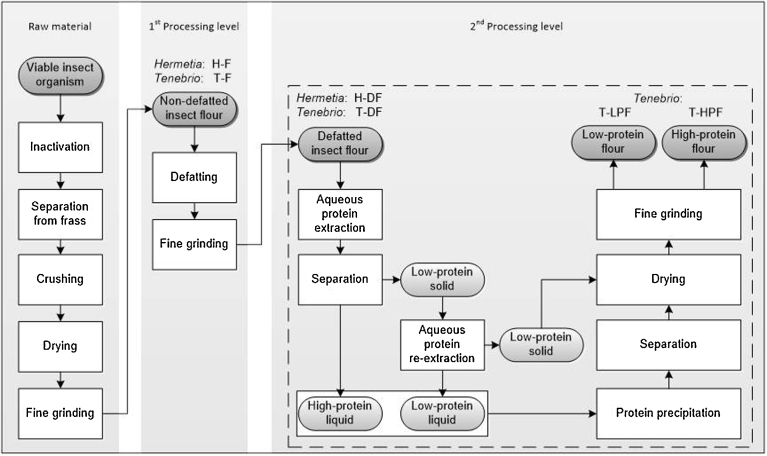
T. molitor larvae, purchased from a local breeder (Futtertier-Shop.de, Eisenhüttenstadt, Germany) and H. illucens larvae, purchased from Hermetia Baruth GmbH (Baruth, Germany), served as test material. A schematic depiction of processing and fractionation of the insect larvae is given in Fig. 1. Larvae were separated from frass by sieving, then packaged in freezer bags, subsequently inactivated by freezing and stored at −20 °C.
Fig. 1.
Schematic representation of processing and fractionation of larvae from T. molitor and H. illucens.
Non-defatted insect flours were produced by pureeing frozen larvae with distilled water (1:1 w/w) at 4 °C, followed by freezing at −20 °C, freeze drying (Christ Alpha 1–4, Christ Gefriertrocknungsanlagen, Osterode, Germany) and grinding (Clatronic KSW 3307, Clatronic International GmbH, Kempen, Germany).
Removal of fat from the obtained T. molitor (T-F) and H. illucens (H-F) flours was conducted by a two-step extraction of the fat with hexane. One part of the respective insect flour and five parts of hexane were stirred on a magnet stirrer for 1 h. Following sedimentation of the solids, the hexane-fat-mixture was decanted. The procedure was repeated twice. Residual hexane was removed by evaporation overnight. Subsequent fine grinding of the defatted low-fat fractions using a coffee mill produced defatted T. molitor (T-DF) and H. illucens (H-DF) flours.
A high-protein fraction (T-HPF) was recovered from T-DF by aqueous extraction of the soluble proteins with distilled water (1:25 w/v) adjusted to pH 10 (1 M sodium hydroxide) under stirring (300 rpm) at a constant extraction temperature of 60 °C for 30 min. The recovered extract was centrifuged at 4000 g and 20 °C for 20 min. The clear supernatant was collected and proteins were precipitated by adjusting the pH to 4 (1 M hydrochloric acid). Re-extraction (pH 2, 60 °C, 30 min) and precipitation of the residual proteins from the solids were conducted as previously described. Both protein-rich extracts were centrifuged (4000 g, 20 °C, 20 min). Proteins were frozen (−80 °C), freeze dried, ground and unified. The low-protein fraction (T-LPF) consisted of the insoluble residues recovered during aqueous protein extraction which were unified, freeze dried and fine ground.
2.2. Characterization of quality and techno-functional parameters
2.2.1. Crude protein, crude fat and dry matter content
Crude protein contents (NKjel, conversion factor 6.25) were determined by the Kjeldahl method (Kjeldatherm Turbosog, Titrino plus 848, Gerhardt Analytical Systems, Königswinter, Germany), according to DIN EN 25663 and as described by the Association of German Agricultural Investigation and Research Institutions (VDLUFA, 1976). Crude fat content of the flour fractions was analyzed using the filter bag (Filterbags XT4, ANKOM Technology, New York, USA) method Am 5-04 (AOCS, 1998; AOCS, 2005). Dry matter contents of the insects and insect flour fractions were determined by oven drying (105 °C, 48 h).
2.2.2. Color measurement
To measure the impact of the different processing steps on the color of the insects and insect derived products, the HunterLab-system was used. As described by Bußler et al., (2015), a Minolta spectrophotometer (CM-2600D, Konica Minolta Inc., Osaka, Japan) was set at illuminant D65, 3 mm aperture, and 0° viewing angle taking L-values (brightness), a-values (green–red axis), and b-values (blue–yellow axis) for nine samples of each product. Following Eq. (1), the change in color (ΔE) was calculated, whereas the indices 0 and p indicate measured values of unprocessed (larvae) and processed insects (flour fractions).
| (1) |
Following Eqs. (2) and (3), the browning index (BI) was calculated.
| (2) |
| (3) |
2.2.3. Water (WBC) and oil binding capacity (OBC)
To determine the impact of the processing steps on the WBC of the insect flour fractions the method by Smith and Circle, 1978a, Smith and Circle, 1978b, modified by Quinn and Paton (1979) was applied. Therefore each 0.5 g of the respective insect flour fraction was weighted into centrifuge beakers. Samples were stirred (60 s) with 2.5 mL of water using a propeller stirrer and an overhead agitator (Yellowline®, IKA® OST basic, New Jersey, USA). Following a 20 min centrifugation step, (3900 g) the samples were re-weighed after decanting the supernatant and putting the beaker upside-down on filter paper for 60 min. Following Eq. (4), WBC was calculated.
| (4) |
Whereby, m0 is the initial weight of the sample, m1 is the final weight of the sample and m0,DM is the initial weight of the sample based on dry mass (Bußler et al., 2015; Reinkensmeier et al., 2015).
For the determination of the OBC, the method by Schwenke et al. (1981) was applied. Here, 0.5 g of the respective insect flour fraction was stirred with 2.5 mL of commercial rape seed oil two times for 60 s at 1000 rpm with a five-minute intermission in between. Following centrifugation and re-weighing, OBC was calculated similar to WBC (Eq. (4)).
2.2.4. Emulsifying capacity (EC)
Emulsifying capacity (EC) of the T-DF was tested dependent on the protein concentration and the pH. For this purpose 0.1% protein solutions were prepared at pH 5 and 7 and diluted to final protein concentrations of 0.02, 0.04, 0.06, 0.08 and 0.1%. Each 5 mL of the respective protein solution were put into 50 mL tubes. Rapeseed oil dyed with liquid natural carotene (M = 536.89 g/mol, Carl Roth, Karlsruhe, Germany) was added dropwise using an 20 mL automatic burette (solarus, Hirschmann Laborgeräte, Eberstadt, Germany) under continuous dispersion (9500 rpm, Ultra turrax, IKA, Staufen, Germany). The maximum oil volume emulsified was read off with phase separation. EC was calculated following Eq. (5), whereas voil was the volume of oil emulsified, vps was the volume of protein solution used and cps was the protein concentration of the aqueous phase.
| (5) |
2.3. Characterization of insect proteins and protein properties
2.3.1. Protein solubility
For testing the solubility of the contained insect proteins, 0.2 g of the respective insect flour fraction were weighed into a small beaker. A pH dependent extraction of the insect proteins was conducted by adjusting the pH (2 to 12) of the extracts using 1 M hydrochloric acid or 1 M sodium hydroxide. Thereof resulting deviations in the extraction ratio were protocolled and factored into the calculation of the protein concentration. Sodium chloride was used to adjust the ionic strength of distilled water between 0.05 and 10 M. Extraction under varying temperatures was conducted with preheated distilled water while maintaining the respective temperature in a water bath with a built-in shaker. Extraction was carried out under stirring on a rotary shaker (350 rpm) using 5 mL of the previously described solvents. Protein extracts were centrifuged for 10 min (10,000 g, 4 °C) and the clear supernatant was subsequently analyzed.
The Biuret assay (Robinson and Hogden, 1940) was used for quantitative protein analysis. Biuret reagent was prepared as described elsewhere (Bußler et al., 2015; Bußler et al., 2016).
Bovine serum albumin (Fluka, Buchs, Switzerland, cBiuret = 0–10 mg/mL in 2 mg/mL intervals) served as standard and; the assay consisted of 200 μL of the protein extracts and 800 μL of Biuret reagent reacting for 45 min at ambient temperature. The absorption maximum was measured at 540 nm against a blank value (respective solvent) using an UV/Vis spectrophotometer (BioPhotometer plus, Eppendorf, Hamburg, Germany). The amount of soluble insect protein was related to dry matter and crude protein content (Kjeldahl) of the respective insect flour fraction (Bußler et al., 2015).
2.3.2. Fluorescence measurement
Fluorescence emission spectra were measured using a PerkinElmer LS55 fluorescence spectrometer (Rodgau-Jügesheim, Germany) equipped with a pulsed xenon lamp and a red-sensitive photomultiplier (R928) (Bußler et al., 2015) at an excitation wavelength of 280 nm. The fluorescence spectra were scanned in a wavelength range of 300–550 nm placing a cut-off filter at 290 nm in front of the emission monochromator (slit width 5). As described by Bußler et al. (2015), differences in protein concentration and pH of the samples were equalized by dilution with 0.1 M phosphate buffer (pH 7). Measurement was conducted in triplicate using phosphate buffer as the blank.
2.3.3. Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE)
For determining the molecular weight distribution of the insect proteins SDS-PAGE according to Laemmli (1970) was used. As described by Reinkensmeier et al. (2015), the pooled samples (n = 3) were mixed in a ratio of 1:10 with sample buffer (0.0125 M Tris buffer at pH 6.8 containing 0.005 M EDTA at pH 6.8–7.0, 1% of sodium dodecyl sulphate, 10% of glycerol, 1% of 2-mercaptoethanol and 0.005% of Bromophenol Blue). Denaturation of the proteins was conducted at 95 °C for 3 min prior to analysis. Vertical electrophoresis equipment (Mini-PROTEAN) from Bio-Rad (Bio-Rad Laboratories GmbH, Munich, Germany) were used to prepare the gels. As standard the PageRulerTM Unstained Broad Range Protein Ladder (Thermo scientific, Vilnius, Lithuania) was used. The band intensity of 5 μl/10 μl of the samples separated in 12% T gels was estimated following staining the gels with Coomassie Brilliant blue and quantification was conducted using analysis Software (Quantity One 1-D, version 4.5.2, Bio-Rad, Milan, Italy).
2.4. Microbial analysis
For determining the impact of insect processing on the overall microbial count of the mealworm flour fractions, 3 g of the respective material and 27 g of 0.1% casein–peptone-solution (CPS) were mixed in a sterile filter stomacher bag and homogenized (Bag Mixer Interscience, St. Nome, France) at speed 8 for 2 min. Following serial dilution of the homogenates with CPS in Rotilabo®-microtest plates (96er U-profile, Roth, Germany), 50 μl of each dilution were spread on plate count agar. Following incubation at 30 °C for 72 h the number of colony forming units per g on a dry matter basis (CFU/gDM) was determined with a detection limit of plate count analyses of 200 CFU/gDM. All analyses were carried out at least in triplicates.
2.5. Statistical analysis
Extractions and following analytical steps each were conducted in triplicate (n = 9), total viable counts were determined from three independent samples preparing homogenates in duplicate (n = 6). All data were statistically analyzed (ANOVA) with StatisticaTM for WindowsTM (version 9.0, Statsoft Inc., Tulsa, OK, USA) determining significant differences between means by Turkey’s HSD test (p < 0.05). The mean variability of data was indicated by the standard deviation in the figures.
3. Results and discussion
Processing of T. molitor and H. illucens larvae affected composition, appearance, microbial load as well as techno-functional and protein properties of the recovered insect products. The processability of larvae from both insect species was limited by their high fat contents. Direct processing of frozen or dried larvae into flour was found to be non-practicable due to the thermally induced melting of the contained fat during grinding. In order to avoid any thermal impact during preparation of the flour fractions and to maintain the native properties of the contained proteins, the temperature was kept below 20 °C while performing the procedure described above. Initial experiments indicated that the process route applied was less suitable for achieving the desired process objectives regarding the necessary product properties required for detailed analysis in case of H. illucens. In particular the non-removable fat limited the processability and analysis of the respective flour fractions. For this reason detailed characterization providing reliable results was limited to T. molitor flour fractions.
3.1. Impact of extraction process on yield, composition and color of flour fractions from T. molitor and H. illucens
Composition of T. molitor and H. illucens larvae is shown in Table 1. Containing comparable amounts of water and fat, the protein content in T. molitor larvae was 22.1% higher compared to H. illucens larvae. T. molitor larvae had a dry matter content of 34.9% and contained 53.8% of crude protein and 20.0% of crude fat on a dry basis. Dependent on the growth stage of T. molitor larvae, Ghaly and Alkoaik (2009) reported dry matter contents ranging from 38.5 to 41.9%, crude protein contents between 24.3 and 27.6% and crude fat contents from 12.0 to 12.5% on a fresh weight basis, respectively. The results were comparable to moisture, crude protein and crude fat contents reported by Yi et al. (2013). On a dry basis, the protein and fat contents of the yellow mealworms were in the ranges of 63.3–68.9 and 29.8–31.2%, respectively. With a moisture content of 30%, H. illucens larvae contained 31.7% crude protein and 21.1% crude fat on a dry basis. Compared to findings reported in the literature, protein and fat content were lower. Booram et al. (1976) reported that the H. illucens larvae consisted of 42% crude protein and 35% crude fat whereas Kroeckel et al. (2012) found 54.1 ± 1.1% crude protein and 13.4 ± 0.7% crude fat, respectively. The dry matter contents of the different flour fractions increased with increasing degree of processing. The crude protein content of the T. molitor larvae was increased by 4%, 10.8% and 14.4% in the T-F, the T-DF and the T-HPF. The T-LPF had a residual protein content of 11.2%. Production of H-F and H-DF increased the crude protein content by 3.0 and 13.2%, respectively. Here, the initial fat content of the larvae was reduced by 1.1 and 12.3%. Although insects flours contained nearly the same quantity of fat, defatting of the H-F was less effective.
Table 1.
Means (±sd) of yield, dry matter (DM), crude protein (CP), crude fat content (CF), browning indices (BI), and change in color (ΔE) of larvae from T. molitor and H. illucens and different flour fractions produced from it as well as total viable count (TVC) of the Tenebrio flour fractions. Different letters indicate significant (p < 0.05) differences between means.
| Flour fraction | Yield [%] | DM [g/g] | CP [g/gDM] | CF [g/gDM] | BI [−] | ΔE [−] | TVC [log CFU/g] |
|---|---|---|---|---|---|---|---|
| Tenebrio molitor | |||||||
| Larvae | – | 34.9a (±1.2) | 53.8a (±1.0) | 20.0a (±1.0) | 52.8a (±8.7) | – | 8.1a (±0.1) |
| T-F | 96 | 83.8b (±0.9) | 57.8b (±1.2) | 19.1a (±1.3) | 26.6b (±6.6) | 5.4a (±0.5) | 7.9a (±0.2) |
| T-DF | 83 | 87.5c (±0.4) | 64.6c (±0.3) | 2.8b (±0.3) | 37.0c (±5.7) | 6.7b (±0.3) | 7.0b (±0.5) |
| T-HPF | 22 | 96.5d (±0.2) | 68.2d (±0.3) | 0.4c (±0.0) | 19.0d (±4.2) | 15.0c (±0.6) | 4.3c (±0.2) |
| T-LPF | 21 | 98.9e (±0.1) | 11.2e (±0.2) | 2.2d (±0.3) | 53.7e (±5.6) | 9.1d (±0.2) | 6.2d (±0.1) |
| Hermetia illucens | |||||||
| Larvae | – | 30.0a (±1.2) | 31.7a (±0.5) | 21.1a (±0.7) | 27.8a (± 7.1) | – | – |
| H-F | 82 | 84.1b (±0.7) | 34.7b (±0.2) | 20.0b (±0.8) | 19.2b (± 5.2) | 10.2a (± 0.4) | – |
| H-DF | 73 | 87.0c (±0.3) | 44.9c (±1.4) | 8.8c (±0.1) | 52.8c (± 8.7) | 7.7b (± 0.6) | – |
Processing of the insect larvae further affected visual appearance of the flour fractions produced. Changes in color are summarized in Table 1. In general the T. molitor larvae was darker compared to H. illucens. This is confirmed by the higher browning index of the whole T. molitor larvae. Grinding of the mealworms to non-defatted flour slightly increased the browning index and induced a change in color compared to the unprocessed larvae, whereas the non-defatted flour from H. illucens larvae appeared lighter. Defatting with hexane led to a less brownish color of the flours produced from both insect species. Protein extraction from defatted T-DF, subsequent precipitation, drying and grinding produced the dark brown colored T-HPF and the lighter T-LPF. The color of the different insect flour fractions seems to be related to the protein content, whereas the browning index was found to be dependent on the fat content.
Following aqueous extraction of T. molitor proteins in the pH range from 2 to 12, the protein extracts had a light yellow color in the acidic pH range (2–6), a light brown color at pH 7, and a dark brown color in the alkaline pH range (8–12). In addition, the color of the residue fraction was similar to that of the supernatant fractions. This visual observation indicated that chemical reactions took place during protein extraction under varying conditions. Preliminary experiments showed that color formation was most likely due to enzymatic browning reactions and also depended on the protein concentration of the respective extract.
3.2. Impact on techno-functional properties
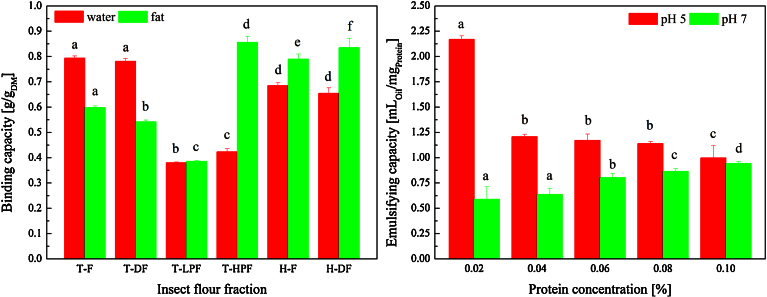
Processing of the T. molitor and H. illucens larvae affected techno-functional properties of the flour fractions produced. WBC and OBC of the flour fractions recovered during protein isolation from T. molitor are depicted in Fig. 2. No significant impact of the defatting step on WBC was observed whereas WBC of the T-HPF and T-LPF were significantly decreased by 0.41 g/gDM and 0.37 g/gDM, respectively. A slight decrease in OBC by 0.05 g/gDM was observed by defatting of the T. molitor flour. The OBC of T-LPF was significantly decreased by 0.21 g/gDM whereas it was increased by 0.26 g/gDM regarding the T-HPF.
Fig. 2.
Water and fat binding capacities (left) on a dry basis [g/gDM] of the T. molitor flour fractions (T-F = T. molitor flour, T-DF = defatted T. molitor flour, T-LPF = low-protein fraction, T-HPF = high-protein fraction) and emulsifying capacities [mLOil/mgProtein] of defined protein solutions prepared from defatted T. molitor flour (right) in dependency of the protein concentration (0.02–0.1%) and the pH (4 and 10) of the aqueous phase. Different letters indicate significant (p < 0.05) differences between means.
In case of H. illucens, defatting of the flour did also not result in significant changes in WBC, whereas OBC was marginally increased by 0.05 g/gDM. Up to now, no comparable research is reported in literature. Yi et al. (2013) investigated foamability, foam stability and gelation of soluble proteins from five insect species and found poor foaming capacities at pH 3, 5, 7, and 10, but the formation of gels at a concentration of 30% w/v with gelation temperature ranging from about 51 to 63 °C for all insect species at pH 7. With regard to food applications, WBC is related with the ability to retain water against gravity, and includes bound water, hydrodynamic water, capillary water and physically entrapped water. The amount of water associated to proteins is closely linked to its amino acids profile, increases with the number of charged residues (Kuntz and Kauzmann, 1974) and strongly depends on protein conformation, hydrophobicity, pH, temperature, ionic strength and protein concentration (Damodaran, 1997).
Emulsion capacity (EC) denotes the maximum amount of oil that can be emulsified under specified conditions by a unit weight of the protein. In this study, the EC of protein solutions prepared from T-DF was investigated under varying protein concentration and pH of the aqueous phase (Fig. 2) and was found to be highly dependent on these two parameters. Emulsification at pH 5 led to an EC of 2.35 mLOil/mgProtein. It decreased with increasing protein concentration. At pH 7 the EC of a 0.02 mg/mL protein solution was significantly lower (0.64 mLOil/mgProtein) and increased to 0.87. Emulsification characteristics of proteins for instance are affected by their surface hydrophobicity as it influences the ability for the protein to adsorb to the oil side of the interface. Greater disintegration typically leads to higher emulsion capacities (Kim et al., 2005). Properties of adsorbed layers at oil-water interfaces has been explained on the basis of a ‘molten globule state’ concept of globular proteins such as α-lactalbumin and β-lactoglobulin. It was found that partially denatured state of globular proteins that retains the secondary structure but not the tertiary structure of the native protein (i.e. increased flexibility of molecules) explains their behavior at an oil-water interface. Some level of partial protein denaturation or a change in molecular charge distribution may lead to the exposure of buried hydrophobic amino acids to the surface. In this case, proteins re-align at the interface in order to position their surface hydrophobic amino acids within the oil phase and hydrophilic amino acids within the aqueous phase. Further, surface charge of the protein influences protein solubility within the aqueous phase. High electrostatic repulsion between oil droplets tends to lead to greater emulsion stability, whereas under pH conditions close to the protein’s isoelectric point (or high ionic strength) droplet flocculation/aggregation may dominate eventually leading to coalescence and instability. Regarding the EC of T. molitor protein this could be an explanation for the higher EC at pH 5 at lower protein concentrations. However, the decreased EC at pH 7 in comparison cannot be explained and needs to be further investigated.
3.3. Protein solubility and structure
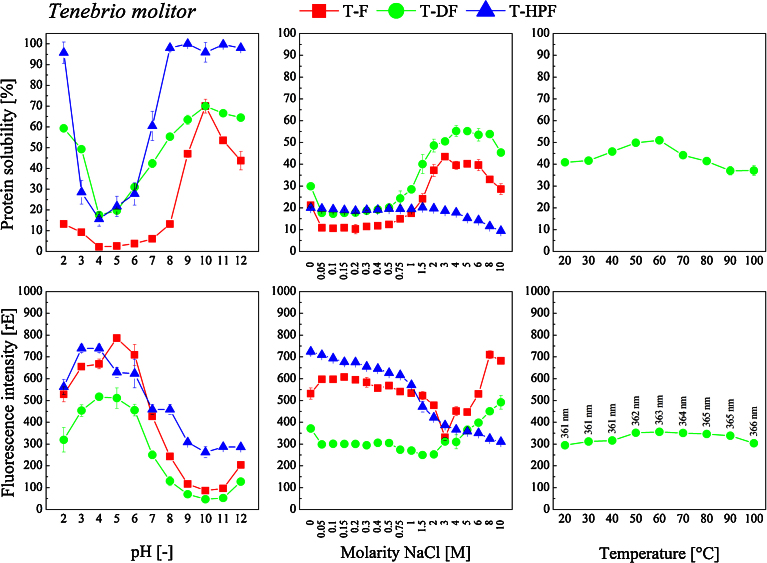
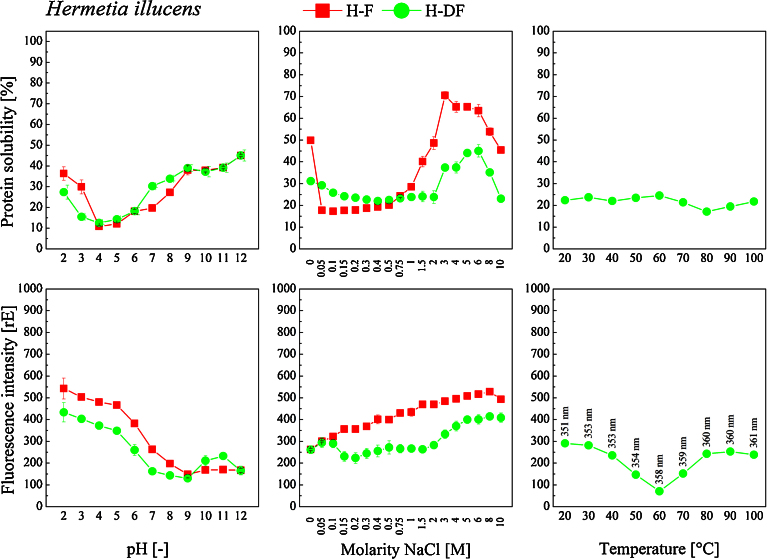
Solubility of the proteins contained in both insect species was found to be highly dependent on the pH during extraction process (Fig. 3 and Fig. 4, top). The insect proteins had their isoelectric point (pI) in the region around pH 4. Regarding T-F, proteins showed highest solubility in the alkaline region at pH 10. Except for this pH, defatting of the flour led to increased protein yields over the entire pH range but especially in the acidic region at pH 2 and 3. Protein yield of H-F was significantly lower. H-F proteins had a higher solubility in the alkaline region at pH 12 and also in the acidic region at pH 2. Hexane extraction of the fat did not lead to increased yields in soluble protein; on the contrary, protein solubility was even decreased at low pH values.
Fig. 3.
Soluble portion (top) and fluorescence intensity (bottom) of proteins extracted from T. molitor flour fractions (T-F: T. molitor flour, T-DF: defatted T. molitor flour, and T-HPF: protein fraction) in dependency of the solvent at different pH (2 to 12, left), ionic strength (0 to 10 M NaCl, center) and temperature (20 to 100 °C, right) during extraction. Protein solubility [%] is presented in relation to the total protein content analyzed via Kjeldahl method. Protein concentrations of the extracts were equalized prior to fluorescence measurement.
Fig. 4.
Soluble portion (top) and fluorescence intensity (bottom) of proteins extracted from H. illucens flour fractions (H-F: H. illucens flour, and H-DF: defatted H. illucens flour) in dependency of the solvent pH (2 to 12, left), ionic strength (0 to 10 M NaCl, center) and temperature (20 to 100 °C, right) during extraction. Protein solubility [%] is presented in relation to the total protein content analyzed via Kjeldahl method. Protein concentrations of the extracts were equalized prior to fluorescence measurement.
For all insect flour fractions the pI was found to be in the region of pH 4. For a great number of proteins, their pI values are in the range of 3.5 and 6.5. At extreme acidic or basic pH values, the protein may unfold exposing more hydrophobic groups. This can be documented by the exposition of the hydrophobic tryptophan residues as measured by the increased fluorescence intensity (Fig. 3 and Fig. 4, bottom). Some insect proteins were reported to have a pI of about 5. For instance, the pI of proteins from silkworm (Bombyx mori) and spider (Nephila edulis) were found to be in the region between pH 4.37–5.05, and 6.47, respectively (Foo et al., 2006). Here, for both insect species the solubility of proteins extracted from the non-defatted flours was slightly reduced by increasing the ionic strength from 0 to 0.4 M (Fig. 3 and Fig. 4, top). Further increasing the molarity of NaCl to 4 and 3 maximized the protein solubility to 55 (T-F) and 70% (H-F), respectively. With regard to the defatted insect flours, solubility curves were similar. Maximum protein solubility of 43% for both insect species was reached at a NaCl molarity of 3 in case of T-DF and of 6 in case of H-DF. In general, defatting led to a reduction in protein solubility. The solubility curve of the T-HPF significantly differed from those obtained for the other flour fractions. For up to a NaCl molarity of 3, no relevant impact on protein solubility was detected, whereas it decreased with further increasing NaCl molarity to 10. Consequently, increasing the ionic strength of the solvent affects the insect protein solubility, but an increase can only be achieved at high salt concentrations.
Increasing the temperature (Fig. 3 and Fig. 4, top) during protein extraction from 20 to 60 °C significantly increased the protein yields by 20 (T-DF) and 10% (H-DF). In general, protein solubility is increased at temperatures between 50 and 60 °C. In case of the insect proteins, elevated extraction temperatures increased their solubility. This may be attributed to weakened interactions between the proteins and other components as for instance fat. In all of the trials, the presence of non-protein impurities needs to be taken into consideration which may limit protein solubility as proteins may form complexes with lipids or nucleic acids that prevent their full solubilization.
Excited at a wavelength of 280nm, tryptophan emits light in the region between 300 and 350 nm- Changes in tryptophan fluorescence can indicate changes of the conformation and three-dimensional structure of proteins as well as the exposure of the hydrophobic amino acid residues (Gießauf et al., 1995; Vivian and Callis, 2001). In this study, fluorescence spectra of the protein extracts recovered from the insect flour fractions were analyzed. Differences in protein fluorescence maxima (and quantum yields) are most likely caused by various ratios of two or more discrete classes of tryptophan residues contained in proteins on the one hand. On the other hand, the extracted proteins have to be considered as a mixture of several protein components. Following Konev (1967), tryptophyls inside the protein in a low-polar hydrophobic microenvironment are characterized by a short wavelength position of the fluorescent maximum (ʎ = 331 nm), while tryptophyls on the surface of a protein in a high-polar aqueous microenvironment are characterized by a large Stokes shift (ʎ = 350 nm). Burstein et al. (1973) also reported that tryptophan residues located at the surface of proteins emit light in higher wavelength regions than those located in the core of proteins. Regarding the recorded insect protein fluorescence spectra (Fig. 3 and Fig. 4, bottom), no fluorescence maximum in the region around 330 nm could be detected. All of the T. molitor protein extracts were characterized by a fluorescence maximum at around 350 nm whereas it was found at 360 nm in case of H. illucens proteins.
Aromatic tryptophan residues are often located in the hydrophobic core of proteins, at the interface between two protein domains/subdomains, or at the subunit interface in oligomeric protein systems and become more exposed to solvent upon disruption of the protein’s tertiary or quaternary structure (Bußler et al., 2015). Exposure of tryptophan surroundings to a more polar environment can be one of the reasons for the observed losses of fluorescence emission. The results of the fluorescence measurements suggest the occurrence of structural changes of the insect proteins under varying extraction conditions. In case of varying the pH during extraction, the increase of tryptophan fluorescence intensity with decreasing solubility of T. molitor and H. illucens proteins is most likely caused by the exposure of hydrophobic residues from the core to the environment of the protein (Fig. 3 and Fig. 4). The two types of binding influenced by pH changes are salt bridges and hydrogen bonding. Whereas an increase in pH leads to the formation of a neutral —NH2 group from —NH3+ ions, a decrease in pH forms neutral —COOH groups from —COO− ions. In both cases the ionic attraction is eliminated, and the protein molecule unfolds explaining the different fluorescence emission spectra. In case of H. illucens protein extracts, a different behavior was observed. Here, a decrease in protein solubility was not accompanied by an increase in fluorescence emission intensity. There are several effects that need to be taken into consideration. Osysko and Muíño (2011) reported measurements of fluorescence quantum yields of tryptophan, tryptophanylaspartate and tryptophanylarginine in aqueous solutions over a wide range of pH, aiming to test the excitation of quenching in tryptophan caused by energy loss due to an electron transfer from the aromatic system of tryptophan to one of the amides in the protein backbone. Low pH conditions result in a net positive charge for the terminal amine, whereas high pH conditions lead to a net negative charge for the terminal carboxyl. Consequently, increasing (decreasing) electron transfer rates and low (high) quantum yields is to be expected, as a low pH will enhance the probability of electron transfer and thus cause a lower quantum yield, whereas a high pH will decrease the probability of electron transfer resulting in larger quantum yields. We observed that high pH results in a high quantum yield which may be caused by the negatively charged carboxyl inducing very low efficiency for the electron transfer. In turn, low pH results in a low quantum yield.
Varying the extraction temperature induced comparable results regarding the relation between protein solubility and fluorescence intensity. Here again, the increase in T. molitor protein solubility was accompanied by a decrease in fluorescence emission intensity (Fig. 3) whereas this correlation was not observed in case of H. illucens protein (Fig. 4). But the maximum emission wavelength shifted to higher values with increasing temperature. This shift was more pronounced in T. molitor protein extracts (from 351 to 361 nm) and less in H. illucens protein extracts (from 361 to 366 nm).
3.4. Protein composition
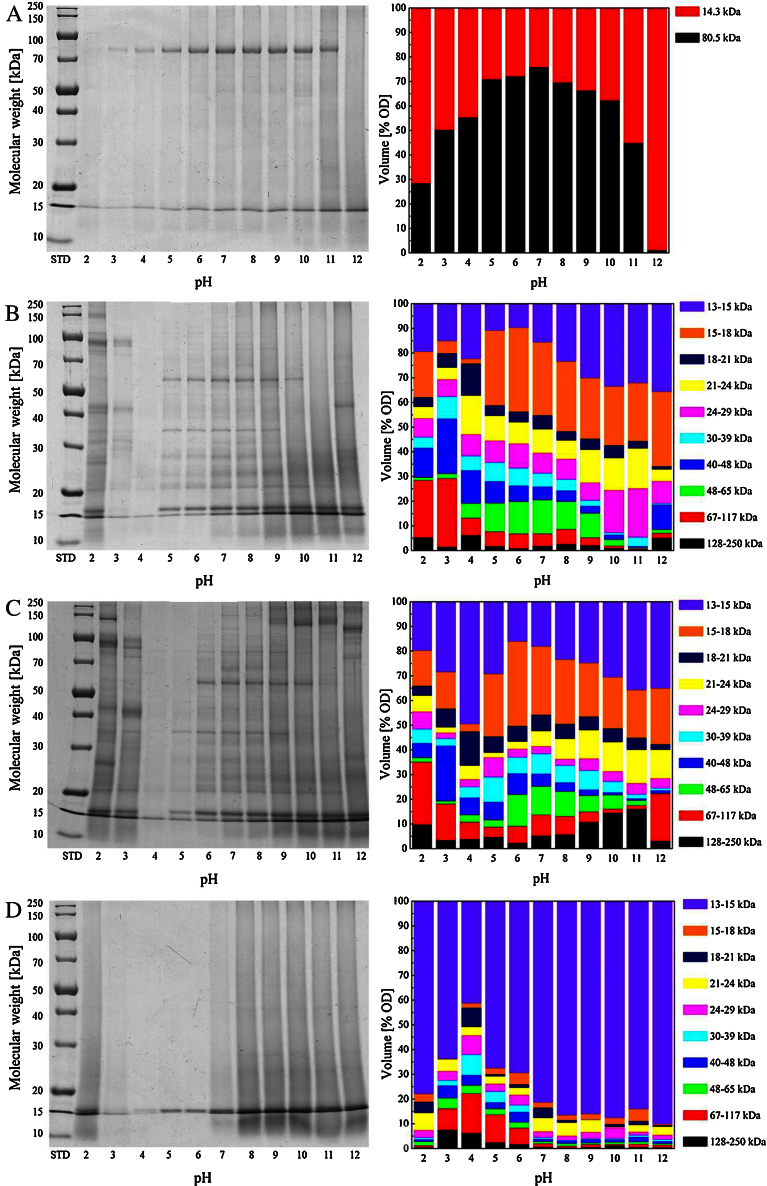
Separation of the T. molitor and H. illucens proteins via SDS-PAGE using 12% T gels resulted in wide ranges of protein bands (Fig. 5A to D). Quantitative evaluation regarding the protein solubility confirms the results obtained by the Biuret assay depicted in Fig. 3 and Fig. 4. In protein extracts recovered from defatted H-F, two major bands characterized by molecular weights of 14.3 kDa and 80.5 kDa, were dominant (Fig. 5A). At pH 7 the protein extract was composed of 75.9% high molecular weight (HMW) fraction and 24.1% low molecular weight (LMW) fraction. Decreasing the pH to 2 led to a gradual increase in the LMW fraction for up to 71.5% whereas increasing the pH to 12 increased it to 98.9%. It is probable that defatting with hexane in combination with extreme pH conditions during protein extraction led to a partial or almost complete proteolysis of the 80.5 kDa band to 14.3 kDa or even lower.
Fig. 5.
Electrophoretic separation (left) and relative composition (right) of soluble T. molitor and H. illucens protein fractions (H-DF: defatted H. illucens flour (A), T-F: T. molitor flour (B), T-DF: defatted T. molitor flour (C), and T-HPF: T. molitor high protein fraction (D)) depending on the pH value of the extraction solution, n = 3.
With regard to T. molitor flour fractions, ten major groups of protein bands could be distinguished (Fig. 5B to D), namely bands 13–15 kDa, 15–18 kDa, 18–21 kDa, 21–24 kDa, 24–29 kDa, 30–39 kDa, 40–48 kDa, 48–65 kDa, 67–117 kDa and 128–250 kDa. The percentage distribution of the protein bands in the aqueous extracts varied upon flour fraction used and extraction conditions applied. As already described by Bußler et al. (2016), the bands observed in the range between 14 and 32 kDa could possibly originate from cuticle proteins with molecular weights predominantly between 14 and 30 kDa (Andersen et al., 1995) or chymotrypsin-like proteinase (24 kDa) (Elpidina et al., 2005), whereas the bands observed ranging from 32 to 95 kDa could possibly originate from enzymes and other proteins, e.g. melanization-inhibiting protein (43 kDa), β-glycosidase (59 kDa), trypsin-like proteinases (59 kDa), and melanization-engaging types of protein (85 kDa) (Cho et al., 1999; Ferreira et al., 2001; Prabhakar et al., 2007; Zhao et al., 2005). The bands with molecular weight >95 kDa could possibly be linked to vitellogenin-like protein with a molecular weight of 160 kDa (Lee et al., 2000). With respect to the protein fractions extracted from T-F mainly the proportions of high- and low-molecular fractions were affected by alteration of the solvent pH. Protein fractions characterized by high molecular weights in the range of 67–250 kDa were found to dominate the protein extracts at pH 2 and 3, as they accounted for almost 30% of the total soluble proteins. Adjusting the pH to 2 and 3 increased the solubility of protein fractions in the range between 40 and 250 kDa whereas the amount of the 48–65 kDa and 13–18 kDa fractions increased with pH values from 5 to 8. Furthermore the proportion of LMW fractions in the range of 13–29 kDa increased with alkalization of the solvent pH to 12. Defatting of the T-F significantly affected the protein composition over the entire pH range (Fig. 5C). The percentage proportion of LMW fractions ranging from 13 to 18 kDa was slightly decreased by 4% at pH 2 whereas it was more than doubled (increase from 20 to 43% and from 24.5 to 52.5%) at pH 3 and 4, respectively. In the pH range from 5 to 12 the amount of this protein fraction varied between 41 and 66% prior to and between 48 and 58% following defatting with hexane. Except for the extracts recovered at pH 3 and 4, the proportion of the HMW fraction with a molecular weight in the range between 67 and 250 kDa was higher in the extracts recovered from T-DF. This may be attributed to protein agglomerates formed. The relatively high amounts of the protein fraction with molecular weights ranging from 24 to 29 kDa which were between 17.3 and 19.8% at pH 10 and 11 in the T-F were reduced to 4.2 to 4.5% upon defatting of the flour.
Isolation of the T. molitor proteins from T-DF at pH 10 and 2 completely changed the protein composition over the entire pH range (Fig. 5D). The LMW fractions with molecular weights from 13 to 15 kDa accounted for the largest share of soluble T. molitor proteins. At pH 12 90.1% of the proteins were found to have a molecular weight in this range. At pH 2 and between pH 7 and 10 the percentage proportion ranged from 77.9% to 86.5% and was reduced at pH 3 (63.7%), 5 (67.5%) and 6 (68.4%). In the pH range of lowest protein solubility (pH 4) the percentage proportion amounted to 41.2%. However, the proportion of HMW fractions (67–250 kDa), which was infinitesimally low at pH 2 due to acid hydrolysis and in the pH range between 7 and 12, amounted to 22.3%. Furthermore, this protein fraction was contained in relevant amounts at pH 3 (16.2%), 5 (13.8%) and 6 (8.4%). The high amount of LMW fractions may be attributed to the proteolytic degradation of proteins during isolation which may be triggered by intrinsic enzymes of the mealworms or of microorganisms.
3.5. Microbial safety
The T. molitor larvae were highly contaminated with microorganisms (Table 1). Pureeing, freeze-drying and grinding of the larvae at low temperatures in order to produce the T-F led to an insignificant log-reduction of the total viable count (TVC) by 0.1 (±0.1). Hexane extraction of the fat significantly reduced the TVC by 1.1 (±0.5) log cycles. The most effective process step in decreasing the microbial load was the preparation of the T-HPF via aqueous extraction at pH 10 and 2, precipitation of the soluble proteins at pH 4, freeze drying and grinding. Compared to whole T. molitor larvae, the TVC of the T-HPF was significantly reduced by 3.8 (±0.0) log cycles. Regarding the low-protein fraction, the TVC was found to be quite high with a log-reduction of 1.9 (±0.0) compared to the raw material. As expected, the process applied is not appropriate for the production of microbially safe incest flour fractions. Extreme pH conditions applied did apparently inactivate microorganisms, only less effectively. For the production of microbial safe insect flours fractions, the application of effective inactivation processes as reported by Rumpold et al. (2014) will be necessary and needs to be studied extensively.
4. Conclusion
The results of this study indicate that edible insects as T. molitor and H. illucens can be utilized to prepare protein-rich intermediates to be used in the production of food and feed. Despite the solubility characteristics, which were shown to be specific for the insect species used, required processing parameters seem to be similar to those of plant proteins enabling the usage of traditional methods on protein processing. In case of T. molitor, T-F was found to be highly soluble at alkaline pH values. The proteins contained in T-DF and T-HPF were also highly soluble at pH 2. Defatting (T-DF) and isoelectric precipitation (T-HPF) increased the concentration of T. molitor proteins by 11 and 15%, respectively.
These results further indicate that it is advisable to prepare fat-reduced and protein enriched fractions such as T-DF and H-DF. Further research is required to address bioavailability issues especially with regard to the distribution of amino acid profiles and bioavailability of essential amino acids. The results further indicate that the techno-functional properties can be effectively manipulated, but further research is needed to identify specific tools for tailoring them. It appears that protein composition of the samples may influence the functional properties. Finally, the potential of by-products, as for instance T-LPF and the extracted insect fat providing further options for functionalized added-value products needs to be taken into consideration. Alternative methods could be used for fat removal, thereby omitting environmentally unfriendly use of organic solvents.
Also with respect to microbial safety issues, a combination of several physical separation methods or the application of different thermal treatments may be more suitable techniques for preparing protein rich intermediates rather than extensive isolation procedures. The results obtained in this study clearly underline the importance of tailored process design, especially of the defatting step, when exploiting insects as an alternative protein source and therefore represent an important step towards the development of sustainable and microbiologically safe rearing, harvest and post-harvest processing technologies as well as protein recovery procedures to ensure high food and feed quality.
Declarations
Author contribution statement
Sara Bußler: Conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; wrote the paper.
Birgit Rumpold: Conceived and designed the experiments; analyzed and interpreted the data; wrote the paper.
Elisabeth Jander, Harshadrai M. Rawel: Performed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data.
Oliver Schlueter: Conceived and designed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Additional information
No additional information is available for this paper.
Acknowledgements
Lucie Guérin from ONIRIS, College of Veterinary Medicine, Food Science & Engineering in Nantes, France is gratefully acknowledged for supporting experimental trails of this study during her internship at the Leibniz-Institute for Agricultural Engineering Potsdam-Bornim e.V.
References
- Andersen S.O., Rafn K., Krogh T.N., Hojrup P., Roepstorff P. Comparison of larval and pupal cuticular proteins in Tenebrio molitor. Insect Biochem. Mol. Biol. 1995;25(2):177–187. doi: 10.1016/0965-1748(94)00048-m. [DOI] [PubMed] [Google Scholar]
- American Oil Chemists’ Society . American Oil Chemists’ Society; Champaign, IL: 1998. Official methods and recommended practices of the AOCS. [Google Scholar]
- American Oil Chemists’ Society . American Oil Chemists’ Society; Champaign, IL: 2005. Official methods and recommended practices of the AOCS. [Google Scholar]
- Balzan S., Fasolato L., Maniero S., Novelli E. Edible insects and young adults in a north-east Italian city an exploratory study. Br. Food J. 2016;118(2):318–326. [Google Scholar]
- Barroso F.G., de Haro C., Sánchez-Muros M.-J., Venegas E., Martínez-Sánchez A., Pérez-Bañón C. The potential of various insect species for use as food for fish. Aquaculture. 2014;422–423:193–201. [Google Scholar]
- Booram C.V., Norton G.L., Baker R.W., Hale O.M. vol. 75. ASAE Technical Paper; St. Joseph, Illonois: 1976. (The value of Hermetia illucens as an alternative protein source). [Google Scholar]
- Burstein E.A., Vedenkina N.S., Ivkova M.N. Fluorescence and the location of tryptophan residues in protein molecules. Photochem. Photobiol. 1973;18(4):263–279. doi: 10.1111/j.1751-1097.1973.tb06422.x. [DOI] [PubMed] [Google Scholar]
- Bußler S., Steins V., Ehlbeck J., Schlüter O. Impact of thermal treatment versus cold atmospheric plasma processing on the techno-functional protein properties from Pisum sativum ‘Salamanca’. J. Food Eng. 2015;167(Part B):166–174. [Google Scholar]
- Bußler S., Rumpold B.A., Fröhling A., Jander E., Rawel H.M., Schlüter O.K. Cold atmospheric pressure plasma processing of insect flour from Tenebrio molitor: Impact on microbial load and quality attributes in comparison to dry heat treatment. Innov. Food Sci. & Emerg. Technol. 2016;36:277–286. [Google Scholar]
- Cho M.Y., Choi H.W., Moon G.Y., Kim M.H., Kwon T.H., Homma K.-i. An 86 kDa diapause protein 1-like protein is a component of early-staged encapsulation-relating proteins in coleopteran insect, Tenebrio molitor larvae 1. FEBS Lett. 1999;451(3):303–307. doi: 10.1016/s0014-5793(99)00608-0. [DOI] [PubMed] [Google Scholar]
- Cilia M., Fish T., Yang X., McLaughlin M., Thannhauser T.W., Gray S. A comparison of protein extraction methods suitable for gel-based proteomic studies of aphid proteins. J. Biomol. Tech. 2009;20(4):201–215. [PMC free article] [PubMed] [Google Scholar]
- Damodaran S. Food proteins: an overview. In: Damodaran S., Paraf A., editors. Food Proteins and Their Applications. Marcel Dekker; New York: 1997. pp. 57–100. [Google Scholar]
- Del Valle F.R., Mena M.H., Bourges H. An investigation into insect protein. J. Food Process. Preserv. 1982;6(2):99–110. [Google Scholar]
- Durst P., Johnson D., Leslie R., Shono K. FAO, Regional Office for Asia and the Pacific; Chiang Mai, Thailand: 2010. Forest Insects as Food: Humans Bite Back. Proceedings of a Workshop on Asia-pacific Resources and Their Potential for Development 19–21 February 2008. [Google Scholar]
- EFSA Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015;13(10):4257. [Google Scholar]
- Elpidina E.N., Tsybina T.A., Dunaevsky Y.E., Belozersky M.A., Zhuzhikov D.P., Oppert B. A chymotrypsin-like proteinase from the midgut of Tenebrio molitor larvae. Biochimie. 2005;87(8):771–779. doi: 10.1016/j.biochi.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Ferreira A.H.P., Marana S.R., Terra W.R., Clélia F. Purification molecular cloning, and properties of a β-glycosidase isolated from midgut lumen of Tenebrio molitor (Coleoptera) larvae. Insect Biochem. Mol. Biol. 2001;31(11):1065–1076. doi: 10.1016/s0965-1748(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Foo C.W.P., Bini E., Hensmann J., Knight D.P., Lewis R.V., Kaplan D.L. Role of pH and charge on silk protein assembly in insects and spiders. Appl. Phys. A. 2006;82:223–233. [Google Scholar]
- Ghaly A.E., Alkoaik F.N. The yellow mealworm as a novel source of protein. Am. J. Agric. Biol. Sci. 2009;4(4):319–331. [Google Scholar]
- Gießauf A., Steiner E., Esterbauer H. Early destruction of tryptophan residues of apolipoprotein B is a vitamin E-independent process during copper-mediated oxidation of LDL. Biochim. Biophys. Acta. 1995;1256:221–232. doi: 10.1016/0005-2760(95)00024-7. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Decker E.A., McClements D.J. Influence of protein concentration and order of addition on thermal stability of beta-lactoglobulin stabilized n-hexadecane oil-in-water emulsions at neutral pH. Langmuir. 2005;21(1):134–139. doi: 10.1021/la048019t. [DOI] [PubMed] [Google Scholar]
- Konev S.V. Plenum; New York: 1967. Fluorescence and Phosphorescence of Proteins and Nucleic Acids. [Google Scholar]
- Kroeckel S., Harjes A.G.E., Roth I., Katz H., Wuertz S., Susenbeth A. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima) Aquaculture. 2012;364–365:345–352. [Google Scholar]
- Kuntz I.D., Jr, Kauzmann W. Hydration of proteins and polypeptides. In: Anfinsen J.T.E.C.B., Frederic M.R., editors. Vol. 28. Academic Press; 1974. pp. 239–345. (Advances in Protein Chemistry). [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. [10.1038/227680a0] Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K.M., Lee K.Y., Choi H.W., Cho M.Y., Kwon T.H., Kawabata S.-i. Activated phenoloxidase from Tenebrio molitor larvae enhances the synthesis of melanin by using a vitellogenin-like protein in the presence of dopamine. Eur. J. Biochem. 2000;267(12):3695–3703. doi: 10.1046/j.1432-1327.2000.01402.x. [DOI] [PubMed] [Google Scholar]
- Makkar H.P.S., Tran G., Heuzé V., Ankers P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014;197:1–33. [Google Scholar]
- Mariod A., Abdelwahab S., Ibrahim M., Mohan S., Elgadir M.A., Ain N. Preparation and characterization of gelatins from two sudanese edible insects. J. Food Sci. Eng. 2011;1(1):45. [Google Scholar]
- Mariod A.A. Insect oil and protein: biochemistry: food and other uses: review. Agric. Sci. 2013;4(9B):76–80. [Google Scholar]
- Nowak V., Persijn D., Rittenschober D., Charrondiere U.R. Review of food composition data for edible insects. Food Chem. 2016;193:39–46. doi: 10.1016/j.foodchem.2014.10.114. [DOI] [PubMed] [Google Scholar]
- Osysko A.P., Muíño P.L. Fluorescence quenching of tryptophan and tryptophanyl dipeptides in solution. J. Biophys. Chem. 2011;2(3):316–321. [Google Scholar]
- Prabhakar S., Chen M.S., Elpidina E.N., Vinokurov K.S., Smith C.M., Marshall J. Sequence analysis and molecular characterization of larval midgut cDNA transcripts encoding peptidases from the yellow mealworm, Tenebrio molitor L. Insect Mol. Biol. 2007;16(4):455–468. doi: 10.1111/j.1365-2583.2007.00740.x. [DOI] [PubMed] [Google Scholar]
- Quinn J.R., Paton D. A practical measurement of water hydration capacity of protein materials. Cereal Chem. 1979;56(1):38–40. [Google Scholar]
- Reinkensmeier A., Bußler S., Schlüter O., Rohn S., Rawel H.M. Characterization of individual proteins in pea protein isolates and air classified samples. Food Res. Int. 2015;76(Part 1):160–167. [Google Scholar]
- Robinson H.W., Hogden C.G. The biuret reaction in the determination of serum proteins. J. Biol. Chem. 1940;135:707–725. [Google Scholar]
- Rumpold B.A., Fröhling A., Reineke K., Knorr D., Boguslawski S., Ehlbeck J. Comparison of volumetric and surface decontamination techniques for innovative processing of mealworm larvae (Tenebrio molitor) Innovative Food Sci. Emerg. Technol. 2014;26:232–241. [Google Scholar]
- Rumpold B.A., Schlüter O.K. Potential and challenges of insects as an innovative source for food and feed production. Innovative Food Sci. Emerg. Technol. 2013;17:1–11. [Google Scholar]
- Sánchez-Muros M.-J., Barroso F.G., Manzano-Agugliaro F. Insect meal as renewable source of food for animal feeding: a review. J. Cleaner Prod. 2014;65:16–27. [Google Scholar]
- Schösler H., Boer J.d., Boersema J.J. Can we cut out the meat of the dish? Constructing consumer-oriented pathways towards meat substitution. Appetite. 2012;58(1):39–47. doi: 10.1016/j.appet.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Schwenke K.D., Prahl L., Rauschal E., Gwiazda S., Dąbrowski K., Rutkowski A. Functional properties of plant proteins. Part 2. Selected physicochemical properties of native and denatured protein isolates from faba beans, soybeans, and sunflower seed. Food/Nahrung. 1981;25(1):59–69. [Google Scholar]
- Smith A.K., Circle S.J. In: Smith A.K.: Chemical composition of the seed. Circle S.J., editor. AVI PUB; 1978. [Google Scholar]
- Smith A.K., Circle S.J., editors. Chemical composition of the seed. The Avi Publishing Company, Inc; Westport, Connecticut, USA: 1978. [Google Scholar]
- Van Huis A., Itterbeeck J., Klunder H., Mertens E., Halloran A., Muir G. FAO Forestry Paper 171. 2013. Edible insects: future prospects for food and feed security. [Google Scholar]
- VDLUFA . VDLUFA Methodenbuch III. In: VDLUFA-Verlag, editor. vol. 3. VDLUFA-Verlag; Bonn: 1976. p. 2190. (Band III Die chemische Untersuchung von Futtermitteln). [Google Scholar]
- Vivian J.T., Callis P.R. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys. J. 2001;80(5):2093–2109. doi: 10.1016/S0006-3495(01)76183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Lakemond C.M.M., Sagis L.M.C., Eisner-Schadler V., van Huis A., van Boekel M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013;141(4):3341–3348. doi: 10.1016/j.foodchem.2013.05.115. [DOI] [PubMed] [Google Scholar]
- Zhao M., Söderhäll I., Park J.W., Ma Y.G., Osaki T., Ha N.C. A novel 43-kDa protein as a negative regulatory component of phenoloxidase-induced melanin synthesis. J. Biol. Chem. 2005;280(26):24744–24751. doi: 10.1074/jbc.M504173200. [DOI] [PubMed] [Google Scholar]